Abstract
Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr) exerts multiple effects on viral and host cellular activities during viral infection, including nuclear transport of the proviral integration complex, induction of cell cycle G2 arrest, and cell death. In this report, we show that a fission yeast chaperone protein Hsp16 inhibits HIV-1 by suppressing these Vpr activities. This protein was identified through three independent genome-wide screens for multicopy suppressors of each of the three Vpr activities. Consistent with the properties of a heat shock protein, heat shock-induced elevation or overproduction of Hsp16 suppressed Vpr activities through direct protein-protein interaction. Even though Hsp16 shows a stronger suppressive effect on Vpr in fission yeast than in mammalian cells, similar effects were also observed in human cells when fission yeast hsp16 was expressed either in vpr-expressing cells or during HIV-1 infection, indicating a possible highly conserved Vpr suppressing activity. Furthermore, stable expression of hsp16 prior to HIV-1 infection inhibits viral replication in a Vpr-dependent manner. Together, these data suggest that Hsp16 inhibits HIV-1 by suppressing Vpr-specific activities. This finding could potentially provide a new approach to studying the contribution of Vpr to viral pathogenesis and to reducing Vpr-mediated detrimental effects in HIV-infected patients.
Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr), a virion-associated protein of about 13 kDa, is highly conserved among HIV, simian immunodeficiency virus, and other lentiviruses (51, 52). Increasing evidence suggests that Vpr plays multiple roles during the viral life cycle including a possible role in promoting viral pathogenesis. For instance, Vpr displays several distinct activities in host cells. These include cytoplasmic-nuclear shuttling and nuclear localization to the nuclear membrane (25), induction of cell cycle G2 arrest (24), and cell killing (48). The cell cycle G2 arrest induced by Vpr is thought to suppress human immune functions by preventing T-cell clonal expansion (40) and to provide an optimized cellular environment for maximal levels of viral replication (22). The cytoplasmic-nuclear shuttling is believed to participate in nuclear transport of the viral preintegration complex (15, 25, 43). In addition, Vpr induces cell death. The biological significance of this effect is unclear at present, but it may contribute to the depletion of CD4+ T cells in HIV-infected patients (32, 41, 47). Consistent with the multiple roles Vpr plays during the viral life cycle, infections with Vpr-defective viruses in rhesus monkeys, chimpanzees, or human subjects are often associated with slower disease progression, and the mutant vpr often reverts back to the wild type (20, 22, 27, 47, 61). In vitro, Vpr is a moderate viral activator that contributes to a noticeable increase (two- to fourfold) in viral replication in some proliferating cells (22, 53, 59).
Early mutational analysis of the Vpr activities indicated that three of the Vpr-specific activities such as localization to the nuclear membrane, induction of cell cycle G2 arrest, and cell death are functionally independent of each other and have been demonstrated in a wide range of eukaryotic cells (9, 18). Consistent with this conservation, Vpr acts very similarly in fission yeast (Schizosaccharomyces pombe) and mammalian cells, making fission yeast a particularly useful model for studying Vpr activities (17, 62, 64). To further understand the potential roles of HIV-1 Vpr in the viral life cycle and to identify possible cellular factors antagonizing these Vpr effects, we used the fission yeast model system to launch three independent genome-wide searches for cellular proteins that are capable of suppressing each of these Vpr activities. Among the limited number of Vpr suppressors identified, a small heat shock protein (Hsp16) was found to specifically antagonize the Vpr activities of nuclear membrane localization, induction of cell cycle G2 arrest, and cell death.
In this report, we describe specific suppression of the Vpr-related activities by Hsp16 and the conservation of this suppression in human cells and in the context of viral infections. In addition, we demonstrate that Hsp16 reduces viral replication in proliferating host cells in a Vpr-dependent manner.
(Part of this study was conducted by S. Innis and P. Reed in partial fulfillment of the requirements for the degree of Master of Science at Northwestern University.)
MATERIALS AND METHODS
Cell growth and gene expression in fission yeast cells.
Genotypes and sources of S. pombe strains and plasmids used in this study are summarized in Table 1. Gene induction under the control of the fission yeast nmt1 promoter in liquid medium has been described previously (36, 60). Briefly, cells containing the plasmid with the nmt1 promoter were first grown to stationary phase in the presence of 20 μM thiamine. Cells were then washed three times with distilled water, diluted to a final concentration of approximately 2 × 105 cells/ml in 10 ml of the appropriately supplemented Edinburgh minimal medium (EMM) with or without thiamine. Cells were examined 24 h after gene induction. All cells were normally grown at 30°C with constant shaking at 200 rpm unless otherwise specified.
TABLE 1.
Fission yeast strains, human cell lines, plasmids, and HIV-1 viral stocks
| Strains, cell lines, plasmids, and viral stocks | Genotype and characteristics | Source or reference |
|---|---|---|
| S. pombe strains | ||
| SP223 | Wild type, h−, ade6-216, leu1-32, ura4-294 | ATCC |
| Q812 | Wild type, h−, leu1-32, ura4-294 | 49 |
| Q1649 | Wild type, h−, leu1-32, ura4-294, gfp-hsp16 | 49 |
| RE076 | SP223 with single copy of F34Ivpr integrated at ura4 locus; used for measuring Vpr-induced cell cycle G2 delay | 19 |
| RE007 | SP223 with single copy of vpr integrated at ura4 locus; used for determining Vpr-induced cell killing | This study |
| RE078 | SP223 with single copy of gfp-vpr integrated at ura4 locus; used for determining nuclear localization of Vpr | This study |
| Mammalian cell lines | ||
| HeLa | A cervical epithelial cell line | ATCC |
| H9 | CD4+ cells derived from PBMC for high-yield permissive growth with HIV-1 | 44 |
| CEM-SS | CD4+ cells derived from human T4-lymphoblastoid cells | 37 |
| 293T | An embryonic kidney fibroblast cell line | ATCC |
| 293T-632 | A 293T cell line expressing pVgRxR and pCMV-EBNA1 | 66 |
| Plasmids | ||
| pYZIN | Fission yeast expression vector with an inducible nmt1 promoter and a leu1 selectable marker | 63 |
| pYZ3N | Same as pYZ1N but with a 5′ GFP-tag | 63 |
| pSF173 | Fission yeast expression vector with an inducible nmt1 promoter and a 5′ HA-tag, ura4 selectable | ATCC |
| pZY1 | Mammalian expression vector with a muristerone A-inducible promoter; geneticin-resistant | 66 |
| pZH1 | Mammalian expression vector with a muristerone A-inducible promoter; hygromycin-resistant | 66 |
| pCDNA3.1 | Mammalian expression vector with a CMV promoter; hygromycin-resistant | Invitrogen |
| HIV-1 viral stocks | ||
| HIV-1LAI Vpr (+) | HIV-1 viral laboratory (subtype B) strain LAI | 4 |
| HIV-1NL4-3 Vpr (+) | Vpr-positive NL4-3-based HIV-1 vector pNLHX | 8 |
| HIV-1NL4-3 Vpr (−) | Same as above; vpr gene is mutated at the start codon from ATG to GTG | 43 |
| HIV-1 (VSV-G) Vpr (+) | Vpr-positive NL4-3-based HIV-1 vector. HIV-1 env gene is replaced by VSV-G gene | 8 |
| HIV-1 (VSV-G) Vpr (−) | Same as above; vpr gene is mutated at the start codon from ATG to GTG | 43 |
Cell growth and gene expression in mammalian cells.
Characteristics and sources of mammalian cell lines and plasmids used in this study are summarized in Table 1. CD4+ H9 and CEM-SS cells were grown in RPMI 1640 medium, and 293T cells were maintained in Dulbecco's modified Eagle medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal calf serum and 100 U of penicillin-streptomycin per ml. The 293T-632 strain is a stable zeocin-resistant cell line (66) expressing a heterodimer of the modified ecdysone receptor (VgEcR) and the retinoid X receptor. The heterodimer binds a hybrid ecdysone response element (E/GRE) only in the presence of the synthetic analogue of ecdysone, muristerone A (38, 50). The transfected 293T-632 cells expressing vpr and Vpr suppressors were selected with Geneticin (750 μg/ml; Mediatech) for vpr cloned in the pZY1 plasmid and hygromycin (200 μg/ml) for genes cloned in the pZH1 plasmid (66). Gene induction from the muristerone A-inducible mammalian promoter has been described previously (66).
Viral infections.
