Abstract
Aims/Introduction
To investigate the ability of human amniotic fluid stem cells (hAFSCs) to differentiate into insulin‐producing cells.
Materials and Methods
hAFSCs were induced to differentiate into pancreatic cells by a multistep protocol. The expressions of pancreas‐related genes and proteins, including pancreatic and duodenal homeobox‐1, insulin, and glucose transporter 2, were detected by polymerase chain reaction and immunofluorescence. Insulin secreted from differentiated cells was tested by enzyme‐linked immunosorbent assay.
Results
hAFSCs were successfully isolated from amniotic fluid that expressed the pluripotent markers of embryonic stem cells, such as Oct3/4, and mesenchymal stem cells, such as integrin β‐1 and ecto‐5′‐nucleotidase. Here, we first obtained the hAFSCs that expressed pluripotent marker stage‐specific embryonic antigen 1. Real‐time polymerase chain reaction analysis showed that pancreatic and duodenal homeobox‐1, paired box gene 4 and paired box gene 6 were expressed in the early phase of induction, and then stably expressed in the differentiated cells. The pancreas‐related genes, such as insulin, glucokinase, glucose transporter 2 and Nkx6.1, were expressed in the differentiated cells. Immunofluorescence showed that these differentiated cells co‐expressed insulin, C‐peptide, and pancreatic and duodenal homeobox‐1. Insulin was released in response to glucose stimulation in a manner similar to that of adult human islets.
Conclusions
The present study showed that hAFSCs, under selective culture conditions, could differentiate into islet‐like insulin‐producing cells, which might be used as a potential source for transplantation in patients with type 1 diabetes mellitus.
Keywords: Amniotic fluid‐derived stem cells, Diabetes mellitus, Insulin‐producing cells
Introduction
Multipotent CD117+ stem cells isolated from amniotic fluid share many common features with both embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs)1, 2, 3, 4. Although CD117+ cells make up <1% of total cells isolated from the amniotic fluid, these cells are highly capable of proliferating in vitro, even in the absence of feeder cells. Human amniotic fluid stem cells (hAFSCs) can amplify for more than 300 generations and still maintain the stability of karyotypes3. hAFSCs are also able to differentiate into cells of all three germ layers in vitro without forming teratomas in vivo. MSCs isolated from bone marrow usually begin to differentiate at approximately the 30th generation in vitro, whereas hAFSCs still maintain a high proliferation rate at the same generation5. Meanwhile, the telomere length is longer, and the chromosomes are more stable in hAFSCs compared with MSCs at the same generation5. hAFSCs are relatively easy to access with few ethical issues. Their high genetic stability offers advantages of low tumorigenicity and low immunogenic activity. Therefore, hAFSCs could be used as a source for multipotent stem cells.
Type 1 diabetes mellitus is an autoimmune disease characterized by destruction of the islets β‐cells of Langerhans in the endocrine pancreas6. Stem cells, including hAFSCs, could serve as an appropriate cell source to generate new β‐cells. Recent studies have shown that insulin‐secreting cells can be generated from ESCs7, 8, 9, 10, induced pluripotent stem cells11 or adult stem cells12, 13, 14. However, these cells have low differentiation rates, are more likely to form teratomas and might be subject to immune rejection15, 16. Recent studies have also shown that hAFSCs can differentiate into adipocytes, osteoblasts, chondrocytes and neural‐like cells in vitro 17, 18, 19. Zou et al.20 found that small interfering ribonucleic acid pancreatic and duodenal homeobox‐1 (Pdx‐1)‐transfected CD44+/CD105+ human amniotic fluid cells could not fully differentiate into β‐cell‐like cells, suggesting that Pdx‐1 played an important role in the induction of CD44+/CD105+ human amniotic fluid cells into pancreatic β‐cell‐like cells in vitro. Recent evidence has shown that hAFSCs can be modified to a β‐cell phenotype by overexpression of pancreatic transcription factors, such as Pdx‐1 21. In addition, Gage et al.22 recently showed that overexpression of six different transcription factors, Pdx‐1, neurogenin‐3 (Ngn3), V‐maf musculoaponeurotic fibrosarcoma oncogene homolog A (Mafa), paired box gene 6 (Pax6), neurogenic differentiation (NeuroD) and insulin gene enhancer protein 1 (Isl‐1), from adenoviral vectors could reprogram human amniotic fluid cells in vitro towards a β‐cell phenotype. However, these induced β‐cells mainly relied on the expression of exogenous genes through a viral genomic reprogramming approach, and the expression of insulin could not be considerably increased in vitro. Trovato et al.23 showed that under experimental conditions, used in their study, cultured hAFSCs failed to differentiate into β‐cells. Thus, an efficient strategy to direct hAFSCs differentiation into insulin‐producing cells, improve differentiation efficiency and promote maturation of insulin‐producing cells is urgently required.
Recently, we showed that human adipose tissue‐derived stromal cells could differentiate into insulin‐producing cells through a non‐viral genomic reprogramming approach24. Based on this, we further investigated whether this protocol was sufficient to promote hAFSCs differentiation into insulin‐producing cells, and promote maturation of differentiated insulin‐producing cells. Carnevale et al.17 recently showed that hAFSCs and dental pulp stem cells could differentiate into insulin‐producing cells with a multistep method under appropriate stimuli, such as trans‐retinoic acid, zinc sulphate and selenium. In our study, we found that hAFSCs could also differentiate into insulin‐producing cells with a different method in response to appropriate stimuli, such as epidermal growth factor (EGF), basic fibroblast growth factor (bFGF) and exendin‐4. In conclusion, we developed a multistep method, using a non‐viral genomic reprogramming approach, to direct differentiation of hAFSCs into insulin‐producing cells.
