Summary
We evaluated the transcriptional expression of dual‐specificity protein phosphatase 23 (DUSP23) in CD4+ T cells from 30 systemic lupus erythematosus (SLE) patients and 30 healthy controls. DUSP23 mRNA levels were considerably higher in the patient group: 1490 ± 1713 versus 294·1 ± 204·2. No association was found between DUSP23 mRNA expression and the presence of typical serological and clinical parameters associated with SLE. Meaningful statistical values were obtained in the patient group between the levels of DUSP23 and integrin subunit alpha L (ITGAL), perforin 1 (PRF1) and CD40L. Similarly, transcript levels of different DNA methylation‐related enzymes [DNA methylation‐related enzymes (DNMT1, DNMT3A, DNMT3B, MBD2, and MBD4)] were also correlated positively with the expression of DUSP23. In an attempt to counteract the hypomethylation status of the promoters of certain genes known to be over‐expressed in SLE, it is possible that DUSP23 acts as a negative regulatory mechanism which ultimately silences the transcription of these epigenetically regulated genes by triggering an increase in the expression of different DNMTs.
Keywords: DNMTs, DUSP23; epigenetics; lupus
Introduction
Systemic lupus erythematosus (SLE) is a complex systemic autoimmune disease. Both environmental factors and genetic background seem to play crucial roles in the pathogenesis of this disorder. Genome‐wide scan studies have mapped many significant lupus predisposing gene loci 1. One of these regions is located on human chromosome 1 (1q21–23) 2. This region contains Fc receptors for immunoglobulin G (FCGR), such as FCGR2A, FCGR2B, FCGR3A and FCGR3B, as well as FasL and C‐reactive protein (CRP); interestingly, many variants of these genes have been associated with SLE in multiple populations 3, 4, 5, 6, 7. Dual‐specificity protein phosphatase 23 (DUSP23) is another gene located at chromosome 1q23 8, and it therefore may potentially be considered as a candidate gene for SLE susceptibility.
Protein phosphorylation mediated by kinases modulates several biological mechanisms, such as protein interactions, protein degradation, metabolism and signal transduction. In order to achieve a correct cellular homeostasis, phosphorylated proteins must be dephosphorylated by phosphatases. Interestingly, some reports have established a relationship between the presence of SLE and some single nucleotide polymorphisms (SNPs) found in genes coding for particular phosphatases such as protein tyrosine phosphatase, non‐receptor (PTPN)11 and PTPN22 9, 10. Other authors have also demonstrated that mRNA and protein levels of another phosphatase, protein phosphatase 2A (PP2A), as well as the activity of the catalytic subunit (PP2Ac), are increased in T cells isolated from SLE patients compared with healthy controls 11.
Dual‐specificity protein phosphatases (DUSPs) can dephosphorylate serine, tyrosine and threonine residues. DUSP23 is a low molecular weight DUSP which lacks the N‐terminal domain of mitogen‐activated protein kinase phosphatases (MKPs). DUSP23 and other low molecular weight DUSPs, such as DUSP3 and DUSP22, are structurally similar to the vaccinia virus VH1 phosphatase 12, 13, 14. DUSP3, DUSP22 and VH1 participate in both the interferon (IFN) and interleukin‐signalling pathways by dephosphorylating signal transducer and activator of transcription (STAT) proteins 15, 16. Elevated IFN‐α activity is detected frequently in the sera of patients with SLE 17. Furthermore, patients with SLE display a specific mRNA expression pattern of interferon‐dependent genes in their leucocytes, termed ‘the interferon signature’ 18, 19.
All these findings prompted us to investigate whether the expression of another potential candidate gene for SLE susceptibility that codes for a phosphatase, DUSP23, could somehow be altered in these patients.
Subjects and methods
Subjects
Data were collected from 30 Spanish individuals (six men and 24 women; mean age = 33 years, range = 20–50 years) who suffered from SLE. An ethnically matched random healthy control population (blood donors) was also included into the study (n = 30, 17 men and 13 women; mean age = 38 years, range = 21–66 years). Subjects’ written consent was obtained according to the Declaration of Helsinki, and the design of the work conformed to standards applied currently in Spain 20. All the SLE patients fulfilled at least four of the American College of Rheumatology criteria 21. Laboratory parameters were evaluated as described previously 22. SLE activity was assessed by the SLE disease activity index (SLEDAI) and those with a SLEDAI equal to or higher than 6 were considered to have active disease 23. Eight patients were not receiving any drug at the time of sample withdrawal. For the remaining patients, treatment was achieved by prescribing one or more of the following drugs: corticosteroids, non‐steroidal anti‐inflammatory drugs (NSAIDs), chloroquine, mycophenolate, azathioprine, tacrolimus, leflunomide and etanercept (see Table 1).
Table 1.