To test the effect of Hsp16 on Vpr-induced cell cycle G2 arrest in the context of viral infection, we pseudotyped HIV-1 with a vesicular stomatitis virus G (VSV-G) envelope and infected 293T cells carrying the muristerone A-inducible hsp16-expressing vector. For pseudotyping, Vpr-positive and Vpr-negative matching infectious pNLHX clones (57) were cotransfected into 293T cells together with the VSV-G-expressing plasmid pHEF-VSVG obtained from the National Institutes of Health (NIH) AIDS Reagent Program (8). A total of 3 × 106 cells were infected with the VSV-G-pseudotyped Vpr-positive or Vpr-negative HIV-1 vector, and the cell cycle profile of infected cells was analyzed 48 h after infection. The Vpr-negative construct had a single base mutation at the start codon of the vpr open reading frame, which changes ATG to GTG (43). The infected cells were normalized by reverse transcriptase (RT) activity to 3 × 106 cpm of RT activity per 106 cells.
To evaluate the potential suppressive effect of Hsp16 on cell killing caused by HIV infection, CD4+ H9 cells (44) that stably express a plasmid control and Hsp16 were established. A total of 3 × 106 to 5 × 106 of these H9 cells were either mock infected or infected with 2.0 × 103 times the 50% tissue culture infective dose of HIV-1LAI. Equal infection of cells was further verified by measuring viral RNA levels 24 h after viral infection by using the Roche Monitor assay, according to the manufacturer's instructions. Prior to preparing the cells for RNA determination, the infected cells were washed three times with 20× phosphate-buffered saline (PBS) to remove the cell-free virus. These measurements showed that the viral RNA loads were 7.65 × 105 copies/ml for H9 without Hsp16 and 6.92 × 105 copies/ml for H9 cells with Hsp16. Since variation of this assay is normally within threefold, the viral RNA data indicated that both cells were infected with approximately equal levels of virus.
To determine the potential effects of Hsp16 on viral replication, the Vpr-positive and Vpr-negative matching infectious pNLHX clones (57) were used to infect CD4+ H9 and CEM-SS cells. Equal levels of infection of the cells were verified by measuring viral RNA levels 24 h after viral infection by using the Roche Monitor assay according to the manufacturer's instructions. Viral replication was determined in CD4+ H9 and CEM-SS cells by determining p24 antigen levels with a commercially available HIVAG-1 polyclonal antigen kit (Abbott Laboratories, Abbott Park, Ill.).
Measurement of the Vpr-specific activities in fission yeast.
All of the functional assays used to measure Vpr-specific activities, i.e., cell cycle G2 arrest, nuclear localization, and cell death induced by Vpr, have been described previously (9, 60, 65). Vpr-induced cell elongation, which is commonly known as the “cdc phenotype” (Fig. 1A) (19, 28, 35, 39), is the result of cell cycle G2 delay. As described previously (19), this cell elongation phenotype was used as an initial marker for Vpr-induced G2 arrest in this study. Cell images were first captured on a Leica microscope, and cell length was measured individually with the captured images by using OpenLab software according to manufacture's instructions. The statistical significance of cell length variation under different conditions was determined by using the t test for paired two samples for means. For example, in thiamine-containing growth medium (vpr gene expression is off [vpr-off]), fission yeast cells with a vpr plasmid are of normal length, with an average length of 8 to 12 μm (64), but in thiamine-free medium (vpr-on), cells average 15 to 18 μm in length after approximately 24 to 30 h of gene induction (19). As a result, vpr-expressing cells either stop cell division when the wild-type vpr (RE007) is expressed (60) or show a delay in entering mitosis when the mutant F34Ivpr (RE076) is expressed (19). The effect of Vpr on cell proliferation can be further determined by growth kinetics analyses (60). Colony formation on agar plates was used as a measure of Vpr-induced cell death, which has been described previously (65). Briefly, S. pombe cells containing the pYZ1N::vpr plasmid were first grown on a selective leucine-free EMM plate under vpr-repressing conditions. A loopful of viable cells was then streaked onto vpr-inducing or vpr-repressing EMM plates and incubated at 30°C for 3 to 4 days. No colonies or small colonies on the vpr-inducing EMM plates and normal growth on the vpr-repressing EMM plates indicate Vpr-induced cell killing (9, 63). Nuclear localization of Vpr was followed with a green fluorescent protein (GFP)-tagged Vpr (9, 65). Cultures of cells with plasmids expressing GFP or the GFP-Vpr fusion proteins were prepared the same way as described above for the measurement of G2 arrest. Cellular localization was determined 18 to 24 h after vpr gene induction on a Leica fluorescence microscope.
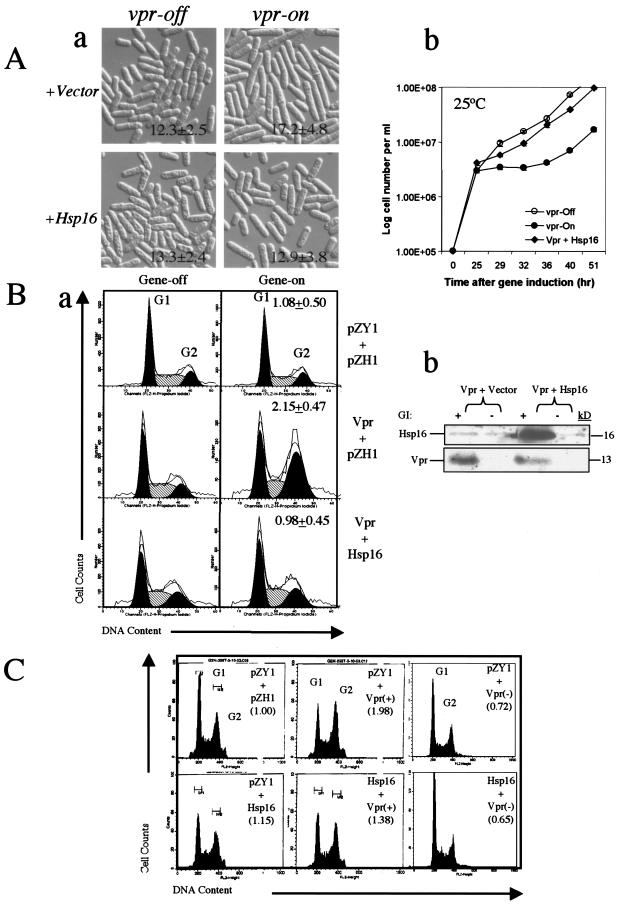
FIG. 1.
Overexpression of hsp16 suppresses Vpr-induced cell cycle G2 arrest in fission yeast and human noninfected and HIV-infected cells. (A) Suppression of Vpr-induced G2 arrest by Hsp16 as measured in fission yeast by Vpr-induced cell elongation (a) and growth delay (b) in RE076 cells. Cell length was measured after 21 h of gene induction at 30°C. Growth kinetics was measured at 25°C. (B) Suppression of Vpr-induced G2 arrest by Hsp16 in human 293T cells. (a) The extent of Vpr-induced G2 arrest, measured by flow cytometric analysis, was quantified by the relative G2 to G1 ratio between gene-repressing and gene-expressing cells 72 h after gene induction. A relative G2/G1 ratio close to 1 indicates no significant difference between the gene-on and gene-off cultures. A relative ratio of >1 suggests increased G2 cells in the gene-on culture (66). (b) Western blotting shows specific expression of HIV-1 vpr and the fission yeast hsp16 gene in 293T-632 cells. GI, gene induction. (C) Suppression of Vpr-induced G2 arrest by Hsp16 in 293T cells infected with VSV-G pseudotyped HIV-1NL4-3. Vpr-positive or Vpr-negative HIV-1NL4-3 (57) was used to infect hsp16-expressing cells. Cell cycle profiles were measured by flow cytometric analysis 48 h after viral infection. Cells were infected with viruses adjusted to 3 × 106 cpm of RT activity per 106 cells.
Measurement of the Vpr-specific activities in mammalian cells.
All mammalian transfections were conducted by electroporation on a Bio-Rad Gene Pulser II, according to the manufacturer's protocols. A total of 5 to 10 μg of DNA was normally used per transfection. To test the potential effects of the Vpr suppressors on nuclear membrane localization and transport of Vpr, a 1:9 ratio of the gfp-vpr carrying plasmid to the suppressor gene-carrying plasmids was used to maximize the suppressor gene expression in each of the vpr-expressing cells. Cells were washed with PBS 24 h after transfection, fixed on coverslips for 10 min in 4% paraformaldehyde, and rinsed in PBS. The coverslips were then inverted and mounted on glass slides with mount medium H1200 containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratory Inc). To measure nuclear import of Vpr, 400 nM leptomycin B (LMB) was added to the medium for 1 h prior to cell fixation according to a procedure previously described (45). It is of note that a wide range of LMB concentrations from nanomolar to micromolar could be used to inhibit the nuclear export of proteins. Even though a concentration as low as 5 nM LMB has been used to block nuclear export of a GFP-pyruvate kinase (PK)-Vpr fusion protein (45), we used 400 nM here to ensure nuclear localization of Vpr.
