Abstract
Background
The risk and outcomes of stroke in patients with chronic obstructive pulmonary disease exacerbations (COPDe) remain unclear. We examined whether patients with COPDe faced increased risk of stroke or post-stroke outcomes.
Methods
Using Taiwan’s National Health Insurance Research Database, we identified 1918 adults with COPDe and selected comparison cohorts of 3836 adults with COPD no exacerbations and 7672 adults without COPD who were frequency matched by age and sex in 2000–2008 (Study 1). Stroke event was identified during 2000–2013 follow-up period. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke associated with COPDe were calculated. In a nested cohort study (Study 2) of 261686 new-diagnosed stroke patients in 2000–2009, we calculated adjusted odds ratios (ORs) and 95% CIs of adverse events after stroke in patients with COPDe.
Results
Patients with COPDe had increased stroke incidence, with an adjusted HR of 1.28 (95% CI, 1.03–1.59). In the Study 2, COPDe were associated with post-stroke mortality (OR, 1.34, 95% CI 1.20–1.52), epilepsy (OR, 1.43; 95% CI, (1.22–1.67), and pneumonia (OR, 1.50; 95% CI, 1.39–1.62). Previous intubation for COPD and inpatient admissions due to COPD were factors associated with post-stroke adverse events.
Conclusion
Patients who have had COPDe face increased risks of stroke and post-stroke adverse events.
Introduction
Chronic obstructive pulmonary disease (COPD) was the fourth leading cause of death worldwide in 2008, and it is expected to move to third place by 2020 [1–3]. The worldwide prevalence of COPD (physiologically defined) is 3–11%, and approximately 4267500 COPD-related deaths occur in 2013 among 188 countries [4,5]. In United States, the medical costs attributable to COPD and its sequelae were estimated at $32.1 billion in 2010 and projected to reach $49 billion in 2020 [6].
Stroke was the second leading cause of death worldwide in 2010. It causes serious long-term disability and associated costs; in 2010 U.S. economic losses from stroke cost an estimated $73.7 billion [7]. A multicenter study reported ten leading risk factors that accounted for more than 90% of global stroke risk: cardiac disease, diabetes, alcohol intake, hypertension, current smoking, abdominal obesity, unhealthy diet, lack of exercise, psychosocial stress and depression [8]. Other risk factors associated with stroke require further study.
Previous studies have reported that patients with COPD no exacerbations (COPDne) or COPDe are at increased risk of stroke [9,10]. However, the risk of stroke for patients with COPDe is not completely understood. Previous research was limited by short-term follow-up, lack of adequate control group, and poor adjustment for potential confounders [9,10]. In addition, the impact of previous COPDe on mortality and complications after stroke remain unknown. Based on claims data from Taiwan’s National Health Research Database, we conducted two nationwide cohort studies to assess the risk of stroke and post-stroke mortality and complications in patients with COPDe.
Methods
Ethical approval
Reimbursement claims used in this study were collected from Taiwan’s National Health Insurance Research Database, which is available for research access [11–13]. To protect personal privacy, the database was decoded and patient identifications were scrambled for further public access for research. This study was evaluated and approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB-201605049) and E-DA Hospital (EDA-JIRB-2014012).
Study design and population
We used Taiwan’s National Health Insurance Research Database to perform the current two studies. Source of data and details of this national database was described in our previous reports [11–13]. With using the one million representative sample of Taiwan’s National Health Insurance Research Database, we conducted a retrospective cohort study (Study 1) of 3634 patients with newly diagnosed COPDe (defined as COPD patients receiving emergency care or inpatient care, treated with steroid and/or antibiotics). With frequency matching by age and sex (case-control ratio = 1:2), 7268 patients with COPD (defined as at least two visits for outpatient care with physician’s primary diagnosis without exacerbations) were also identified as exposure cohort. For comparison, 14,536 frequency-matched (case-control ratio = 1:4) persons without COPD (no physician’s diagnoses of COPD) were selected. These three cohorts aged ≥20 years were established between January 1, 2000, and December 31, 2008, and then followed up until December 31, 2013. The inclusion criteria of COPDe were based on previous studies [10,14]. We calculated person-years during the follow-up period for each participant until the diagnosis of stroke or until being censored because of death, withdrawal from the insurance system, or loss to follow-up. The non-COPD group included the remaining people who did not experience COPD throughout follow-up.