Patients’ demographics, clinical characteristics and treatment received at the time of sample withdrawal.
| Age | Sex | Arthritis | Cutaneous lesions | Pleuritis | Pericarditis | Neurological manifestations | Nephropathy | SLEDAI | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 28 | F | Yes | Yes | Yes | No | No | Yes | 16 | Mycophenolate, prednisone |
| 32 | M | Yes | No | No | No | No | No | 4 | Nothing |
| 44 | F | Yes | Yes | Yes | Yes | Yes | Yes | 20 | Prednisone |
| 34 | M | Yes | Yes | Yes | Yes | No | Yes | 16 | Mycophenolate, prednisone, chloroquine |
| 27 | F | Yes | Yes | Yes | Yes | No | Yes | 4 | Prednisone, chloroquine, mycophenolate |
| 22 | F | Yes | Yes | No | No | No | Yes | 18 | Nothing |
| 43 | F | Yes | No | No | No | No | No | 4 | NSAIDs |
| 50 | F | Yes | Yes | No | No | No | Yes | 6 | Mycophenolate, prednisone, beclometasone |
| 44 | F | Yes | No | No | Yes | No | Yes | 24 | Prednisone, mycophenolate |
| 33 | F | Yes | No | Yes | Yes | No | No | 4 | Prednisone, chloroquine |
| 23 | F | Yes | Yes | No | No | No | No | 2 | Prednisone, chloroquine, azathioprine |
| 20 | F | Yes | Yes | No | No | Yes | No | 4 | Prednisone |
| 20 | F | Yes | No | No | No | No | No | 6 | Prednisone, tacrolimus, leflunomide |
| 21 | M | Yes | Yes | Yes | No | Yes | Yes | 24 | Prednisone, mycophenolate |
| 40 | M | Yes | Yes | No | Yes | No | Yes | 16 | Chloroquine, mycophenolate |
| 46 | F | Yes | Yes | No | No | No | No | 6 | Nothing |
| 48 | F | Yes | Yes | No | No | No | Yes | 2 | Nothing |
| 25 | F | Yes | No | No | No | No | Yes | 16 | Nothing |
| 34 | F | Yes | Yes | No | No | No | Yes | 2 | Nothing |
| 35 | F | Yes | Yes | No | No | No | no | 2 | Nothing |
| 24 | F | Yes | Yes | No | No | No | no | 2 | Chloroquine |
| 26 | F | Yes | Yes | No | No | No | Yes | 6 | Prednisone |
| 26 | F | Yes | Yes | Yes | No | No | No | 2 | Prednisone, mycophenolate |
| 28 | M | Yes | Yes | Yes | Yes | No | Yes | 16 | Prednisone, mycophenolate, etanercept |
| 25 | M | Yes | Yes | Yes | No | No | Yes | 0 | Prednisone, chloroquine |
| 45 | F | Yes | Yes | No | No | No | No | 6 | Chloroquine |
| 39 | F | Yes | Yes | Yes | Yes | No | Yes | 25 | Prednisone, mycophenolate |
| 47 | F | Yes | Yes | no | Yes | No | No | 12 | Nothing |
| 33 | F | No | No | No | No | Yes | No | 10 | Prednisone |
| 28 | F | No | No | No | No | No | Yes | 12 | Prednisone |
Blood samples from patients who were not receiving any medication were withdrawn at disease onset. M = male; F = female; NSAIDs = non‐steroidal anti‐inflammatory drugs; SLEDAI = systemic lupus erythematosus disease activity index.
Isolation of peripheral blood mononuclear cells (PBMCs) and CD4+ T cells
A total of 20 ml of ethylenediamine tetraacetic acid (EDTA)‐K3‐preserved venous peripheral blood was withdrawn from both patients and controls. PBMCs were obtained by Hystopaque‐1077 (Sigma, Madrid, Spain) density gradient centrifugation. CD4+ T cells were isolated by negative selection with the CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the enriched CD4+ was evaluated by flow cytometry; the purity of CD4+ T was generally higher than 90%.
RNA isolation and real‐time quantitative–polymerase chain reaction (RT–PCR)
Total RNA from CD4+ T cells was isolated using the Ultraspec RNA isolation system (Biotecx Laboratories Inc., Huston, TX, USA). Reverse transcription (RT) was carried out with the QuantiTect Reverse Transcription kit (Qiagen, Izasa, Spain) and cDNA was amplified using the QuantiTect Multiplex PCR kit (Qiagen), according to the manufacturer's instructions. DUSP23 was quantified using the Taqman Gene expression assay (Hs00367783_m1) from ThermoFisher Scientific (Waltham, MA, USA). Primers (forward: 5′‐TCACCCACACTGTGCCCATCTACGA‐3′, reverse: 5′‐CAGCGGAACCGCTCATTGCCAATGG‐3') and probe (Yakima Yellow‐5′‐ATGCCCTCCCCCATGCCATCCTGCGT‐3′‐BHQ1) sequences of the reference gene (β‐actin) were from Eurofins MWG Synthesis GmbH (Ebersberg, Germany). Reactions for determining the expression of DUSP23 and β‐actin were carried out as duplex PCR. All reactions were run in duplicate in MicroAmp optical 96‐well plates sealed with optical adhesive covers (ThermoFisher Scientific) on an ABI PRISM 7000 Sequence Detection System. Negative controls (in which water instead of cDNA was added) were also run in each plate. Furthermore, contamination of the RNA samples by genomic DNA was excluded by an analysis without prior cDNA conversion, i.e. excluding reverse transcriptase from the RT reaction. Each assay included a standard curve for both genes. The standard curve was constructed with serial dilutions of RT products corresponding to different concentrations of total RNA from a reference cell line (Jurkat). Expression was compared to the standard curve and reported in an equivalent quantity of total RNA from the reference. Normalization of RNA amounts was performed using β‐actin expression analysed with the same procedure. Finally, expression ratios between DUSP23 and β‐actin were calculated.
