Abstract
The HIV-1 Nef protein was analyzed for apoptotic structural motifs that interact with the CXCR4 receptor and induce apoptosis in CD4+ lymphocytes. Two apoptotic motifs were identified. One centered on Nef amino acids (aa) 50 to 60, with the overlapping 20-mer peptides retaining about 82% of the activity of the full Nef protein. The second centered on aa 170 to 180, with the overlapping 20-mer peptides retaining about 30% of the activity of the full protein. Significant apoptotic abilities were observed for 11-mer motif peptides spanning aa 50 to 60 and aa 170 to 180, with a scrambled version of the 11-mer motif peptide corresponding to aa 50 to 60 showing no apoptotic ability. Hallmarks of apoptosis, such as the formation of DNA ladders and caspase activation, that were observed with the full-length protein were equally evident upon exposure of cells to these motif peptides. A CXCR4 antibody and the endogenous ligand SDF-1α were effective in blocking Nef peptide-induced apoptosis as well as the physical binding of a fluorescently tagged Nef protein, while CCR5 antibodies were ineffective. The CXCR4-negative cell line MDA-MB-468 was resistant to the apoptotic peptides and became sensitive to the apoptotic peptides upon transfection with a CXCR4-expressing vector. A fluorescently tagged motif peptide and Nef protein displayed physical binding to CXCR4-transfected MDA-MB-468 cells, but not to CCR5-transfected cells. The removal of the apoptotic motif sequences from the full-length protein completely eliminated the ability of Nef to induce apoptosis. However, these modified Nef proteins still retained the ability to enhance viral infectivity. Thus, specific sequences in the Nef protein appear to be necessary for Nef protein-induced apoptosis as well as for physical interaction with CXCR4 receptors.
The reduction and killing of lymphocytes by retroviruses have traditionally been directly linked to the viral load, and the depletion process is induced by viral infectivity (21, 104). However, an alternative scenario (bystander effect) posits that lymphocyte killing leading to depletion is a result of apoptosis and that apoptosis predominantly occurs in uninfected, bystander cells, with a distinct lack of cell killing in the productively infected cells themselves (3, 23, 80). Many studies have contributed to the bystander effect premise that the longevity of infected cells is due to intracellularly expressed Nef protein (9, 23, 44, 107, 108). Alternatively, a second premise of the bystander effect scenario directly implicates viral proteins (i.e., Nef) and/or indirectly implicates virally stimulated cellular factors as mediators of bystander cell apoptosis (4, 10, 30-33, 40, 41, 45, 49, 50, 59, 61, 63, 72, 80-82, 93, 98, 109).
We have previously shown that the Nef proteins expressed by human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) efficiently induce apoptosis in T-cell lines, peripheral blood mononuclear cells (PBMCs), and other cell lines (60). Receptor-ligand and antibody competition studies, as well as receptor insertion experiments with cell lines lacking CXCR4 expression, revealed that the chemokine receptor CXCR4 is the surface receptor involved in Nef-induced apoptosis. These studies and others (41, 47, 91; also our unpublished data) directly showed that exogenous Nef protein is secreted extracellularly at concentrations that could contribute to the CD4+ lymphocyte depletion that occurs prior to and during the onset of AIDS. The body of evidence from patient, primate, and transgenic animal studies suggests that soluble Nef protein causes pathogenic effects, including T-cell depletion (3, 15, 28, 30-33, 36, 45, 49, 50, 63, 65, 68, 74, 88, 98). Thus, there is enough evidence to directly implicate the Nef protein in bystander cell death leading to CD4+-T-cell depletion, and we have identified the receptor through which Nef induces an apoptotic signal in T cells. The next step is to determine the mechanics of this receptor-ligand interaction that lead to programmed cell death. This is one step toward the development of therapeutics that can antagonize pathogenesis due to Nef and can prolong or possibly halt the progression toward AIDS.
Chemokines are a superfamily of small, cytokine-like proteins that induce cytoskeletal rearrangement and firm adhesion to endothelial cells and that are involved in directional migration (chemotaxis) through interactions with G-protein-coupled receptors. The chemokine receptor-ligand pair CXCR4-SDF-1α is unique in that SDF-1α is the only known ligand for this receptor (70, 76, 97, 112). The pair induces strong chemotactic efficacy for leukocytes in vitro and highly potent chemoattraction in vivo (8, 7, 70, 76, 97, 112). Both CXCR4- and SDF-1α-deficient mice display perinatal lethality because of profound defects in embryonic development of the hematopoietic, cardiovascular, and nervous systems (70, 76, 97, 112). These phenotypic changes are mediated by the disrupted migration of embryonic progenitor cells into the appropriate microenvironment. This suggests that SDF-1α-CXCR4 interactions are vital for the migration of nonhematopoietic as well as hematopoietic cells in vivo. Furthermore, in vivo studies using neutralizing antibodies to CXCR4 implicate this receptor in the homing and repopulation of human stem cells into the bone marrow of SCID mice (83). Finally, CXCR4 (or fusin, as it was originally named) has been shown to be a coreceptor for HIV-1 (7, 38).
For the CXC class of chemokines, and specifically for SDF-1α, important residues for receptor binding have been identified at the N terminus and in the loop region (RFFESH) following the two disulfide bridges (16-19, 27, 69, 105). The N-terminal region appears to be the most critical receptor binding site (18). These two sites appear to be unstructured in the solution structure of SDF-1α (27, 37). However, short N-terminal peptides of SDF-1α have been found to have most or part of the activity of the full SDF-1α chemokine (69).
The HIV-1 gp120 V3 loop has been shown to be a principal determinant of chemokine receptor specificity for CCR5 and CXCR4 (12, 13, 20, 96, 101, 106). One study suggested that chemokines and the C2-V3-C3 region of gp120 have a common origin (90). A second study identified a region located between the bases of the V1/V2 and V3 loops (86), which the authors suggested had remarkable amino acid conservation among CCR5- and CXCR4-tropic envelopes. The authors also suggested that this site represents a generic chemokine receptor binding domain that is capable of interacting with multiple chemokine receptors. However, the accumulated literature is unclear as to the exact motif(s) on gp120 that is involved in binding to the chemokine receptors during entry. Although gp120 has also been shown to induce apoptosis in many cell lines through CXCR4 (6, 24, 25, 42, 52, 53, 56, 58, 61, 64, 73, 87, 103), there is no literature that defines the apoptotic motif(s) or the mechanics of this receptor-ligand interaction.
The Nef protein is a 25- to 34-kDa N-terminally myristylated protein that is expressed by HIV-1, HIV-2, and SIV coding mRNAs (51). The protein is 205 amino acids (aa) long and is thus amenable to a full analysis to identify the apoptotic motif(s). For this study, we analyzed the Nef protein to determine the structural motifs on the protein that interact with the CXCR4 receptor and to show that they physically interact with CXCR4. Two apoptotic motifs were identified in Nef, and they appear to be necessary for the induction of apoptosis through CXCR4 by the full-length protein.
MATERIALS AND METHODS
Proteins and antibodies.
SDF-1α was obtained from Chemicon (Temecula, Calif.). vMIP-II was obtained from R&D Systems (Minneapolis, Minn.). The following antibodies were used: (i) monoclonal mouse anti-human fusin clone 12G5, also called mIgG2a[CXCR4] (Research Diagnostics Inc., Flanders, N.J.); (ii) a monoclonal mouse anti-human CCR5 antibody (Pharmingen, San Diego, Calif.); (iii) a monoclonal mouse anti-human CD4 antibody (American Bio-Technologies, Inc., Cambridge, Mass.); (iv) mouse immunoglobulin G (IgG) (Sigma, St. Louis, Mo.); (v) a monoclonal mouse anti-caspase 3 antibody (Active Motif, Carlsbad, Calif.); (vi) a rabbit anti-HIV-1 Nef antiserum (NIH AIDS Research and Reference Reagent Program, Rockville, Md.); (vii) goat anti-mouse IgG heavy plus light chains (H+L) labeled with horseradish peroxidase (Pierce, Rockford, Ill.); and (viii) goat anti-rabbit IgG(H+L) labeled with horseradish peroxidase (Pierce). A set of 20 HIV-1 Nef 20-aa peptides, each with a 10-aa overlap, was obtained from the NIH AIDS Research and Reference Reagent Program (Fig. 1A). These peptides were named by the number of their last amino acid, from N20 (Nef amino acids 1 to 20) through N205 (Nef amino acids 190 to 205). Fluorescently labeled Nef protein was obtained from Intracel Corporation (Carlsbad, Calif.). Eleven-amino-acid Nef peptides (Nef 50-60/Motif 1 [M1] and Nef 170-180/Motif 2 [M2]) encompassing the identified apoptotic motifs, an 11-aa randomly scrambled version of M1 (Nef scrambled motif 1 [sM1]), and the same peptides with fluorescent tags were obtained from Sigma Genosys (Houston, Tex.). The fluorescent tag on both the full-length Nef protein and the peptides was N-terminal fluorescein (NHS-ester; Flc). Ceramide was obtained from Sigma. The soluble gp120 protein from HIV-1 IIIb was obtained from Intracel and was found to be >90% pure, as estimated by Coomassie blue gel staining. gp120 was expressed from a baculovirus expression system and was full length and glycosylated. It was also shown that the protein is recognized by an anti-gp120 antibody in enzyme-linked immunosorbent assays, Western blots, and capture assays.
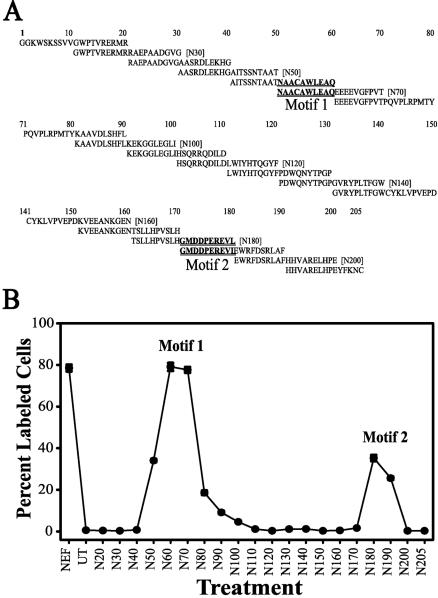
FIG. 1.
Analysis of HIV-1 Nef protein for an apoptotic motif(s). (A) A set of 20-mer peptides, each with a 10-aa overlap, spanning the 205 aa of the KNFS Nef protein were obtained from the AIDS Reagent Program. (B) Jurkat cell cultures were untreated (UT) or treated with a 10-ng/ml (5 to 6 nM) concentration of each of the Nef peptides (N20 to N205) or 100 ng (4.3 nM; Nef) of the Nef protein/ml for 24 h at 37°C. The cultures were then fixed and analyzed for apoptosis by a TUNEL assay. The y axis denotes percentages of the total cells that were TUNEL positive, and the x axis denotes the full Nef protein, untreated cells, or the peptides, starting with aa 1 to 20 (N20) and ending with aa 190 to 205 (N205). The error bars show the standard errors of the measurements, and the results are a compilation of at least three independent experiments. The peaks of apoptosis are denoted motif 1 and motif 2. The corresponding sequence region is denoted by the underlined sequences in panel A.
Nef-expressing plasmid.