In the stroke cohort from the National Health Insurance Research Database, consisting of all prevalent and incident stroke patients in 2000–2009 from the total population of 23 million people in Taiwan, we identified 261686 new-onset stroke patients with hospitalization (Study 2). There were 60854 stroke patients had previous COPD and 11,828 stroke patients had previous COPDe. We compared post-stroke acute myocardial infarction, gastrointestinal bleeding, epilepsy, pneumonia, urinary tract infection, medical expenditure, length of hospital stay, intensive care unit stay, and mortality for a 30-day period after stroke for stroke patients with pre-stroke COPDne, COPDe, and no COPD.
Measures and definition
We identified income status by defining low-income patients as those qualifying for waived medical copayment verified by Taiwan Bureau of National Health Insurance. Whether the surgery was performed in a teaching hospital and the types of surgery and anesthesia were also recorded. We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to define chronic obstructive pulmonary disease (ICD-9-CM 491, 492, 496), stroke (ICD-9-CM 430–438), preoperative medical diseases, and post-stroke complications. Coexisting medical conditions that were determined from medical claims for the 24-month pre-stroke period included hypertension (ICD-9-CM 401–405), mental disorders (ICD-9-CM 290–319), diabetes (ICD-9-CM 250), liver cirrhosis (ICD-9-CM 571), hyperlipidemia (ICD-9-CM 272.0, 272.1, and 272.2), congestive heart failure (ICD-9-CM 428), traumatic brain injury (ICD-9-CM 800–804, 850–854), anemia (ICD-9-CM 280–285), atrial fibrillation (ICD-9-CM 427.31), and peripheral vascular disease (ICD-9-CM 443). Renal dialysis was identified by administration code (D8, D9). People who visited outpatient care for obesity and smoking cessation were identified in this study. In-hospital 30-day mortality after the index surgery was considered the primary outcome, and post-stroke epilepsy (ICD-9-CM 345), pneumonia (ICD-9-CM 480–486), acute myocardial infarction (ICD-9-CM 410), gastrointestinal bleeding (ICD-9-CM 578), urinary tract infection (ICD-9-CM 599.0) were considered secondary outcomes in the nested cohort study. Length of hospital stay during stroke admission was also analyzed.
Statistical analyses
We compared sociodemographic factors (such as age, sex, and low income), coexisting medical conditions (such as hypertension, mental disorders, diabetes, liver cirrhosis, hyperlipidemia, congestive heart failure, traumatic brain injury, anemia atrial fibrillation, peripheral vascular disease, renal dialysis, epilepsy, obesity, and smoking cessation), and medications (such as anticoagulant, anti-platelet agents, and lipid-lowering agents), for people with COPDne, with COPDe, or with no COPD using the chi-square tests. The adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke associated with COPDe were calculated by using multivariate Cox proportional hazard models. In the further stratified analysis, the adjusted HRs and 95% CIs of stroke in patients with COPDne and COPDe were also calculated in both sexes and all age groups.
In the nested cohort study, the chi-square tests were used to compare differences relating to sociodemographics, coexisting medical conditions, and medications between stroke patients without COPD and with previous COPDe. By using multivariate logistic regressions, we calculated adjusted odds ratios (ORs) and 95% CIs of the risks of adverse events after stroke, including acute myocardial infarction, gastrointestinal bleeding, epilepsy, pneumonia, urinary tract infection, increased medical expenditure, prolonged length of stay in intensive care unit, and mortality for stroke patients with previous COPDne and COPDe.
Results
After matching by age and sex among cohorts with COPDne, COPDe and without COPD (Table 1), patients with COPDe showed higher proportions of having low-income status, hypertension, mental disorders, diabetes, liver cirrhosis, hyperlipidemia, congestive heart failure, traumatic brain injury, anemia, atrial fibrillation, and peripheral vascular disease than people without COPD (all p<0.0001). Use of medications such as anticoagulant, anti-platelet agents, lipid-lowering agents, methylxanthines, beta2-adrenergic agonist, systemic corticosteroids, anticholinergics, and inhaled corticosteroids were also higher in patients with COPDe than in those without COPD (all p<0.0001).
