Abstract
Background
The aim of this study was to identify risk factors associated with mortality in patients re‐admitted to an intensive care unit (ICU) after initial recovery from major lung resection.
Methods
We retrospectively reviewed the case records of all patients who underwent major lung resection between February 2011 and May 2013. A total of 1916 patients underwent major resection surgery for various lung diseases, 63 (3.3%) of which required ICU admission after initial recovery. We analyzed preoperative and perioperative data, including ICU factors and outcomes.
Results
The patient group included 57 men (90.5%) with a mean age of 65.3 years. Pathologic diagnosis was malignancy in 92.1% of patients, while 7.9% had benign disease. Open thoracotomy was performed in 84.1%, whereas minimally invasive approaches were performed in 15.9%. In‐hospital mortality occurred in 16 (25.4%) patients. Patients were classified as either survivors (n = 47, 74.6%) or non‐survivors (n = 16, 25.4%). The most common reason for ICU readmission was pulmonary complication (n = 50, 79.4%). Thirty‐one patients (49.2%) required mechanical ventilation, seven (11.1%) required extracorporeal membrane oxygenation, and three (4.8%) required renal support. Multivariate analysis showed that acute respiratory distress syndrome (ARDS) and delirium were independent risk factors for in‐hospital mortality. In addition, delirium frequently occurred in patients with ARDS.
Conclusion
ARDS and delirium were independent risk factors for in‐hospital mortality in patients who were readmitted to the ICU after major lung resection. Future studies are needed to determine if the prevention of delirium and ARDS can improve postoperative outcomes for patients with lung cancer.
Keywords: ARDS (acute respiratory distress syndrome), delirium, intensive care unit (ICU)
Introduction
Common reasons for intensive care unit (ICU) readmission are pulmonary, cardiac, gastrointestinal, and neurologic complications.1 Hospital mortality is significantly higher in patients who are readmitted to the ICU.1 Many studies have investigated ICU readmission after cardiac, general, and orthopedic surgery.2, 3, 4, 5, 6 The prevalence, cause, and mortality of hospital readmission after lung resection have been previously discussed; however, few studies have evaluated readmission after pulmonary resection.7, 8 Song et al. conducted a study on ICU admission in patients with thoracic tumors, including lung cancer and esophageal cancer. They reported that the rate of ICU readmission after thoracic oncology surgery was 8.6%, while in‐hospital mortality was 33.0%.9 The risk factors associated with in‐hospital mortality were a high Acute Physiology and Chronic Health Evaluation (APACHE) score and prolonged ventilation.
The purpose of our study was to define the prevalence, pattern, and predictive risk factors for ICU mortality after initial recovery from major lung resection.
Methods
We retrospectively reviewed the medical records of 1906 consecutive patients who underwent major lung resection for lung diseases at Samsung Medical Center, Korea, between February 2011 and May 2013. Major pulmonary resection included pneumonectomy, bilobectomy, and lobectomy. The Sungkyunkwan University institutional review board approved the study.
The same thoracic surgery team evaluated all patients and preoperative evaluations were performed according to a standardized protocol. This included chest radiography (CXR), chest computed tomography (CT), bronchoscopy, pulmonary function testing, electrocardiography, and positron emission tomography‐CT (PET‐CT) scans. Echocardiography and a quantitative ventilation/perfusion scan were performed when appropriate. Patients with lung cancer also underwent brain magnetic resonance imaging (MRI).
Patients undergoing pulmonary resection were analyzed according to demographics, comorbidities, type of operative procedure, diagnosis requiring or resulting from pulmonary resection, postoperative complications, hospital mortality, length of hospital stay (LOS), and death after hospital discharge.
Pneumonectomy and bilobectomy were performed through a standard posterolateral thoracotomy. Lobectomy was performed through a standard posterolateral thoracotomy or with video‐assisted thoracoscopic surgery. Perioperative antimicrobial prophylaxis with second‐generation cephalosporins (cefotiam, cefuroxime, and cefotetan) was routinely administered. Most patients were extubated at the end of the operation or shortly after arrival in the post‐anesthesia care unit and were then transferred to the ICU. Patients who required mechanical ventilation were transferred directly to the ICU, where a mechanical ventilator was applied. Patients who had undergone lobectomies were transferred to the general ward at the discretion of the surgeons. We defined this postoperative ICU stay as the first admission in the ICU. Postoperative pain was controlled with continuous intravenous or epidural analgesia.