Materials and Methods
Cell culture and phenotype analysis of hAFSCs
hAFSCs were isolated from the amniotic fluid as previously described by De Coppi et al.3 Samples of amniotic fluid were collected by amniocentesis during routine prenatal diagnosis for female patients at the Second Affiliated Hospital of Jilin University, Changchun, China. Written consent was obtained from all participants. This study was approved by the research ethics committee of Jilin University.
Samples were centrifuged at 200 g, then the pellets were resuspended and seeded in uncoated T75 culture flasks at a concentration of 1 × 106 cells/mL in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco, Gaithersburg, MD, USA). After 2 days, the plates were washed with phosphate buffered saline (PBS) to remove the non‐adherent cells, and the adherent cells were cultivated in a preconfluence condition. The medium was then replaced every third day. Cells were passaged at 1:3 every 4–5 days on reaching 80% confluence, and then prepared for use in the following experiments.
The phenotypes of hAFSCs were analyzed using flow cytometry. The cells were incubated with antibodies for CD105, CD29, CD117, CD73, CD166, CD34, OCT3/4, CD45, CD40, CD80, CD86, SSEA‐1 and human leukocyte antigen‐D related (eBioscience, San Diego, CA, USA) conjugated to either fluorescein isothiocyanate or phycoerythrin. Mouse immunoglobulin G conjugated to fluorescein isothiocyanate or phycoerythrin was used as the negative control. Flow cytometric data were acquired using a BD FACSCalibur and analyzed using CellQuest Software (BD, San Jose, CA, USA).
Multilineage differentiation of hAFSCs
For differentiation into adipogenic and osteogenic cells, hAFSCs were plated at a density of 1 × 104 cells/mL in a six‐well plate. At 80% confluence, the cells were cultured in adipogenic or osteogenic inducing medium for 2 weeks. The adipogenic medium contained DMEM, 10% FBS, 5 × 10−4 mmol/L isobutyl‐methylxanthine, 1 × 10−6 mol/L dexamethasone, 1 × 10−5 mol/L insulin and 2 × 10−4 mol/L indomethacin, and the osteogenic medium contained DMEM, 10% FBS, 0.1 μmol/L dexamethasone, 5 × 10−5 mol/L ascorbate‐2‐phosphate and 0.2 mol/L glycerophosphate. The medium was changed every third day. Adipogenic differentiation was assessed using an Oil Red O stain, whereas the osteogenic differentiation was confirmed by Alizarin Red staining.
To induce neurogenic differentiation, the cells were plated at a density of 5 × 103 cells/mL in a 24‐well plate. The induced medium contained insulin–transferrin–selenium, 10 ng/mL bFGF (Promega, Madison, WI, USA) and 20 ng/mL brain‐derived neurotrophic factor (BDNF; Sigma, St. Louis, MO, USA). Immunocytochemistry was used to detect the expression of neurone‐specific enolase after 2 days.
A three‐stage culture strategy was used to induce hAFSCs differentiation into insulin‐producing cells. Briefly, cells in the logarithmic growth phase of the third passage were digested with trypsin, then seeded at 5 × 104 cells/mL on poly‐lysine‐coated six‐well plates for 3 days in low‐glucose DMEM (5.5 × 10−3 mol/L glucose) culture medium with 10% FBS. At stage 2, cells were cultured in high‐glucose DMEM (2.5 × 10−2 mol/L glucose), supplemented with 5% FBS, 1 × 10−2 mol/L nicotinamide (Sigma‐Aldrich, St. Louis, MO, USA) and 20 ng/mL bFGF for 7 days. At stage 3, for maturation, the cells were cultured in low‐glucose DMEM with 5% FBS, 20 ng/mL EGF (Invitrogen), 1 × 10−2 mol/L nicotinamide and 50 ng/mL exendin‐4 for 2 weeks. The medium was changed every 3 days thereafter. Cells cultured in medium without inducers were used as controls. All media were from Gibco BRL.
Reverse transcription polymerase chain reaction analysis
Total ribonucleic acid was isolated from undifferentiated hAFSCs and from cells in stages 1–3 using Trizol (Invitrogen). Single‐stranded complementary deoxyribonucleic acid was synthesized from 1 μg of total ribonucleic acid using reverse transcriptase (Takara, Dalian, China) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was carried out using Taq deoxyribonucleic acid polymerase (Takara). PCR products were electrophoresed in 1% agarose gels. The primer sequences and the lengths of the products are shown in Table 1. All primers were synthesized using Shanghai Sangon Biological Engineering Technology (Shanghai, China).
Table 1.
Polymerase chain reaction primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | accacagtccatgccatcac | tccaccaccctgttgctgta |
| Insulin | agcctttgtgaaccaacacc | gctggtagagggagcagatg |
| Pdx‐1 | gatgaagtctaccaaagctcacgc | gatgaagtctaccaaagctcacgc |
| Glut2 | accctggttttcactgtcatcactg | gctttgattcttccaagtgtgtcc |
| Glucokinase | aagaaggtgatgagacggatgc | catctggtgtttggtcttcacg |
| Nestin | caagaaccactggggtctgt | tcccacctctgttgacttcc |
| Ngn3 | ggtagaaaggatgacgcctc | ccgagttgaggtcgtgcat |
| NeuroD | gaacgcagaggaggactcac | gtggaagacatgggagctgt |
| Nkx6.1 | gttcctcctcctcctcttcctc | aagatctgctgtccggaaaaag |
| Pax6 | ccgagagtagcgactccag | cttccggtctgcccgttc |
| Pax4 | gtgggcagtatcctgattcagt | tgtcactcagacacctttctgg |
GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; Glut2, glucose transporter 2; NeuroD, neurogenic differentiation; Ngn3, neurogenin‐3; Nkx6.1, NK6 homeobox 1; Pax, paired box; Pdx‐1, pancreatic and duodenal homeobox‐1.