To measure Vpr-induced G2 arrest in vpr-inducible 293T cells or cells infected with VSV-G-pseudotyped HIV-1, flow cytometric analysis was carried out as previously described with minor modifications (66). Briefly, approximately 106 293T-632 cells transfected with both pZH1-vpr and pZY1-carrying genes of interest were plated into 25 cm2 flasks and grown under selection by zeomycin (400 μg/ml), Geneticin (750 μg/ml), and hygromycin (200 μg/ml) for 5 to 7 days prior to vpr gene induction. Muristerone A (1.0 μM) was added to cell cultures to induce gene expression as previously described (66). Cells were collected in 1 ml of PBS with 5 mM EDTA, fixed by the addition of 2.5 ml of cold 100% ethanol, and stored at 4°C for at least 15 h. The cells were washed twice with PBS-5 mM EDTA and treated with RNase A (2 μg/ml) at 37°C for 30 min prior to propidium iodide staining. Propidium iodide was added to a final concentration of 10 μg/ml, and flow cytometric analysis was carried out after a 1-h incubation on ice. The DNA content of the transfected cells was determined on a Becton-Dickinson flow cytometer by using Cellquest software, and cell cycle profiles were calculated and drawn by using a ModFit LT 3.0 program (Varity Software House). Vpr-induced cell death in HIV-infected CD4+ H9 cells was evaluated either by trypan blue staining, a dye that specifically recognizes dead cells (Sigma), or with a commercial colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)2 2,5-diphenyl tetrazolium bromide] assay, which measures cell viability, used according to the manufacturer's instructions (Boehringer Mannheim).
Searches for multicopy Vpr suppressors in fission yeast.
To search for multicopy suppressors of Vpr-induced G2 arrest, an S. pombe expression cDNA library was transformed into the RE076 strain, which carries a single integrated copy of F34Ivpr (19). The use of RE076 in measuring Vpr-induced G2 arrest is based on the fact that a single amino acid substitution at residue 34 of Vpr (F34I) eliminates vpr-induced cell death but does not affect the induction of cell cycle G2 delay in either mammalian or fission yeast cells (9, 19, 54). Vpr-induced cell elongation in RE076 indicates a G2 arrest and was used as an initial marker for the screens. Genes causing a reduction of cell length back to the normal length or shorter (i.e., <12 μm) in the vpr-expressing RE076 strain were considered as potential suppressor candidates. Out of approximately 2.0 × 104 transformants individually screened under the microscope, a limited number of Vpr suppressor candidates were identified and characterized (19). The suppression effects were confirmed by reintroducing the corresponding cDNA-carrying plasmids back into the parental strain, and the putative gene functions were identified by BLAST homology searches of S. pombe genome databases.
A similar strategy was also used to screen for multicopy suppressors of Vpr-induced cell death or nuclear localization of Vpr. For each screen, more than 2.0 × 104 transformants were screened and tested. Strains with a single integrated copy of the wild-type vpr (RE007) or gfp-vpr (RE078) were used as the tester strains for these two Vpr activities. The criterion used to identify suppressors of Vpr-induced cell death was the ability of a fission yeast transformant to form colonies of normal size on the vpr-expressing agar plate as previously described (60, 63). Potential Vpr suppressors for nuclear localization were individually screened under the fluorescent microscope by looking for displacement of Vpr from its localization on the nuclear membrane.
Fluorescence microscopy.
A Leica DMR fluorescence microscope equipped with a high performance charge-coupled device camera (Hamamatsu) and OpenLab software (Improvision, Inc., Lexington, Mass.) was used for all imaging analysis. For the observation of GFP, we used a Leica L5 filter, which has an excitation of 480 nm (range, 460 to 500 nm) and emission of 527 nm (range, 512 to 542 nm). For DNA staining, cells were counterstained with 1 μg/ml DAPI, which was observed with a Leica A8 filter with an excitation of 360 nm (range, 340 to 380 nm) and emission of 470 nm (range, 450 to 490 nm).
Other molecular and cellular techniques.
The induction of cellular heat shock responses was conducted as previously described (26, 49). Briefly, cultures were first grown as mentioned above to fully express Vpr and then exposed to either an acute heat shock at 45°C for 15 min or grown at high temperature (36°C) for the indicated period of time. Coimmunoprecipitations were conducted by essentially following a standard procedure (http://www.bio.uva.nl/pombe/handbook).
RESULTS
Suppression of Vpr-induced cell cycle G2 arrest by overexpression of fission yeast small heat shock protein Hsp16.
To understand the molecular mechanisms underlying the ability of Vpr to induce cell cycle G2 arrest, we launched a genome-wide screening for multicopy suppressors of Vpr-induced G2 arrest. The screen was carried out in fission yeast strain RE076 carrying a single integrated copy of an inducible F34Ivpr gene in the S. pombe chromosome (19) (Table 1). As shown in Fig. 1A (panel a, top row), expression of F34Ivpr in S. pombe strain RE076 induced the cell elongation typical of a cell cycle G2 delay, which is commonly known as the cdc phenotype (19, 28, 39). Cell length measurements 21 h after gene induction indicated that vpr-expressing cells had an average length of 17.2 ± 4.8 μm (n = 200) during log phase growth (19), which is significantly longer (P < 0.001) than normal cells and vpr-repressing cells, with an average length of 12.3 ± 2.5 μm (Fig. 1A, panel a, top left) (19). However, the Vpr-induced G2 delay was strongly suppressed when a cDNA clone encoding hsp16 was expressed from the nmt1 promoter in the same vpr-expressing RE076 strain (Fig. 1A, panel a, bottom rows). Coexpression of vpr with hsp16 resulted in no significant increase (P = 0.124 for two-tails; n = 200) in cell length compared to the lengths of cells without expression of the hsp16 gene. Suppression of Vpr-induced cell cycle delay by hsp16 was further confirmed by its ability to prevent Vpr-induced growth delay, a result of the G2 delay (Fig. 1A, panel b) (60, 65). A normal vpr-repressing cell culture grown at 25°C reaches stationary phase after about 45 h with a doubling time of about 3 to 4 h. In contrast, a significant growth delay was observed when the vpr gene was fully expressed 20 h after initiating gene induction in the thiamine-depleted (vpr-expressing) medium. The difference in growth rate was more than 1 log unit, and the vpr-expressing cells did not reach stationary phase until 60 h later (Fig. 1A). In contrast, this growth delay was eliminated when hsp16 was expressed in the vpr-expressing RE076 cells. The growth kinetics of these two cultures were very similar to the kinetics of the cell cultures without vpr gene expression, indicating that expression of this small heat shock protein suppressed the growth inhibition caused by Vpr.
To test whether suppression of Vpr-induced G2 arrest by Hsp16 is conserved in mammalian cells, we expressed Hsp16 in 293T cells. To measure the Vpr-induced G2 arrest in mammalian cells, we adapted an inducible vpr gene expression system in a human embryonic kidney 293T-632 cell line (66). In this system, vpr gene expression is induced by adding 1.0 μM muristerone A (66). As shown in Fig. 1B (panel a), addition of muristerone A to the control cell line 293T-632 not containing Vpr did not affect the cell cycle profile 72 h after gene induction (G2/G1 ratio of 1.08 ± 0.5). In contrast, a significant increase in the G2/G1 ratio (2.15 ± 0.47) was observed when the vpr gene was expressed, indicating an accumulation of G2 cells in the vpr-expressing cultures (66). Coexpression of fission yeast hsp16 together with vpr blocked Vpr-induced G2 arrest (Fig. 1B, panel a). A relative G2/G1 ratio of 0.98 ± 0.45 was observed for the Hsp16 culture. Western blot analysis showed that both Vpr and Hsp16 were properly produced and that Hsp16 did not significantly affect the protein level of Vpr, indicating a specific suppression of Vpr-induced G2 arrest by Hsp16 (Fig. 1B, panel b).
To test whether the suppressive effects of Hsp16 on Vpr-induced G2 arrest observed in fission yeast and mammalian cells also applies in the context of viral infection, we used VSV-G-pseudotyped HIV-1 viruses to infect 293T cells (Fig. 1C) (8, 57). Both Vpr-positive and Vpr-negative viruses used for viral infection are isogenic except that Vpr-negative virus carries a single base mutation at the start codon of the vpr open reading frame which changes ATG to GTG (43). The 293T cells that stably expressed the control vector pZY1 (top row) or Hsp16 (bottom row) were infected. As shown in Fig. 1C, coexpression of the vectors pZY1 and pZH1 did not affect the cell cycle profile of 293T cells. Infection with Vpr-positive virus caused a significant shift of the G2/G1 ratio to 1.98, indicating a cell cycle G2 shift due to viral infection (Fig. 1C). In contrast, infection of the same 293T cells with Vpr-negative virus did not affect the cell cycle profile (top right), indicating that the G2 shift was caused specifically by Vpr. Consistent with our findings in fission yeast and mammalian cells, with overproduction of Hsp16 (bottom middle) the cell cycle profile at least in part reverted back to normal, with a relative G2/G1 ratio of 1.38. Together, these data suggest that the suppression of Vpr-induced cell cycle G2 arrest by Hsp16 is a conserved activity between human and fission yeast cells.