Patients who underwent a lobectomy were managed in the ICU for one night, while patients who underwent a pneumonectomy or bilobectomy were managed in the ICU for more than two days. Patients were instructed to carry out deep breathing exercises and incentive spirometry during the postoperative period.
Reasons for readmission were classified into seven categories according to the admitting diagnosis: pulmonary (acute respiratory distress syndrome [ARDS], acute lung injury, pneumonia, atelectasis, lobar torsion, upper airway obstruction, and chylothorax); cardiac (atrial fibrillation, bradycardia, myocardial infarction, stress‐induced cardiomyopathy); gastrointestinal (GI bleeding); neurologic (cerebral infarction, cerebral hemorrhage); renal (acute kidney injury); psychiatric (delirium); and miscellaneous (postoperative bleeding). Indications for transfer to the ICU are shown in Table 1.
Table 1.
Indications for transfer to ICU
| Indications for transfer to ICU |
|---|
| Cardiovascular problem |
|
| Respiratory problem |
|
| Neurological problem |
|
| Psychiatric problem |
| Delirium: need for respiratory care |
| Renal problem |
| Acute renal failure: need for CRRT |
| Procedure |
| Bronchoscopy for toileting (if unenforceable in bronchoscopy laboratory relating to a hemodynamic problem, or testing facilities) |
| Miscellaneous |
| Chest tube drainage: Initial drainage >1 L, 150 cc/hour that last more than four hours, bloody color change |
ABGA, arterial blood gas analysis; ARDS, acute respiratory distress syndrome; BTS, blood tinged sputum; CRRT, continuous renal replacement therapy; HR, heart rate; ICU, intensive care unit; SBP, systolic blood pressure; SpO2, peripheral capillary oxygen saturation.
Patients in the survival and non‐survival groups were compared with regard to demographics, comorbidities, type of operative procedure, diagnosis requiring or resulting from pulmonary resection, postoperative complications, and LOS.
Statistical analysis
Data were analyzed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) and JMP 11 (SAS institute, Cary, NC, USA) statistical software. Categorical variables were compared using the x2 or Fisher's exact tests, and continuous variables were compared using the Student's t or Mann–Whitney U tests as appropriate. P values less than 0.05 were considered significant. Continuous variables with a normal distribution were compared by the unpaired Student's t‐test and those without a normal distribution were compared by the Mann–Whitney U test. The risk of in‐hospital mortality associated with selected factors was evaluated using stepwise binary logistic regression analysis to estimate odds ratios (OR) and their 95% confidence intervals (CI). Variables with a P value <0.1 on univariate analysis were subsequently entered in a multivariate logistic regression analysis model to identify independent risk factors for in‐hospital mortality in the patients readmitted to the ICU after major lung resection. P < 0.05 was considered statistically significant.
Results
A total of 1906 consecutive patients underwent major lung resection for lung diseases. Thirty‐two (1.7%) patients died during the first ICU admission, 1874 patients were transferred to the general ward after initial recovery from the ICU, and 1811 patients (95%) were discharged without significant complications. Sixty‐three patients (3.3%) required readmission to the ICU after initial recovery. Forty‐seven patients survived and 16 died. The in‐hospital mortality rate was 2.5% (Fig 1).
Figure 1.
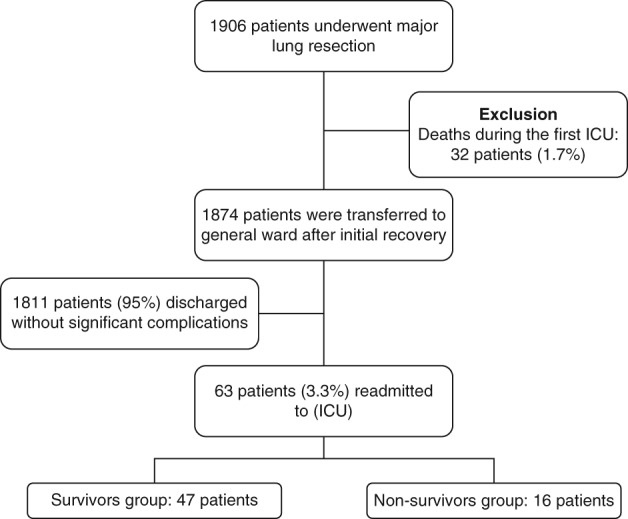
Flow diagram of patients included in the study. ICU, intensive care unit.
