Abstract
We present a rare case of newly diagnosed Evans syndrome associated with lung papillary adenocarcinoma in which the patient showed prompt restoration of blood cell count and long‐lasting complete remission of Evans syndrome after lung cancer resection. Detailed investigation led to a diagnosis of Evans syndrome. In the first year of the disease, left lower lung papillary adenocarcinoma was diagnosed. Pulmonary lobectomy and three courses of chemotherapy were performed. Six months after the initial visit, the primary lung cancer and the autoimmune diseases appeared to be well controlled. We hypothesized that our patient's initial presentation of hematological manifestation was a paraneoplastic phenomenon associated to her underlying malignancy. This rare case report illustrates the unique relationship between primary lung cancer and the development of paraneoplastic Evans syndrome.
Keywords: Evans syndrome, lung adenocarcinoma, paraneoplastic syndrome
Introduction
Paraneoplastic syndromes (PNS) are defined as tumor‐associated indirect systemic effects. Cancer patients, especially those in late stage, may display various hematological manifestations, but Evans syndrome is a rare PNS in malignant solid tumors. We present a rare case of newly diagnosed secondary Evans syndrome associated with pulmonary papillary carcinoma in which the patient showed prompt restoration of peripheral blood cell count and long‐lasting complete remission of Evans syndrome after lung cancer resection and chemotherapy.
Case report
A 39‐year‐old non‐smoking woman presented to the Tianjin Medical University General Hospital on 10 May 2013 with palpitation and fatigue that had persisted for a year, and cough and expectoration that had persisted for two months. On admission, the patient exhibited a mild degree of pallor; however, no other abnormality was observed. The results of peripheral blood cell count were as follows: red blood cell 1.87 × 1012/L, hemoglobin 65 g/L, reticulocyte 5.6%, white blood cell 4.43 × 109/L, 60% neutrophils, 27% lymphocytes, and platelet 35 × 109/L. The D‐dimer level was 1128 ng/mL. Vitamin B12, folic acid, and ferritin levels were normal. Further liver chemistry studies showed elevated levels of aspartate aminotransferase 89 U/L, γ‐glutamyltransferase 87 U/L, lactate dehydrogenase 321 U/L, total bilirubin 23.5 μmol/L, and indirect bilirubin 16.6 μmol/L. Urine routine and renal function tests were negative. The CD55 and CD59 levels in blood cells indicated normal. No abnormality was found during congenital hemolysis tests. A direct Coombs’ test for immunoglobulin G was positive. Cold agglutinins and cryoglobulins were not detected in the serum. The patient was serologically negative for other immunologic autoantibodies. Bone marrow aspiration was indicated, with an increased ratio of erythroid series and increased megakaryocytes, and a biopsy of the bone marrow from the iliac bone had a normal appearance on pathologic examination (Fig 1). Results from chromosome testing were normal, at 46 XX. Based on the laboratory findings, the patient was diagnosed with Evans syndrome.
Figure 1.
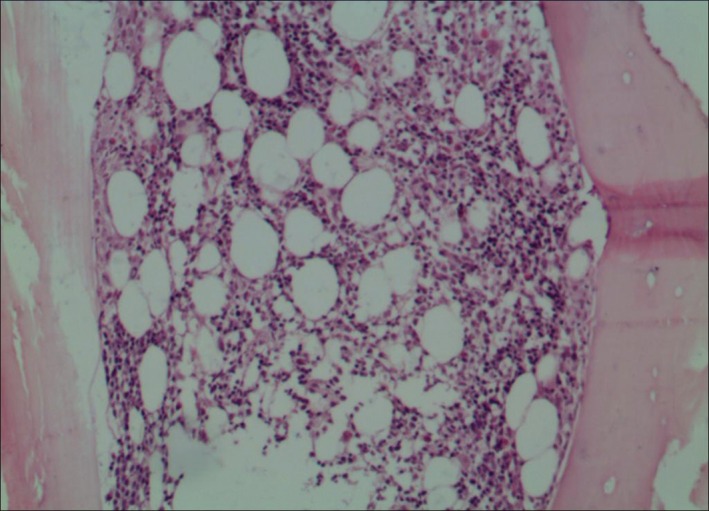
Microscopic view of the bone marrow biopsy specimen. Pathologic diagnosis showed hyperplasia of granulocyte series, erythroid series, and megakaryocytes (hematoxylin‐eosin ×10).