We cloned the nef gene from pNL4-3 by PCR, using a forward primer (5′-GGG GGG AAG CCT CAT ATG GGT GGC AAG TGG TCA AAA AGT AGT GT-3′) engineered to contain an NdeI restriction site while retaining the start codon and a reverse primer (5′-GGA GGG CTG CAG TCA GTG ATG GTG ATG GTG ATG TCC GCC GGA TCC ACC GCA GTT CTT GAA GTA CTC CGG-3′) designed to contain a linker region followed by a hexahistidine tag, a stop codon, and a PstI restriction site. The amplified sequence was ligated into a pRSETB expression vector that was previously digested with the NdeI and PstI restriction enzymes. This digestion removed the N-terminal hexahistidine tag, the T7 gene 10 leader, and the Xpress epitope from the pRSETB vector. The recombinant plasmid, which contained a C-terminal hexahistidine tag, was referred to as pRSETB-Nef.
Expression and purification of recombinant HIV-1 Nef.
The full-length recombinant HIV-1 Nef protein was bacterially expressed in Escherichia coli BL21 (DE3) cells from the Nef expression plasmid pRSETB-Nef as previously described (60). Purification of expressed recombinant HIV-1 Nef was performed by use of a His-Bind kit (Novagen, Madison, Wis.) by which the Nef protein was affinity purified through binding of the C-terminal hexahistidine tag to nickel-charged tentacle methacrylate polymeric beads. Impurities were removed from the Ni-bound protein by a gradient of increasing imidazole concentrations. The final product was >95% pure, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The monomeric form of purified recombinant HIV-1 Nef protein resolved at approximately 32 kDa, with a lower concentration of the dimeric form occurring at approximately 50 kDa (data not shown). A protein analysis using the more sensitive method of surface-enhanced laser desorption-ionization mass spectrometry under nondenaturing conditions revealed the existence of Nef protein monomers, dimers, and trimers (data not shown). This suggests that the recombinant protein possessed stabilizing secondary and tertiary structures that normally exist in the native protein.
Development of Nef knockout clones. (i) pNefΔM1.
Clones expressing the deletion mutant Nef 52-61 were constructed by spliced overlap extension mutagenesis from the viral clone pNL4-3 KFS. The outer oligonucleotide primers were 5′CCT AGA AGA ATA AGA CAG GGC and 5′GCA GTT CTT GAA GTA CTC CGG. The internal PCR primers were 5′AAT ACA GCA GCT AAC GAG GAG GAA GAG GTG and 5′CAC CTC TTC CTC CTC GTT AGC AGC TGC TGT ATT.
(ii) pNefΔM1M2.
Clones expressing a double knockout were constructed by spliced overlap extension mutagenesis from the NefΔM1 clone. The outer oligonucleotide primers were 5′CCT AGA AGA ATA AGA CAG GGC and 5′GCA GTT CTT GAA GTA CTC CGG. The internal PCR primers were 5′CCT GTG AGC CTG CAT GAG TGG AGG TTT GAC and 5′GTC AAA CCT CCA CTC ATG CAG GCT CAC AGG.
Expression of Nef variant clones.
HEK 293 cells (107) were transfected with 6 μg of plasmid DNA from the HIV-1 vectors described above by use of the Effectene transfection reagent (Qiagen) according to the manufacturer's recommendations. The cultures were then allowed to grow and express the appropriate Nef protein in the medium for 48 to 96 h. The conditioned medium was then collected, analyzed for Nef protein, and used for assays.
Cell types.
Jurkat and H9 cells are CD4+-T-cell lines derived from human T-cell leukemia and human cutaneous T-cell lymphoma cells, respectively, and were obtained from the NIH AIDS Research and Reference Reagent Program. Fresh human PBMCs were obtained from Cambrex Bio Science (Walkersville, Md.). MDA-MB-468 and MDA-MB-231 cells were derived from human breast adenocarcinoma and human breast carcinoma cells, respectively, and were obtained from the American Type Culture Collection (Manassas, Va.). HEK 293 cells were derived from a human primary embryonic kidney transformed with adenovirus type 5 and were obtained from the NIH AIDS Research and Reference Reagent Program.
Cell culturing.
Jurkat, H9, MDA-MB-468, and MDA-MB-231 cells were sustained in RPMI 1640 medium (Invitrogen, Palo Alto, Calif.) supplemented with 10% heat-inactivated fetal bovine serum, streptomycin (100 U/ml), penicillin (100 U/ml), l-glutamine (2 mM), and HEPES buffered saline solution (30 μM). Alternatively, the cells were treated with various concentrations of and for various time periods with purified recombinant HIV-1 Nef or eukaryotic cell conditioned medium containing the Nef protein expressed from transfected Nef constructs at 37°C. HEK 293 cells were maintained in Dulbecco's modified Eagle's medium (Gibco, Carlsbad, Calif.) supplemented with 10% heat-inactivated fetal bovine serum and gentamicin (100 U/ml).
TUNEL assay.
Cultures were assayed for apoptosis by use of a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay as described previously (57). The cells were visualized by epifluorescence on a computer-controlled microscope system (Carl Zeiss, Thornwood, N.Y.). Microscopic images were processed with a charge-coupled device camera (MC 100 SPOT 60910; Photonic Science, East Sussex, United Kingdom). Further image processing was conducted with Image-Pro Plus 2.0 software (Media Cybernetics, Silver Spring, Md.).
DNA ladder formation and agarose gel electrophoresis.
A hallmark of apoptosis is internucleosomal cleavage, leading to the generation of discrete DNA fragments that are multiples of approximately 100 bp. For an assay of DNA ladder formation, Jurkat cell cultures were either untreated or treated with extracellular HIV-1 Nef for 24 h at 37°C. Nuclear extracts were obtained as described previously (57). Briefly, cellular and nuclear membranes were ruptured with lysis buffer (1.0% Nonidet P-40 [NP-40; Sigma], 50 mM Tris-HCl [pH 7.5], 20 mM EDTA) at room temperature for 2 min. The nuclear extracts were centrifuged at 15,340 × g for 20 min at 4°C, and then the RNAs were removed by RNase A (Sigma) digestion at 37°C for 2 h. All of the protein present was eliminated from the mixture by a proteinase K (Promega, Madison, Wis.) treatment at 56°C for 2 h. DNA precipitation was initiated by an overnight treatment with 4 M ammonium acetate and a 0.7 volume of isopropanol at −20°C. DNA purification was achieved by performing several 70% ethanol washes, followed by centrifugation at 15,340 × g for 30 min. Purified DNAs were air dried, resuspended in sterile water, and quantitatively and qualitatively analyzed by spectrophotometry (HP 8453 spectrophotometer; HP ChemStation, Palo Alto, Calif.). Twenty micrograms of DNA from each treatment was prepared in gel loading buffer that gave a final concentration of 0.02% bromophenol blue, 5% glycerol, 0.1% SDS, and 50 μg of ethidium bromide. The buffered DNA samples were resolved in a neutral 1.5% agarose gel at 6 V/cm for 4 h. The molecular weight marker used was the One kb Plus DNA ladder from Invitrogen.
Protein assay.
Jurkat cell cultures were examined for the activation of caspase 3. Jurkat cell cultures were either untreated or treated with extracellular HIV-1 Nef for 24 and 48 h. Cellular extracts were obtained by disrupting cells with lysis buffer (0.5% Triton X-100, 0.15 M NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 100 μg of aprotinin in 100 ml of phosphate-buffered saline) at room temperature for 2 min. After SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes by electroblotting, washed in Tris-buffered saline (20 mM Tris-HCl, 0.25 M NaCl, pH 7.5) supplemented with 0.01% (wt/vol) Tween 20, blocked for nonspecific binding with 5% (wt/vol) dry milk, and then probed with antibodies in 5% (wt/vol) dry milk. Caspase 3 was assayed with a mouse anti-caspase 3 antibody (Active Motif) as the primary antibody and an anti-mouse antibody as the secondary antibody. The mouse anti-caspase 3 antibody recognized both inactive or pro-caspase 3 and the larger catalytic subunit of active caspase 3. The Nef protein was visualized by use of a rabbit anti-HIV-1 Nef antiserum (NIH AIDS Research and Reference Reagent Program) as the primary antibody and goat anti-rabbit IgG(H+L) labeled with horseradish peroxidase (Pierce) as the secondary antibody. The visualization of protein-antibody complexes was achieved by incubation in ECL Plus Western blotting detection system solutions (Amersham Biosciences, Piscataway, N.J.) followed by exposure to photographic film (BioMax film; Fisher Scientific, Pittsburgh, Pa.).
Transfection.
Cells in log phase were pelleted and resuspended in RPMI 1640 (without serum) at a concentration of 4.5 × 106 cells per 400-μl aliquot. A 2.5-μg sample of CXCR4 plasmid DNA (Pc-Fusin; NIH AIDS Research and Reference Reagent Program) or CCR5 plasmid DNA (Pc-CCR5; NIH AIDS Research and Reference Reagent Program) was added to each aliquot of cells, transferred to 0.4-cm-electrode-gap-width electroporation cuvettes (Bio-Rad, Hercules, Calif.), and electroporated at 250 V with a 960-μF capacitance pulse and 200 Ω of resistance (Gene Pulser; Bio-Rad). The cells were allowed to rest at room temperature for 10 min, transferred to microcentrifuge tubes with 400 μl of fresh RPMI, and pelleted at 3,000 rpm for 5 min to remove debris. The cells were resuspended in 1 ml of RPMI (with serum) on plates and were incubated at 37°C for 48 h to allow expression of the transfected gene. We determined the average transfection efficiency of the MDA-MB-468 cell line to be about 80%. This was done by use of the pHcRed1 clone (BD Biosciences Clontech, Palo Alto, Calif.), which is a red fluorescing version of green fluorescent protein.
Infectivity assays.
Viruses containing the mutant Nef proteins were produced through transcomplementation of a clone containing a nef deletion with the Nef constructs, as previously described (66, 67). These viruses were tested in a single-round multinuclear activation of a galactosidase indicator (MAGI) infectivity assay to examine the effect that deletion of the apoptotic motif(s) had on viral infectivity.
Binding assay.
MDA-MB-468 cells in log phase were harvested, diluted to a concentration of 106 cells, and electroporated as described above. A fluorescently tagged peptide or Nef protein was diluted to 100 ng/ml in a nonfluorescent phosphate buffer, 0.1 M potassium phosphate mono/dibasic, pH 7.4 (PanVera Corp., Madison, Wis.), and 1 ml of the ligand solution was added to the plates and allowed to incubate for 1 h at 37°C. The first supernatant was removed from the plate, and the remaining unbound fluorescence was monitored with a fluorescence spectrometer (650-40 fluorescence spectrometer; Perkin-Elmer, Oak Brook, Ill.) with the excitation wavelength set at 488 nm and the emission wavelength set at 530 nm. The spectrometer was zeroed with pure nonfluorescent phosphate buffer. A positive control consisting of 1 ml of the fluorescently tagged ligand solution, unexposed to cell monolayers, was measured to obtain the total fluorescence available from a fixed concentration of tagged ligand. The cell monolayers were washed once with nonfluorescent phosphate buffer and then were treated with a 1-ml solution of proteinase K (50 μg/ml) (EM Science, Inc., Gibbstown, N.J.) for 2 h at 37°C. Subsequently, the second supernatant, containing the fluorescent tag from the bound peptide, was removed from the cell monolayers and counted as described above with a fluorescence spectrometer. The fluorescence data for each treatment condition were assayed for the ratio of unbound peptide (first supernatant measurement) to bound peptide (second supernatant measurement).
Data analysis.
Numerical and graphical analyses of all data obtained were performed by using SigmaPlot 8 software.
RESULTS
Identification of Nef apoptotic motifs.