The mean age was 65.30 ± 7.14 years (range 44–76). Fifty‐seven (90.5%) patients were male. Sixteen patients died in the ICU. Subjects were divided into two groups (survival and non‐survival groups) and the patient characteristics of each group are shown in Table 1. There were no significant differences in demographics, diagnosis, pulmonary function tests, comorbidities, preoperative chemotherapy, preoperative radiotherapy, extent of procedure, and procedure approach type between the groups (Table 2).
Table 2.
Characteristics of patients readmitted to the ICU after lung resection
| Characteristics | Survival group (n = 47) | Non‐survival group (n = 16) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age (year) | 65.45 ± 7.27 | 64.88 ± 6.96 | 0.785 |
| Gender | |||
| Male | 42 (89.4) | 15 (93.8) | 0.606 |
| Smoking status | |||
| Missing | 2 (4.3) | 0 (0) | 0.766 |
| Never smoker | 8 (17.0) | 2 (12.5) | |
| Quit >2 years before surgery | 16 (34.0) | 7 (43.8) | |
| Quit <2 years before surgery | 21 (44.7) | 7 (43.8) | |
| Present smoker | 0 (0) | 0 | |
| Pulmonary function test | Stu t‐test | ||
| FEV1 (L) | 2.21 ± 0.66 | 2.19 ± 0.55 | 0.928 |
| FEV1 (%) | 75.67 ± 18.35 | 75.31 ± 18.96 | 0.947 |
| FVC (L) | 3.35 ± 0.98 | 3.12 ± 0.71 | 0.406 |
| FVC (%) | 82.78 ± 19.98 | 77.88 ± 18.55 | 0.393 |
| DLCO (%) | 72.91 ± 21.52 | 67.07 ± 16.76 | 0.341 |
| Diagnosis | |||
| Lung cancer | 43 (91.5) | 14 (87.5) | 0.123 |
| Mesothelioma | 0 (0) | 1 (6.3) | |
| Pulmonary tuberculosis | 3 (6.4) | 0 (0) | |
| Aspergilloma | 0 (0) | 1 (6.3) | |
| IPF | 1 (2.1) | 0 (0) | |
| History of CAD or CVA | |||
| CAD | 7 (14.9) | 2 (12.5) | 0.813 |
| CVA | 4 (8.5) | 1 (6.3) | 0.773 |
| Renal dysfunction | 0 (0) | 1 (6.3) | 0.084 |
| Treatment factors | |||
| Treated with chemotherapy | 9 (19.1) | 5 (31.3) | 0.315 |
| Treated with radiotherapy | 8 (17.0) | 4 (25.0) | 0.483 |
| Type of resection | |||
| Lobectomy | 34 (72.3) | 11 (68.8) | 0.916 |
| Bilobectomy | 5 (10.6) | 2 (12.5) | |
| Pneumonectomy | 7 (14.9) | 3 (18.8) | |
| Bilateral lung transplantation | 1 (2.1) | 0 (0) | |
| Lymph node dissection | |||
| Yes | 41 (87.2) | 14 (87.5) | 0.978 |
| No | 6 (12.8) | 2 (12.5) | |
| Approach type | |||
| Open thoracotomy | 38 (80.9) | 15 (93.8) | 0.223 |
| Minimal invasive surgery | 9 (19.1) | 1 (6.3) | |
| ICU event | |||
| Cardiac complication | 17 (32.2) | 8 (50) | 0.329 |
| Delirium | 4 (8.5) | 7 (43.8) | 0.004 |
| BPF | 3 (6.4) | 2 (14.3) | 0.344 |
| APACHE IV score | 32.324 | 45.693 | <0.001 |
| Mechanical support | |||
| Mechanical ventilation | 19 (40.4) | 12 (75.0) | 0.017 |
| ECMO support | 1 (2.1) | 6 (37.5) | <0.001 |
| Renal support | 1 (2.1) | 2 (12.5) | 0.092 |
CAD, coronary artery disease; CVA, cerebrovascular accident; DLCO, diffusing capacity for carbon monoxide; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ICU, intensive care unit.