A chest computed tomography (CT) scan on admission showed a 3.0 × 2.6 cm2 mass in the lower lobe of the left lung, with no mediastinal or intrapulmonary lymphadenopathy (Fig 2a). Tumor markers were subsequently detected and all returned negative. A bronchoscopy revealed secretions throughout the airways with no visible masses. Bronchopulmonary lavage was negative for any infectious or malignant process. After two weeks of antibacterial and antifungal therapy, the chest CT indicated no improvement. After consulting the chest surgery department, CT‐guided percutaneous lung biopsy was performed. Histopathologic examination of the biopsy specimen showed pulmonary adenocarcinoma. Single‐photon emission bone and cranium CT scans demonstrated no lesions.
Figure 2.
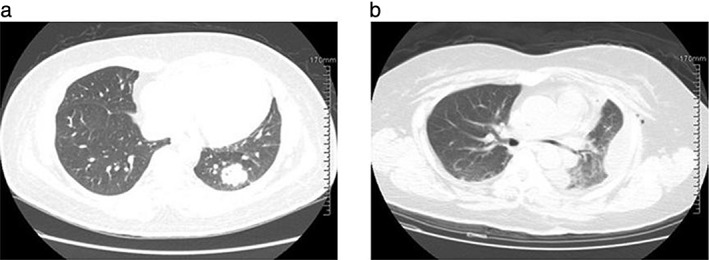
Chest computed tomography reveals (a) a 3.0 × 2.6 cm2 soft tissue density shadow in the lower lobe parenchyma of the left lung field, and (b) the lower lobe of the left lung resection.
As a result of these findings, a diagnosis of lung cancer with Evans syndrome was made. The patient was treated with intravenous gamma globulin combined with component blood transfusion therapy. She safely underwent a left lower pulmonary lobectomy and systemic lymphadenectomy. Histological subtyping was papillary predominant invasive adenocarcinoma (Fig 3). In the subsequent months, she underwent adjuvant chemotherapy with one cycle of gemcitabine/cisplatin and two cycles of docetaxel/nedaplatin. One month after the completion of chemotherapy, the peripheral blood cell count increased to normal and reticulocyte, bilirubin, and lactate dehydrogenase levels all returned to normal range. The patient was followed‐up every three months for 10 months after the surgery, and chest CT (Fig 2b), cranial magnetic resonance imaging, and skeleton emission CT indicated no relapse or metastasis. The blood cell count and hemolysis index were normal.
Figure 3.
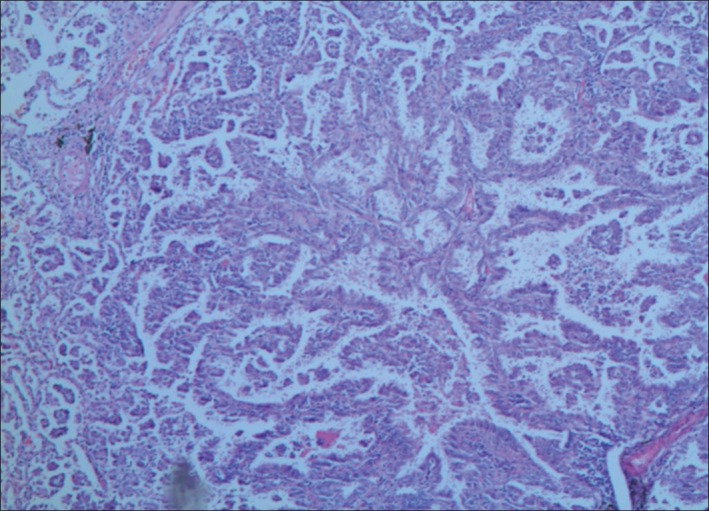
Microscopic view of the lung biopsy specimen. Pathologic diagnosis showed pulmonary adenocarcinoma (hematoxylin‐eosin ×10).