Table 1. Sociodemographic factors and coexisting medical conditions in people with and without COPD.
| No COPD N = 7672 |
COPDne N = 3836 |
COPDe N = 1918 |
P valuea | P valueb | ||||
|---|---|---|---|---|---|---|---|---|
| Sex | n | (%) | n | (%) | n | (%) | 1.0000 | 1.0000 |
| Female | 2800 | (36.5) | 1400 | (36.5) | 700 | (36.5) | ||
| Male | 4872 | (63.5) | 2436 | (63.5) | 1218 | (63.5) | ||
| Age, years | 1.0000 | 1.0000 | ||||||
| 40–49 | 1712 | (22.3) | 856 | (22.3) | 428 | (22.3) | ||
| 50–59 | 2664 | (34.7) | 1332 | (34.7) | 666 | (34.7) | ||
| 60–69 | 2240 | (29.2) | 1120 | (29.2) | 560 | (29.2) | ||
| ≥70 | 1056 | (13.8) | 528 | (13.8) | 264 | (13.8) | ||
| Low income | 128 | (1.7) | 115 | (3.0) | 116 | (6.1) | <0.0001 | <0.0001 |
| Medical conditions | ||||||||
| Hypertension | 3133 | (40.8) | 1969 | (51.3) | 990 | (51.6) | <0.0001 | 0.8374 |
| Mental disorder | 2120 | (27.6) | 1747 | (45.5) | 922 | (48.1) | <0.0001 | 0.0698 |
| Diabetes | 1585 | (20.7) | 907 | (23.6) | 511 | (26.6) | <0.0001 | 0.0129 |
| Liver cirrhosis | 921 | (12.0) | 801 | (20.9) | 346 | (18.0) | <0.0001 | 0.0110 |
| Hyperlipidemia | 1061 | (13.8) | 746 | (19.5) | 274 | (14.3) | <0.0001 | <0.0001 |
| Congestive heart failure | 252 | (3.3) | 278 | (7.3) | 282 | (14.7) | <0.0001 | <0.0001 |
| Traumatic brain injury | 410 | (5.3) | 299 | (7.8) | 241 | (12.6) | <0.0001 | <0.0001 |
| Anemia | 417 | (5.4) | 322 | (8.4) | 145 | (7.6) | <0.0001 | 0.2747 |
| Atrial fibrillation | 95 | (1.2) | 106 | (2.8) | 81 | (4.2) | <0.0001 | 0.0032 |
| PVD | 166 | (2.2) | 161 | (4.2) | 58 | (3.0) | <0.0001 | 0.0284 |
| Renal dialysis | 123 | (1.6) | 69 | (1.8) | 39 | (2.0) | 0.3920 | 0.5364 |
| Epilepsy | 59 | (0.8) | 50 | (1.3) | 33 | (1.7) | 0.0003 | 0.2110 |
| Obesity | 32 | (0.4) | 23 | (0.6) | 14 | (0.7) | 0.0003 | 0.5598 |
| Smoking cessation | 7 | (0.1) | 8 | (0.2) | 7 | (0.4) | 0.0215 | 0.2727 |
| Number of medical conditions | <0.0001 | 0.0074 | ||||||
| 0 | 2310 | (30.1) | 618 | (16.1) | 287 | (15.0) | ||
| 1 | 2326 | (30.3) | 994 | (25.9) | 494 | (25.8) | ||
| 2 | 1637 | (21.3) | 931 | (24.3) | 425 | (22.2) | ||
| 3 | 889 | (11.6) | 695 | (18.1) | 342 | (17.8) | ||
| ≥4 | 510 | (6.7) | 598 | (15.6) | 370 | (19.3) | ||
| Anticoagulant | 241 | (3.1) | 208 | (5.4) | 161 | (8.4) | <0.0001 | <0.0001 |
| Anti-platelet agents | 3340 | (43.5) | 2353 | (61.3) | 1234 | (64.3) | <0.0001 | 0.0269 |
| Lipid-lowering agents | 2252 | (29.4) | 1464 | (38.2) | 712 | (37.1) | <0.0001 | 0.4419 |
| Methylxanthines | 85 | (1.1) | 499 | (13.0) | 596 | (31.1) | <0.0001 | <0.0001 |
| Beta2-adrenergic agonist | 2849 | (37.1) | 2750 | (71.7) | 1686 | (87.9) | <0.0001 | <0.0001 |
| Systemic corticosteroids | 439 | (5.7) | 977 | (25.5) | 1200 | (62.6) | <0.0001 | <0.0001 |
| Anticholinergics | 3712 | (48.4) | 3250 | (84.7) | 1732 | (90.3) | <0.0001 | <0.0001 |
| Inhaled corticosteroids | 3812 | (49.7) | 2878 | (75.0) | 1623 | (84.6) | <0.0001 | <0.0001 |
COPD = chronic obstructive pulmonary disease; COPDe = chronic obstructive pulmonary disease exacerbations; COPDe = chronic obstructive pulmonary disease with no exacerbations. PVD = peripheral vascular disease.
