With the accelerated aging of society, the number of elderly patients with diabetes continues to rise in Japan1. The elderly have specific health problems that vary widely among individuals. In particular, susceptibility to severe hypoglycemia is a hallmark of elderly diabetes2, 3. Severe hypoglycemia not only impairs cognitive function4 but can also increase the risk of cardiovascular events5. Against this background, the Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes was launched in April 2015. As a first step toward developing ‘Clinical Practice Guidelines for the Management of Elderly Patients with Diabetes’, the Joint Committee launched an in‐depth debate on glycemic targets for elderly diabetes.
Over a series of discussions, the Joint Committee drew on the available reports on glycemic targets for the elderly, which included the Japanese Elderly Diabetes Intervention Trial (J‐EDIT) study6, the American Diabetes Association (ADA)/American Geriatrics Society (AGS) Consensus report7, International Diabetes Federation (IDF)'s Global Guideline8, and other relevant reports in the pertinent literature, and developed the ‘Glycemic Targets for Elderly Patients with Diabetes’ described in Figure 1 shown below.
The Joint Committee's consensus is summarized as follows:
The glycemic target is to be determined for each elderly patient by taking into account the patient's background characteristics and health status (e.g., age, cognitive function, physical function [basic and instrumental activities of daily living {ADL}]), comorbidities, risk for severe hypoglycemia, and life expectancy;
The lower limit of the glycemic target is to be targeted to ensure safer glycemic control in those likely to be at risk of severe hypoglycemia; and
The Joint Committee's recommendations allow the glycemic targets to be adjusted to exceed or fall below the glycemic targets suggested in the Table to ensure patient‐centered care while providing the glycemic targets and their lower limits as a basis for glycemic control in elderly patients with diabetes.
Figure 1.
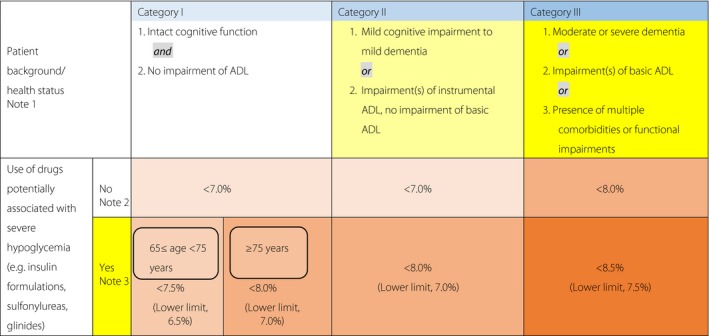
- Refer to the Japan Geriatrics Society website9, 10 for the evaluation of the cognitive function, basic ADL (e.g., self‐care abilities such as dressing, transferring, bathing, and toileting), and instrumental ADL (e.g., abilities to maintain an independent household such as shopping, meal preparation, taking medication, and handling finances,). In end‐of‐life care, priority is to be given to preventing significant hyperglycemia and subsequent dehydration and acute complications through appropriate therapeutic measures.
- As in other age groups, the glycemic target is set at <7.0% in the elderly for preventing diabetic complications. However, this could be set at <6.0% for those thought likely to achieve glycemic control through diet and exercise therapy alone or those likely to achieve glycemic control with drug therapy without adverse reactions, or 8.0% for those in whom intensifying therapy may prove difficult. In either case, no lower limit is specified for the glycemic target. A glycemic target of <8.5% may be allowed in patients thought to be in category III and therefore at risk of developing adverse reactions to multi‐drug combination therapy or in those with serious comorbidities or poor social support.
- In patients in whom priority should be given to preventing the onset/progression of diabetic complications due to their duration of disease, the glycemic target or its lower limit may be set for each elderly patient with appropriate measures in place to prevent severe hypoglycemia. Current treatments are to be continued in those less than 65 years of age despite their HbA1c values falling below their glycemic target or lower limit while on therapy, but care needs to be taken to monitor these patients for potential severe hypoglycemia. Glinides may be classified as drugs unlikely to be associated with severe hypoglycemia, as the onset of severe hypoglycemia varies depending on the type and amount of glinide used in a particular patient relative to the patient's glucose level.