Transcript levels of DNA methylation‐related enzymes (DNMT1, DNMT3A, DNMT3B, MBD2, MBD4, integrin subunit alpha L (ITGAL), perforin 1 (PRF1), killer‐cell immunoglobulin‐like receptor (KIR2DL4), CD70 and CD40L) were detected as described previously 24, 25, 26.
Genomic DNA extraction and measurement of DNA deoxymethylcytosine (dmC) content by enzyme‐linked immunosorbent assay (ELISA)
DNA dmC content was measured by means of an ELISA developed in our laboratory, as described previously 24.
Statistical analysis
The Mann–Whitney U‐test was used to compare values. Spearman's correlation was used to examine the relationship between two continuous variables. P‐values less than 0·05 were considered significant. All analyses were performed with the Prism GraphPad version 5.0 software.
Results
In order to evaluate the mRNA levels of DUSP23 in SLE CD4+ T cells we performed quantitative real‐time PCR assays. As β‐actin expression does not change in lupus T cells 27, we decided to choose this gene as a control to normalize mRNA levels. DUSP23 normalized values were considerably higher in the patient group than in the healthy control population: 1490 ± 1713 versus 294·1 ± 204·2, and these findings were statistically significant (P < 0·0001) (see Fig. 1). Of note, transcript levels varied widely among individuals. Men and women had similar transcript levels of DUSP23 in the control group (295·7 ± 232·3 versus· 292·2 ± 169·7, P = 0·645). Although not statistically significant, female SLE patients showed higher levels than male SLE patients (1879·29 ± 2725·22 versus 1392·39 ± 1426·80, respectively, P = 0·979). When age was taken into account, we did not observe any correlation with respect to DUSP23 mRNA levels either in the control or in the patient group.
Figure 1.
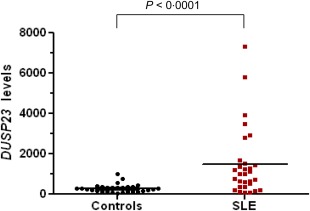
Comparison of dual‐specificity protein phosphatase 23 (DUSP23) transcript levels between SLE patients and healthy controls. [Colour figure can be viewed at wileyonlinelibrary.com]
We then assessed whether or not DUSP23 transcript levels were correlated with the main laboratory findings used to diagnose SLE patients. Several Spearman's correlation tests were performed. Statistically significant differences were not found between levels of DUSP23 mRNA and lymphocyte counts, titres of anti‐dsDNA, C3 and C4 complement levels and CH50 activity levels. Similarly, differences between patients grouped according to the presence or absence of lymphopenia, anti‐dsDNA or hypocomplementaemia were not statistically significant. Furthermore, patients with thrombocytopenia, leucopenia or haemolytic anaemia did not show different levels of DUSP23 when the comparison was established with those patients without these serological findings (see Table 2a).
Table 2.
Distribution of dual‐specificity protein phosphatase 23 (DUSP23) transcript levels in systemic lupus erythematosus (SLE) patients (mean ± standard deviation) according to the values of several serological features: high anti‐dsDNA titres: > 15 IU/ml; low complement C3 counts: < 85 mg/dl; low complement C4 counts: < 10 mg/dl; low CH50 activity: < 34 U/ml; low lymphocyte counts: < 1·2 × 109 cells/l; low platelet counts: < 150 × 109 platelets/l; low leucocyte counts: < 4·1 × 109 cells/l; presence of anti‐ribonucleoprotein (anti‐RNP) antibodies: > 12 U/ml; presence of anti‐Smith (anti‐Sm) antibodies: > 12 U/ml; presence of anti‐SSA/Ro antibodies: > 12 U/ml; presence of anti‐SSB/La antibodies: > 12 U/ml; presence of anti‐cardiolipin (aCL) immunoglobulin (Ig)G antibodies: > 15 GPL/ml ; presence of anti‐microsomal antibodies: > 35 IU/ml; presence of anti‐thyroglobulin antibodies: > 40 IU/ml.