a Chi-square tests for people had no COPD, COPDne, and COPDe.
b Chi-square tests between COPDne and COPDe.
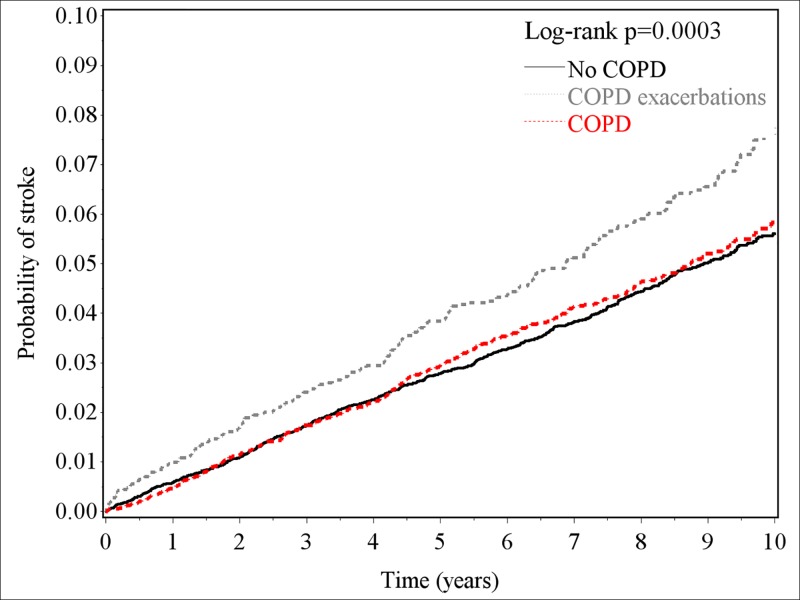
Table 2 shows that higher incidence of stroke was found in patients with previous COPDe than those without COPD (7.6 vs. 5.8 per 1000 person-years, p<0.0001) during the follow-up period. The corresponding HR of stroke associated with COPDe was 1.28 (95% CI, 1.03–1.59). The risk of stroke was not associated with COPDne during the follow-up period. The association between COPDe and stroke risk was significant in males (HR, 1.37; 95% CI, 1.06–1.77) and patients aged 50–69 years (HR, 1.44; 95% CI, 1.00–2.07). The probabilities of stroke at one, five and ten years after patients suffering COPDe were 1%, 4% and 8%, respectively (Fig 1). Compared with non-COPD cohort, patients with COPDe had increased probability of having stroke (log-rank test, p<0.0001), however, there was no significant difference between COPDne and non-COPD patients. The difference between non-COPD and COPDe increased with the follow-up time increased.
Table 2. Risk of stroke in association with previous COPD exacerbations by sex and ageb.