Disclosure
Masakazu Haneda has received speaker honoraria from pharmaceutical companies Tanabe Mitsubishi Pharma Corporation, Boehringer Ingelheim GmbH, Taisho Toyama Pharmaceutical Co. Ltd., and Astellas Pharma Inc., research fundings from Astellas Pharma Inc., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and MSD Co. Ltd., Nobuya Inagaki has received speaker honoraria from pharmaceutical companies Boehringer Ingelheim GmbH, Sanofi Co. Ltd., Merck Sharp & Dohme, Astellas Pharma Inc., and Takeda Pharmaceutical Co. Ltd., research fundings from Merck Sharp & Dohme,, and Tanabe Mitsubishi Pharma Corporation, donations from Astellas Pharma Inc., Daiichi‐Sankyo Co. Ltd., Tanabe Mitsubishi Pharma Corporation, Takeda Pharmaceutical Co. Ltd., Merck Sharp & Dohme, Boehringer Ingelheim GmbH, Japan Tabacco Inc., Ono Pharmaceutical Co. Ltd., Dainippon Sumitomo Parma Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Pfizer Japan Inc., Sanofi Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., and Kissei Pharmaceutical Co. Ltd., Hirotaka Watada has received speaker honoraria from pharmaceutical companies Astellas Pharma Inc., AstraZeneca, Kowa Pharmaceutical Co. Ltd., Sanofi Co. Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharma Co. Ltd., Novo Nordisk Pharma Ltd., Boehringer Ingelheim GmbH, Tanabe Mitsubishi Pharma Corporation, MSD Co. Ltd., and Dainippon Sumitomo Parma Co. Ltd., research fundings from Novartis Pharma Co. Ltd., Eli Lilly Japan Co. Ltd., and Taisho Toyama Pharmaceutical Co. Ltd., donations from MSD Co. Ltd., Astellas Pharma Inc., AstraZeneca, Ono Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Sanofi Co. Ltd., Daiichi‐Sankyo Co. Ltd., Dainippon Sumitomo Parma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Tanabe Mitsubishi Pharma Corporation, Boehringer Ingelheim GmbH, Novo Nordisk Pharma Ltd., Pfizer, and Mochida Pharmaceutical Co. Ltd., and endowed department by Takeda Pharmaceutical Co. Ltd., and MSD Co. Ltd., Ryo Suzuki has received subsidies from Novo Nordisk Pharma Ltd., and Sanofi Co. Ltd., Hideki Ito has no conflict of interest, Atsushi Araki has received speaker honoraria from pharmaceutical companies Merck Sharp & Dohme, Dainippon Sumitomo Parma Co. Ltd., Kyowa Hakko Kirin Co. Ltd., AstraZeneca, Astellas Pharma Inc., Eli Lilly Japan Co. Ltd., Ono Pharmaceutical Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co. Ltd., and Boehringer Ingelheim GmbH, donation from Daiichi‐Sankyo Co. Ltd., Takashi Sakurai has received donation from Ono Pharmaceutical Co. Ltd., Koutaro Yokote has received speaker honoraria from pharmaceutical companies Astellas Pharma Inc., AstraZeneca, Daiichi‐Sankyo Co. Ltd., Dainippon Sumitomo Parma Co. Ltd., Eizai Co. Ltd., Kowa Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Mochida Pharmaceutical Co. Ltd., MSD Co. Ltd., Boehringer Ingelheim GmbH, Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Sanofi Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Shionogi Pharmaceutical Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Tanabe Mitsubishi Pharma Corporation,, research funding from Astellas Pharma Inc., donations from Astellas Pharma Inc., Brystol‐Meyers Co. Ltd., Daiichi‐Sankyo Co. Ltd., Dainippon Sumitomo Parma Co. Ltd., Eli Lilly Japan Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Mochida Pharmaceutical Co. Ltd., MSD Co. Ltd., Boehringer Ingelheim GmbH, Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Shionogi Pharmaceutical Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Tanabe Mitsubishi Pharma Corporation, Teijin Pharma Ltd., and Toyama Chemical Co. Ltd., endowed department by MSD Co. Ltd.