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti‐dsDNA | C3 | C4 | CH50 | Lymphocyte counts | |||||
|
Normal n = 5 |
High n = 24 |
Normal n = 7 |
Low n = 22 |
Normal n = 10 |
Low n = 19 |
Normal n = 9 |
Low n = 20 |
Normal n = 11 |
Low n = 19 |
| 1283·97 ± 1063·94 | 1352·39 ± 1631·03 | 1082·79 ± 925·62 | 1422·62 ± 1690·02 | 1096·35 ± 1017·19 | 1469·15 ± 1756·11 | 1137·73 ± 1053·94 | 1431·88 ± 1721·54 | 1484·30 ± 1598·38 | 1492·94 ± 1818·82 |
| Platelet counts | Leucocyte counts | Haemolytic anaemia | |||
|---|---|---|---|---|---|
|
Normal n = 23 |
Low n = 7 |
Normal n = 17 |
Low n = 13 |
Presence n = 2 |
Absence n = 28 |
| 1614·92 ± 1935·60 | 1078·58 ± 433·38 | 1499·41 ± 1498·42 | 1477·17 ± 2024·16 | 805·87 ± 262·40 | 1538·62 ± 1764·08 |
| (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti‐RNP | Anti‐Sm | Anti‐SSA/Ro | Anti‐SSB/La | aCL | |||||
|
Presence n = 8 |
Absence n = 21 |
Presence n = 8 |
Absence n = 21 |
Presence n = 11 |
Absence n = 18 |
Presence n = 4 |
Absence n = 25 |
Presence n = 4 |
Absence n = 25 |
| 696·95 ± 580·96 | 1585·79 ± 1714·56 | 859·85 ± 653·99 | 1523·74 ± 1734·74 | 1632·65 ± 2122·97 | 1162·12 ± 1061·39 | 1119·94 ± 1596·02 | 1375·90 ± 1552·76 | 758·17 ± 574·44 | 1,433·78 ± 1622·36 |
| Lupus anti‐coagulant | Anti‐thyroglobulin* | Anti‐microsomals** | |||
|---|---|---|---|---|---|
|
Presence n = 3 |
Absence n = 26 |
Presence n = 9 |
Absence n = 16 |
Presence n = 8 |
Absence n = 17 |
| 2732·51 ± 4002·32 | 1179·99 ± 1043·33 | 2172·23 ± 1120·50 | 1041·17 ± 1731·36 | 2132·65 ± 1298·08 | 1126·33 ± 1678·41 |
*P = 0·001; **P = 0·037.
Similarly, patients who had different representative antibodies of the disease other than anti‐dsDNA, such as anti‐ribonucleoprotein (anti‐RNP), anti‐Smith (anti‐Sm), anti‐SSA/Ro, anti‐SSB/La, anti‐cardiolipin (aCL) or lupus anti‐coagulant (LA), did not show statistically significant different levels of DUSP23 when compared with those who did not test positive for these antibodies. Only those with anti‐thyroglobulin and/or anti‐microsomal antibodies had statistically significant high titres of DUSP23 (2172·23 ± 1120·50 versus 1041·17 ± 1731·36, P = 0·001; 2132·65 ± 1298·08 versus 1126·33 ± 1678·41, P = 0·037, respectively) (see Table 2b).
The presence of particular clinical manifestations, such as arthritis, nephritis, cutaneous lesions (including Raynaud's phenomenon, oral ulcers or just photosensitivity), pleuritis, pericarditis or neurological manifestations, was not associated with the presence of either higher or lower DUSP23 transcript levels. With regard to the disease activity, non‐statistically significant correlations were observed between DUSP23 transcript levels and SLEDAI values. Patients with a SLEDAI > 6 had higher DUSP23 mRNA levels than patients with a SLEDAI ≤ 6 (1760·52 ± 2049·94 versus 1083·65 ± 970·37, respectively) but it was not statistically significant.
We did not find statistically significant differences with regard to mRNA DUSP23 levels when we compared patients who had not received any drug at the time of sample withdrawal with those who had received some medication (1467·83 ± 1295·91 versus 1497·75 ± 1868·71, P = 0·65).
We also evaluated the associations between the expression of DUSP23 and the expression levels of five of the most comprehensively studied SLE epigenetically regulated genes, namely ITGAL, PRF1, KIR2DL4, CD70 and CD40L. Furthermore, we also carried out correlation analyses of the transcript expression of DUSP23 with the levels of several DNA methylation‐related enzymes and the global DNA methylation status. As seen in Fig. 2, meaningful statistical values were obtained in the patient group between the levels of DUSP23 and ITGAL, PRF1 and CD40L. Conversely, transcript levels of DUSP23 did not correlate with the expression of KIR2DL4 and CD70 (r = 0·016, P = 0·933; and r = 0·024, P = 0·899, respectively). Of note, 14 patients and 17 healthy controls did not express KIR2DL4 in their CD4+ T cells. In the control group, a statistically significant correlation was observed only between DUSP23 and ITGAL (r = 0·436, P = 0·016).
Figure 2.