Quantitative real‐time PCR was carried out using the default thermocycler program for all genes: 3 min of pre‐incubation at 94°C, followed by 40 cycles for 30 s at 94°C, 20 s at 60°C and 45 s at 72°C. Individual real‐time PCR reactions were carried out in 20‐μL volumes in a 96‐well plate (Applied Biosystems, London, UK) containing 6 μL diethylpyrocarbonate water, 1 μL of sense and antisense primers (1 × 10−5 mol/L), and 10 μL SYBR Green with ROX plus 2 μL of sample. Each experiment was repeated in triplicate, and quantitative PCR analysis was carried out in triplicate. A homogenized sample from human fetal intestine was used as a positive control. The primer sequences and the lengths of the products are shown in Table 2.
Table 2.
Real‐time polymerase chain reaction primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | agaaggctggggctcatttg | aggggccatccacagtcttc |
| Pdx‐1 | tgaagtctaccaaagctcacg | tcttgatgtgtctctcggtca |
| Insulin | ctacctagtgtgcggggaac | agctggtagagggagcagatg |
| Glut2 | tgcccacaatctcatactcaa | tacagacagggaccagagcat |
| Pax6 | tgtccaacggatgtgtgagta | tcccgcttatactgggctatt |
GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; Glut2, glucose transporter 2; Pax, paired box; Pdx‐1, pancreatic and duodenal homeobox‐1.
Immunofluorescence
The undifferentiated and differentiated cells were fixed for 20 min with 4% paraformaldehyde in PBS at room temperature, washed three times with PBS, and then permeabilized with 0.1% triton X‐100 for 5 min and 5% bovine serum albumin (to block non‐specific binding) for 30 min. Cells were incubated overnight at 4°C with primary antibodies, including rabbit anti‐insulin, rabbit anti‐C‐peptide, rabbit anti‐glucagon (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti‐insulin (Boster, Wuhan, China) and mouse anti‐Pdx‐1 (Abcam, Cambridge, UK) antibodies. Cells were then washed with PBS, and incubated with corresponding secondary antibodies (fluorescein isothiocyanate or Cy3‐conjugated goat anti‐rabbit or anti‐mouse immunoglobulin G antibodies) for 30 min at 37°C; 4, 6‐diamidino‐2‐phenylindole was used for nucleus staining.
C‐peptide release assay by enzyme‐linked immunosorbent assay
Secretion of insulin/C‐peptide in culture supernatants was measured using the commercial ELISA Kit (Linco, Norcross, MO, USA) according to the manufacturer's instructions. Briefly, the differentiated cells were washed thoroughly three times with PBS containing 2 mg/mL bovine serum albumin, and then incubated with 2.5 mmol/L glucose or 27.5 mmol/l glucose for 2 h. Cell culture supernatant was collected to measure the secreted insulin/C‐peptide. Media from the undifferentiated cells were used as the control. These experiments were carried out in triplicate. Reactivity was measured at 450 nm, using a plate reader (BioTek, Winooski, VT, USA). Values were converted to C‐peptide concentrations using references supplied by the manufacturer.
Statistical analysis
Data are presented as mean ± standard deviation. The Student's t‐test was used for between two‐group analyses. One‐way analysis of variance (anova) was used to compare data among three or more groups. Differences with a P‐value of <0.05 were considered statistically significant.
Results
Characterization of hAFSCs
hAFSCs were successfully isolated from the amniotic fluid. The stem cell markers and lineage‐specific antigens of the subcultured hAFSCs were examined by flow cytometry. The results showed that hAFSCs expressed MSC markers, such as CD105, CD29, CD166 and CD73, and ESCs pluripotent markers, such as Oct4 and SSEA‐1. Importantly, these cells expressed CD117, the specific marker for hAFSCs (Figure 1). These cells did not express hematopoietic stem/progenitor cell marker CD34, endothelial cell marker CD45, T cell‐/B cell‐associated cell surface antigens CD40, or co‐stimulatory molecules CD80/CD86. Meanwhile, we did not detect expression of cell transplantation‐related human leukocyte antigen‐D related antigens on the cell surface.
Figure 1.
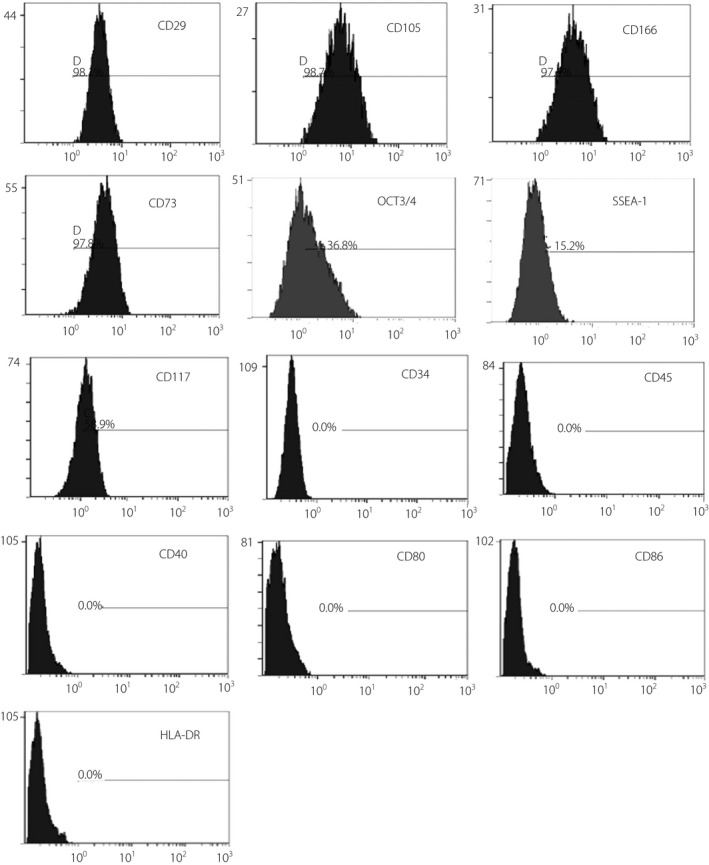
The surface phenotypes of human amniotic fluid stem cells determined by flow cytometry. Undifferentiated cells expressed CD29, CD105, CD73, CD166, OCT3/4, SSEA‐1 and CD117, but not CD45, CD34, CD40, CD86, CD80 and HLA‐DR.