We have previously shown that exogenous HIV-1 Nef can induce apoptosis in cells expressing the CXCR4 receptor, including lymphocytes (60). It had previously been shown by members of our laboratory and by others that HIV-1 gp120-induced apoptosis is mediated through the chemokine receptors CXCR4 and CCR5 (56, 58, 64, 73, 110). However, the mechanics by which either of these molecules interacts with CXCR4 to induce apoptosis are not clear. Peptides derived from SDF-1α have been shown to retain much of the binding and signaling activity of the full molecule (54). Thus, we speculated that peptides of Nef might retain some of the apoptotic activity of the full protein, and the Nef protein is small enough to be amenable to a full overlapping peptide analysis. A set of 20-mer peptides with 10-aa overlaps spanning the Nef protein (205 aa) (Fig. 1A) was obtained from the NIH AIDS Research and Reference Reagent Program. Jurkat cell cultures were exposed to each of the Nef peptides at 10 ng/ml (5 to 6 nM) for 24 h. The cultures were subsequently screened for apoptosis by a TUNEL assay (Fig. 1B). The first three N-terminal peptides, spanning aa 1 to 20 (N20), aa 11 to 30 (N30), and aa 21 to 40 (N40), induced only the background levels of apoptosis observed for an untreated control (Fig. 1B, compare the untreated point to points 20, 30, and 40). Peptide-driven apoptosis was observed beginning with the peptide spanning aa 31 to 50 (N50), which induced some apoptosis, peaking with the peptides spanning aa 41 to 60 (N60) and aa 51 to 70 (N70) (Fig. 1A, motif 1), and then dropping off to background levels with the peptide spanning aa 81 to 100 (N100) (Fig. 1A). Background levels of apoptosis were observed in cultures treated with peptides spanning aa 81 to 100 (N100) through aa 151 to 170 (N170) (Fig. 1A). A second, smaller apoptotic peak was observed for the peptides spanning aa 161 to 180 (N180) and aa 171 to 190 (N190) (Fig. 1A, motif 2). The C-terminal peptides spanning aa 181 to 200 (N200) and aa 1 to 205 (N205) (Fig. 1A) induced only background apoptotic levels. Thus, two regions in the HIV-1 Nef protein that induce apoptosis were identified (Fig. 1). The major peak, centering on aa 50 to 60 (motif 1 [M1]), induced >80% of the apoptotic levels of the full protein. A minor peak was also identified, centering on aa 170 to 180 (motif 2), which induced about 30% of the apoptotic levels of the full protein.
Characterization of peptide-induced apoptosis.
The classic markers used for the identification of apoptosis were observed in the Nef peptide-treated Jurkat cells. Caspase 3 is a late (or effector) caspase, being generated from its pro-nonenzymatic form through initiator caspase-mediated cleavage (22, 84, 94, 95, 99) by caspase 8 or 9. Caspase 3 is often referred to as the executioner, as its presence authenticates the occurrence of apoptosis and confirms that the cell is destined, or “programmed,” for cell death (22, 84, 94, 95, 99). The high-molecular-mass procaspase 3 (32 kDa) was observed after both 24 and 48 h of Nef peptide treatment. The occurrence of the catalytic subunits of active caspase 3 as well as the ceramide-positive control (Fig. 2A) was observed after a 24-h exposure of Jurkat cell cultures to Nef peptides. Extensive expression of active caspase 3 (17 kDa) was detected at 48 h posttreatment (Fig. 2A). No similar induction was observed when Jurkat cells were untreated or were exposed to HIV-1 gp120 IIIb or sM1 after both 24 and 48 h of exposure. The 50-kDa tubulin band was assayed as a gel loading control.
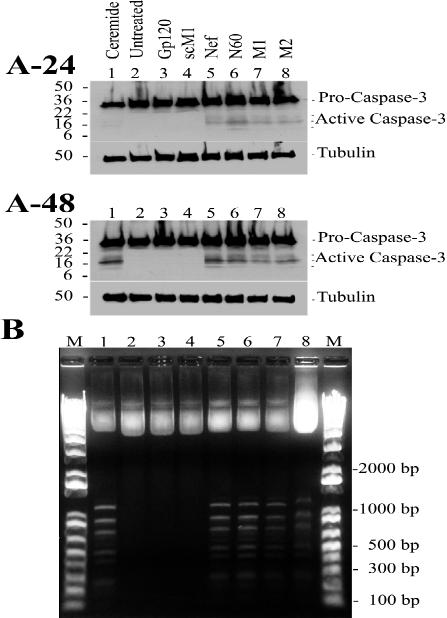
FIG. 2.
Identification of hallmarks of apoptosis. (A) Jurkat cell cultures were exposed to ceramide (lane 1; 23.4 μM), no treatment (lane 2), gp120 IIIb (lane 3; 0.22 nM), sM1 (lane 4; 4.2 nM), Nef protein (lane 5; 4.3 nM), N60 (lane 6; 5.8 nM), M1 (lane 7; 4.2 nM), or M2 (lane 8; 4.3 nM) for 24 h (A-24) or 48 h (A-48). Twenty micrograms of total protein was loaded per lane. Caspase 3 activation was tested by Western blot analysis. The high-molecular-mass band (32 kDa) was procaspase 3, and the large catalytic subunit of active caspase 3 was 17 kDa. Tubulin (50 kDa) was the gel loading control. Prestained SDS-PAGE standards (broad range) (Bio-Rad) were used as molecular weight markers. The image was formatted with Adobe Illustrator 8.0 software. (B) DNAs from Jurkat cell extracts were resolved by agarose gel electrophoresis, stained with ethidium bromide, and visualized by exposure to UV light. Pictures were taken with the DP12 microscope digital camera system (Olympus Optical Co., Ltd., Melville, N.Y.), and the image was generated with Adobe Photoshop 5.0 software and formatted with Adobe Illustrator 8.0 software. The results are typical representations of at least two independent experiments. The lanes are as follows: the One kb Plus DNA ladder marker (lanes M), ceramide (lane 1; 23.4 μM, 24 h), untreated cells (lane 2; 24 h), gp120IIIb (lane 3; 0.22 nM, 24 h), sM1 (lane 4; 4.2 nM, 24 h), Nef protein (lane 5; 4.3 nM, 24 h), N60 (lane 6; 5.8 nM, 24 h), M1 (lane 7; 4.2 nM, 24 h), and N180 (lane 8; 5.7 nM, 24 h). Note that the treatments shown at the top of panel A-24, corresponding to lanes 1 to 8, apply similarly for lanes 1 to 8 of panels A-48 and B.
DNA bands with approximately 100-bp differences in molecular size in an agarose gel provide evidence of extensive internucleosomal cleavage of the genomic DNA, which is a common phenomenon in apoptotic cells. Additional confirmation of peptide-induced apoptosis was obtained by the detection of DNA ladders in Jurkat cell cultures exposed to Nef peptides (Fig. 2B). No similar induction was observed in untreated Jurkat cells or in cells exposed to HIV-1 gp120 IIIb or sM1 after both 24 and 48 h of exposure.
Specificity of peptide interaction with CXCR4.
We have previously shown that Nef-induced apoptosis acts through the CXCR4 receptor (60). We showed that the breast tumor cell line MDA-MB-468, which does not express CXCR4, is refractory to Nef-induced apoptosis. When Pc-Fusin, which contains the gene coding for the CXCR4 receptor, was transfected into and expressed in MDA-MB-468 cells, the transfected line became susceptible to Nef-induced apoptosis (Fig. 3A). To determine if Nef peptides act in a similar fashion to the full-length protein, we transfected MDA-MB-468 cultures with Pc-Fusin, an empty vector (pCR3.1), or a CCR5 cDNA clone. At 48 h posttransfection, the cultures were treated with (i) HIV-1 Nef protein (N), (ii) an 11-aa peptide encompassing either apoptotic motif 1 (the M1 peptide) or apoptotic motif 2 (the M2 peptide), and (iii) the Nef peptide spanning aa 111 to 130 (N130), which does not induce apoptosis (Fig. 1). The cells were treated for 24 h and subsequently assayed for apoptosis by a TUNEL assay (Fig. 3A). Untransfected MDA-MB-468 cells, as well as cells transfected with the empty vector or the CCR5 cDNA clone, were refractory to all Nef-induced apoptosis (Fig. 3A, bars 1 to 5, 11 to 14, and 15 to 18). In contrast, MDA-MB-468 cells expressing CXCR4 were susceptible to Nef-induced apoptosis (Fig. 3A, bars 7, 8, and 10). N130 had no effect on untransfected or Pc-Fusin-transfected MDA-MB-468 cells (Fig. 3A, compare bars 4 and 9). All of the other nonapoptotic peptides described in Fig. 1B were also not apoptotic in CXCR4-transfected MDA-MB-468 cells (data not shown).
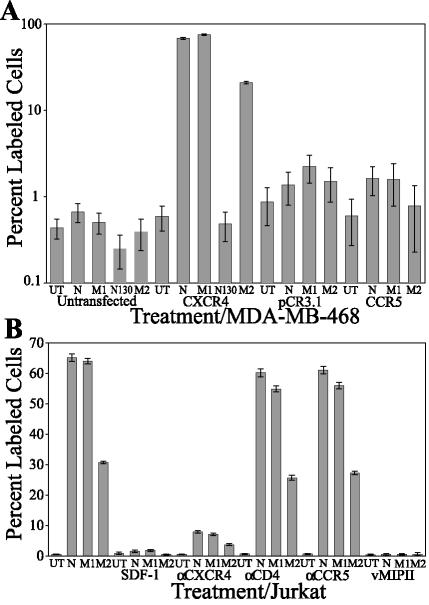
FIG. 3.
Specificity of Nef peptide-induced apoptosis to CXCR4. (A) MDA-MB-468 cultures were either untransfected or transiently transfected with a vector, CCR5, or CXCR4 cDNA clone. Bars 1 to 5 represent experiments performed on untransfected MDA-MB-468 cells, bars 6 to 10 represent experiments performed on Pc-Fusin (CXCR4)-transfected MDA-MB-468 cells, bars 11 to 14 represent experiments performed on pCR3.1-transfected MDA-MB-468 cells, and bars 15 to 18 represent experiments performed on CCR5-transfected MDA-MB-468 cells. At 48 h posttransfection, the cultures were treated for 24 h as follows: for bars 1, 6, 11, and 15, cells were treated with medium (UT); for bars 2, 7, 12, and 16, cells were treated with Nef protein (N; 4.3 nM); for bars 3, 8, 13, and 17, cells were treated with M1 (4.2 nM); for bars 4 and 9, cells were treated with the 20-mer peptide N130 (5.7 nM); and for bars 5, 10, 14, and 18, cells were treated with M2 (4.3 nM). In the graph, the y axis is shown on a log scale to allow viewing of the treatments that did not induce apoptosis. (B) Jurkat cell cultures were pretreated with medium (bars 1 to 4), SDF-1α (bars 5 to 8; 4.7 nM), CXCR4 antibody (bars 9 to 12; 5 μg/ml), CD4 antibody (bars 13 to 16; 10 ng/ml), CCR5 antibody (bars 17 to 20; 5 μg/ml), or vMIP-II (bars 21 to 24; 5.5 nM). Subsequently, a subset of those cultures were treated with medium (UT; bars 1, 5, 9, 13, 17, and 21), Nef protein (N; bars 2, 6, 10, 14, 18, and 22; 4.3 nM), M1 (bars 3, 7, 11, 15, 19, and 23; 4.2 nM), or M2 (bars 4, 8, 12, 16, 20, and 24; 4.3 nM). The error bars show the standard errors of the measurements, and the results are a compilation of at least three independent experiments.