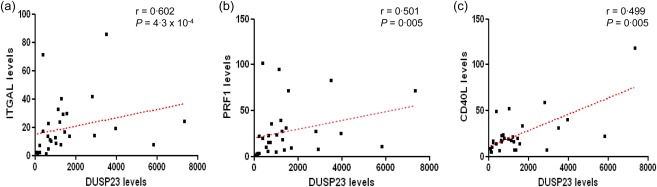
Correlations between transcripts levels of dual‐specificity protein phosphatase 23 (DUSP23) and integrin subunit alpha L (ITGAL) (a), DUSP23 and perforin 1 (PRF1) (b), and DUSP23 and CD40L (c) in CD4+ T cells of patients affected with SLE. Correlations are indicated by the Spearman's rank correlation coefficient (r) and the P‐value. [Colour figure can be viewed at wileyonlinelibrary.com]
Correlations between the expression of DUSP23 and DNMT1, DNMT3A, DNMT3B, MBD2 and MBD4 and the CD4+ T cell global DNA methylation status can be seen in Table 3. When studying the patient group we could detect that transcript levels of all the enzymes were correlated positively with the expression of DUSP23, which was not observed in the control group. Conversely, although DUSP23 expression in SLE patients tended to correlate negatively with the global DNA methylation status of CD4+ T cells, it was not statistically meaningful.
Table 3.
Correlation study between the transcript expression of dual‐specificity protein phosphatase 23 (DUSP23) and different DNA methylation‐related enzymes (DNMT1, DNMT3A, DNMT3B, MBD2 and MBD4) and the CD4+ T cell global DNA methylation status.
| DNMT1 | DNMT3A | DNMT3B | MBD2 | MBD4 | DNA dmC content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | |
| DUSP23 | r = 0·233 | r = 0·376 | r = 0·083 | r = 0·529 | r = 0·337 | r = 0·966 | r = 0·263 | r = 0·465 | r = 0·333 | r = 0·586 | r = 0·264 | r = −0·205 |
| P = 0·224 | P = 0·048 | P = 0·668 | P = 0·004 | P = 0·092 | P = 2·2 ×10−8 | P = 0·161 | P = 0·010 | P = 0·072 | P = 0·001 | P = 0·166 | P = 0·29 | |
Statistically significant correlations are indicated by the P‐value and the Spearman's rank correlation coefficient (r) highlighted in bold type.
Discussion
In this work, we have found that CD4+ T cells from SLE patients show enhanced levels of DUSP23 expression. Gender‐ and age‐related differences in DUSP23 transcripts levels were not observed, either in the control group or in the SLE population. DUSP23 transcript levels were not associated with any of the laboratory parameters and clinical features used currently to diagnose and evaluate the clinical course of SLE. Only those patients with anti‐thyroglobulin and/or anti‐microsomal antibodies had statistically significant high titres of DUSP23. Of note, patients with these antibodies also showed high levels of ITGAL (see our previous study 28). As DUSP23 expression correlated with the expression of ITGAL, it is possible that such an association may indeed be reflecting a true relationship with ITGAL.
Patients with a SLEDAI score > 6 tended to have a higher expression of DUSP23 when compared to patients with a low score. SLEDAI is a weighted, cumulative index of lupus disease activity that includes different parameters. That we did not find any independent statistically significant correlation with such variables may be due to the reduced number of patients with whom we resulted after stratifying them into each group. Alternatively, it is possible that the tendency observed with SLEDAI was found because it measures several variables at once. Thus, from our results we may conclude that DUSP23 may somehow reflect the severity of the disease when it is based on the simultaneous presence of different clinical and laboratory findings.
Clearly, the type of treatment received by the patients could account for these results. Statistically significant differences with regard to mRNA DUSP23 levels were not found between those patients who had not received any drug and those who had received some medication. Therefore, it seems that the high transcript DUSP23 levels observed in our patients may indeed reflect the disease status, i.e. they would not be the consequence of some drug administration.
T cell DNA demethylation plays an important role in the pathogenesis of SLE. Several studies have shown that DNA extracted from T cells of SLE patients is globally hypomethylated when compared to DNA from normal T cells 24, 29, 30. Some authors believe that it may be due to a defective extracellular receptor‐associated kinase (ERK) pathway. Signalling via this pathway is decreased in CD4+ T cells from SLE patients and causes decreased DNA methyltransferase (DNMT) expression 31, 32. Other authors, however, believe that the DNA hypomethylation observed in SLE may be caused by an over‐expression of proposed DNA demethylating enzymes, such as MBD2 and MBD4 24, 33, 34. When acting upon specific promoters, this DNA demethylation causes, in turn, the transcript over‐expression of certain genes in SLE patients, such as ITGAL, PRF1, KIR2DL4, CD70 and CD40L 35, 36, 37, 38, 39.