Adipogenic differentiation
The adipogenic potential of hAFSCs was assessed after treating cells with adipogenic induction medium. Lipid vacuoles were noticeable under light microscopy and visualized by Oil Red O staining as early as 10 days after adipogenic induction (Figure 2b). After 3 weeks of culture with osteogenic induction medium, hAFSCs showed nodular structures and the presence of a dense extracellular matrix, evidenced by positive Alizarin Red staining (Figure 2c). The undifferentiated hAFSCs were fibroblastic in shape (Figure 2a).
Figure 2.
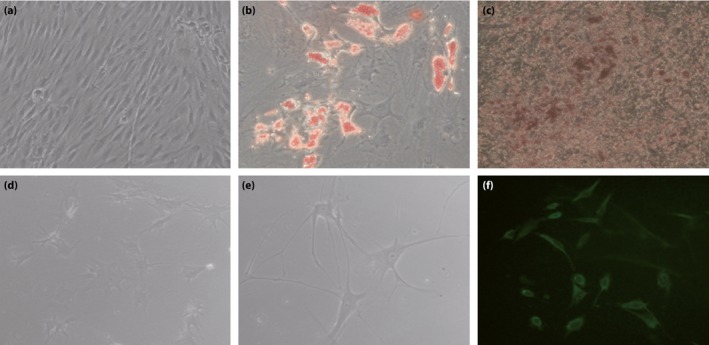
Multilineage differentiation ability of human amniotic fluid stem cells (hAFSCs). (a) Undifferentiated hAFSCs (×200). (b) Oil Red staining of hAFSCs differentiated into adipogenic lineage (×300). (c) Alizarin staining of hAFSCs differentiated into osteogenic lineage (×200). Morphological features of hAFSCs for neurogenic differentiation (d) at 24 h after induction (×200) and (e) at 48 h after induction, which showed neuron‐like morphology (×300). (f) Representative neurone‐specific enolase immunofluorescence on differentiated hAFSCs (×200).
Neurogenic differentiation
There was a significant change in cell morphology after 24 h of culture in the induction media (Figure 2d). The cytoplasm started to shrink and the cell body extended out of a plurality of small protuberances. After 48 h, the cell neurite gradually changed into a dendrite‐like structure, which was similar to the phenotype of a neuron‐like cell (Figure 2e). Immunofluorescence showed that the cells expressed the marker of mature neurons, neurone‐specific enolase (Figure 2f).
Induced differentiation of hAFSCs into islet‐like clusters
The morphological characteristics of hAFSCs at stages 1–3 are shown in Figure 3. At stage 1, hAFSCs were expanded and the cell morphology did not change significantly. At stage 2, hAFSCs changed from fibroblast‐like to a polygon shape approximately 7 days after the induction medium was added, and began to gather and form cell aggregates. At stage 3, islet‐like cell clusters were formed at 15 days (Figure 3a–e).
Figure 3.
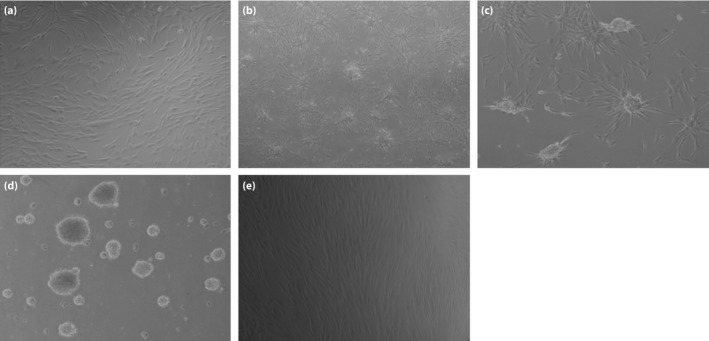
Morphological changes of human amniotic fluid stem cells (hAFSCs) during β‐cell differentiation. (a) Morphological features of hAFSCs after 3 days induction (×100). (b) The differentiated hAFSCs cells began to gather after 8–10 days of induction (×40). (c) The differentiated hAFSCs cells began to form islet‐like cell clusters after 20 days of induction (×100). (d) The islet‐like cell clusters fully formed after 25 days of induction (×100). (e) The undifferentiated hAFSCs were spindle‐shaped and fibroblast‐like (×100).
Identification of the pancreatic precursor cells and mature pancreatic cells
Real‐time PCR analysis showed that pancreatic differentiation of early transcription factor Pdx‐1 began to appear on day 5, and was stably expressed during the process of induction (Figure 4a). Pax6 was expressed in the early stage of differentiation, and was elevated with increased incubation time. On day 10, the expression of pancreas‐associated genes, insulin and glucose transporter 2 (Glut2), was detected, which reached a peak on day 23 (Figure 4a).
Figure 4.