Suppression of the Vpr-induced G2 arrest by Hsp16 mimics cellular heat shock response.
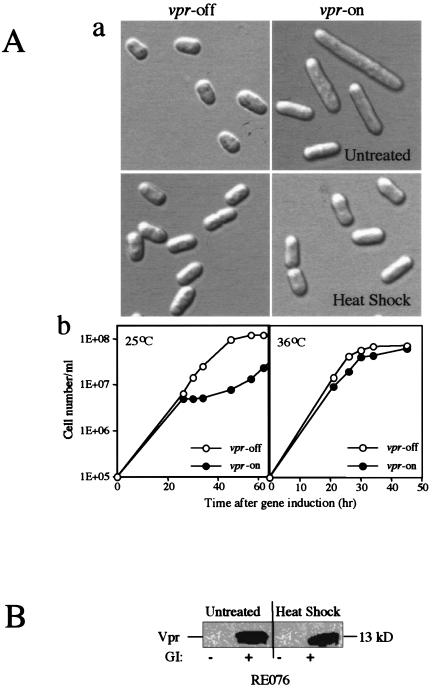
We next investigated the molecular mechanism underlying the inhibiting effects of Hsp16 on Vpr. Since the mRNA levels of Hsp16 increase up to 60-fold when S. pombe cells are subject to heat shock (49), we investigated whether heat shock could suppress Vpr. Vpr-expressing RE076 cells were first grown at 30°C for 21 h to allow full expression of vpr. Cells were then heat shocked at 45°C for 15 min, and both the treated and untreated cells were allowed to recover for three additional hours after heat shock treatment at 30°C prior to observation. Consistent with our earlier findings (Fig. 1A, panel a) (19), expression of vpr in the untreated controls induced longer cells in comparison to the non-vpr-expressing cells (Fig. 2A, panel a). In contrast, no significant difference was found between the vpr-on and vpr-off cells in the heat-treated cell cultures. Tests of cell viability indicated that the brief heat treatment of S. pombe cells at 45°C did not comprise cell viability. Thus, these data suggest that heat shock, indeed, suppresses Vpr-induced cell elongation (Fig. 2A, panel a). However, this acute heat shock caused only a transient effect on Vpr activity, as the heat-treated vpr-expressing cells became longer after extended incubation at 30°C (data not shown). To test whether constant high temperature incubation would sustain this suppression, a time course growth experiment was conducted (Fig. 2A, panel b). While expression of F34Ivpr induced a growth delay at 25°C. this delay was completely eliminated when the same vpr-expressing cells were grown at 36°C (Fig. 2A, panel b). Western blot analysis further showed that Vpr proteins were properly produced in the heat-treated RE076 cells at a level similar to that in the untreated cells, indicating that Vpr protein production was unaffected by heat shock (Fig. 2B). Thus, suppression of the Vpr-induced G2 arrest by Hsp16 indeed mimics the cellular heat shock response.
FIG. 2.
Suppression of Vpr-induced cell cycle G2 arrest by Hsp16 mimics cellular heat shock responses. (A) Induction of cellular heat shock responses suppresses Vpr-induced G2 arrest as indicated by prevention of the Vpr-induced cell elongation (a) and growth delay (b). Cellular heat shock responses were triggered either by an acute heat treatment (45°C for 15 min) or constant high temperature at 36°C. (B) Induction of cellular heat shock responses does not affect the protein levels of Vpr as indicated by Western blot analysis. GI, gene induction.
Suppression of Vpr-induced cell death by overexpression of Hsp16.
Following the G2 arrest induced by Vpr, cells undergo a process of cell death (46, 48, 65). Many studies have reported that Vpr induces apoptosis in human cells (3, 48, 59). However, Vpr seems to induce more cell death than can be accounted for solely by apoptosis (59). Indeed, Vpr-induced cell death has been shown to be either mitochondrial dependent (46, 48) or mitochondrial independent (29, 59, 62, 65). We therefore investigated whether Hsp16 can protect cells from the overall cell death caused by Vpr.
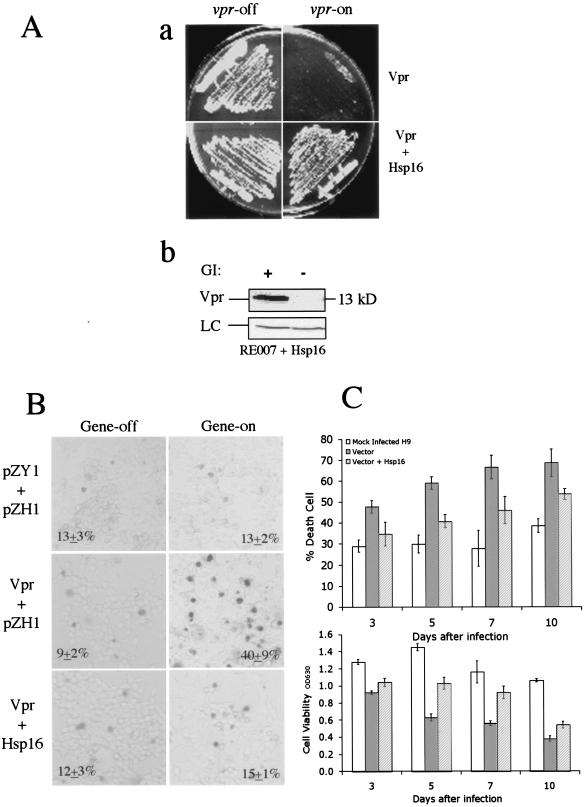
In fission yeast, Vpr-induced cell death can normally be detected by the inability of vpr-expressing cells to form colonies on an inducing agar plate (9, 65). For example, cells without vpr gene expression (vpr-off) grow normally on a repressing plate (Fig. 3A, panel a). In contrast, only very small colonies or no colonies could be seen on an inducing plate where vpr is expressed (Fig. 3A, panel a). Earlier studies showed that the absence of colonies is due to rapid induction of cell death by wild-type Vpr (60, 63). However, when hsp16 was coexpressed with vpr, the cells regained their ability to form colonies of normal size, indicating that overexpression of hsp16 suppressed Vpr-induced cell death (Fig. 3A, panel a). Consistent with this observation, we have also identified hsp16, through an independent screen for multicopy suppressors, as a strong suppressor for Vpr-induced cell death (data not shown). Moreover, suppression of Vpr-induced cell death by Hsp16 was specific as overexpression of another S. pombe small heat shock protein, hsp9, did not restore colony formation (data not shown). To test whether Hsp16 interferes with the expression of the vpr gene, Western blot analysis was performed to determine the Vpr protein levels in hsp16-expressing cells. While no Vpr was detected when the vpr gene was repressed in thiamine-containing medium (Fig. 3A, panel b), a band corresponding to the Vpr 13-kDa protein was found in cells coexpressing hsp16 (Fig. 3A, panel b), indicating Vpr production was not affected by Hsp16.
FIG. 3.
Overexpression of hsp16 suppresses Vpr-induced cell death in fission yeast and human noninfected and HIV-infected cells. (A) Suppression of Vpr-induced cell death in RE007 cells as shown by colony forming ability on EMM selective agar under the vpr-inducing (vpr-on) and vpr-repressing (vpr-off) conditions (a). Plates are shown after 3 to 4 days of incubation at 30°C. Overproduction of Hsp16 in RE007 does not affect the protein levels of Vpr as detected by anti-Vpr serum in a Western blot analysis (b). RE007 cells expressing hsp16 were collected 24 h after gene induction at 30°C. GI, gene induction; LC, protein loading control. (B) Suppression of Vpr-induced cell death by Hsp16 in human 293T cells. Deadcells were detected 72 h after gene induction by trypan blue straining. Gene-off, no muristerone A was added; Gene-on, 1.0 μM muristerone A was added to induce gene expression. (C) Suppressive effects of Hsp16 on cytopathicity induced by HIV-1 infection in H9 cells. Top panel shows cell death induced by HIV-1 infection. Bottom panel measures cell viability during the course of the experiments. H9 cells were either mock infected (left columns) or HIV-1LAI-infected cells carrying empty vector (middle columns) or a vector expressing hsp16 (right columns). Cell death and viability of the infected cells were examined 3, 5, 7, and 10 days after infection. The percentage of cell death induced by HIV-1 infection was quantified by trypan blue straining. Cell viability was determined by measuring the optical density at 630 nm (OD630) by using a commercial MTT assay (Boehringer Mannheim).