| N | Events | PY | Incidence | HR (95% CI)a | |
|---|---|---|---|---|---|
| No COPD | 7672 | 385 | 65986 | 5.8 | 1.00 (reference) |
| COPDne | 3836 | 194 | 34636 | 5.6 | 0.97 (0.88–1.06) |
| COPDe | 1918 | 120 | 15812 | 7.6 | 1.28 (1.03–1.59) |
| Female | |||||
| No COPD | 2800 | 131 | 25046 | 5.2 | 1.00 (reference) |
| COPDne | 1400 | 58 | 12947 | 4.5 | 0.94 (0.80–1.10) |
| COPDe | 700 | 34 | 6124 | 5.6 | 1.13 (0.76–1.68) |
| Male | |||||
| No COPD | 4872 | 254 | 40939 | 6.2 | 1.00 (reference) |
| COPDne | 2436 | 136 | 21688 | 6.3 | 0.98 (0.88–1.09) |
| COPDe | 1218 | 86 | 9688 | 8.9 | 1.37 (1.06–1.77) |
| 40–49 years | |||||
| No COPD | 1712 | 39 | 16721 | 2.3 | 1.00 (reference) |
| COPDne | 856 | 18 | 8434 | 2.1 | 0.85 (0.63–1.14) |
| COPDe | 428 | 18 | 3932 | 4.6 | 1.29 (0.71–2.37) |
| 50–59 years | |||||
| No COPD | 2664 | 122 | 24162 | 5.0 | 1.00 (reference) |
| COPDne | 1332 | 72 | 12362 | 5.8 | 1.04 (0.90–1.21) |
| COPDe | 666 | 44 | 5858 | 7.5 | 1.44 (1.00–2.07) |
| 60–69 years | |||||
| No COPD | 2240 | 148 | 19046 | 7.8 | 1.00 (reference) |
| COPDne | 1120 | 71 | 10153 | 7.0 | 0.96 (0.83–1.11) |
| COPDe | 560 | 48 | 4597 | 10.4 | 1.37 (0.97–1.92) |
| ≥70 years | |||||
| No COPD | 1056 | 76 | 6057 | 12.5 | 1.00 (reference) |
| COPDne | 528 | 33 | 3687 | 9.0 | 0.92 (0.75–1.13) |
| COPDe | 264 | 10 | 1426 | 7.0 | 0.65 (0.33–1.30) |
CI = confidence interval; COPD = chronic obstructive pulmonary disease; COPDe = chronic obstructive pulmonary disease exacerbations; COPDe = chronic obstructive pulmonary disease with no exacerbations; HR, hazard ratio; PY = person-years
a Full model adjusted for all covariates listed in Table 1 except for respiratory medications.
b Compared with non-COPD controls, the HR of hemorrhagic stroke, ischemic stroke, and other stroke for COPD patients without exacerbations were 0.87 (95% CI, 0.72–1.07), 0.97(95% CI, 0.87–1.09), 1.04(95% CI, 0.83–1.29), respectively; the corresponding HR for COPD patients with exacerbations were 1.42 (95% CI, 0.91–2.22), 1.27(95% CI, 0.97–1.66), and 1.09 (95% CI, 0.60–1.96), respectively.
Fig 1. Kaplan-Meier curves for probabilities of having stroke in the study cohorts with COPDne, COPDe, and no COPD.
Compared with patients without COPD (Table 3), stroke patients with previous COPDe had higher proportions of males, older people, low-income people, other stroke, stay in teaching hospital, hypertension, mental disorders, diabetes, congestive heart failure, traumatic brain injury, dementia, hyperlipidemia, anemia, atrial fibrillation, liver cirrhosis, peripheral vascular disease, renal dialysis, obesity, smoking cessation, anti-platelet agents, lipid-lowering agents, and anticoagulants, methylxanthines, beta2-adrenergic agonist, systemic corticosteroids, anticholinergics, and inhaled corticosteroids (all p<0.01).
Table 3. Characteristics of hospitalized stroke patients with and without COPD.
| Number | No COPD | COPDne | COPDe | P valuea | P valueb | |||
|---|---|---|---|---|---|---|---|---|
| 230553 | 38997 | 7924 | ||||||
| Gender | n | (%) | N | (%) | n | (%) | <0.0001 | <0.0001 |
| Female | 101287 | (43.9) | 13431 | (34.4) | 2071 | (26.1) | ||