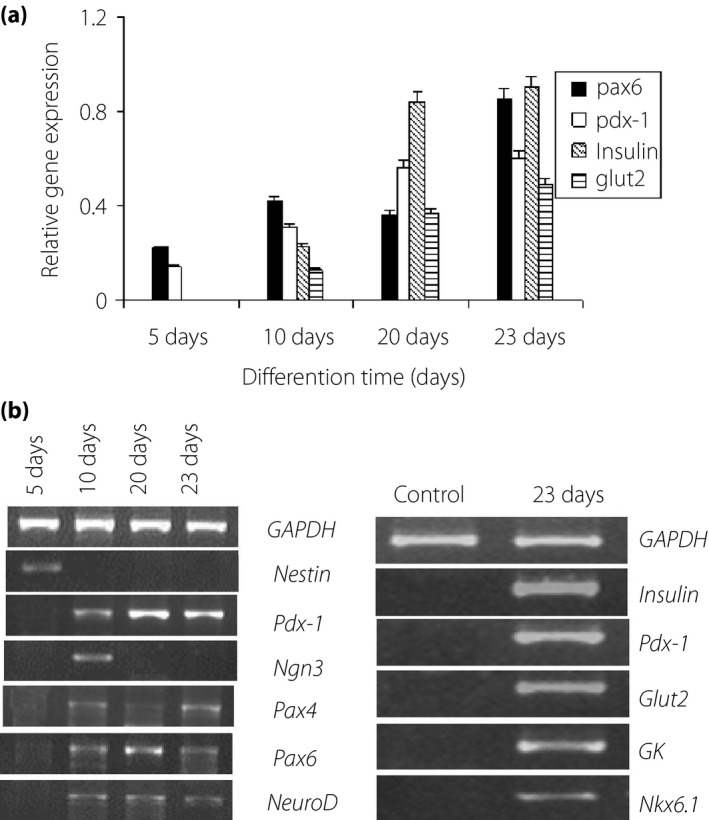
Detection of islet cell‐related genes in differentiated cells. (a) The expressions of pancreatic lineage genes of differentiated human amniotic fluid stem cells were analyzed by quantitative reverse transcription polymerase chain reaction. (b) Reverse transcription polymerase chain reaction was used to detect the gene expression.
Reverse transcription PCR analysis showed that genes, such as Pdx‐1, Ngn3 and Pax4, were expressed in undifferentiated and differentiated hAFSCs. Ngn3 was expressed in the early stage of differentiation, but not in terminally differentiated cells, whereas Pdx‐1, Pax6, Pax4 and NeuroD expression was maintained in the induced cells (Figure 4b). We also detected the expression of nestin in the early stage of differentiation, but not in mature cells. The differentiated cells expressed the genes related to islet development, such as Pdx‐1, insulin, Glut2, GK and Nkx6.1 (Figure 4b). Immunofluorescence assay showed that the differentiated hAFSCs were positively staining for C‐peptide and insulin, but only a small number of cells expressed glucagon. A substantial proportion of Pdx‐1‐positive cells co‐expressed insulin/C‐peptide, and almost all insulin‐expressing cells co‐expressed C‐peptide. However, there was no insulin/glucagon co‐expression in the cells (Figure 5).
Figure 5.
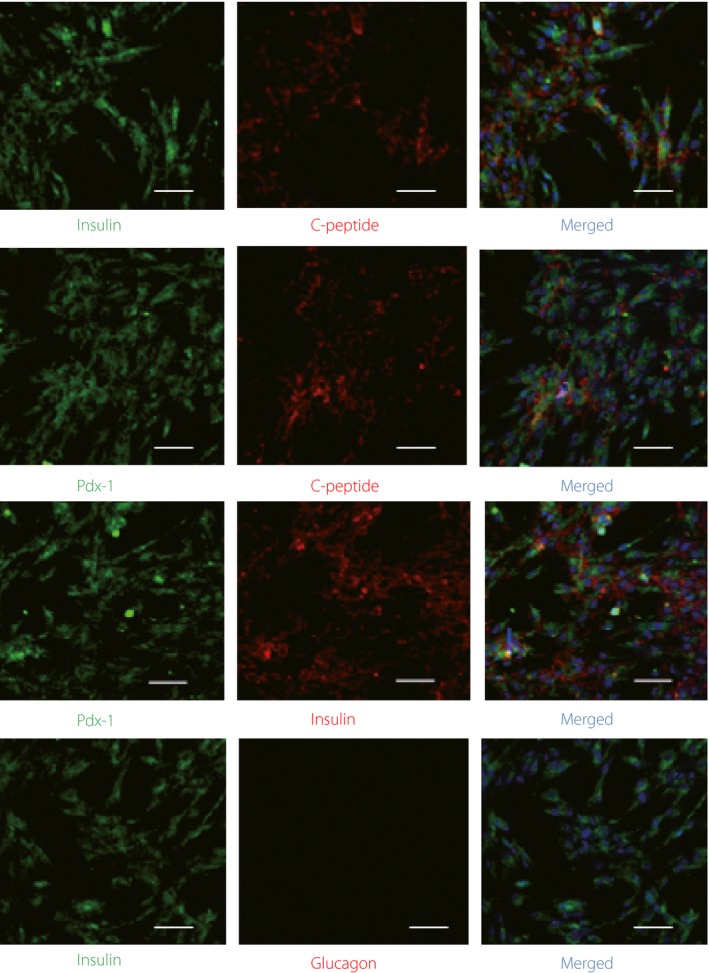
Pancreatic β‐cell characteristics of differentiated human amniotic fluid stem cells at the final maturation stage. Immunofluorescence staining of differentiated human amniotic fluid stem cells by anti‐duodenal homeobox‐1 (Pdx‐1), anti‐insulin, anti‐glucagon and anti‐C‐peptide antibodies. Scale bar, 100 μm.
Functional analysis of the induced cells
To further clarify the function of differentiated hAFSCs, the concentrations of insulin in the culture media were quantitatively measured using the human C‐peptide enzyme‐linked immunosorbent assay kit, as C‐peptide is secreted in equimolar amounts to insulin. Generally, high C‐peptide production indicates high insulin production, and vice versa25. As shown in Figure 6a, the release of C‐peptide in the induction medium increased with the prolongation of induction time from 0 to 20 days.
Figure 6.