Thirty‐one patients required mechanical ventilation; 19 patients survived and 12 died. Mechanical ventilation was more commonly used in the non‐survival group (75.0% vs. 40.4%; P = 0.017). The mean duration of mechanical ventilation was 13.45 ± 13.00 days, and this was significantly shorter in the survival group (8.89 ± 12.43 vs. 20.67 ± 10.76, P = 0.011).
Seven patients required extracorporeal membrane oxygenation (ECMO) support. More patients in the non‐survival group required ECMO support (37.5% vs. 2.1%; P < 0.001). The mean duration of ECMO support was 13.86 ± 7.10 days, and only one patient survived. The duration of ECMO support was 17 days (13.33 ± 7.63), and six patients died.
We observed no significant differences in the total LOS, length of the first ICU stay, or time to readmission between the survival and non‐survival groups. The length of the readmission ICU stay was significantly shorter in the survival group (6.72 ± 13.07 vs. 26.19 ± 10.19; P < 0.001).
Causes of readmission to the intensive care unit
The main causes for ICU readmission are listed in Table 3. The most common cause for ICU readmission was pulmonary complication, which affected 49 of 63 (77.8%) patients. The most common pulmonary complication was ARDS, which affected 32 of 49 (65.3%) patients. Seventeen patients were diagnosed with ARDS of unknown origin, while 15 patients were diagnosed with ARDS as a result of pneumonia. The second‐most common cause for ICU readmission was cardiovascular complication, which affected eight out of 63 patients (12.7%). The most common cardiovascular complication was atrial fibrillation, which affected seven of the eight (87.5%) patients.
Table 3.
Main causes of ICU transfer
| Causes of ICU transfer | Number (n = 63) | % |
|---|---|---|
| Pulmonary complications | 49 | 77.8 |
| ARDS of unknown origin | 17 | 27.0 |
| ARDS due to pneumonia | 15 | 23.8 |
| Pneumonia | 7 | 11.1 |
| Atelectasis with dyspnea | 8 | 12.7 |
| Lobar torsion | 1 | 1.6 |
| Chylothorax | 1 | 1.6 |
| Cardiovascular complication | 8 | 12.7 |
| Atrial fibrillation | 7 | 11.1 |
| Sinus bradycardia | 1 | 1.6 |
| Neurological complication | 2 | 3.2 |
| Cerebral infarction | 2 | 3.2 |
| Gastrointestinal complication | 1 | 1.6 |
| GI bleeding | 1 | 1.6 |
| Psychiatric complication | 0 | 0 |
| Delirium | 0 | 0 |
| Renal complication | 0 | 0 |
| Acute kidney injury | 0 | 0 |
| Miscellaneous | 3 | 4.8 |
| Postoperative bleeding | 3 | 4.8 |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
The causes of ICU readmission were compared between the groups (Table 4). In the survival group, the most common cause of ICU readmission was ARDS (of unknown origin or as a result of pneumonia), which affected 18 of 47 (38.3%) patients. The second most common cause of ICU readmission was cardiac arrhythmia, which affected 17 of 47 patients (32.2%). In the non‐survival group, the most common cause was ARDS (with or without pneumonia), which affected 14 of 16 (87.5%) patients. The second‐most common causes were pneumonia and cardiac arrhythmia, which affected eight of 16 (50%) patients.
Table 4.
Causes of ICU transfer
| Reason for ICU readmission | Survival group (n = 47) | Non‐survival group (n = 16) | P |
|---|---|---|---|
| Pulmonary complication | |||
| ARDS (by unknown origin and pneumonia) | 18 (38.3%) | 14 (87.5%) | <0.001 |
| Pneumonia | 15 (31.9%) | 8 (50%) | 0.236 |
| Bronchopleural fistula | 3 (6.4%) | 2 (14.3%) | 0.344 |
| Cardiovascular complication | |||
| Cardiac arrhythmia | 17 (32.2%) | 8 (50%) | 0.329 |
| Psychiatric complication | |||
| Delirium | 4 (8.5%) | 7 (43.8%) | 0.004 |
| Renal complication | |||
| Renal failure | 1 (2.1%) | 2 (12.5%) | 0.092 |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Delirium occurred in 17.5% of patients and was significantly more common in the non‐survival compared with the survival group (43.8% vs. 8.5%; P = 0.001).