Discussion
Paraneoplastic syndromes are defined as tumor‐associated indirect systemic effects. Given their rarity, it is difficult to determine prevalence; however, one study estimated PNS prevalence of up to 8% in cancer patients.1 Primary bronchogenic carcinoma is one of the most common malignant tumors. PNS occur in approximately 10% of patients with lung cancer.2 Adenocarcinoma is the most common histologic subtype of lung cancer in most countries, accounting for almost half of all lung cancers.3 The patient in our case was a middle‐aged, non‐smoking woman with a family history of lung cancer. Devarakonda et al. reported that nearly 10% of all lung cancers worldwide are diagnosed in people who have never smoked.4 Lung cancer in this group is more frequent in women and is almost always associated with lung adenocarcinoma histology.4 The most common PNS of solid tumors involves nervous system dysfunction. Hematologic autoimmune disease, such as autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP), are the most common PNS of lymphoid neoplasms; however, secondary Evans syndrome is a much rarer PNS in malignant solid tumors.5, 6, 7, 8, 9 Puthenparambil et al. reported that out of 52 cases, nine cases of paraneoplastic AIHA were caused by lung cancer.10 All nine cases were non‐small cell lung cancer; in solid cancers, about two‐thirds of the antibodies were warm and one‐third were cold.10 Krauth et al. identified 15 cases of paraneoplastic ITP caused by lung cancer in 68 solid cancer cases, and four of the 15 cases were adenocarcinoma.11
Cancer patients, especially those in late stage, may display various hematological manifestations, such as cytopenia and coagulation dysfunction. The mechanisms of the syndrome include hematopoietic raw material deficiency, marrow infiltration of tumor cells, anemia of chronic disease, microangiopathic hemolytic anemia, bone marrow fibrosis, and chemotherapy or radiation‐induced bone marrow suppression. Some PNS may be attributed to remote effects of the tumor, such as an ectopic production of bioactive humoral factors (hormones, cytokines) by tumor cells or immune cross‐reactivity by effector cells of antitumor immune response between tumor and normal host tissues.12 The mechanisms by which cancers induce autoimmunity are broken down into the following categories: disruption of central tolerance, peripheral immune dysregulation, and alteration of self‐antigens.13 In a case report describing a patient with PNS‐AIHA, antibodies had formed against the tumor antigens and cross‐reacted with the erythrocyte antigen: band 3.14
The paraneoplastic autoimmune process may affect the nervous system, cutaneous tissue, musculoskeletal system, hematopoietic cells, endocrine system or kidneys.15, 16 Immune‐mediated hematological PNS include AIHA, anti‐FVIII antibodies, antiphospholipid antibodies, autoimmune thrombocytopenia, and others.17, 18 Hematological manifestations may occur prior to or concurrent with cancer, or well after the end of treatment, as a sign of recurrence or during complete remission of the cancer.19, 20
The diagnosis and treatment of PNS is a very challenging issue. An important characteristic of tumor‐related PNS is the influence of anti‐tumor therapy on symptoms. Partial or complete responses to chemotherapy or radiotherapy have been observed. The therapeutic regimen of paraneoplastic Evans syndrome is focused on treating the tumor, which is the protopathy, while the Evans syndrome, which occurs prior to the tumor, is mainly treated with glucocorticoid and intravenous gamma globulin. In general, patients with hematologic syndromes have been reported to have a poor prognosis.2
Hematological PNS in solid tumors have received little attention, thus there are many unanswered questions. We believe that careful laboratory studies in patients with PNS may provide interesting insights into the biology of malignant tumors – in particular, the interaction of the tumor with the immune system – and may provide some clues to indicate immune therapy of solid cancers.
In conclusion, we present a rare case of newly diagnosed secondary Evans syndrome associated with pulmonary papillary carcinoma in which the patient showed prompt restoration of peripheral blood cell count and long‐lasting complete remission of Evans syndrome after lung cancer resection. While some patients attend hospital because of hematological symptoms, such as cytopenia and coagulation dysfunction, the possibility of extramedullary tumors should be considered. Hematology and oncology practitioners should have an increased awareness of PNS so that they can take prompt and proper courses of action for the earlier recognition and diagnosis of malignancies, potentially leading to more positive outcomes for patients.
Acknowledgments
The authors thank proofreaders and editors for assistance in the preparation of the manuscript.
Disclosure
No authors report any conflict of interest.