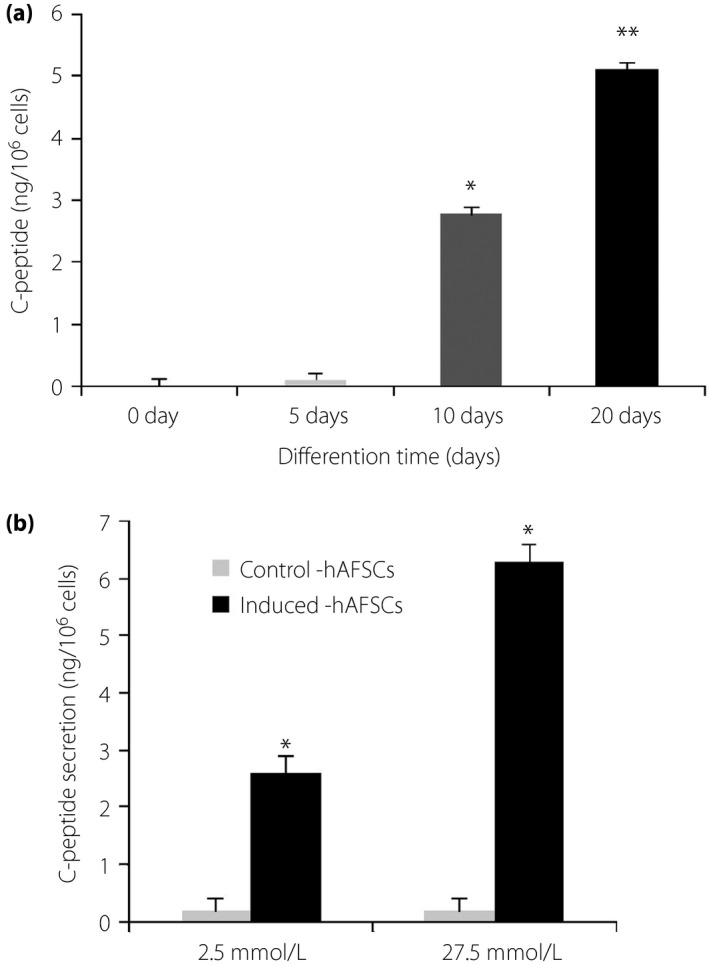
Characterization of human amniotic fluid stem cells (hAFSCs) differentiation. (a) C‐peptide release in the medium from 0 to 20 days of differentiating cultures. Specific enzyme‐linked immunosorbent assay was used to detect C‐peptide (anova followed by Dunnett's test: *P < 0.05, **P < 0.01, ***P < 0.001, vs 0 days). (b) Glucose‐stimulated C‐peptide release from the induced cells.
In order to confirm whether insulin secretion was in response to changes in glucose stimulation, we carried out an enzyme‐linked immunosorbent assay to detect C‐peptide release in the presence of low (2.5 × 10−3 mol/L) and high (2.75 × 10−2 mol/L) glucose. These cells secreted C‐peptide at average concentrations of 2.61 ± 0.2 ng/106 cells and 6.28 ± 1.13 ng/106 cells at the low‐ and high‐glucose challenge, respectively (Figure 6b). There was a 2.4‐fold increase in insulin secretion in response to the high‐glucose concentration. In contrast, C‐peptide concentrations in media from the undifferentiated hAFSCs were very low under both glucose concentrations. These results suggest that hAFSCs are in fact capable of differentiating into functional insulin‐producing cells.
Discussion
In the present study, we successfully isolated hAFSCs from amniotic fluid. These cells expressed a majority of biomarkers related to MSCs and ESCs, which were in line with the characteristics of hAFSCs described previously26, 27. The isolated hAFSCs expressed SSEA‐1, which was detected in the primary cells isolated from amniotic fluid for the first time. SSEA‐1, an antigenic epitope defined as Lewis X carbohydrate, is expressed by preimplantation mouse embryos, teratocarcinoma stem cells and mouse embryonic stem cells28, 29. The function of SSEA‐1 in the amniotic fluid is unknown, although it might bind to growth factors and modulate stem cell differentiation30. Cell type and quantity of amniotic fluid cells change at various stages of differentiation. As pregnancy progresses, the ratio and multiplication capacity of living cells in the amniotic fluid differs. Thus, considering the expression of SSEA1 in amniotic fluid, it might be that these cells are forming progenitor cell niches.
The isolated hAFSCs differentiated into adipocytes and osteoblasts on induction. These findings are in accordance with a previous study3. Compared with human adipose tissue‐derived stromal cells, the differentiation efficiency of hAFSCs towards adipocytes is not very high, induction time is longer and lipid droplet is not obvious. However, the mechanisms are unclear. In the process of differentiation into neuronal cells, we found an obvious change in hAFSCs morphology, and the hAFSCs formed neuron‐like cells in a relatively short time after induction. hAFSCs showed typical neuron‐like protrusions earlier than human adipose tissue‐derived stromal cells, which were induced to differentiate into neuron‐like cells using the same methods24. Mature neurons cannot proliferate, and therefore, damaged neurons would inevitably cause defects in certain functions of nervous system. In the present study, we found that cells passaged with trypsin and ethylenediaminetetraacetic acid could still maintain morphology and survival time in vitro for a relatively long period (more than 4 weeks). hAFSCs can migrate to damaged areas, and express markers of immature nerve and glial cells when transplanted into the rat brain after injury31. At the same time, hAFSCs can also promote regeneration of peripheral nerves and secrete neurotrophic factors32. Therefore, it is feasible to transplant hAFSCs for treatment of neuronal damage.
In the present study, we successfully generated functional insulin‐producing cells from hAFSCs using a multistep in vitro differentiation procedure. To promote cell differentiation, low‐glucose medium was replaced with the high‐glucose medium supplemented with bFGF and nicotinamide. It is well known that high‐glucose is a crucial factor for differentiating stem cells into insulin‐producing cells33. bFGF has been reported to mediate the suppression of sonic hedgehog signaling in the posterior foregut, which is required for initiation of pancreas gene expression34. Nicotinamide can induce differentiation and increase β‐cell mass in cultured human fetal pancreatic cells35, as well as protect cells from prolonged exposure to high concentrations of glucos36. At stage 3, EGF and exendin‐4 were added as inducers. EGF has been identified as an important factor for human pancreatic lineage cell expansion and maturation, and improved the proliferation of Pdx‐1‐positive cells37. Exendin‐4 can promote pancreatic progenitor cells differentiation into islet cells, which was evident by the expression of genes related to mature islet cells, such as insulin and Glut2 37.