Risk factors for in‐hospital mortality
Univariate analysis showed that the following five variables were associated with in‐hospital mortality: readmission as a result of ARDS (P < 0.001), delirium (P = 0.004), APACHE IV score (P < 0.001), mechanical ventilation (P = 0.017), and ECMO support (P < 0.001). Multivariate analysis identified two variables independently associated with in‐hospital mortality: readmission as a result of ARDS (OR 8.6, CI 1.96~61.1, P = 0.003) and delirium (OR 5.5, CI 1.22~28.4, P = 0.026).
Readmission to the ICU as a result of ARDS was significantly more common in the non‐survival group (87.5% vs. 38.3%; P < 0.001). Delirium occurred in 17.5% of patients and was also significantly more common in the non‐survival group (43.8% vs. 8.5%; P = 0.001).
Acute respiratory distress syndrome, delirium, and in‐hospital mortality
We further classified patients into two groups: ARDS vs. non‐ARDS. There were 32 patients with ARDS and 31 patients without ARDS. Delirium occurred in two (6.5%) patients without ARDS and nine (28.1%) with ARDS. Patients with ARDS had a higher prevalence of delirium than patients without ARDS (28.1% vs. 6.5%; P = 0.023). Six (66.7%) ARDS patients died when delirium occurred, while eight (34.8%) ARDS patients died without experiencing delirium. However, there was no significant difference in the in‐hospital mortality rate between the delirium and non‐delirium groups in patients with ARDS (Table 5).
Table 5.
ARDS, delirium, and in‐hospital mortality
| Prevalence of delirium | |||
|---|---|---|---|
| ARDS group (n = 32) | Non‐ARDS group (n = 31) | P | |
| Delirium | 9 (28.1%) | 2 (6.5%) | 0.023 |
| In‐hospital mortality of delirium in patients with ARDS | |||
| Patients with ARDS (n = 9) | Patients without ARDS (n = 23) | P | |
| Death | 6 (66.7%) | 8 (34.8%) | 0.102 |
ARDS, acute respiratory distress syndrome.
Patient outcomes
Sixteen patients died in the ICU, yielding a 25.4% in‐hospital mortality rate and a 0.84% overall operative mortality rate during the study period (total overall operative mortality rate: 2.45%). These deaths comprised 14 lung cancer patients (87.5%), one aspergilloma patient (6.3%), and one mesothelioma patient (6.3%). The main cause of in‐hospital mortality was ARDS (n = 14). The remaining 47 patients were discharged from the ICU to the ward.
Discussion
Morbidity and mortality rates after pulmonary resection remain high in spite of significant advances in preoperative evaluation, surgical technique, anesthetic management, and perioperative care.10
Several authors have studied hospital readmission and mortality rates after pulmonary resection surgery. Handy et al. reported hospital readmission and mortality rates of 18.9% and 11.6% after pulmonary resection, respectively.8 The most common cause of readmission was pulmonary complication. Varela et al. reported a hospital readmission rate of 6.9% and a mortality rate of 6.0%.11 The most common cause of readmission was pleural empyema.
Few papers have documented ICU readmission after pulmonary resection. Desiderio and Downey reported ICU readmission and in‐hospital mortality rates of 3.1% and 29%, respectively.12 Song et al. reported an 8.6% ICU readmission rate after thoracic oncology surgery with an in‐hospital mortality rate of 33.0%.9 The most common cause of ICU readmission in Song et al.'s study was pulmonary complication, and the most common pulmonary complication was ARDS. The risk factors associated with in‐hospital mortality were high APACHE score and prolonged ventilation.
Song et al. targeted thoracic tumors, including lung and esophageal cancers;9 however, our study targeted only lung diseases, including lung cancer. The overall postoperative mortality rate in our study was 2.5% (48/1906), the mortality rate during the first ICU (routine postoperative ICU admission) was 1.7% (32/1906), and the mortality rate after initial recovery was 0.9% (16/1874). The rate of ICU readmission was 3.3% after major lung resection surgery, and these patients had an in‐hospital mortality rate of 25.4% (16/63). The risk factors associated with in‐hospital mortality were ARDS and delirium.