In previous studies our laboratory has demonstrated that SLE patients have significantly less CD4+ T cell DNA dmC content than healthy controls, and we have also found out that MBD2 and MBD4 mRNA levels are considerably higher in SLE 24. Interestingly, we also observed that our patients showed a negative correlation between global DNA methylation indices and MBD2 and MBD4 transcript levels. Based on these inverse correlations, we postulated the idea that both enzymes might have a direct and active role on the genomewide DNA hypomethylation observed in CD4+ T cells from SLE patients. Conversely, we did not observe differences in transcript levels for DNMT1, DNMT3A and DNMT3B between patients and controls 25. Nevertheless, simultaneous association of low complement counts with lymphopenia, high titres of anti‐dsDNA antibodies or a high SLEDAI resulted in the increase of at least one of the three DNA methyltransferases. Therefore, we believe that it is possible that patients are reacting indirectly to an underlying DNA hypomethylation status by increasing mRNA levels of DNA methyltransferases when the disease is definitely active.
It is well known that DNMT1 is up‐regulated by signals transmitted through the ERK and c‐Jun N‐terminal kinase (JNK) pathways 40. Sunahori et al. 41 have found that a phosphatase, protein phosphatase 2A (PP2Ac), is over‐expressed in SLE T cells. The increased PP2A expression and activity dephosphorylate the mitogen‐activated protein kinase (MEK) pathway, resulting in decreased ERK phosphorylation and decreased DNMT1 activity. Another phosphatase which also seems to inhibit signalling through the ERK and JNK pathways (and consequently leads to a decrease in DNMT1 and DNMT3A levels) is protein phosphatase 5 (PP5). CD4+ CD28– T cells express high levels of PP5, and this cell subset is increased notably in SLE patients 42, 43. Together, these observations indicate that the enhanced expression of particular phosphatases may impair these signalling pathways and may contribute to human lupus by decreasing DNA methylation, which eventually causes gene dysregulation and autoreactivity.
The mammalian genome encodes a large number of dual‐specificity phosphatases (DUSP), many of which act as mitogen‐activated protein kinase (MAPK) phosphatases. They may have specificity for particular MAPKs, such as ERK, JNK and p38. Thus, they become important regulators of MAPK signalling when these MAPKs are activated (and consequently, phosphorylated) in response to extracellular stimuli. Previous works have produced conflicting results on whether DUSP23 may dephosphorylate ERK or stimulate and activate JNK and p38. Wu et al. 8 showed that DUSP23 can dephosphorylate ERK1 in vitro, whereas Takagaki et al. 14 found that DUSP23 could not alter the activation of ERK (i.e. it did not act as a phosphatase) but rather enhanced the activation (i.e. phosphorylation) of JNK and p38 in COS‐7 cells induced by sorbitol. The latter study also demonstrated a higher level of phosphorylation of kinases MKK4 and MKK6, and it must be pointed out that this activation, as well as that experienced by JNK and p38, was not dependent upon the enzymatic activity of DUSP23. This being so, the in‐vitro effect carried out by DUSP23 on ERK may not be physiologically relevant, and we can infer that in‐vivo DUSP23 may perhaps act as a scaffold to allow MKK binding to JNK or p38 or, alternatively, it may participate in the inhibition of an as‐yet unidentified regulator which affects the JNK/p38 MAPK pathways negatively. If the latter hypothesis is correct, activation of JNK by DUSP23 would probably activate the synthesis of DNMT1. To support this idea further, it is important to point out that in the present work we found positive correlations between the transcript levels of DUSP23 and DNMTs in our SLE population.
Although DUSP23 transcript levels did not correlate negatively with the degree of global DNA methylation observed in SLE patients, DUSP23 expression did correlate with the expression of ITGAL, PRF1 and CD40L, three genes whose promoters are known to be hypomethylated in these patients and whose expression is linked directly to the expression of DNMTs 26. Conversely, DUSP23 transcript levels did not correlate with the levels of KIR2DL4 and CD70. In a previous study we did not find over‐expression of KIR2DL4 mRNA in our patients 26. It should be kept in mind that few patients and controls showed any expression of KIR2DL4 and, therefore, reliable conclusions cannot be drawn. With regard to CD70, although over‐expressed in our patients (as ITGAL, PRF1 and CD40L), we did not find any indication that DNMTs may be linked to its regulation 26. Therefore, we believe that it is possible that other mechanisms participate in its regulation. As demonstrated by other authors, both aberrant histone modifications within the CD70 promoter, as well as certain regulatory factors, may contribute to the increase of CD70 expression in SLE 44, 45.
In the present work we have established that DUSP23 transcript levels not only correlate directly with the levels of MBD2 and MBD4 (which may be responsible for the global DNA hypomethylation observed in CD4+ T cells from SLE patients), but are also associated positively with the mRNA expression of DNMT1, DNMT3A and DNMT3B. As DUSP23 seems to have the capacity to activate the JNK pathway, we believe that the enhanced expression detected in SLE patients may be triggered to induce the expression of DNMTs in an attempt to counterbalance the DNA hypomethylation status of the promoter of certain genes, such as ITGAL, PRF1 and CD40L. The lack of correlation between the mRNA levels of CD70 and the levels of DUSP23 would reinforce the idea that DUSP23 is aimed to be enhanced to induce enzymes which will act only on genes whose promoters are known to be susceptible to the action of DNMTs. Thus, although not over‐expressed, DNMTs would increase their levels to try to methylate (i.e. silence) the promoters of those genes whose over‐expression would be detrimental to the subject's health.