In our differentiation protocol, we observed expression of Pdx‐1 in the early stage of differentiation. The expression of Pdx‐1 is essential for pancreas development, as both exocrine and endocrine components of the pancreas are developed from Pdx‐1 + cells38. In Pdx‐1 − mice, the development of the pancreas is blocked at a very early stage. In addition, we detected co‐expression of Pdx‐1 with Pax4, Pax6 and Ngn3. It has been reported that Ngn3, which is critical for pancreatic endocrine development, is expressed only transiently in vivo 39. Ngn3 expression in our isolated hAFSCs suggests that a large proportion of these cells might still be at the endocrine progenitor stage, awaiting additional signals to develop into mature cells. Several types of homeodomain proteins, such as Pax4 and Pax6, which were found in the present study, have been shown to be important for pancreatic endocrine cells differentiation. These data show that our approach successfully induced pancreatic progenitor differentiation from hAFSCs.
In the present study, we used exendin‐4 instead of glucagon‐like peptide‐1 to induce Pdx‐1 + pancreatic progenitors into mature pancreatic insulin‐producing cells. Exendin‐4 is a native glucagon‐like peptide‐1 analog with an insulinotropic property, and the effect of exendin‐4 is fourfold greater than the effect of glucagon‐like peptide‐1. Exendin‐4 can induce differentiation of human ESC and induced pluripotent stem cells into pancreatic insulin‐producing cells37. In the present study, the expression of insulin, Glut2, Nkx6.1, and glucokinase in differentiated hAFSCs showed that the cells expressed characteristics of mature insulin‐producing cells. The expression of insulin and Glut2 was maintained and temporally increased during cell passaging, showing that hAFSCs were committed to β‐cell‐like differentiation. C‐peptide was co‐expressed with Pdx‐1, but we did not detect insulin/glucagon co‐expression in differentiated hAFSCs. These findings were consistent with the hormone expression pattern of mature islets40. The concentrations of secreted insulin increased with differentiation, suggesting that the differentiation process we described in the present study is somehow similar to pancreatic development in vivo.
We further studied whether or not these insulin‐producing hAFSCs were able to respond to changes in glucose concentration in vitro. The results showed that differentiated hAFSCs secreted more insulin in response to glucose stimulation, in a manner similar to the adult human islets. However, the amount of insulin was lower than that produced by adult islet cells37. This could be due to fewer insulin‐positive cells in our preparations as compared with adult human pancreatic islets. However, the long‐term insulin secretory response of hAFSCs requires further investigation. Interestingly, we observed that some C‐peptide‐positive cells in hAFSCs co‐expressed glucagon (data not shown). The existence of such cells has been reported with both mouse41 and human fetal pancreas42. It is unclear at this time whether these cells represent pancreatic endocrine progenitors or an immature cell type belonging to the fetal stage of pancreas development.
In summary, hAFSCs isolated from amniotic fluid were differentiated into insulin‐producing cells, and these differentiated cells secreted insulin in response to glucose stimulation with features similar to those of pancreatic β‐cells. The present study suggests another non‐pancreatic, low‐invasive source of cells for islet regeneration, and a possible new therapeutic strategy for the treatment of type 1 diabetes mellitus.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This research was supported by National Natural Science Foundation of China (31540082). We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
J Diabetes Investig 2017; 8: 34–43
Contributor Information
An‐Hui Wei, Email: weian_hui@126.com.
Jin‐Lan Jiang, Email: jiangjl2016@163.com.
References
- 1. Tsai MS, Lee JL, Chang YJ, et al Isolation of human multipotent mesenchymal stem cells from second‐trimester amniotic fluid using a novel two‐stage culture protocol. Hum Reprod 2004; 19: 1450–1456. [DOI] [PubMed] [Google Scholar]
- 2. Prusa AR, Marton E, Rosner M, et al Oct‐4‐expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 2003; 18: 1489–1493. [DOI] [PubMed] [Google Scholar]
- 3. De Coppi P, Bartsch G Jr, Siddiqui MM, et al Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25: 100–106. [DOI] [PubMed] [Google Scholar]
- 4. Tsai MS, Hwang SM, Chen KD, et al Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells 2007; 25: 2511–2523. [DOI] [PubMed] [Google Scholar]
- 5. Sessarego N, Parodi A, Podestà M, et al Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 2008; 93: 339–346. [DOI] [PubMed] [Google Scholar]
- 6. Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965; 14: 619–633. [DOI] [PubMed] [Google Scholar]
- 7. D'Amour KA, Bang AG, Eliazer S, et al Production of pancreatic hormone‐ ‐expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24: 1392–1401. [DOI] [PubMed] [Google Scholar]
- 8. Jiang J, Au M, Lu K, et al Generation of insulin‐producing islet‐like clusters from human embryonic stem cells. Stem Cells 2007a; 25: 1940–1953. [DOI] [PubMed] [Google Scholar]
- 9. Jiang W, Shi Y, Zhao D, et al In vitro derivation of functional insulin‐producing cells from human embryonic stem cells. Cell Res 2007b; 17: 333–344. [DOI] [PubMed] [Google Scholar]
- 10. Cai J, Yu C, Liu Y, et al Generation of homogeneous Pdx1+ pancreatic progenitors from human ES cell‐derived endoderm cells. J Mol Cell Biol 2010; 2: 50–60. [DOI] [PubMed] [Google Scholar]
- 11. Tateishi K, He J, Taranova O, et al Generation of insulin‐secreting islet‐like clusters from human skin fibroblasts. J Biol Chem 2008; 283: 31601–31607. [DOI] [PubMed] [Google Scholar]