The ability of the Nef protein to induce apoptosis can be blocked by pretreatment with the ligand SDF-1α or with an antibody to CXCR4 (Fig. 3B) (60). To determine if the Nef peptides displayed similar characteristics to the full-length Nef protein, we performed a competition assay (Fig. 3B). Jurkat cell cultures were either untreated or pretreated with the competitive ligand SDF-1α, vMIP-II, which is a human herpesvirus 8-encoded protein that has been shown to interact with CXCR4 (111), or antibodies to the receptors involved in HIV infection, specifically CXCR4, CCR5, and CD4. Subsequently, these cultures were untreated or treated with the HIV-1 Nef protein, the M1 peptide, or the M2 peptide for 24 h and then assayed by TUNEL (Fig. 3B). Untreated cultures displayed little background apoptosis (Fig. 3B, bar 1 [0.51%]). The pretreatment of cultures with SDF-1α or vMIP-II blocked Nef-induced effects, reducing apoptosis to background levels in these cultures (Fig. 3B, compare bars 1 to 4 with bars 5 to 8 and bars 21 to 24, respectively). For example, apoptosis in M1-treated cells (Fig. 3B, bar 3 [47.6%]) was blocked by SDF-1α and vMIP-II, which reduced the amount of apoptosis to 0.49% (bar 7) and 0.12% (bar 23), respectively. Pretreatment with an anti-CXCR4 antibody significantly blocked Nef-induced apoptosis in the cultures, although it did not reduce the amount of apoptosis to background levels (Fig. 3B, compare bars 1 to 4 with bars 9 to 12). In M1-treated cells (Fig. 3B, bar 3 [47.6%]), apoptosis was significantly blocked by the anti-CXCR4 antibody, although it was not reduced to background levels (Fig. 3B, bar 11 [5.97%]). Alternatively, pretreatment with an anti-CD4 or anti-CCR5 antibody had no effect on Nef-induced apoptosis (Fig. 3B, compare bars 1 to 4 with bars 13 to 16 and bars 17 to 20). Pretreatment with an anti-CD4 antibody had no significant effect on M1-induced apoptosis (Fig. 3B, compare bar 3 [47.6%] with bar 15 [40.1%]).
Analysis of apoptotic motifs.
The previously described evidence showed that a 20-mer Nef peptide overlapping aa 50 to 60 (motif 1) accounts for the major portion of apoptosis. We wanted to narrow down the effective region that drives apoptosis. Therefore, two 11-mer peptides spanning motif 1 were made, with one having the correctly ordered sequence (Fig. 1A, TNAACAWLEAQ [motif 1]) and the other having the same amino acids scrambled in a random order (sM1; ALAETCQNAWA). Jurkat cell cultures were untreated, exposed to approximately equimolar concentrations of the Nef protein (100 ng/ml; 4.3 nM) and the 20-mer Nef peptide N60 (10 ng/ml; 5.8 nM), or exposed to the M1 or sM1 peptide (5 ng/ml; 4.2 nM) for 24 h (Fig. 4A). The cultures were subsequently screened for apoptosis by a TUNEL assay. The untreated controls displayed background levels of apoptosis (Fig. 4A, UT [0.56%]). The 11-mer M1 peptide induced 82% of the apoptosis observed with the full-length Nef protein (Fig. 4, compare bar 4 [44.1%] with bar 2 [63%]). In contrast, the sM1 peptide induced only the background levels of apoptosis observed for the untreated control (Fig. 4A, compare bar 5 [1.08%] with bar 1 [0.56%]). Therefore, it appears that Nef aa 50 to 60 (M1) are sufficient to induce an apoptotic signal.
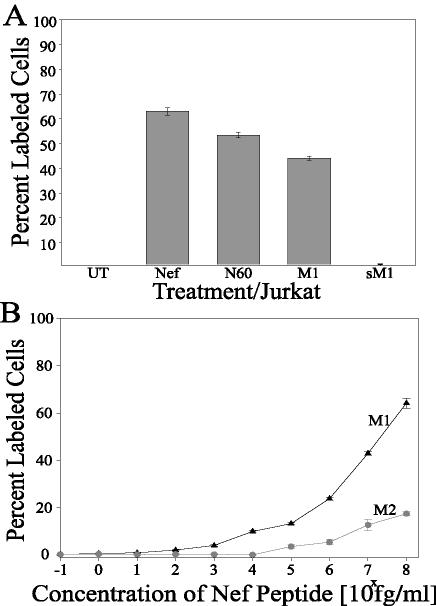
FIG. 4.
Analysis of apoptotic motifs. (A) Specificity of Nef-induced apoptosis to the identified motif regions. Jurkat cell cultures were treated with the specified Nef peptides for 24 h and subsequently fixed and assayed for apoptosis by TUNEL. Bar 1 represents the results from Jurkat cells treated with medium (UT), bar 2 represents Jurkat cells treated with Nef protein (N; 4.3 nM), bar 3 represents Jurkat cells treated with the 20-mer peptide N60 (5.8 nM), bar 4 represents treatment with M1 (4.2 nM), and bar 5 represents treatment with sM1 (4.2 nM). (B) Dose-response analysis of apoptotic motifs. Jurkat cell cultures were treated with either the M1 peptide or M2 peptide at different concentrations and subsequently fixed and assayed for apoptosis by TUNEL. The peptide concentrations ranged from 1 fg/ml (0.849 fM) to 100 ng/ml (84.9 nM). The error bars show the standard errors of the measurements, and the results are a compilation of at least three independent experiments.
We examined the apoptotic potency of the Nef motifs. Jurkat cultures were exposed to increasing concentrations of either the M1 peptide or the M2 peptide and were subsequently analyzed for apoptosis (Fig. 4B). Nef M1 displayed an apoptotic effect significantly above the background starting at 103 fg/ml (1 pg/ml; 0.849 pM), with an increasing induction of apoptosis throughout the entire range of concentrations analyzed above 0.849 pM (Fig. 4B, M1 curve). The 50% effective concentration (EC50) for M1-induced apoptosis was calculated to be 39.6 ng/ml (33.6 nM). In contrast, the Nef M2 peptide required a much larger dosage to induce an apoptotic effect significantly above background, starting at 105 fg/ml (100 pg/ml; 84.9 pM). Again, an increasing induction of apoptosis was observed throughout the entire range of concentrations analyzed above 84.9 pM (Fig. 4B, M2 curve). However, the EC50 was not reached at the highest concentration examined, 100 ng/ml (84.9 nM). The full-length Nef protein displayed an EC50 of 390.4 ng/ml (16.77 nM) (60), and the EC50 for SDF-1α chemotactic activity is 5 nM (18). Thus, M1 appeared to be quite potent apoptotically, and M2 appeared to be approximately 100-fold less potent than M1.
Deleting the Nef motifs from the full Nef protein.
To confirm that the peptides identified in our assays were important for the induction of apoptosis in the context of the full protein, we created deletion mutants. One construct had the nucleic acid sequences corresponding to aa 51 to 61 (motif 1) removed from pNL4-3 KFS and was named pNefΔM1. The second construct had nucleic acid sequences corresponding to aa 51 to 61 (motif 1) and aa 170 to 180 (motif 2) removed from pNL4-3 KFS and was named pNefΔM1M2. These constructs were transfected and expressed in HEK 293 cells, and the conditioned supernatants were collected and analyzed. We previously showed that the Nef protein is secreted into the extracellular medium from transfected cells (60).
To determine if the deletion mutants retained functionality, we tested the ability of each protein to enhance the infectivity of a virus with a deletion of nef (NL4-3KFSΔNef). NL4-3KFSΔNef was transcomplemented with a NefΔM1 (Fig. 5A, bar 4), NefΔM1M2 (Fig. 5A, bar 5), or wild-type Nef expression plasmid. The resulting virions were then examined in a single-round MAGI infectivity assay. The infectivities of the viruses that were transcomplemented with the mutated Nef proteins were well above those of the parent virus with a deletion of nef (Fig. 5A, bar 2) and were not significantly different from those of viruses that were transcomplemented with wild-type Nef (Fig. 5A, bar 3). Thus, the mutants were functional for an enhancement of infectivity, suggesting that there is no significant effect of the deletions on Nef's ability to enhance infectivity. Furthermore, all of the constructs were found to express similar amounts of Nef protein into the culture medium (Fig. 5B).
FIG. 5.
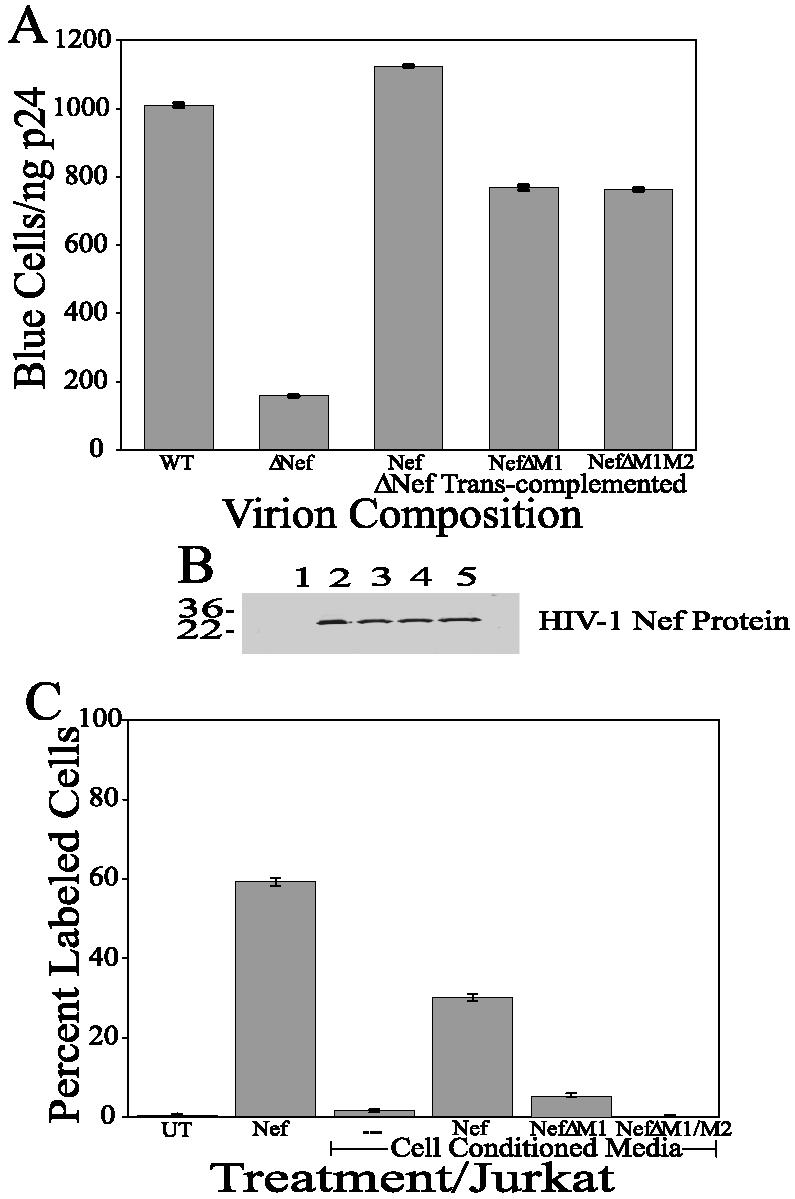
Relationship between Nef modifications and changes in Nef-induced apoptosis. (A) The changes in Nef-induced apoptosis are not the results of instability of the resultant Nef protein. Virions were transcomplemented with mutant Nef proteins, and the resultant viruses were analyzed for infectivity and the ability of the Nef protein to enhance infectivity. Bar 1 represents the infectivity of the wild-type HIV-1 virus in a MAGI assay; bar 2 represents the infectivity of a virus with a deletion of Nef; bars 3, 4, and 5 represent the infectivities of viruses with a deletion of Nef that were transcomplemented with normal Nef (bar 3), NefΔM1 (bar 4), or NefΔM1M2 (bar 5). The error bars show the standard errors of the measurements, and the results are a compilation of at least three independent experiments. (B) The changes in Nef-induced apoptosis are not the results of variations in Nef protein expression. A Western blot analysis with 40 μl of HEK 293 conditioned medium was performed by using an anti-Nef antibody. Lane 1: untransfected HEK 293 cell conditioned medium; lane 2: HIV Nef protein (1 μg); lane 3: HIV-1 Nef-transfected HEK 293 cell conditioned medium; lane 4: HIV-1 NefΔM1-transfected HEK 293 cell conditioned medium; lane 5: HIV-1 NefΔM1M2-transfected HEK 293 cell conditioned medium. (C) Deletion of apoptosis motifs in the Nef protein eliminates Nef-induced apoptosis. Jurkat cell cultures were treated with conditioned cell supernatants from HEK 293 cells expressing various Nef variants to analyze the effect of the changes in Nef on Nef-induced apoptosis. Bar 1 represents the results for Jurkat cells treated with medium (UT); bar 2 represents treatment with Nef protein (N; 4.3 nM); bar 3 represents treatment with conditioned medium from untransfected HEK 293 cells; bar 4 represents treatment with 40 μl of conditioned medium from pNL4-3 KFS-transfected HEK 293 cells; bar 5 represents treatment with 40 μl of conditioned medium from pNefΔM1-transfected HEK 293 cells; and bar 6 represents treatment with 40 μl of conditioned medium from pNefΔM1M2-transfected HEK 293 cells. The error bars show the standard errors of the measurements, and the results are a compilation of at least three independent experiments.