Disclosure
The authors declare that no disclosures exist.
Author contributions
E. B. designed the work, isolated the CD4+ T cells, performed the RT–PCRs, measured the DNA dmC content, analysed the results and wrote the paper; L. F. extracted the DNA and the RNA; J. O. R. collected samples from SLE patients and healthy controls; and M. V. T. supervised the work.
References
- 1. Castro J, Balada E, Ordi‐Ros J, Vilardell‐Tarres M. The complex immunogenetic basis of systemic lupus erythematosus. Autoimmun Rev 2008; 7:345–51. [DOI] [PubMed] [Google Scholar]
- 2. Moser KL, Neas BR, Salmon JE et al Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African‐American pedigrees. Proc Natl Acad Sci USA 1998; 95:14869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magnusson V, Johanneson B, Lima G, Odeberg J, Alarcon‐Segovia D, Alarcon‐Riquelme ME. Both risk alleles for FcgammaRIIA and FcgammaRIIIA are susceptibility factors for SLE: a unifying hypothesis. Genes Immun 2004; 5:130–7. [DOI] [PubMed] [Google Scholar]
- 4. Su K, Wu J, Edberg JC et al A promoter haplotype of the immunoreceptor tyrosine‐based inhibitory motif‐bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol 2004; 172:7186–91. [DOI] [PubMed] [Google Scholar]
- 5. Aitman TJ, Dong R, Vyse TJ et al Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 2006; 439:851–5. [DOI] [PubMed] [Google Scholar]
- 6. Wu J, Metz C, Xu X et al A novel polymorphic CAAT/enhancer‐binding protein beta element in the FasL gene promoter alters Fas ligand expression: a candidate background gene in African American systemic lupus erythematosus patients. J Immunol 2003; 170:132–8. [DOI] [PubMed] [Google Scholar]
- 7. Edberg JC, Wu J, Langefeld CD et al Genetic variation in the CRP promoter: association with systemic lupus erythematosus. Hum Mol Genet 2008; 17:1147–55. [DOI] [PubMed] [Google Scholar]
- 8. Wu Q, Li Y, Gu S et al Molecular cloning and characterization of a novel dual‐specificity phosphatase 23 gene from human fetal brain. Int J Biochem Cell Biol 2004; 36:1542–53. [DOI] [PubMed] [Google Scholar]
- 9. Quaio CR, Dutra RL, Brasil AS, Pereira AC, Kim CA, Bertola DR. A possible role of different PTPN genes in immune regulation. Scand J Immunol 2012; 75:540–1. [DOI] [PubMed] [Google Scholar]
- 10. Stanford SM, Bottini N. PTPN22: the archetypal non‐HLA autoimmunity gene. Nat Rev Rheumatol 2014; 10:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sunahori K, Juang YT, Kyttaris VC, Tsokos GC. Promoter hypomethylation results in increased expression of protein phosphatase 2A in T cells from patients with systemic lupus erythematosus. J Immunol 2011; 186:4508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual‐specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 2009; 418:475–89. [DOI] [PubMed] [Google Scholar]
- 13. Alonso A, Burkhalter S, Sasin J et al The minimal essential core of a cysteine‐based protein‐tyrosine phosphatase revealed by a novel 16‐kDa VH1‐like phosphatase, VHZ. J Biol Chem 2004; 279:35768–74. [DOI] [PubMed] [Google Scholar]
- 14. Takagaki K, Satoh T, Tanuma N et al Characterization of a novel low‐molecular‐mass dual‐specificity phosphatase‐3 (LDP‐3) that enhances activation of JNK and p38. Biochem J 2004; 383:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoyt R, Zhu W, Cerignoli F, Alonso A, Mustelin T, David M. Cutting edge: selective tyrosine dephosphorylation of interferon‐activated nuclear STAT5 by the VHR phosphatase. J Immunol 2007; 179:3402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Najarro P, Traktman P, Lewis JA. Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J Virol 2001; 75:3185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 1979; 301:5–8. [DOI] [PubMed] [Google Scholar]
- 18. Baechler EC, Batliwalla FM, Karypis G et al Interferon‐inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 2003; 100:2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baechler EC, Batliwalla FM, Reed AM et al Gene expression profiling in human autoimmunity. Immunol Rev 2006; 210:120–37. [DOI] [PubMed] [Google Scholar]
- 20. Vollmann J, Winau R. Informed consent in human experimentation before the Nuremberg code. BMJ 1996; 313:1445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 22. Bujan S, Ordi‐Ros J, Paredes J et al Contribution of the initial features of systemic lupus erythematosus to the clinical evolution and survival of a cohort of Mediterranean patients. Ann Rheum Dis 2003; 62:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum 1992; 35:630–40. [DOI] [PubMed] [Google Scholar]