- 12. Lechner A, Habener JF. Bone marrow stem cells find a path to the pancreas. Nat Biotechnol 2003; 21: 755–766. [DOI] [PubMed] [Google Scholar]
- 13. Xie QP, Huang H, Xu B, et al Human bone marrow mesenchymal stem cells differentiate into insulinproducing cells upon microenvironmental manipulation in vitro. Differentiation 2009; 77: 483–491. [DOI] [PubMed] [Google Scholar]
- 14. Ai C, Todorov I, Slovak ML, et al Human marrow‐derived mesodermal progenitor cells generate insulin‐secreting islet‐like clusters in vivo. Stem Cells Dev 2007; 16: 757–770. [DOI] [PubMed] [Google Scholar]
- 15. Drukker M, Benvenisty N. The immunogenicity of human embryonic stem‐derived cells. Trends Biotechnol 2004; 22: 136–141. [DOI] [PubMed] [Google Scholar]
- 16. Draper JS, Pigott C, Thomson JA, et al Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat 2002; 200: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carnevale G, Riccio M, Pisciotta A, et al In vitro differentiation into insulin‐producing β‐cells of stem cells isolated from human amniotic fluid and dental pulp. Dig Liver Dis 2013; 45: 669–676. [DOI] [PubMed] [Google Scholar]
- 18. Tsai MS, Hwang SM, Tsai YL, et al Clonal amniotic fluid‐derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 2006; 74: 545–551. [DOI] [PubMed] [Google Scholar]
- 19. Kakishita K, Nakao N, Sakuragawa N, et al Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6‐hydroxydopamine lesions. Brain Res 2003; 980: 48–56. [DOI] [PubMed] [Google Scholar]
- 20. Zou G, Liu T, Zhang L, et al Induction of pancreatic β‐cell‐like cells from CD44+/CD105+ human amniotic fluids via epigenetic regulation of the pancreatic and duodenal homeobox factor 1 promoter. DNA Cell Biol 2011; 30: 739–748. [DOI] [PubMed] [Google Scholar]
- 21. Chun SY, Mack DL, Moorefield E, et al Pdx1 and controlled culture conditions induced differentiation of human amniotic fluid‐derived stem cells to insulin‐producing clusters. J Tissue Eng Regen Med 2015; 9: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gage BK, Riedel MJ, Karanu F, et al Cellular reprogramming of human amniotic fluid cells to express insulin. Differentiation 2010; 80: 130–139. [DOI] [PubMed] [Google Scholar]
- 23. Trovato L, De Fazio R, Annunziata M, et al Pluripotent stem cells isolated from human amniotic fluid and differentiation into pancreatic beta‐cells. J Endocrinol Invest 2009; 32: 873–876. [DOI] [PubMed] [Google Scholar]
- 24. Wei AH, Wang WJ, Mu XP, et al Enhanced differentiation of human adipose tissue‐derived stromal cells into insulin‐producing cells with glucagon‐like peptide‐1. Exp Clin Endocrinol Diabetes 2012; 120: 28–34. [DOI] [PubMed] [Google Scholar]
- 25. Marques RG, Fontaine MJ, Roger J. C‐peptide: much more a byproduct of insulin biosynthesis. Pancreas 2004; 29: 231–238. [DOI] [PubMed] [Google Scholar]
- 26. Perin L, Sedrakyan S, Giuliani S, et al Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One 2010; 5: e9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moorefield EC, McKee EE, Solchaga L, et al Cloned, CD117‐selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One 2011; 6: e26535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knowles BB, Aden DP, Solter D. Monoclonal antibody detecting a stage‐specific embryonic antigen (SSEA‐1) on preimplantation mouse embryos and teratocarcinoma cells. Curr Top Microbiol Immunol 1978; 81: 51–53. [DOI] [PubMed] [Google Scholar]
- 29. Knowles BB, Rappaport J, Solter D. Murine embryonic antigen (SSEA‐1) is expressed on human cells and structurally related human blood group antigen I is expressed on mouse embryos. Dev Biol 1982; 93: 54–58. [DOI] [PubMed] [Google Scholar]
- 30. Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt‐1. Dev Biol 2006; 291: 300–313. [DOI] [PubMed] [Google Scholar]
- 31. Pan HC, Cheng FC, Chen CJ, et al Post‐injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci 2007; 14: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 32. Pan HC, Yang DY, Chiu YT, et al Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J Clin Neurosci 2006; 13: 570–575. [DOI] [PubMed] [Google Scholar]
- 33. Tang DQ, Cao LZ, Burkhardt BR, et al In vivo and in vitro characterization of insulin‐producing cells obtained from murine bone marrow. Diabetes 2004; 53: 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev 1998; 12: 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang L, Li S, Hatch H, et al In vitro trans‐differentiation of adult hepatic stem cells into pancreatic endocrine hormone‐producing cells. Proc Natl Acad Sci USA 2004; 99: 8078–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otonkoski T, Beattie GM, Mally MI, et al Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Investig 1993; 92: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang D, Jiang W, Liu M, et al Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin‐producing cells. Cell Res 2009; 19: 429–438. [DOI] [PubMed] [Google Scholar]
- 38. Stoffers DA, Zinkin NT, Stanojevic V, et al Pancreatic agenesis attribute able to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 1997; 15: 106–110. [DOI] [PubMed] [Google Scholar]
- 39. Schwitzgebel VM, Scheel DW, Conners JR, et al Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000; 127: 3533–3542. [DOI] [PubMed] [Google Scholar]
- 40. Murtaugh LC. Pancreas and beta‐cell development: from the actual to the possible. Development 2007; 134: 427–438. [DOI] [PubMed] [Google Scholar]
- 41. Alpert S, Hanahan D, Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell 1988; 53: 295–308. [DOI] [PubMed] [Google Scholar]
- 42. De Krijger RR, Aanstoot HJ, Kranenburg G, et al The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol 1992; 153: 368–375. [DOI] [PubMed] [Google Scholar]