To determine the effects of the mutations on the ability of the full Nef protein to induce apoptosis, we examined the Nef protein secreted into the extracellular medium in our apoptosis assay. Jurkat cell cultures were exposed for 24 h to (i) normal medium, (ii) bacterially expressed Nef protein (100 ng/ml; 4.3 nM), (iii) conditioned medium from untransfected HEK 293 cells, (iv) conditioned medium from wild-type Nef-transfected cells, (v) conditioned medium from pNefΔM1-transfected cells, and (vi) conditioned medium from pNefΔM1M2-transfected cells. These cultures were subsequently screened for apoptosis by a TUNEL assay (Fig. 5C). Jurkat cells treated with normal medium and conditioned medium from untransfected HEK 293 cells displayed only background levels of apoptosis (Fig. 5C, bar 1 [0.75%] and bar 3 [1.6%]). Jurkat cells treated with the bacterial Nef protein at 100 ng/ml displayed significant apoptosis (Fig. 5C, bar 2 [59.2%]), as did those treated with conditioned medium from wild-type Nef-transfected cells (Fig. 5C, bar 4 [30.2%]). Apoptosis in Jurkat cells treated with conditioned medium from pNefΔM1-transfected cells was reduced more than fivefold compared to Jurkat cells treated with conditioned medium from normal Nef-transfected cells (Fig. 5C, compare bar 5 [5.6%] with bar 4 [30.2%]). However, the amount of apoptosis induced by conditioned medium from pNefΔM1-transfected cells on Jurkat cells was still threefold higher than the background levels observed in Jurkat cells treated with conditioned medium from untransfected cells (Fig. 5C, compare bar 5 [5.6%] with bar 3 [1.6%]). Apoptosis dropped to background levels in Jurkat cells treated with conditioned medium from pNefΔM1M2-transfected cells (Fig. 5C, compare bar 6 [0.46%] with bar 3 [1.6%]). As shown in Fig. 1, we observed some apoptosis induced by the N50 peptide. The total elimination of apoptosis upon the deletion of both M1 (aa 51 to 61) and M2 (aa 170 to 180) negates the evidence above suggesting a third apoptotic site.
Effect on primary lymphocytes.
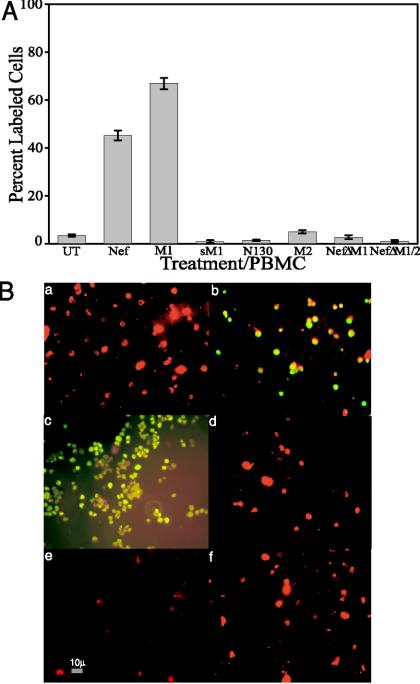
Previously, we showed that the soluble Nef protein induced apoptosis in unstimulated human PBMCs at levels similar to that observed in other cell lines (60). The data described above suggested that an examination of the apoptotic effect of the Nef peptides and the ΔNef proteins would yield similar results to those observed in cultured cells. Unstimulated human PBMCs were treated with Nef protein, M1, sM1, N130, M2, NefΔM1, or NefΔM1M2 (Fig. 6). A concentration of 100 ng of Nef/ml of medium exhibited 45.5% (Fig. 6A, bar 2) apoptosis, compared to only 3.42% (Fig. 6A, bar 1) in untreated cells and 1.39% (Fig. 6A, bar 5) in PBMCs treated with N130. M1 induced 66.81% (Fig. 6A, bar 3) apoptosis compared to 0.89% (Fig. 6A, bar 4) apoptosis in PBMCs treated with sM1, and the second apoptotic motif, M2, induced 4.94% (Fig. 6A, bar 6) apoptosis in PBMCs. Cell-conditioned medium containing NefΔM1 induced 2.71% (Fig. 6A, bar 7) apoptosis in the PBMC cultures, displaying a 17-fold reduction in the ability to induce apoptosis compared to the wild-type bacterial Nef protein. The elimination of both motifs in NefΔM1M2 reduced the ability of that protein to induce apoptosis another threefold, to 0.97% (Fig. 6A, bar 8).
FIG. 6.
PBMCs were either untreated or exposed to Nef (4.3 nM), various Nef peptides (M1 and sM1, 4.2 nM; M2, 4.3 nM), or ΔNef proteins (40 μl) for 24 h. (A) Apoptotic cells were detected by TUNEL, and then percentages of apoptosis in treated cells were compared to the levels in untreated cells. Data from two experiments performed in triplicate with 12 individually treated cell sets were pooled to generate average values and were used to determine standard errors. (B) PBMCs processed for TUNEL (FITC; green) were then stained by using a CD4 primary antibody and a secondary antibody tagged with Texas Red (red). Typical combined FITC-Texas Red images of fields of PBMCs are shown. (a) Untreated; (b) bacterial Nef protein, 4.3 nM; (c) M1, 4.2 nM; (d) sM1, 4.2 nM; (e) M2, 4.3 nM; (f) NefΔM1M2, 40 μl. The images were taken via fluorescence microscopy and arranged with Adobe Photoshop 5.0.2 software. Magnification, ×400.
Nef protein- or peptide-treated PBMCs were processed for TUNEL (fluorescein isothiocyanate [FITC]; green) and then stained by use of a CD4 primary antibody and a secondary antibody tagged with Texas Red (red). Figure 6B shows typical fields of PBMCs that were either untreated (a) or treated with bacterial Nef protein (b), M1 (c), sM1 (d), M2 (e), or NefΔM1M2 (f). The images are combined FITC-Texas Red images. As can be seen in the figure, in the Nef protein (b), M1 (c), and M2 (e) panels there are many CD4+ cells (red) that also display TUNEL staining (green), which indicates which CD4+ cells are apoptotic (yellow). Alternatively, the untreated (a), sM1 (d), and NefΔM1M2 (f) panels show no TUNEL staining (green) and thus show only CD4+ cells (red). We observed similar Nef motif-induced apoptotic effects on CD8+ T cells (data not shown). Thus, the apoptotic Nef motifs display the ability to kill unstimulated human lymphocytes.
Nef interactions with CXCR4.
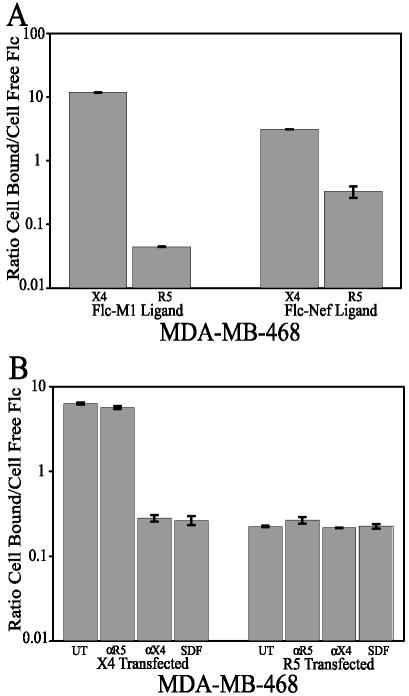
We already generated data that suggested, but did not confirm, that Nef physically interacts with the CXCR4 chemokine receptor (60). A fluorescent-ligand binding assay was used to examine the ability of the Nef protein and the M1 peptide to interact with CXCR4. The breast tumor cell line MDA-MB-468, which we previously used to show the involvement of CXCR4 in Nef-induced apoptosis, was used in this assay. MDA-MB-468 cells, which normally are refractory to Nef-induced apoptosis, become sensitive when they are transfected with Pc-Fusin, which expresses CXCR4 (60). MDA-MB-468 cultures were transfected with Pc-Fusin or Pc-CCR5 and incubated for 48 h to allow transient expression. These cultures were then exposed to a fluorescently tagged Nef protein or M1 peptide. The first supernatants, which contained unbound fluorescently tagged ligand, were removed from the cultures and assayed for their fluorescence levels. The cultures were treated with proteinase K to strip off the cell membrane-bound ligand (fluorescently tagged), and the second supernatant was assayed for fluorescence levels. The resultant data were plotted for each condition to obtain a bound fluorescence/unbound fluorescence ratio. This directly measured the ability of the fluorescently tagged ligand to interact with the cell monolayer (Fig. 7). If the ligand bound to the cell membrane, one would expect to obtain a ratio of >1, favoring bound molecules over unbound molecules. Alternatively, if the ligand was unbound (not binding to the cell membrane), a ratio of <1 should be observed, favoring unbound molecules over bound molecules. MDA-MB-468 cells transfected with Pc-CCR5 displayed a very low ratio, with Flc-M1 giving a ratio of 0.04 (Fig. 7A, bar 2) and Flc-Nef giving a ratio of 0.11 (Fig. 7A, bar 4). Alternatively, MDA-MB-468 cells transfected with Pc-Fusin and expressing CXCR4 had very high ratios, with Flc-M1 giving a ratio of 11.9 (Fig. 7A, bar 1), and Flc-Nef giving a ratio of 6.7 (Fig. 7A, bar 3). This clearly shows that both the Nef protein and the Nef M1 peptide only physically interact with these cells when the CXCR4 receptor is on the cell surface.
FIG. 7.