J Diabetes Investig 2017; 8: 126–128
In 2016, the Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes published one part of the committee report in ‘Treatment Guide for Diabetes 2016–2017; 98′ (Bunkodo, Tokyo, Japan, 2016) and ‘Practice Guideline for the Treatment for Diabetes in Japan 2016; 447–448’ (Nankodo, Tokyo, Japan, 2016) in Japanese. This is the English version of that report, and has been jointly published in Diabetology International(the official English journal of JDS), Geriatrics and Gerontology International (the official English journal of the JGS), and Journal of Diabetes Investigation (the official journal of the Asian Association for the Study of Diabetes).
Members of the JDS/JGS Joint Committee on Improving Care for Elderly Patients with Diabetes. JDS: Masakazu Haneda (Head, Professor Emeritus, Visiting Professor, Asahikawa Medical University, Asahikawa, Japan), Nobuya Inagaki (Professor, Department of Diabetes, Endocrinology and Nutrition, Kyoto University Graduate School of Medicine, Kyoto, Japan), Ryo Suzuki (Assistant Professor, Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan), Hirotaka Watabe (Professor, Department of Metabolism and Endocrinology, Juntendo University, Graduate School of Medicine, Tokyo, Japan), JGS: Hideki Ito (Head, C.E.O., Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo, Japan), Atsushi Araki (Executive Director, Department of Internal Medicine, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo, Japan), Takashi Sakurai (Director, Center for Comprehensive Care and Research on Memory Disorders, National Center for Geriatrics and Gerontology, Aichi, Japan), Koutaro Yokote (Professor, Department of Medicine, Chiba University, Graduate School of Medicine, Chiba, Japan).
References
- 1. National Health/Nutrition Survey 2012 : Summary of survey results. Part 1: Current status of diabetes. Ministry of Health, Labor and Welfare, pp 32–34, 2012(in Japanese) [Google Scholar]
- 2. Huang ES, Laiteerapong N, Liu JY, et al Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014; 174: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geller AI, Shehab N, Lovegrove MC, et al National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014; 174: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitmer RA, Karter AJ, Yaffe K, et al Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goto A, Arah OA, Goto M, et al Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 6. Araki A, Iimuro S, Sakurai T, et al Long‐term multiple risk factor interventions in Japanese elderly diabetic patients: The Japanese Elderly Diabetes Intervention Trial (J‐EDIT)—study design, baseline characteristics, and effects of intervention. Geriatr Gerontol Int 2012; 12(Suppl. 1): 8–17. [DOI] [PubMed] [Google Scholar]
- 7. Kirkman MS, Briscoe VJ, Clark N, et al Diabetes in older adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IDF global guideline . Managing older people with type 2 diabetes: Glucose control management and targets. pp 30–36, 2013.
- 9. Japanese Geriatrics Society . Methodology for evaluation of cognitive function and diagnosis of dementia—A useful tool for geriatric care. (in Japanese) http://www.jpn-geriat-soc.or.jp/tool/index.html. Accessed December 1, 2016.
- 10. Methodology for evaluation of ADL . Methodology for evaluation of cognitive function and diagnosis of dementia—A useful tool for geriatric care. (in Japanese) http://www.jpn-geriat-soc.or.jp/tool/index.html. Accessed December 1, 2016.
- 11. The Japan Geriatrics Society, Japan Agency for Medical Research and Development. Guidelines for Medical Treatment and its Safety in the Elderly 2015, Medical View Co., Ltd; Tokyo, 2015. (in Japanese) [Google Scholar]
- 12. Kojima T, Mizukami K, Tomita N, et al Report of the committee: screening Tool for Older Persons’ Appropriate Prescriptions in Japanese (STOPP‐J) – Report of the Japan Geriatrics Society Working Group on “Guidelines for Medical Treatment and its Safety in the Elderly”. Geriatr Gerontol Int 2016; 16: 983–1001. Published online 5 Sep, 2016. [DOI] [PubMed] [Google Scholar]