References
- 1. Pelosof LC, Gerber DE. Paraneoplastic syndromes: An approach to diagnosis and treatment. (Published erratum appears in Mayo Clin Proc 2011; 86: 364.) Mayo Clin Proc 2010; 85: 838–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanaji N, Watanabe N, Kita N et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014; 5: 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol 2015; 16: e342–51. [DOI] [PubMed] [Google Scholar]
- 5. Lechner K, Chen YA. Paraneoplastic autoimmune cytopenias in Hodgkin lymphoma. Leuk Lymphoma 2010; 51: 469–74. [DOI] [PubMed] [Google Scholar]
- 6. Wakata N, Kiyozuka T, Konno S et al. Autoimmune thrombocytopenic purpura, autoimmune hemolytic anemia and gastric cancer appeared in a patient with myasthenia gravis. Intern Med 2006; 45: 479–81. [DOI] [PubMed] [Google Scholar]
- 7. Kubota T, Daibata M, Kobayashi M, Taguchi H. [Malignant lymphoma of the lung associated with Evans' syndrome: Report of a case.] Nihon Kokyuki Gakkai Zasshi 2002; 40: 965–9. (In Japanese.) [PubMed] [Google Scholar]
- 8. Watanabe T, Matsumura Y, Minowa M et al. Remission of newly diagnosed immune thrombocytopenia after lung cancer resection. Ann Thorac Surg 2014; 97: e105–7. [DOI] [PubMed] [Google Scholar]
- 9. Xing L, Wang H, Qu W, Fang F, Dong QE, Shao Z. Lung papillary adenocarcinoma complicated with paraneoplastic autoimmune hemolytic anemia: A case report. Thorac Cancer 2014; 5: 82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puthenparambil J, Lechner K, Kornek G. Autoimmune hemolytic anemia as a paraneoplastic phenomenon in solid tumors: A critical analysis of 52 cases reported in the literature. Wien Klin Wochenschr 2010; 122: 229–36. [DOI] [PubMed] [Google Scholar]
- 11. Krauth MT, Puthenparambil J, Lechner K. Paraneoplastic autoimmune thrombocytopenia in solid tumors. Crit Rev Oncol Hematol 2012; 81: 75–81. [DOI] [PubMed] [Google Scholar]
- 12. Banat GA, Tretyn A, Pullamsetti SS et al. Immune and inflammatory cell composition of human lung cancer stroma. PLoS One 2015; 10 (9): e0139073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maverakis E, Goodarzi H, Wehrli LN, Ono Y, Garcia MS. The etiology of paraneoplastic autoimmunity. Clin Rev Allergy Immunol 2012; 42: 135–44. [DOI] [PubMed] [Google Scholar]
- 14. Kamesaki T. [Autoimmune hemolytic anemia as a paraneoplastic syndrome associated with solid tumors.] Rinsho Ketsueki 2015; 56: 778–84. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 15. Tai P, Yu E, Joseph K, Miale TA. Review of autoimmune diseases associated with cancer. Front Biosci (Elite Ed) 2010; 2: 122–6. [DOI] [PubMed] [Google Scholar]
- 16. Yeung SC, Habra MA, Thosani SN. Lung cancer‐induced paraneoplastic syndromes. Curr Opin Pulm Med 2011; 17: 260–8. [DOI] [PubMed] [Google Scholar]
- 17. Miyajima S, Taguchi Y, Tanaka E et al. [A case of pulmonary adenocarcinoma accompanied by minimal change nephrotic syndrome, antiphospholipid syndrome and warm‐type autoimmune hemolytic anemia.] Nihon Kokyuki Gakkai Zasshi 2006; 44: 631–5. (In Japanese.) [PubMed] [Google Scholar]
- 18. Loh KP, Kansagra A, Asik A, Ali S, Dahiya S. Paraneoplastic autoimmune hemolytic anemia in ovarian cancer: A marker of disease activity. Rare Tumors 2015; 7: 5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paraschiv B, Diaconu CC, Toma CL, Bogdan MA. Paraneoplastic syndromes: The way to an early diagnosis of lung cancer. Pneumologia 2015; 64: 14–9. [PubMed] [Google Scholar]
- 20. Agatsuma T, Koizumi T, Yasuo M et al. [A squamous cell lung cancer patient who developed immune hemolytic anemia after gemcitabine and docetaxel administration.] Gan To Kagaku Ryoho 2009; 36: 1145–7. (In Japanese.) [PubMed] [Google Scholar]