Analysis of CXCR4 binding. MDA-MB-468 cultures were transfected with Pc-Fusin or Pc-CCR5 and allowed to transiently express the specific receptor. (A) The cultures were then exposed to a fluorescently tagged (with Flc) ligand, either the Nef protein (4.3 nM) or M1 (4.2 nM) peptide, for 1 h at 37°C. The first supernatants, containing unbound fluorescently tagged ligand, were removed and assayed for fluorescence levels. The cultures were treated with proteinase K to strip off the cell membrane-bound ligand (fluorescently tagged), and these second supernatants were assayed for fluorescence levels. The resultant data were plotted for each condition to obtain a bound fluorescence (first supernatant)/unbound fluorescence (second supernatant) ratio. Bars 1 and 3 represent CXCR4-transfected MDA-MB-468 cells, and bars 2 and 4 represent CCR5-transfected MDA-MB-468 cells. Bars 1 and 2 represent cultures exposed to the fluorescently tagged motif 1 peptide; bars 3 and 4 represent cultures exposed to the fluorescently tagged Nef protein. (B) The cultures were exposed to tagged Nef protein (4.3 nM) alone or tagged Nef protein with one of the following competitive ligands: SDF-1α (4.7 nM), CXCR4 antibody (5 μg/ml), or CCR5 antibody (5 μg/ml). The supernatants were collected and assayed as described above. Bars 1 to 4 represent CXCR4-transfected MDA-MB-468 cells, and bars 5 to 8 represent CCR5-transfected MDA-MB-468 cells. Bars 1 and 5 represent cells exposed to tagged Nef alone, bars 2 and 6 represent cells exposed to tagged Nef and CCR5 antibody, bars 3 and 7 represent cells exposed to tagged Nef and CXCR4 antibody, and bars 4 and 8 represent cells exposed to tagged Nef and SDF-1α. The error bars show the standard errors of the measurements, and the results are a compilation of at least two independent experiments.
To further show that the Nef protein interacts physically with CXCR4, we combined the binding assay with a competition assay using known CXCR4 ligands. The binding assay was used as described above, with one variation. During exposure to the tagged Nef ligand, either an anti-CXCR4 antibody, an anti-CCR5 antibody, or SDF-1 was added at a high concentration. Competitive ligands will block any potential interaction of CXCR4 with the tagged Nef ligand, driving the derived high ratios down toward favoring the unbound state. In fact, that was what was observed. MDA-MB-468 cells transfected with Pc-Fusin with no competitive ligand gave a very high ratio of 6.3 (Fig. 7B, bar 1), as expected, as did the same ligand competing with the anti-CCR5 antibody, displaying a ratio of 5.6 (Fig. 7B, bar 2). In contrast, Pc-Fusin-transfected cultures exposed to the tagged ligand and competing with the anti-CXCR4 antibody or SDF-1 displayed very low ratios of 0.28 (Fig. 7B, bar 3) for the anti-CXCR4 antibody and 0.27 (Fig. 7B, bar 4) for SDF-1. Low ratios were observed for all conditions for Pc-CCR5-transfected cells (Fig. 7B, bars 5 to 8), as expected for cells not expressing CXCR4. These data support and extend the evidence that the Nef protein and the M1 peptide interact with these cells by physically interacting with the CXCR4 receptor.
DISCUSSION
The evidence presented above describes the initial steps in defining the mechanics of Nef-mediated apoptosis. Using overlapping peptides that spanned the entire length of the Nef protein, we have identified two motifs within the HIV-1 Nef protein that are required for the induction of apoptosis through CXCR4. One major peak of apoptotic activity centered on aa 50 to 60 of Nef (motif 1) and could be narrowed down to an 11-mer peptide which was almost as effective (82%) at inducing apoptosis as the full protein. A second, minor peak of apoptotic activity was identified, centering on aa 170 to 180 (motif 2), with a 20-mer peptide containing this motif found to induce about 30% of the apoptotic activity of the full protein. The removal of the residues corresponding to either motif 1 alone or both motifs 1 and 2 from the complete Nef protein (NefΔM1 or NefΔM1M2) resulted in a significant reduction in the apoptosis kinetics of the resultant proteins compared to those in Jurkat cells treated with normal Nef. Neither deletion changed the protein's ability to enhance infectivity or to be expressed extracellularly. Interestingly, there is some literature suggesting that under certain conditions (in neuronal cells) SDF-1 can induce apoptosis through the CXCR4 receptor (53). Similarly, the HIV protein gp120 has been shown to induce apoptosis through the CXCR4 receptor in certain cell lines (6, 24, 25, 42, 52, 53, 56, 58, 61, 64, 73, 87, 103). In multiple unpublished experiments using soluble gp120, we have observed no gp120-induced apoptotic effect on Jurkat cells, H9 cells, or human PBMCs, although we found that the same protein induced significant apoptosis in other CXCR4+ cell types (57; unpublished data). Interestingly, when we transfected Jurkat cells with Pc-Fusin, we observed a rescue of gp120-induced apoptosis (data not shown). This suggests either (i) a fundamental difference in the lymphocytic CXCR4 receptor or the CXCR4-stimulated apoptotic signaling pathway in lymphocytes or (ii) some type of dose-response mechanism wherein the numbers of CXCR4 receptors on lymphocytes are too low to induce an apoptotic signal with gp120 as a ligand but are sufficient for a Nef-induced effect. The evidence on gp120-induced apoptosis in lymphocytes is conflicting, with some studies suggesting that soluble gp120 alone is sufficient to induce apoptosis in lymphocytes (6, 87), while other studies suggest that special conditions are required for gp120-induced apoptosis (5, 26, 55, 89). Thus, the literature is not clear about what effect gp120 has on lymphocytes, although we clearly saw no apoptotic effect of gp120, as evidenced by several different apoptotic markers. The evidence presented here suggests that (i) there are specific motifs on the Nef protein that can induce apoptosis; (ii) these motifs are necessary to cause apoptosis; and (iii) since a Nef protein with both motifs removed retained other activities, it is not likely that a gross structural rearrangement explains the loss of apoptotic activity.
A two-site model of chemokine-receptor interaction has been proposed for SDF-1 and CXCR4 in which the amino terminus of the chemokine receptor plays a major role in the initial binding of SDF-1, while an interaction of SDF-1 with the loops of CXCR4 transmits an activation signal (2, 29, 43, 75, 92). The determination of the nuclear magnetic resonance structure of SDF-1α and accompanying analyses of SDF-1α mutants (27) and SDF-1α-derived peptides (54) provided a model for the interaction of SDF-1α and CXCR4 that was confirmed by other studies (34). In this model, during an initial docking step, the N-terminal region of CXCR4 interacts with the RFFESH motif comprised of aa 12 to 17 of SDF-1α. This step leads to conformational changes that allow the subsequent interaction of CXCR4 with the N-terminal residues of SDF-1α (aa 1 to 11; KPVSLSYRCPC), triggering a functional response. Interestingly, peptides consisting of SDF-1α aa 1 to 17 retain much of the activity of the full chemokine, showing that the sequence structure that is important for interacting with CXCR4 is among the first 17 aa (37, 54, 69, 102).
Recognition and affinity between chemokines and their receptors are thought to be initiated by charge complementarity (48, 62). SDF-1α is one of the most basic chemokines, with an overall charge of +8. The CXCR4 receptor's extracellular region has a net charge of −9 (111). Additionally, for other chemokines it has been shown that a combination of a hydrophobic surface surrounded by ionic residues is a common characteristic (48, 62). SDF-1α has a surface with a high positive potential adjacent to an extended hydrophobic crevice (111). Interestingly, the V3 loop of T-cell-tropic gp120 has a high net positive charge overall and relative to the V3 loops of M-tropic strains (78). The Nef apoptotic motifs identified here do not fit this amino acid sequence characteristic, as they have little charge and what charge they do have is acidic. Furthermore, sequence comparisons made between the two Nef apoptotic motifs (aa 50 to 60 and 170 to 180) and the V3 loop of gp120, SDF-1α, and vMIP-II showed no obvious relationship (data not shown). Finally, no obvious sequence similarities were found by a comparison of either Nef motif with the full sequence of the HIV-2 or SIV Nef protein. However, this initial work to identify motifs will allow subsequent studies, which are currently in progress, to map the Nef motif amino acids that are critical for (i) CXCR4 binding and (ii) interactions with CXCR4 leading to apoptosis.
A recent paper has described gp120- and SDF-1-induced CXCR4 cell surface interactions, as examined by fluorescence resonance energy transfer (FRET) (100). Toth et al. suggested that CXCR4 dimerization is involved in SDF-1α- and gp120-induced signaling events (100). The gp120 IIIb-induced increase in CXCR4-associated FRET was dependent on the expression of CD4 and was decreased by an AMD3100 cotreatment. Interestingly, in the absence of CD4, gp120 IIIb actually decreased the amount of CXCR4-associated FRET. The authors of that study suggested that the binding of gp120 to CXCR4 might not fully reproduce the repertoire of signaling events that are normally produced by the natural ligand, SDF-1α. Their hypothesis could similarly describe Nef-induced signaling through CXCR4. This aberrant signaling could have deleterious consequences on cell survival.
In our previous work, molecular competition analysis revealed that SDF-1α, the endogenous ligand for CXCR4, as well as anti-CXCR4 antibodies, was protective against Nef protein-induced apoptosis. Although this suggested that these CXCR4 ligands physically block the ability of the Nef protein to interact with the CXCR4 receptor, we could not eliminate the possibility that both SDF-1α and the anti-CXCR4 antibody induce a protective signal through CXCR4 that competes with Nef-induced apoptosis and that the Nef protein interacts with some as yet unknown surface factor. However, the physical binding data presented here show that Nef and the Nef apoptotic peptides only interact with the cell membranes of MDA-MB-468 cells when CXCR4 is on the cell membrane. Furthermore, SDF-1α and the anti-CXCR4 antibody physically blocked the ability of the Nef protein to interact with the cell membranes of Pc-Fusin-transfected MDA-MB-468 cells. We obtained similar results by using flow cytometry to analyze Nef or Nef apoptotic peptide binding to CXCR4 (data not shown). The data clearly suggest that it is this physical competition for interaction with the receptor that eliminates the ability of Nef or Nef apoptotic peptides to induce apoptosis. Thus, this evidence makes a strong case that Nef directly interacts with the CXCR4 receptor and induces apoptosis through this interaction.
As discussed above, an alternate hypothesis (the bystander effect) to explain T-lymphocyte depletion which is supported by an increasing amount of evidence is that this depletion is a result of programmed cell killing or apoptosis (39). This hypothesis directly implicates viral proteins or indirectly implicates virally stimulated cellular factors as mediators of apoptosis. In fact, many studies implicate Nef either as a mediator of infected cell longevity or as a mediator of bystander cell death (4, 23, 30-33, 41, 44-46, 49, 50, 59, 60, 63, 79-82, 93, 98, 107, 108). The validity of soluble Nef protein inducing bystander effects hinges on (i) a soluble Nef protein that induces cell killing in the extracellular environment and (ii) a weak immune response to the Nef protein, or at least to the Nef apoptotic sequences, that does not block Nef-induced apoptosis.
Data from our previous experiments (60), the results discussed in this paper, and the results of other studies (41, 47, 71, 91) clearly show that Nef can induce apoptosis in T cells and support the existence of soluble Nef protein in the extracellular environment. We have already discussed several potential mechanistic scenarios that might explain Nef secretion (60).