A similar suppressive activity was also observed when Vpr-induced cell death was evaluated in mammalian 293T cells by staining dead cells with trypan blue (Fig. 3B). Cell death in the vpr-expressing cells was examined 72 h after vpr gene induction. A minimal amount of cell death (9 to 13%) was observed in all of the cell cultures without vpr gene induction (Fig. 3B). A similar background level of cell death (13% ± 2%) was also observed in the 293T-632 control cells treated with 1.0 μM muristerone A (top right). In contrast, a significant increase in dead cells staining with trypan blue (40% ± 9%) was found in the vpr-expressing cell culture (Fig. 3B, second row). However, cell death was reduced to near background levels (15% ± 1%) when hsp16 was coexpressed with vpr. Thus, expression of fission yeast hsp16 appears to suppress Vpr-induced cell death in human 293T cells (Fig. 3B, bottom right).
To evaluate the potential suppressive effect of Hsp16 on cell killing caused by HIV infection, CD4+ H9 cells were mock infected (Fig. 3C) or infected with HIV-1LAI plus a vector control or HIV-1LAI plus the vector expressing hsp16. Equal levels of HIV-1 infection of these cells were confirmed by determination of viral RNA levels 24 h after infection. The viral RNA loads were 7.65 ×105 copies/ml for H9 without Hsp16 and 6.92 × 105 copies/ml for H9 cells with Hsp16, respectively. Since variations of the Roche HIV-1 monitor assay are normally within threefold, the viral RNA load data indicated that both cell groups were infected with approximately equal numbers of viruses. Cell death caused by HIV-1 was evaluated 3, 5, 7, and 10 days after viral infection by staining with trypan blue (Fig. 3C, top) or by measuring cell viability with the MTT assay (Fig. 3C, bottom). Significant suppression of cytotoxicity caused by HIV-1 infection was observed in cells that stably produced Hsp16. For example, the background cell death ranged from 29 to 38% in mock-infected cells (Fig. 3C). A steadily increasing percentage of dead cells (47 to 69%) was observed in HIV-infected cells with the control vector from day 3 to day 10 after viral infection. In contrast, an approximately 27% reduction in cell death was observed in the hsp16-expressing cells 3 days after viral infection, and a 22 to 31% reduction was observed from 5 to 10 days after viral infection. Similar suppression by Hsp16 was also found when the MTT assay was used to determine cell viability (Fig. 3C). For example, cell viability decreased from 28 to 64% from day 3 to day 10 after viral infection. From 30 to 39% more viable cells were recovered in hsp16-expressing cells at 5 to 10 days even though there was no significant recovery of cell viability at 3 days. Thus, overproduction of Hsp16 confers at least partial protection in the context of viral infection.
Hsp16 interferes with nuclear membrane localization and nuclear import of Vpr by direct protein-protein interaction.
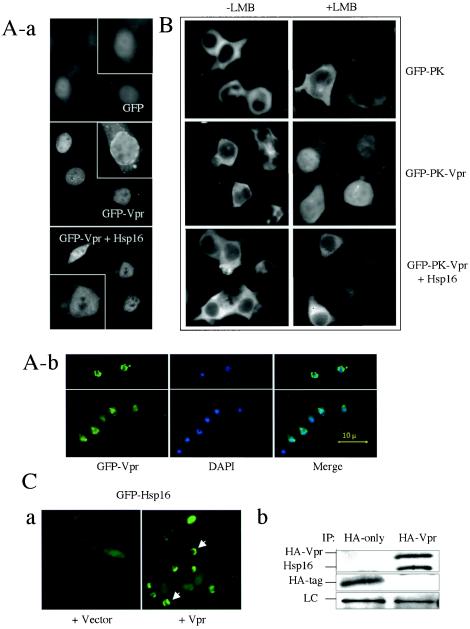
HIV-1 Vpr localizes at higher levels on the nuclear membrane relative to nucleus and cytoplasm in both yeast and mammalian cells (9, 54). The biological significance of Vpr accumulation on the nuclear membrane is at present unclear. Earlier studies demonstrated that Vpr binds to importin α on the nuclear membrane (42, 54), indicating a potential role for Vpr in nuclear targeting, possibly by regulating the docking of the preintegration complex to the nuclear pore complex. Earlier studies showed that HSP70 competes with Vpr for its binding to importin α (2) and that overexpression of HSP70 displaces Vpr from the nuclear membrane (unpublished data). This raises the possibility that Hsp16 may also be able to displace Vpr from the nuclear membrane. To test this possibility, GFP-tagged Vpr was used to monitor Vpr localization onto the nuclear membrane in HeLa cells (58). When the gfp gene was expressed by itself in HeLa cells, a relatively uniform GFP distribution was observed throughout the cells, with somewhat stronger nuclear accumulation. However, the nuclear rim was not clearly visible (Fig. 4A, panel a, top). In contrast, an easily distinguishable nuclear membrane localization of GFP-Vpr was detected even though Vpr was also detected in the cytoplasm and nucleus similar to GFP alone (panel a, middle). Consistent with earlier findings (54, 56), this observation indicates preferential localization of some Vpr to the nuclear membrane (54, 58). However, when hsp16 was coexpressed with vpr, the unique nuclear rings formed by GFP-Vpr disappeared (panel a, bottom), suggesting that Hsp16 may be able to prevent Vpr localization to the nuclear membrane in HeLa cells. Consistent with these observations, we have independently identified the same effect of Hsp16 on Vpr in fission yeast cells through a genome-wide screening for multicopy suppressors of Vpr localization on the nuclear membrane (Fig. 4A, panel b). In fission yeast cells, Vpr localizes almost exclusively to the nuclear rim with little cytoplasmic distribution and with the center of nucleus clear (Fig. 4A, panel b, top) (9). As shown in Fig. 4A (panel b, bottom), overexpression of hsp16 prevented localization of Vpr on the nuclear membrane; instead, it localized within the nucleus and also had somewhat increased distribution throughout the cytoplasm. Together, these observations suggest that Hsp16 may interfere with the nuclear localization of Vpr onto the nuclear membrane.
FIG.4.
Overexpression of hsp16 interferes with nuclear membrane localization and nuclear import of Vpr through direct protein-protein interaction. (A) Hsp16 diminishes nuclear membrane localization of Vpr in HeLa cells (a) and fission yeast (b). Note that Vpr localization on the nuclear membrane in HeLa cells can be distinguished as a clear ring surrounding the nucleus in the GFP-Vpr panel. However, this nuclear ring formed by GFP-Vpr was not clearly visible in GFP alone or GFP-Vpr+Hsp16 panels. Single-cell inserts in each panel show a magnified view of the presence or absence of nuclear membrane localization of Vpr. Nuclear membrane localization of Vpr was visualized by the expression of the GFP-Vpr fusion protein 24 h after transfection in HeLa cells. Cells containing the pYZ1N vector show Vpr localization on the nuclear membrane (panel b, top); cells containing pYZ1N-hsp16 show enhanced distribution of Vpr in the nucleus and cytoplasm (panel b, bottom). Nuclear membrane localization of Vpr was visualized 24 h after gene induction in fission yeast. DAPI staining was used to show nuclei. (B) Hsp16 blocks nuclear entry of Vpr in 293T cells. Nuclear import of Vpr was examined 24 h after transfection and 1 h after the addition of 400 nM LMB. (C) Potential interaction of Vpr with Hsp16. Panel a shows the effect of Vpr on subcellular localization of GFP-Hsp16 in strain Q1649. Note that expression of HIV-1 vpr changes the subcellular distribution of GFP-Hsp16 from evenly distributed (left) to a pattern that is similar to Vpr distribution, as indicated by arrows (right). Panel b shows the coimmunoprecipitation of Hsp16 with HA-tagged Vpr. Both anti-HA and anti-Hsp16 antibodies were used in Western blot analysis. LC, protein loading control.
Since Vpr cycles between the nucleus and cytoplasm in mammalian cells (15, 45), the increased cytoplasmic localization of Vpr in cells expressing Hsp16 could either be due to the enhancement of nuclear export or to the inhibition of nuclear import of Vpr. To distinguish these two possibilities, a GFP-PK-Vpr fusion protein system was used to monitor nuclear import or export of Vpr (45). In this system, LMB was used to block nuclear export of Vpr. A wide range of concentrations of LMB (from nanomolar to micromolar) has been used in various studies to examine nuclear export of proteins. A concentration as low as 5 nM LMB was shown to block nuclear export of Vpr (45). We used 400 nM to ensure nuclear localization of Vpr constructs. A GFP-PK fusion protein was used as a control since it does not enter the nucleus regardless of LMB treatment (Fig. 4B). The GFP-PK-Vpr fusion, on the other hand, accumulates in the nucleus upon the LMB treatment (Fig. 4B, middle row, right), thus providing a specific assay for measuring nuclear import of Vpr (45). By using this assay, we tested the possible effect of Hsp16 on the nuclear import of Vpr. Relatively clear nuclei indicating low levels of GFP-PK-Vpr were found when the fission yeast hsp16 was coexpressed in the LMB-treated cells, suggesting that expression of Hsp16 at least partially suppressed the nuclear import of Vpr (Fig. 4B, bottom).