Acute respiratory distress syndrome is a critical complication after major pulmonary resection. Despite extensive progress in intensive care (low‐dose steroid therapy, low‐tidal‐volume ventilation strategy, and ECMO), ARDS remains a major cause of mortality in the ICU.13, 14, 15 Previous studies have reported the prevalence of ARDS after lung resection as 2.2~3.9% and by procedure as: pneumonectomy 3.8~7.9%, lobectomy/bilobectomy 1.9~2.96%, and sublobar resection 0.7~3.2%.16, 17, 18 The mortality rate has been reported as 40~64.6%.16, 17, 18 ECMO is increasingly used to treat high mortality ARDS. The Conventional Ventilatory Support versus ECMO for severe Adult Respiratory Failure (CESAR) trial showed that ECMO‐based management improves survival.15 However, little research has been published of the effects of ECMO in patients with ARDS after lung resection surgery. Seo et al. reported an in‐hospital survival rate after ECMO for postoperative ARDS of 18.8%. Prolonged pre‐ECMO ventilator support was a risk factor associated with in‐hospital mortality.19 In our study, seven patients received ECMO‐based management. However, only one (14.3%) patient survived. We do not know why the survival rate of ECMO‐based management is lower for patients with ARDS occurring after major lung resection surgery. In our center, ECMO is administered to ARDS patients by applying a mechanical ventilator if the peripheral capillary oxygen saturation (SpO2) level is not maintained. ECMO may have been inserted at a late stage, and ECMO was even applied to patients with irreversible lung function. Future studies on this issue are needed and are in progress at our center.
Delirium commonly occurs as a perioperative complication in older adults, with a reported prevalence varying from 0% to 73.5%. Perioperative and postoperative delirium is associated with longer hospital stay, complications, poor recovery, greater cost, and mortality.20, 21 Major risk factors for postoperative delirium include cognitive impairment, severity of illness, visual impairment, and dehydration. Minor risk factors include polypharmacy, catheterization, use of restraints, malnutrition, and any iatrogenic event. Other risk factors are older age, increased number of comorbidities, increased functional dependency, history of falls in the last six months, presurgical and postsurgical pain, and increased white matter pathology.22 Hsieh et al. reported that ARDS is a risk factor for ICU delirium.23 Patients with delirium had greater in‐hospital mortality than patients without delirium. In contrast, Al‐Qadheeb et al. reported no demonstrable relationship between delirium and short‐term in‐hospital mortality.24 In our study, delirium occurred more often in patients with ARDS than in those without ARDS (P = 0.023). However, no significant difference in the rate of in‐hospital mortality was identified between the delirium and the non‐delirium groups in patients with ARDS.
In patients with such risk factors, preventative techniques to avoid postoperative delirium have been effective.25 Strategies for the prevention of delirium include non‐pharmacologic and pharmacologic interventions. Non‐pharmacologic interventions include increased patient mobilization, reduced sleep disruption, orientation reminders, and increased daily social interaction. Pharmacologic intervention includes antipsychotics, cholinesterase inhibitors, melatonin, and alpha‐2 agonists.22
There were several limitations to this study. First, institutional bias could not be avoided because patients were selected from a single institution. Second, the sample size of patients was small. In our study, delirium was an incidental finding with only a small number of cases; therefore, the small but important subgroup of delirious patients was not analyzed independently. We do not know why delirium often occurs in patients with ARDS, although sedative drugs, analgesics, steroids, immobilization, and hypoxia are thought to cause delirium. To investigate this finding, a prospective and multicenter study is required. Hsieh et al. reported that patients with ARDS and delirium had higher mortality.23 However, in our study, there was no significant difference (delirium 66.7% vs. without 34.8%, P = 0.102). We believe that this result is attributed to the small sample size and lack of data points.
In conclusion, ICU readmission after initial recovery from major lung resection was associated with high in‐hospital mortality. Pulmonary complications were the most common cause of ICU readmission after initial recovery from major lung resection (n = 49, 77.8%). ARDS and delirium were independent risk factors for in‐hospital mortality in patients who were readmitted to the ICU after major lung resection. In addition, delirium frequently occurred in patients with ARDS.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors thank our surgical team, research nurse, and nurses in intensive care unit.