There is also evidence that addresses the second premise of a weak immune response to the Nef protein, or at least to the Nef apoptotic sequences, that does not block Nef-induced apoptosis. Fujii et al. found that anti-Nef antibody titers in patient sera were inversely related to the amounts of Nef present and that 5 of 32 patient samples tested had undetectable levels of Nef protein but exhibited the highest titers of anti-Nef antibody (41). This clearly shows that the immune response to Nef varies widely. Anti-Nef antibody responses were also shown to vary significantly among individuals in several other studies (11, 85). Additionally, a large body of data exists describing Nef cytotoxic T lymphocytes and antibody epitopes (for example, see the HIV Molecular Immunology Database [http://hiv-web.lanl.gov/content/immunology]; 1, 14, 35, 77). An analysisof these data yielded overwhelming evidence that the immune response to the Nef regions of aa 50 to 60 and 170 to 180, which we have identified as important for the induction of apoptosis, is at best weak, and probably nonexistent, in primates and mice. However, the same data showed that the Nef protein has several epitopic sequential regions (other than the two mentioned above) that induce strong immune responses. This evidence supports the second premise in that the specific sequential regions that induce apoptosis (aa 50 to 60 and 170 to 180) seem to generate, at best, a weak immune response. This level of response may not be sufficient to block soluble extracellular Nef-induced apoptosis. Thus, we argue that the weak immune response to specific epitopes (motifs 1 and 2), not to the overall Nef protein, is what is important for allowing Nef-driven bystander effects, which lead to the culpability of the soluble Nef protein in HIV pathogenesis.
Ultimately, this line of investigation could lead to the development of therapeutics (e.g., a vaccine) that target the Nef apoptotic motifs. The identified motifs, if attached to an appropriate immunogen and injected into a subject, could cause an induction of a strong immune response against those specific epitopes. This should confer direct protection against viral Nef-induced pathogenesis. A vaccine of this type would not be designed to induce sterilizing immunity but to block the ability of soluble Nef protein to induce apoptosis, specifically in T cells but also in other cell types (e.g., the endothelium). This could alleviate lymphocyte depletion and organ damage and prolong or possibly slow the progression toward AIDS.
Acknowledgments
We thank William Roth for helpful discussions and for editing the manuscript.
This work was supported by NIH/NIGMS/MBRS (grant 58268) and NIH/NCRR/RCMI (grant G12-RR03034). The set of 20 HIV-1 Nef peptides and the rabbit anti-HIV-1 Nef antiserum were obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, Md.).
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja, S. K., J. C. Lee, and P. M. Murphy. 1996. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J. Biol. Chem. 271:225-232. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. W., M. S. Ascher, and H. W. Sheppard. 1998. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: a worst-case dynamic analysis. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 17:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Azad, A. A. 2000. Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem. Biophys. Res. Commun. 267:677-685. [DOI] [PubMed] [Google Scholar]
- 5.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. H. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biard-Piechaczyk, M., V. Robert-Hebmann, V. Richard, J. Roland, R. A. Hipskind, and C. Devaux. 2000. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329-344. [DOI] [PubMed] [Google Scholar]
- 7.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 8.Bleul, C. C., R. C. Fuhlbrigge, J. M. Casasnovas, A. Aiuti, and T. A. Springer. 1996. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boecker, W., H. Buerger, K. Schmitz, I. A. Ellis, P. J. van Diest, H. P. Sinn, J. Geradts, R. Diallo, C. Poremba, and H. Herbst. 2001. Ductal epithelial proliferations of the breast: a biological continuum? Comparative genomic hybridization and high-molecular-weight cytokeratin expression patterns. J. Pathol. 195:415-421. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., Y. K. Yip, I. George, M. Tyorkin, E. Salik, and K. Sperber. 1998. Chronically HIV-1-infected monocytic cells induce apoptosis in cocultured T cells. J. Immunol. 161:4257-4267. [PubMed] [Google Scholar]
- 11.Chen, Y. M., R. H. Lin, C. M. Lee, C. Y. Fu, S. C. Chen, and W. J. Syu. 1999. Decreasing levels of anti-Nef antibody correlate with increasing HIV type 1 viral loads and AIDS disease progression. AIDS Res. Hum. Retrovir. 15:43-50. [DOI] [PubMed] [Google Scholar]
- 12.Cho, M. W., M. K. Lee, M. C. Carney, J. F. Berson, R. W. Doms, and M. A. Martin. 1998. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J. Virol. 72:2509-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 14.Choppin, J., W. Cohen, A. Bianco, J. P. Briand, F. Connan, M. Dalod, and J. G. Guillet. 2001. Characteristics of HIV-1 Nef regions containing multiple CD8+ T cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J. Immunol. 166:6164-6169. [DOI] [PubMed] [Google Scholar]
- 15.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark-Lewis, I., B. Dewald, T. Geiser, B. Moser, and M. Baggiolini. 1993. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc. Natl. Acad. Sci. USA 90:3574-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark-Lewis, I., B. Dewald, M. Loetscher, B. Moser, and M. Baggiolini. 1994. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J. Biol. Chem. 269:16075-16081. [PubMed] [Google Scholar]
- 18.Clark-Lewis, I., K. S. Kim, K. Rajarathnam, J. H. Gong, B. Dewald, B. Moser, M. Baggiolini, and B. D. Sykes. 1995. Structure-activity relationships of chemokines. J. Leukoc. Biol. 57:703-711. [DOI] [PubMed] [Google Scholar]
- 19.Clark-Lewis, I., C. Schumacher, M. Baggiolini, and B. Moser. 1991. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J. Biol. Chem. 266:23128-23134. [PubMed] [Google Scholar]
- 20.Cocchi, F., A. L. DeVico, A. Garzino-Demo, A. Cara, R. C. Gallo, and P. Lusso. 1996. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat. Med. 2:1244-1247. [DOI] [PubMed] [Google Scholar]
- 21.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 22.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 24.Constans, J., M. Seigneur, A. D. Blann, M. Renard, F. Resplandy, J. Amiral, V. Guerin, M. R. Boisseau, and C. Conri. 1998. Effect of the antioxidants selenium and beta-carotene on HIV-related endothelium dysfunction. Thromb. Haemost. 80:1015-1017. [PubMed] [Google Scholar]
- 25.Corasaniti, M. T., S. Piccirilli, A. Paoletti, R. Nistico, A. Stringaro, W. Malorni, A. Finazzi-Agro, and G. Bagetta. 2001. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci. Lett. 312:67-70. [DOI] [PubMed] [Google Scholar]
- 26.Corbeil, J., and D. D. Richman. 1995. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J. Gen. Virol. 76:681-690. [DOI] [PubMed] [Google Scholar]
- 27.Crump, M. P., J. H. Gong, P. Loetscher, K. Rajarathnam, A. Amara, F. Arenzana-Seisdedos, J. L. Virelizier, M. Baggiolini, B. D. Sykes, and I. Clark-Lewis. 1997. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 16:6996-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, and C. Chatfield. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 29.DeMartino, J. A., G. Van Riper, S. J. Siciliano, C. J. Molineaux, Z. D. Konteatis, H. Rosen, and M. S. Springer. 1994. The amino terminus of the human C5a receptor is required for high affinity C5a binding and for receptor activation by C5a but not C5a analogs. J. Biol. Chem. 269:14446-14450. [PubMed] [Google Scholar]
- 30.Dickie, P. 1996. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res. Hum. Retrovir. 12:177-189. [DOI] [PubMed] [Google Scholar]
- 31.Dickie, P. 2000. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res. Hum. Retrovir. 16:777-790. [DOI] [PubMed] [Google Scholar]
- 32.Dickie, P., J. Felser, M. Eckhaus, J. Bryant, J. Silver, N. Marinos, and A. L. Notkins. 1991. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185:109-119. [DOI] [PubMed] [Google Scholar]
- 33.Dickie, P., F. Ramsdell, A. L. Notkins, and S. Venkatesan. 1993. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology 197:431-438. [DOI] [PubMed] [Google Scholar]
- 34.Doranz, B. J., M. J. Orsini, J. D. Turner, T. L. Hoffman, J. F. Berson, J. A. Hoxie, S. C. Peiper, L. F. Brass, and R. W. Doms. 1999. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J. Virol. 73:2752-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draenert, R., M. Altfeld, C. Brander, N. Basgoz, C. Corcoran, A. G. Wurcel, D. R. Stone, S. A. Kalams, A. Trocha, M. M. Addo, P. J. Goulder, and B. D. Walker. 2003. Comparison of overlapping peptide sets for detection of antiviral CD8 and CD4 T cell responses. J. Immunol. Methods 275:19-29. [DOI] [PubMed] [Google Scholar]
- 36.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 37.Elisseeva, E. L., C. M. Slupsky, M. P. Crump, I. Clark-Lewis, and B. D. Sykes. 2000. NMR studies of active N-terminal peptides of stromal cell-derived factor-1. Structural basis for receptor binding. J. Biol. Chem. 275:26799-26805. [DOI] [PubMed] [Google Scholar]
- 38.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 39.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 40.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. Human immunodeficiency virus type 1 Nef protein on the cell surface is cytocidal for human CD4+ T cells. FEBS Lett. 393:105-108. [DOI] [PubMed] [Google Scholar]
- 41.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 393:93-96. [DOI] [PubMed] [Google Scholar]
- 42.Gabuzda, D., and J. Wang. 2000. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J. Neurovirol. 6(Suppl. 1):S24-S32. [PubMed] [Google Scholar]
- 43.Gayle, R. B., III, P. R. Sleath, S. Srinivason, C. W. Birks, K. S. Weerawarna, D. P. Cerretti, C. J. Kozlosky, N. Nelson, T. Vanden Bos, and M. P. Beckmann. 1993. Importance of the amino terminus of the interleukin-8 receptor in ligand interactions. J. Biol. Chem. 268:7283-7289. [PubMed] [Google Scholar]
- 44.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 45.Goudreau, G., S. Carpenter, N. Beaulieu, and P. Jolicoeur. 1996. Vacuolar myelopathy in transgenic mice expressing human immunodeficiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat. Med. 2:655-661. [DOI] [PubMed] [Google Scholar]
- 46.Greenway, A. L., D. A. McPhee, K. Allen, R. Johnstone, G. Holloway, J. Mills, A. Azad, S. Sankovich, and P. Lambert. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 76:2692-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guy, B., Y. Riviere, K. Dott, A. Regnault, and M. P. Kieny. 1990. Mutational analysis of the HIV nef protein. Virology 176:413-425. [DOI] [PubMed] [Google Scholar]
- 48.Hammond, M. E., V. Shyamala, M. A. Siani, C. A. Gallegos, P. H. Feucht, J. Abbott, G. R. Lapointe, M. Moghadam, H. Khoja, J. Zakel, and P. Tekamp-Olson. 1996. Receptor recognition and specificity of interleukin-8 is determined by residues that cluster near a surface-accessible hydrophobic pocket. J. Biol. Chem. 271:8228-8235. [DOI] [PubMed] [Google Scholar]
- 49.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 51.Harris, M. 1996. From negative factor to a critical role in virus pathogenesis: the changing fortunes of Nef. J. Gen. Virol. 77:2379-2392. [DOI] [PubMed] [Google Scholar]