Even though overexpression of hsp16 is able to block the accumulation of Vpr on the nuclear membrane, Hsp16 by itself normally distributes uniformly throughout the fission yeast cell at a low level in the absence of heat shock (Fig. 4C, panel a, left) (49). To examine the cellular distribution of Hsp16 in the presence of Vpr, we expressed vpr in an S. pombe strain (Q1649) in which the hsp16 gene is tagged with GFP and integrated as a single-copy gene into the chromosome (49). In contrast to the uniform subcellular distribution of Hsp16 observed without Vpr, under these conditions an accumulation of Hsp16 is now seen close to the nuclear membrane where Vpr localizes (Fig. 4C, panel a, arrows). This mutual redistribution of Vpr and Hsp16 suggests a potential interaction between these two proteins. To test this possibility, immunoprecipitation was carried out from cells transformed with either a hemagglutinin (HA)-tagged Vpr or a control (HA-tag) by using anti-HA antibody, and both the anti-HA and anti-Hsp16 antibodies were used to detect the coimmunoprecipitation of these two proteins. As shown in Fig. 4C (panel b), a small band corresponding to the HA-tag was found reacting with the anti-HA antibody when only HA-tag was used as a control. Neither Vpr nor Hsp16 was detected in this pull down. However, both Hsp16 and HA-tagged Vpr were detected in the HA-Vpr pull-down products, indicating the association of these proteins within the cell. Together, these observations suggest that Hsp16 interferes with nuclear membrane localization and nuclear import of Vpr through direct protein-protein interaction.
Hsp16 inhibits viral replication in a Vpr-dependent manner.
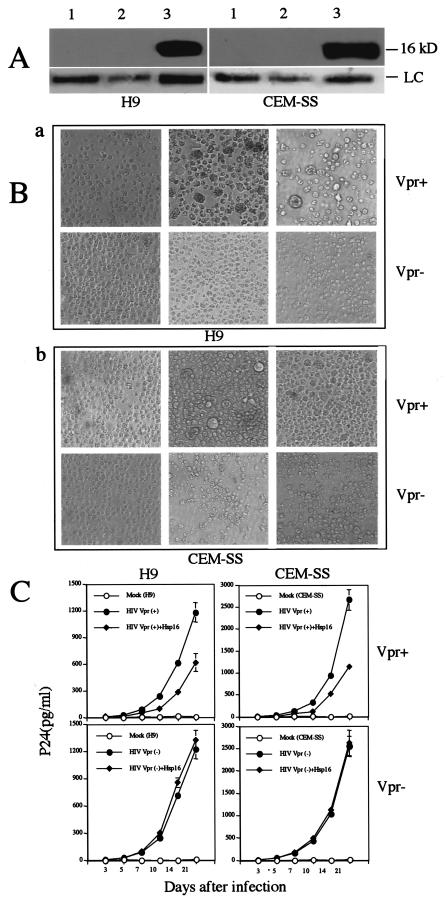
Vpr activities such as G2 arrest and nuclear targeting to the nuclear membrane have been implicated as positive factors for HIV-1 replication (16, 22, 23), and consistent with these activities, Vpr has been shown to increase viral replication up to fourfold in some proliferating T lymphocytes (22, 53, 59). Because Hsp16 counteracts some of these Vpr activities, we expected that Hsp16 might be able to inhibit HIV-1 replication at a comparable level. To test this possibility, we established CD4+ H9 and CEM-SS cell lines stably producing high levels of Hsp16 (Fig. 5A). These H9 or CEM-SS cells were then infected with two matched pNLHX viral clones (55) that differ only in the vpr gene. The Vpr-negative construct has a single base mutation at the start codon of the vpr open reading frame, which changes ATG to GTG (42). Equal levels of infection of the cells were verified by measuring viral RNA levels 24 h after viral infection by using the Roche Monitor assay. Results of these measurements showed that the viral RNA loads varied in the range of log 4.87 ± 0.02 copies/ml for H9 cells infected with Vpr-positive or Vpr-negative viruses, and log 5.05 ± 0.02 copies/ml for CEM-SS cells infected with Vpr-positive or Vpr-negative viruses, respectively. The viral load data indicated that CEM-SS cells were infected with a viral load about 1.5-fold greater than that in H9 cells. However, within each cell type an approximately equal amount of Vpr-positive or Vpr-negative virus was present. HIV-1 infection with virus carrying a wild-type vpr gene typically causes gross enlargement of the infected cells relative to the Vpr-negative virus (29), and transfection with just vpr alone is also sufficient to cause this enlargement (29, 33, 60). Consistently, H9 or CEM-SS cells infected with Vpr-positive viruses, indeed, appeared to be slightly larger than those infected with Vpr-negative cells (Fig. 5B), and the Vpr effect was especially indicated by a small percentage of extremely large cells (top middle). However, the number of these large cells was significantly reduced in the hsp16-expressing cells that were infected with Vpr-positive virus (top right). The observation that expression of hsp16 was able to reduce formation of these large cells and restore nearly normal cell density (data not shown) suggests that Hsp16 does confer at least some degree of protection against Vpr.
FIG.5.
Stable expression of Hsp16 in H9 and CEM-SS cells prior to HIV infection reduces viral replication and HIV-induced cytopathicity in a Vpr-dependent manner. (A) Western blots show high levels of Hsp16 in CD4+ H9 and CEM-SS cells. Lane 1, mock-infected cells; lane 2, HIV-infected cells carrying plasmid pcDNA3.1; lane 3, HIV-infected cells expressing hsp16. Blots show 16-kDa bands reacted to anti-Hsp16 antibody. LC, protein loading control. (B) Stable expression of Hsp16 in H9 and CEM-SS cells blocks the gross enlargement of cells typically induced by Vpr-positive HIV infection (29). Images were taken with equal amount of cells. Gross enlarged cells are of typical effect caused by Vpr (29). (C) Stable expression of Hsp16 in CD4+ H9 and CEM-SS cells inhibits viral replication in Vpr-positive HIV infection; Hsp16 has no effect on viral replication in Vpr-negative HIV infection.
To test the potential effect of Hsp16 on viral replication, p24 antigen was measured in culture supernatants over a period of 14 to 21 days after infection. As shown in Fig. 5C, a consistent but moderate reduction of HIV-1 viral replication was observed in H9 cells expressing hsp16. For example, levels of p24 antigen steadily increased (from 26.0 ± 1.5 to 1,175.3 ± 90.1 pg/ml) in HIV-infected cells expressing the vector control from day 5 to day 21 of HIV-1 infection, indicating successful viral infection (Fig. 5C, top left). However, a 1.8- to 2.6-fold reduction in p24 antigen levels was detected in HIV-infected cells expressing Hsp16 during the same period after viral infection. No detectable p24 antigen was observed in mock-infected cells over the entire experimental period. To ensure that the observed viral inhibition by Hsp16 is not cell line-specific, we also examined CEM-SS cells, another CD4-positive cell line that was derived from T lymphocytes (37). A similar suppressive effect on viral replication (1.8- to 2.9-fold reduction) was observed in the CEM-SS cells that stably express hsp16 genes (Fig. 5C, top right). It was noted that viral replication in CEM-SS cells was higher than in H9 cells. This may be due at least in part to the higher initial viral inoculum for CEM-SS cells.
To further examine whether the inhibitory effect of Hsp16 on viral replication is due to its suppression of Vpr activities or other viral factors, we repeated the same infection experiments in the H9 and CEM-SS cells by using a matching Vpr-defective viral clone that differs only in the vpr gene. The Vpr-negative construct has a single base mutation at the start codon of the vpr open reading frame, which changes ATG to GTG (42). As shown in Fig. 5C (bottom), the kinetics of viral replication were essentially indistinguishable between cells with or without Hsp16, suggesting that the inhibitory effect of Hsp16 on viral replication is specifically through Vpr and thus, Vpr-dependent.
DISCUSSION
A fission yeast small heat shock protein (Hsp16) was identified by three independent genome-wide searches for multicopy suppressors of Vpr nuclear localization and Vpr-induced cell cycle G2 arrest and cell death. Cross-identification of this yeast protein in all three screens suggested that Hsp16 has a global suppressing effect on the Vpr activities. These suppressing effects seem to be a conserved phenomenon as Hsp16 showed similar suppressive activities toward Vpr in S. pombe and mammalian cells (Fig. 1, 3, and 4). Since Hsp16 inhibits all three Vpr activities that are functionally independent of each other (9, 18), the suppressing effect of Hsp16 might target either an early upstream step of the Vpr pathway or act directly on Vpr itself. Our data support the second possibility, as Hsp16 directly associates with Vpr (Fig. 4C, panel b). Hsp16 appears to have a stronger suppressive effect on Vpr in fission yeast than in mammalian cells in the context of viral infection (Fig. 1C and 3C). While this may be related to differences in the levels of Hsp16 and Vpr in fission yeast compared to infection in mammalian cells, it is also possible that this yeast protein may not function in human cells in exactly the same way as in yeast cells. It will thus be of interest to test whether its human counterparts have similar but stronger suppression of Vpr during viral infection.