References
- 1. Rosenberg AL, Watts C. Patients readmitted to ICUs*: A systematic review of risk factors and outcomes. Chest 2000; 118: 492–502. [DOI] [PubMed] [Google Scholar]
- 2. Hannan EL, Racz MJ, Walford G et al. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA 2003; 290: 773–80. [DOI] [PubMed] [Google Scholar]
- 3. Kassin MT, Owen RM, Perez SD et al. Risk factors for 30‐day hospital readmission among general surgery patients. J Am Coll Surg 2012; 215: 322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty‐day readmission rates as a measure of quality: Causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag 2013; 58: 64–76. [PubMed] [Google Scholar]
- 5. Morris MS, Deierhoi RJ, Richman JS, Altom LK, Hawn MT. The relationship between timing of surgical complications and hospital readmission. JAMA Surg 2014; 149: 348–54. [DOI] [PubMed] [Google Scholar]
- 6. Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med 2013; 369: 1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Assi R, Wong DJ, Boffa DJ et al. Hospital readmission after pulmonary lobectomy is not affected by surgical approach. Ann Thorac Surg 2015; 99: 393–8. [DOI] [PubMed] [Google Scholar]
- 8. Handy JR Jr, Child AI, Grunkemeier GL et al. Hospital readmission after pulmonary resection: Prevalence, patterns, and predisposing characteristics. Ann Thorac Surg 2001; 72: 1855–9. [DOI] [PubMed] [Google Scholar]
- 9. Song SW, Lee HS, Kim JH, Kim MS, Lee JM, Zo JI. Readmission to intensive care unit after initial recovery from major thoracic oncology surgery. Ann Thorac Surg 2007; 84: 1838–46. [DOI] [PubMed] [Google Scholar]
- 10. Grichnik KP, D'Amico TA. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Semin Cardiothorac Vasc Anesth 2004; 8: 317–34. [DOI] [PubMed] [Google Scholar]
- 11. Varela G, Aranda JL, Jiménez MF, Novoa N. Emergency hospital readmission after major lung resection: Prevalence and related variables. Eur J Cardiothorac Surg 2004; 26: 494–7. [DOI] [PubMed] [Google Scholar]
- 12. Desiderio D, Downey R. Critical issues in early extubation and hospital discharge in thoracic oncology surgery. J Cardiothorac Vasc Anesth 1998; 12: 3–6. [PubMed] [Google Scholar]
- 13. Lee HS, Lee JM, Kim MS, Kim HY, Hwangbo B, Zo JI. Low‐dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg 2005; 79: 405–10. [DOI] [PubMed] [Google Scholar]
- 14. The Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8. [DOI] [PubMed] [Google Scholar]
- 15. Peek GJ, Mugford M, Tiruvoipati R et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009; 374: 1351–63. [DOI] [PubMed] [Google Scholar]
- 16. Dulu A, Pastores SM, Park B, Riedel E, Rusch V, Halpem NA. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006; 130: 73–8. [DOI] [PubMed] [Google Scholar]
- 17. Kutlu CA, Williams EA, Evans TW, Pastorino U, Goldstraw P. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000; 69: 376–80. [DOI] [PubMed] [Google Scholar]
- 18. Ruffini E, Parola A, Papalia E et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001; 20: 30–6. [DOI] [PubMed] [Google Scholar]
- 19. Seo DJ, Yoo JS, Kim JB et al. Venovenous extracorporeal membrane oxygenation for postoperative acute respiratory distress syndrome. Korean J Thorac Cardiovasc Surg 2015; 48: 180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data‐collection studies. Arch Intern Med 1995; 155: 461–5. [DOI] [PubMed] [Google Scholar]
- 21. Marcantonio ER, Goldman L, Mangione CM et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271: 134–9. [PubMed] [Google Scholar]
- 22. Korc‐Grodzicki B, Root JC, Alici Y. Prevention of post‐operative delirium in older patients with cancer undergoing surgery. J Geriatr Oncol 2015; 6: 60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsieh SJ, Soto GJ, Hope AA, Ponea A, Gong MN. The association between acute respiratory distress syndrome, delirium, and in‐hospital mortality in intensive care unit patients. Am J Respir Crit Care Med 2015; 191: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al‐Qadheeb NS, Balk EM, Fraser GL et al. Randomized ICU trials do not demonstrate an association between interventions that reduce delirium duration and short‐term mortality: A systematic review and meta‐analysis. Crit Care Med 2014; 42: 1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inouye SK, Bogardus ST Jr, Charpentier PA et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340: 669–76. [DOI] [PubMed] [Google Scholar]