- 24. Balada E, Ordi‐Ros J, Serrano‐Acedo S, Martinez‐Lostao L, Vilardell‐Tarres M. Transcript overexpression of the MBD2 and MBD4 genes in CD4+ T cells from systemic lupus erythematosus patients. J Leukoc Biol 2007; 81:1609–16. [DOI] [PubMed] [Google Scholar]
- 25. Balada E, Ordi‐Ros J, Serrano‐Acedo S, Martinez‐Lostao L, Rosa‐Leyva M, Vilardell‐Tarres M. Transcript levels of DNA methyltransferases DNMT1, DNMT3A and DNMT3B in CD4+ T cells from patients with systemic lupus erythematosus. Immunology 2008; 124:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balada E, Castro‐Marrero J, Felip L, Ordi‐Ros J, Vilardell‐Tarres M. Associations between the expression of epigenetically regulated genes and the expression of DNMTs and MBDs in systemic lupus erythematosus. PLoS One 2012; 7:e45897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klinman DM, Mushinski JF, Honda M et al Oncogene expression in autoimmune and normal peripheral blood mononuclear cells. J Exp Med 1986; 163:1292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balada E, Castro‐Marrero J, Felip L, Ordi‐Ros J, Vilardell‐Tarres M. Clinical and serological findings associated with the expression of ITGAL, PRF1, and CD70 in systemic lupus erythematosus. Clin Exp Rheumatol 2014; 32:113–6. [PubMed] [Google Scholar]
- 29. Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 1990; 33:1665–73. [DOI] [PubMed] [Google Scholar]
- 30. Zhu X, Liang J, Li F, Yang Y, Xiang L, Xu J. Analysis of associations between the patterns of global DNA hypomethylation and expression of DNA methyltransferase in patients with systemic lupus erythematosus. Int J Dermatol 2011; 50:697–704. [DOI] [PubMed] [Google Scholar]
- 31. Deng C, Kaplan MJ, Yang J et al Decreased Ras‐mitogen‐activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum 2001; 44:397–407. [DOI] [PubMed] [Google Scholar]
- 32. Gorelik G, Fang JY, Wu A, Sawalha AH, Richardson B. Impaired T cell protein kinase C delta activation decreases ERK pathway signaling in idiopathic and hydralazine‐induced lupus. J Immunol 2007; 179:5553–63. [DOI] [PubMed] [Google Scholar]
- 33. Lei W, Luo Y, Yan K et al Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol 2009; 38:369–74. [DOI] [PubMed] [Google Scholar]
- 34. Luo Y, Li Y, Su Y et al Abnormal DNA methylation in T cells from patients with subacute cutaneous lupus erythematosus. Br J Dermatol 2008; 159:827–33. [DOI] [PubMed] [Google Scholar]
- 35. Richardson BC, Strahler JR, Pivirotto TS et al Phenotypic and functional similarities between 5‐azacytidine‐treated T cells and a T cell subset in patients with active systemic lupus erythematosus. Arthritis Rheum 1992; 35:647–62. [DOI] [PubMed] [Google Scholar]
- 36. Kaplan MJ, Lu Q, Wu A, Attwood J, Richardson B. Demethylation of promoter regulatory elements contributes to perforin overexpression in CD4+ lupus T cells. J Immunol 2004; 172:3652–61. [DOI] [PubMed] [Google Scholar]
- 37. Basu D, Liu Y, Wu A et al Stimulatory and inhibitory killer Ig‐like receptor molecules are expressed and functional on lupus T cells. J Immunol 2009; 183:3481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oelke K, Lu Q, Richardson D et al Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum 2004; 50:1850–60. [DOI] [PubMed] [Google Scholar]
- 39. Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol 2007; 179:6352–8. [DOI] [PubMed] [Google Scholar]
- 40. Rouleau J, MacLeod AR, Szyf M. Regulation of the DNA methyltransferase by the Ras‐AP‐1 signaling pathway. J Biol Chem 1995; 270:1595–601. [DOI] [PubMed] [Google Scholar]
- 41. Sunahori K, Nagpal K, Hedrich CM, Mizui M, Fitzgerald LM, Tsokos GC. The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen‐activated protein kinase/extracellular signal‐regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in T‐cells from controls and systemic lupus erythematosus patients. J Biol Chem 2013; 288:21936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y, Gorelik GJ, Strickland FM, Richardson BC. Decreased ERK and JNK signaling contribute to gene overexpression in ‘senescent’ CD4+CD28– T cells through epigenetic mechanisms. J Leukoc Biol 2010; 87:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopez P, Rodriguez‐Carrio J, Martinez‐Zapico A, Caminal‐Montero L, Suarez A. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J Leukoc Biol 2016; 99:405–12. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Y, Qiu X, Luo Y et al Histone modifications and methyl‐CpG‐binding domain protein levels at the TNFSF7 (CD70) promoter in SLE CD4+ T cells. Lupus 2011; 20:1365–71. [DOI] [PubMed] [Google Scholar]
- 45. Zhao M, Sun Y, Gao F et al Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4+ T cells. J Autoimmun 2010; 35:58–69. [DOI] [PubMed] [Google Scholar]