The suppression of Vpr activities by Hsp16 suggests that Hsp16 might inhibit viral replication, and Hsp16 was found to inhibit viral replication only in Vpr-positive virus (Fig. 5C). However, the inhibition of viral replication by Hsp16 appears to be too large to be accounted for simply by inactivation of Vpr. While a number of reports indicate that HIV-1 with Vpr replicates at 2 to 4 times higher levels in T-cell lines than virus without Vpr (22, 53, 59), others have reported that Vpr plays little or no role in the levels of viral replication in T-cell lines (12, 14, 21). The results shown in Fig. 5C indicate that, under these experimental conditions, Vpr has little effect (<20% decrease) on viral replication (compare the Vpr-negative virus in the bottom panel to the Vpr-positive virus in the top panel). This small decrease in the Vpr-negative virus replication is not sufficient to explain the ≥50% decrease in viral replication after Hsp16 is present but only with Vpr-positive virus. One possible explanation is that the association of Hsp16 with Vpr does not simply inactivate Vpr per se but creates a complex that actively inhibits a step in viral replication. For example, Vpr is ordinarily packaged in the virus (11), but viruses with and without Vpr are apparently assembled with the same efficiency and are equally infectious (Fig. 5C) (12, 14, 21). If the Hsp16-Vpr complex is still recruited during virus assembly but this complex inhibits completion of viral assembly, then production of Hsp16 could inhibit replication via the Hsp16-Vpr complex although Vpr itself does not affect viral assembly or the efficiency of the replication step. Further work is necessary to establish whether the Hsp16-Vpr complex actively inhibits viral replication and, if so, which step or steps of replication are inhibited.
Suppression of Vpr by Hsp16 mimics the effects of cellular heat shock response because heat treatments of vpr-expressing cells suppressed the Vpr-induced growth delay (Fig. 2A), suggesting that Hsp16-mediated suppression of Vpr could be part of the cellular heat shock response. Heat shock proteins are well known for their molecular chaperone activities in protecting cellular proteins under stressful conditions. For example, small heat shock proteins have been shown to form large heteroaggregates and function as molecular chaperones to protect cellular proteins from denaturation in times of stress (31). The aggregation of small heat shock proteins associated with Vpr could prevent Vpr from interacting with other cellular proteins and thereby inhibit its activities. It is conceivable, however, that normal levels of the small heat shock proteins may not be sufficient to entrap Vpr, and thus overproduction of these small heat shock proteins either by expression from a strong promoter on a high-copy-number plasmid or by triggering cellular stress responses is needed to provide sufficient levels. This model is supported by our observations that there is indeed aggregated Hsp16 in vpr-expressing cells around the nuclear periphery where Vpr normally localizes (Fig. 4C, panel a, arrows). However, a more specific functional test such as measuring the chaperone activity of Hsp16 (34) is needed to further substantiate this possibility. It should be mentioned that this model does not explain the increased cytoplasmic localization of Vpr where no obvious Hsp16 aggregation was observed. An alternative and additional mechanism could be that Hsp16 may also inhibit Vpr activities by preventing entry of Vpr to the nucleus (Fig. 4B) (1, 2).
Human small heat shock protein HSP27 is a potential homologue of fission yeast Hsp16 as both proteins share a common C-terminal GXLX4P motif, a hallmark of the small heat shock proteins (10). In addition, anti-HSP27 antibodies weakly cross-reacted with Hsp16, suggesting a certain degree of similarity at the structural level between these two proteins (data not shown). Interestingly, earlier studies showed that HSP27 may play a specific role in resisting HIV infection (6, 7, 13). For example, expression of HSP27 is specifically elevated as early as 3 to 8 h after initial HIV infection (5, 55). Even though the molecular mechanisms underlying the HIV-responsive expression of these heat shock proteins have not been identified, it was surmised that HSP27 could participate in eliciting host innate antiviral immune responses, thus having protective effects against HIV infection (6, 7). Similarly, expression of vpr also elicits a moderate increase of Hsp16 both at translational and transcriptional levels in fission yeast (data not shown), suggesting that expression of this viral protein may be perceived as a stimulus to the activation of Hsp16. Consistent with the idea that the elevation of heat shock protein levels represents a cellular response to counteract the potential viral effect, here we show that overproduction of hsp16 by heat treatment or gene overexpression suppressed Vpr-specific activities. Certainly, it is possible that the Vpr-Hsp16 interaction we observed in fission yeast may not represent what actually happens in mammalian cells. However, both the suppression of Vpr activities by Hsp16 and the heat shock responses appear to be highly conserved activities between S. pombe and mammalian cells, suggesting that a similar scenario, such as that described above for the HIV-HSP27 interaction, might occur in HIV-infected cells. Thus, it will be of interest to test whether HSP27 shows similar inhibitory effects against Vpr. Studies are under way in our laboratories to answer this question.
In summary, we have isolated a yeast chaperone protein that can specifically inhibit the activities of HIV-1 Vpr either as a single viral protein or in the context of viral infection. This is to our knowledge the first protein known to specifically inhibit Vpr in vitro and in vivo. This finding could potentially provide a new approach to study the specific effects of Vpr. The evidence presented here indicates that the suppressive effect of Hsp16 on the HIV-1 Vpr activities is a conserved cellular function, which may implicate a new level of host innate cellular responses to HIV infection. Since the Vpr-specific activities have been linked to such clinical manifestation of AIDS as activation of viral replication (30), suppression of host immune responses (40), and depletion of CD4+ T lymphocytes (47, 48), this finding could potentially provide a new approach to reducing Vpr-mediated detrimental effects in HIV-infected patients by stimulating expression of small heat shock proteins or by administering exogenous small heat shock proteins such as Hsp16.
Acknowledgments
This study was supported by NIH grants AI40891 and GM63080 to R.Y.Z. and AI33776 to M.B. and by the Natural Sciences and Engineering Research Council of Canada (P.G.Y.). E.A. was supported by an NIH Minority Supplement Grant.
We thank Warner Greene for the GFP-PK-Vpr constructs and Sui Huang, Tina Cordero, Csaba Fenyvesvolgyi, and Hannah Koh for technical assistance.