- 52.Herbein, G., U. Mahlknecht, F. Batliwalla, P. Gregersen, T. Pappas, J. Butler, W. A. O'Brien, and E. Verdin. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395:189-194. [DOI] [PubMed] [Google Scholar]
- 53.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. 8:595-598. [DOI] [PubMed] [Google Scholar]
- 54.Heveker, N., M. Montes, L. Germeroth, A. Amara, A. Trautmann, M. Alizon, and J. Schneider-Mergener. 1998. Dissociation of the signalling and antiviral properties of SDF-1-derived small peptides. Curr. Biol. 8:369-376. [DOI] [PubMed] [Google Scholar]
- 55.Holm, G. H., C. Zhang, P. R. Gorry, K. Peden, D. Schols, E. De Clercq, and D. Gabuzda. 2004. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 78:4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang, M. B., and V. C. Bond. 2000. Involvement of protein kinase C in HIV-1 gp120-induced apoptosis in primary endothelium. J. Acquir. Immune. Defic. Syndr. 25:375-389. [DOI] [PubMed] [Google Scholar]
- 57.Huang, M. B., M. Hunter, and V. C. Bond. 1999. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res. Hum. Retrovir. 15:1265-1277. [DOI] [PubMed] [Google Scholar]
- 58.Huang, M. B., M. Khan, M. Garcia-Barrio, M. Powell, and V. C. Bond. 2001. Apoptotic effects in primary human umbilical vein endothelial cell cultures caused by exposure to virion-associated and cell membrane-associated HIV-1 gp120. J. Acquir. Immune Defic. Syndr. 27:213-221. [DOI] [PubMed] [Google Scholar]
- 59.Ikuta, K., M. Kameoka, and R. B. Luftig. 1997. AIDS pathogenesis: the role of accessory gene mutations, leading to formation of long-lived persistently infected cells and/or apoptosis-inducing HIV-1 particles. Virus Res. 52:145-156. [DOI] [PubMed] [Google Scholar]
- 60.James, C. O., M.-B. Huang, M. Khan, M. Garcia-Barrio, M. D. Powell, and V. C. Bond. 2004. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J. Virol. 78:3099-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jekle, A., O. T. Keppler, E. De Clercq, D. Schols, M. Weinstein, and M. A. Goldsmith. 2003. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J. Virol. 77:5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jerva, L. F., G. Sullivan, and E. Lolis. 1997. Functional and receptor binding characterization of recombinant murine macrophage inflammatory protein 2: sequence analysis and mutagenesis identify receptor binding epitopes. Protein Sci. 6:1643-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolicoeur, P., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and Z. Hanna. 1999. A novel mouse model of HIV-1 disease. Leukemia 13(Suppl. 1):S78-S80. [DOI] [PubMed] [Google Scholar]
- 64.Kaul, M., and S. A. Lipton. 1999. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 96:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 66.Khan, M., M. Garcia-Barrio, and M. D. Powell. 2001. Restoration of wild-type infectivity to human immunodeficiency virus type 1 strains lacking nef by intravirion reverse transcription. J. Virol. 75:12081-12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan, M., M. Garcia-Barrio, and M. D. Powell. 2003. Treatment of human immunodeficiency virus type 1 virions depleted of cyclophilin a by natural endogenous reverse transcription restores infectivity. J. Virol. 77:4431-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 69.Loetscher, P., J. H. Gong, B. Dewald, M. Baggiolini, and I. Clark-Lewis. 1998. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J. Biol. Chem. 273:22279-22283. [DOI] [PubMed] [Google Scholar]
- 70.Ma, Q., D. Jones, P. R. Borghesani, R. A. Segal, T. Nagasawa, T. Kishimoto, R. T. Bronson, and T. A. Springer. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 95:9448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macreadie, I. G., L. A. Castelli, A. Lucantoni, and A. A. Azad. 1995. Stress- and sequence-dependent release into the culture medium of HIV-1 Nef produced in Saccharomyces cerevisiae. Gene 162:239-243. [DOI] [PubMed] [Google Scholar]
- 72.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158:1014-1019. [PubMed] [Google Scholar]
- 73.Meucci, O., A. Fatatis, A. A. Simen, T. J. Bushell, P. W. Gray, and R. J. Miller. 1998. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. USA 95:14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monteclaro, F. S., and I. F. Charo. 1996. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J. Biol. Chem. 271:19084-19092. [DOI] [PubMed] [Google Scholar]
- 76.Nagasawa, T., S. Hirota, K. Tachibana, N. Takakura, S. Nishikawa, Y. Kitamura, N. Yoshida, H. Kikutani, and T. Kishimoto. 1996. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382:635-638. [DOI] [PubMed] [Google Scholar]
- 77.Novitsky, V., H. Cao, N. Rybak, P. Gilbert, M. F. McLane, S. Gaolekwe, T. Peter, I. Thior, T. Ndung'u, R. Marlink, T. H. Lee, and M. Essex. 2002. Magnitude and frequency of cytotoxic T-lymphocyte responses: identification of immunodominant regions of human immunodeficiency virus type 1 subtype C. J. Virol. 76:10155-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Brien, W. A., M. Sumner-Smith, S. H. Mao, S. Sadeghi, J. Q. Zhao, and I. S. Chen. 1996. Anti-human immunodeficiency virus type 1 activity of an oligocationic compound mediated via gp120 V3 interactions. J. Virol. 70:2825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okada, H., S. Morikawa, and M. Tashiro. 1998. HIV-1 Nef binding protein expressed on the surface of murine blood cells. Med. Microbiol. Immunol. (Berlin) 186:201-207. [DOI] [PubMed] [Google Scholar]
- 80.Okada, H., R. Takei, and M. Tashiro. 1997. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95 (Fas). FEBS Lett. 414:603-606. [DOI] [PubMed] [Google Scholar]
- 81.Okada, H., R. Takei, and M. Tashiro. 1997. Nef protein of HIV-1 induces apoptotic cytolysis of murine lymphoid cells independently of CD95 (Fas) and its suppression by serine/threonine protein kinase inhibitors. FEBS Lett. 417:61-64. [DOI] [PubMed] [Google Scholar]
- 82.Okada, H., R. Takei, and M. Tashiro. 1998. Inhibition of HIV-1 Nef-induced apoptosis of uninfected human blood cells by serine/threonine protein kinase inhibitors, fasudil hydrochloride and M3. FEBS Lett. 422:363-367. [DOI] [PubMed] [Google Scholar]
- 83.Peled, A., I. Petit, O. Kollet, M. Magid, T. Ponomaryov, T. Byk, A. Nagler, H. Ben Hur, A. Many, L. Shultz, O. Lider, R. Alon, D. Zipori, and T. Lapidot. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283:845-848. [DOI] [PubMed] [Google Scholar]
- 84.Raff, M. 1998. Cell suicide for beginners. Nature 396:119-122. [DOI] [PubMed] [Google Scholar]
- 85.Reiss, P., J. M. Lange, A. de Ronde, F. de Wolf, J. Dekker, C. Debouck, and J. Goudsmit. 1990. Speed of progression to AIDS and degree of antibody response to accessory gene products of HIV-1. J. Med. Virol. 30:163-168. [DOI] [PubMed] [Google Scholar]
- 86.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 87.Roggero, R., V. Robert-Hebmann, S. Harrington, J. Roland, L. Vergne, S. Jaleco, C. Devaux, and M. Biard-Piechaczyk. 2001. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 75:7637-7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salvi, R., A. R. Garbuglia, A. Di Caro, S. Pulciani, F. Montella, and A. Benedetto. 1998. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 72:3646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schols, D., and E. De Clercq. 1996. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J. Virol. 70:4953-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimizu, N., and T. Gojobori. 2000. How can human and simian immunodeficiency viruses utilize chemokine receptors as their coreceptors? Gene 259:199-205. [DOI] [PubMed] [Google Scholar]
- 91.Shutt, D. C., and D. R. Soll. 1999. HIV-induced T-cell syncytia release a two component T-helper cell chemoattractant composed of Nef and Tat. J. Cell Sci. 112:3931-3941. [DOI] [PubMed] [Google Scholar]
- 92.Siciliano, S. J., T. E. Rollins, J. DeMartino, Z. Konteatis, L. Malkowitz, G. Van Riper, S. Bondy, H. Rosen, and M. S. Springer. 1994. Two-site binding of C5a by its receptor: an alternative binding paradigm for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 91:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simard, M. C., P. Chrobak, D. G. Kay, Z. Hanna, S. Jothy, and P. Jolicoeur. 2002. Expression of simian immunodeficiency virus Nef in immune cells of transgenic mice leads to a severe AIDS-like disease. J. Virol. 76:3981-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slee, E. A., C. Adrain, and S. J. Martin. 1999. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 6:1067-1074. [DOI] [PubMed] [Google Scholar]
- 95.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Speck, R. F., K. Wehrly, E. J. Platt, R. E. Atchison, I. F. Charo, D. Kabat, B. Chesebro, and M. A. Goldsmith. 1997. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J. Virol. 71:7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tachibana, K., S. Hirota, H. Iizasa, H. Yoshida, K. Kawabata, Y. Kataoka, Y. Kitamura, K. Matsushima, N. Yoshida, S. Nishikawa, T. Kishimoto, and T. Nagasawa. 1998. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393:591-594. [DOI] [PubMed] [Google Scholar]
- 98.Thomas, F. P., C. Chalk, R. Lalonde, Y. Robitaille, and P. Jolicoeur. 1994. Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J. Virol. 68:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 100.Toth, P. T., D. Ren, and R. J. Miller. 2004. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J. Pharmacol. Exp. Ther. 310:8-17. [DOI] [PubMed] [Google Scholar]
- 101.Trkola, A., T. Ketas, V. N. KewalRamani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tudan, C., G. E. Willick, S. Chahal, L. Arab, P. Law, H. Salari, and A. Merzouk. 2002. C-terminal cyclization of an SDF-1 small peptide analogue dramatically increases receptor affinity and activation of the CXCR4 receptor. J. Med. Chem. 45:2024-2031. [DOI] [PubMed] [Google Scholar]
- 103.Ullrich, C. K., J. E. Groopman, and R. K. Ganju. 2000. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood 96:1438-1442. [PubMed] [Google Scholar]
- 104.Wain-Hobson, S. 1997. Down or out in blood and lymph? Nature 387:123-124. [DOI] [PubMed] [Google Scholar]
- 105.Williams, G., N. Borkakoti, G. A. Bottomley, I. Cowan, A. G. Fallowfield, P. S. Jones, S. J. Kirtland, G. J. Price, and L. Price. 1996. Mutagenesis studies of interleukin-8. Identification of a second epitope involved in receptor binding. J. Biol. Chem. 271:9579-9586. [DOI] [PubMed] [Google Scholar]
- 106.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 107.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon, K., J. G. Jeong, and S. Kim. 2001. Stable expression of human immunodeficiency virus type 1 Nef confers resistance against Fas-mediated apoptosis. AIDS Res. Hum. Retrovir. 17:99-104. [DOI] [PubMed] [Google Scholar]
- 109.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2001. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of trail in primary human macrophages by HIV-1 Tat. J. Biomed. Sci. 8:290-296. [DOI] [PubMed] [Google Scholar]
- 110.Zheng, J., A. Ghorpade, D. Niemann, R. L. Cotter, M. R. Thylin, L. Epstein, J. M. Swartz, R. B. Shepard, X. Liu, A. Nukuna, and H. E. Gendelman. 1999. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 73:8256-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou, N., Z. Luo, J. Luo, D. Liu, J. W. Hall, R. J. Pomerantz, and Z. Huang. 2001. Structural and functional characterization of human CXCR4 as a chemokine receptor and HIV-1 coreceptor by mutagenesis and molecular modeling studies. J. Biol. Chem. 276:42826-42833. [DOI] [PubMed] [Google Scholar]
- 112.Zou, Y. R., A. H. Kottmann, M. Kuroda, I. Taniuchi, and D. R. Littman. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393:595-599. [DOI] [PubMed] [Google Scholar]