REFERENCES
- 1.Agostini, I., S. Popov, T. Hao, J. H. Li, L. Dubrovsky, O. Chaika, N. Chaika, R. Lewis, and M. Bukrinsky. 2002. Phosphorylation of Vpr regulates HIV type 1 nuclear import and macrophage infection. PG-283-8. AIDS Res. Hum. Retrovir. 18:283-288. [DOI] [PubMed] [Google Scholar]
- 2.Agostini, I., S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 259:398-403. [DOI] [PubMed] [Google Scholar]
- 3.Ayyavoo, V., S. Mahalingam, Y. Rafaeli, S. Kudchodkar, D. Chang, T. Nagashunmugam, W. V. Williams, and D. B. Weiner. 1997. HIV-1 viral protein R (Vpr) regulates viral replication and cellular proliferation in T cells and monocytoid cells in vitro. J. Leukoc. Biol. 62:93-99. [DOI] [PubMed] [Google Scholar]
- 4.Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, B. G., Y. Tao, E. Pearson, I. Remer, and M. A. Wainberg. 1995. Altered constitutive and stress-regulated heat shock protein 27 expression in HIV type 1-infected cell lines. AIDS Res. Hum. Retrovir. 11:713-717. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, B. G., and M. A. Wainberg. 1999. Heat shock protein-based therapeutic strategies against human immunodeficiency virus type 1 infection. Infect. Dis. Obstet. Gynecol. 7:80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, B. G., and Z. Wainberg. 2001. Heat shock proteins: novel therapeutic tools for HIV-infection? Expert Opin. Biol. Ther. 1:67-77. [DOI] [PubMed] [Google Scholar]
- 8.Chang, L. J., V. Urlacher, T. Iwakuma, Y. Cui, and J. Zucali. 1999. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 6:715-728. [DOI] [PubMed] [Google Scholar]
- 9.Chen, M., R. T. Elder, M. Yu, M. G. O'Gorman, L. Selig, R. Benarous, A. Yamamoto, and Y. Zhao. 1999. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 73:3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciocca, D. R., S. Oesterreich, G. C. Chamness, W. L. McGuire, and S. A. Fuqua. 1993. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J. Natl. Cancer Inst. 85:1558-1570. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, E. A., G. Dehni, J. G. Sodroski, and W. A. Haseltine. 1990. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 64:3097-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 13.Cullen, B. R., and J. Heitman. 1994. Human immunodeficiency virus. Chaperoning a pathogen. Nature 372:319-320. [DOI] [PubMed] [Google Scholar]
- 14.Dedera, D., W. Hu, N. Vander Heyden, and L. Ratner. 1989. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J. Virol. 63:3205-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzio, P., S. Choe, M. Ebright, R. Knoblauch, and N. R. Landau. 1995. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 69:7909-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder, R. T., Z. Benko, and Y. Zhao. 2002. HIV-1 VPR modulates cell cycle G2/M transition through an alternative cellular mechanism other than the classic mitotic checkpoints. Front. Biosci. 7:D349-D357. [DOI] [PubMed] [Google Scholar]
- 18.Elder, R. T., M. Yu, M. Chen, S. Edelson, and Y. Zhao. 2000. Cell cycle G2 arrest induced by HIV-1 Vpr in fission yeast (Schizosaccharomyces pombe) is independent of cell death and early genes in the DNA damage checkpoint. Viral Res. 68:161-173. [DOI] [PubMed] [Google Scholar]
- 19.Elder, R. T., M. Yu, M. Chen, X. Zhu, M. Yanagida, and Y. Zhao. 2001. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287:359-370. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs, J. S., D. A. Regier, and R. C. Desrosiers. 1994. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retrovir. 10:343-350. [DOI] [PubMed] [Google Scholar]
- 22.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 23.Gummuluru, S., and M. Emerman. 1999. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J. Virol. 73:5422-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzinger, N., M. Bukinsky, S. Haggerty, A. Ragland, V. Kewalramani, M. Lee, H. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphrey, T., and T. Enoch. 1998. Sum1, a highly conserved WD-repeat protein, suppresses S-M checkpoint mutants and inhibits the osmotic stress cell cycle response in fission yeast. Genetics 148:1731-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang, S. M., M. Weeger, C. Stahl-Hennig, C. Coulibaly, G. Hunsmann, J. Muller, H. Muller-Hermelink, D. Fuchs, H. Wachter, M. M. Daniel, R. C. Desrosiers, and B. Fleckenstein. 1993. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 67:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, M., and P. Nurse. 1988. Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 4:287-290. [DOI] [PubMed] [Google Scholar]
- 29.Levy, D. N., L. S. Fernandes, W. V. Williams, and D. B. Weiner. 1993. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell 72:541-550. [DOI] [PubMed] [Google Scholar]
- 30.Levy, D. N., Y. Refaeli, R. R. MacGregor, and D. B. Weiner. 1994. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 91:10873-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindner, R. A., T. M. Treweek, and J. A. Carver. 2001. The molecular chaperone alpha-crystallin is in kinetic competition with aggregation to stabilize a monomeric molten-globule form of alpha-lactalbumin. Biochem. J. 354:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macreadie, I. G., C. K. Arunagiri, D. R. Hewish, J. F. White, and A. A. Azad. 1996. Extracellular addition of a domain of HIV-1 Vpr containing the amino acid sequence motif H(S/F)RIG causes cell membrane permeabilization and death. Mol. Microbiol. 19:1185-1192. [DOI] [PubMed] [Google Scholar]
- 34.Mao, Q., D. Ke, X. Feng, and Z. Chang. 2001. Preheat treatment for Mycobacterium tuberculosis Hsp16.3: correlation between a structural phase change at 60 degrees C and a dramatic increase in chaperone-like activity. Biochem. Biophys. Res. Commun. 284:942-947. [DOI] [PubMed] [Google Scholar]
- 35.Masuda, M., Y. Nagai, N. Oshima, K. Tanaka, H. Murakami, H. Igarashi, and H. Okayama. 2000. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J. Virol. 74:2636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 37.Nara, P. L., and P. J. Fischinger. 1988. Quantitative infectivity assay for HIV-1 and -2. Nature 332:469-470. [DOI] [PubMed] [Google Scholar]
- 38.No, D., T. P. Yao, and R. M. Evans. 1996. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 93:3346-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurse, P., P. Thuriaux, and K. Nasmyth. 1976. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146:167-178. [DOI] [PubMed] [Google Scholar]
- 40.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Y. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 41.Poon, B., J. B. Jowett, S. A. Stewart, R. W. Armstrong, G. M. Rishton, and I. S. Chen. 1997. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J. Virol. 71:3961-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popov, S., M. Rexach, L. Ratner, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 273:13347-13352. [DOI] [PubMed] [Google Scholar]
- 43.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 45.Sherman, M. P., C. M. de Noronha, M. I. Heusch, S. Greene, and W. C. Greene. 2001. Nucleocytoplasmic shuttling by human immunodeficiency virus type 1 Vpr. J. Virol. 75:1522-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shostak, L. D., J. Ludlow, J. Fisk, S. Pursell, B. J. Rimel, D. Nguyen, J. D. Rosenblatt, and V. Planelles. 1999. Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr. Exp. Cell Res. 251:156-165. [DOI] [PubMed] [Google Scholar]
- 47.Somasundaran, M., M. Sharkey, B. Brichacek, K. Luzuriaga, M. Emerman, J. L. Sullivan, and M. Stevenson. 2002. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc. Natl. Acad. Sci. USA 99:9503-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taricani, L., H. E. Feilotter, C. Weaver, and P. G. Young. 2001. Expression of hsp16 in response to nucleotide depletion is regulated via the spc1 MAPK pathway in Schizosaccharomyces pombe. Nucleic Acids Res. 29:3030-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, H. E., H. G. Stunnenberg, and A. F. Stewart. 1993. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature 362:471-475. [DOI] [PubMed] [Google Scholar]
- 51.Tristem, M., C. Marshall, A. Karpas, and F. Hill. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11:3405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tristem, M., A. Purvis, and D. L. Quicke. 1998. Complex evolutionary history of primate lentiviral vpr genes. Virology 240:232-237. [DOI] [PubMed] [Google Scholar]
- 53.Vanitharani, R., S. Mahalingam, Y. Rafaeli, S. P. Singh, A. Srinivasan, D. B. Weiner, and V. Ayyavoo. 2001. HIV-1 Vpr transactivates LTR-directed expression through sequences present within −278 to −176 and increases virus replication in vitro. Virology 289:334-342. [DOI] [PubMed] [Google Scholar]
- 54.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wainberg, Z., M. Oliveira, S. Lerner, Y. Tao, and B. G. Brenner. 1997. Modulation of stress protein (hsp27 and hsp70) expression in CD4+ lymphocytic cells following acute infection with human immunodeficiency virus type-1. Virology 233:364-373. [DOI] [PubMed] [Google Scholar]
- 56.Waldhuber, M. G., M. Bateson, J. Tan, A. L. Greenway, and D. A. McPhee. 2003. Studies with GFP-Vpr fusion proteins: induction of apoptosis but ablation of cell-cycle arrest despite nuclear membrane or nuclear localization. Virology 313:91-104. [DOI] [PubMed] [Google Scholar]
- 57.Westervelt, P., D. B. Trowbridge, L. G. Epstein, B. M. Blumberg, Y. Li, B. H. Hahn, G. M. Shaw, R. W. Price, and L. Ratner. 1992. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J. Virol. 66:2577-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, J., S. Yi, J. W. Wang, T. Hung, and Y. Zhao. 2000. Cell cycle G2 arrest, cell death and nuclear localization induced by human immunodeficiency virus type 1 protein R (Vpr) in cervical cancer cells. Chinese J. Exp. Clin. Virol. 14:223-226. [PubMed] [Google Scholar]
- 59.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao, Y., J. Cao, M. R. G. O'Gorman, M. Yu, and R. Yogev. 1996. Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular functions of fission yeast Schizosaccharomyces pombe. J. Virol. 70:5821-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao, Y., M. Chen, B. Wang, J. Yang, R. T. Elder, X.-Q. Song, M. Yu, and N. Saksena. 2002. Functional conservation of HIV-1 Vpr and variability in a mother-child pair of long-term non-progressors. Viral Res. 89:103-121. [DOI] [PubMed] [Google Scholar]
- 62.Zhao, Y., and R. T. Elder. 2000. Yeast perspectives on HIV-1 Vpr. Front. Biosci. 905-916. [DOI] [PubMed]
- 63.Zhao, Y., R. T. Elder, M. Chen, and J. Cao. 1998. Fission yeast expression vectors adapted for large scale cloning and GFP fusion with positive screening. BioTechniques. 25:438-444. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, Y., and H. B. Lieberman. 1995. Schizosaccharomyces pombe: a model for molecular studies of eukaryotic genes. DNA Cell Biol. 14:359-371. [DOI] [PubMed] [Google Scholar]
- 65.Zhao, Y., M. Yu, M. Chen, R. T. Elder, A. Yamamoto, and J. Cao. 1998. Pleiotropic effects of HIV-1 protein R (Vpr) on morphogenesis and cell survival in fission yeast and antagonism by pentoxifylline. Virology 246:266-276. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, Y., and L. Ratner. 2001. A novel inducible expression system to study transdominant mutants of HIV-1 Vpr. Virology 287:133-142. [DOI] [PubMed] [Google Scholar]