| Male | 129266 | (56.1) | 25566 | (65.6) | 5853 | (73.9) | ||
| Age, years | <0.0001 | <0.0001 | ||||||
| 40–49 | 27378 | (11.9) | 1032 | (2.7) | 96 | (1.2) | ||
| 50–59 | 50929 | (22.1) | 3186 | (8.2) | 362 | (4.6) | ||
| 60–69 | 59344 | (25.7) | 7577 | (19.4) | 1074 | (13.6) | ||
| 70–79 | 61397 | (26.6) | 16071 | (41.2) | 3314 | (41.8) | ||
| ≥80 | 31505 | (13.7) | 11131 | (28.5) | 3078 | (38.8) | ||
| Low income | 9701 | (4.2) | 2354 | (6.0) | 604 | (7.6) | <0.0001 | <0.0001 |
| Types of stroke | <0.0001 | <0.0001 | ||||||
| Hemorrhage | 48159 | (20.9) | 5603 | (14.4) | 1014 | (12.8) | ||
| Ischemia | 142082 | (61.6) | 24313 | (62.4) | 4774 | (60.3) | ||
| Other | 40312 | (17.5) | 9081 | (23.3) | 2136 | (27.0) | ||
| Stay in teaching hospital | 210345 | (91.2) | 33339 | (85.5) | 6526 | (82.4) | <0.0001 | <0.0001 |
| Medical conditions | ||||||||
| Hypertension | 107302 | (46.5) | 22633 | (58.0) | 4430 | (55.9) | <0.0001 | 0.0005 |
| Mental disorder | 34402 | (14.9) | 10402 | (26.7) | 2280 | (28.8) | <0.0001 | 0.0001 |
| Diabetes | 55695 | (24.2) | 9661 | (24.8) | 1826 | (23.0) | 0.0015 | 0.0011 |
| Congestive heart failure | 5683 | (2.5) | 3385 | (8.7) | 1223 | (15.4) | <0.0001 | <0.0001 |
| Traumatic brain injury | 13100 | (5.7) | 3555 | (9.1) | 867 | (10.9) | <0.0001 | <0.0001 |
| Dementia | 6758 | (2.9) | 2623 | (6.7) | 687 | (8.7) | <0.0001 | <0.0001 |
| Hyperlipidemia | 15563 | (6.8) | 3340 | (8.6) | 454 | (5.7) | <0.0001 | <0.0001 |
| Anemia | 4996 | (2.2) | 1668 | (4.3) | 386 | (4.9) | <0.0001 | 0.0185 |
| Atrial fibrillation | 2981 | (1.3) | 1241 | (3.2) | 330 | (4.2) | <0.0001 | <0.0001 |
| Liver cirrhosis | 2606 | (1.1) | 593 | (1.5) | 144 | (1.8) | <0.0001 | 0.0529 |
| PVD | 2305 | (1.0) | 649 | (1.7) | 131 | (1.7) | <0.0001 | 0.9442 |
| Renal dialysis | 4042 | (1.8) | 545 | (1.4) | 111 | (1.4) | <0.0001 | 0.9820 |
| Obesity | 798 | (0.4) | 156 | (0.4) | 29 | (0.4) | 0.2494 | 0.6592 |
| Smoking cessation | 121 | (0.1) | 59 | (0.2) | 7 | (0.1) | <0.0001 | 0.1728 |
| Number of medical conditions | <0.0001 | <0.0001 | ||||||
| 0 | 70929 | (30.8) | 7032 | (18.0) | 1422 | (18.0) | ||
| 1 | 91344 | (39.6) | 13952 | (35.8) | 2612 | (33.0) | ||
| 2 | 46512 | (20.2) | 10486 | (26.9) | 2142 | (27.0) | ||
| 3 | 16322 | (7.1) | 5165 | (13.2) | 1162 | (14.7) | ||
| ≥4 | 5446 | (2.4) | 2362 | (6.1) | 586 | (7.4) | <0.0001 | <0.0001 |
| Lipid-lowering agents | 40662 | (17.6) | 8084 | (20.7) | 1593 | (20.1) | <0.0001 | 0.2090 |
| Anti-platelet agents | 110643 | (48.0) | 26503 | (68.0) | 5869 | (74.1) | <0.0001 | <0.0001 |
| Anticoagulant | 5008 | (2.2) | 1424 | (3.7) | 423 | (5.3) | <0.0001 | <0.0001 |
| Methylxanthines | 736 | (0.3) | 3122 | (8.0) | 1373 | (17.3) | <0.0001 | <0.0001 |
| Beta2-adrenergic agonist | 38200 | (16.6) | 23517 | (60.3) | 6932 | (87.5) | <0.0001 | <0.0001 |
| Systemic corticosteroids | 4121 | (1.8) | 9730 | (25.0) | 5219 | (65.9) | <0.0001 | <0.0001 |
| Anticholinergics | 63782 | (27.7) | 31115 | (79.8) | 7312 | (92.3) | <0.0001 | <0.0001 |
| Inhaled corticosteroids | 55318 | (24.0) | 20939 | (53.7) | 5954 | (75.1) | <0.0001 | <0.0001 |
COPD = chronic obstructive pulmonary disease; COPDe = chronic obstructive pulmonary disease exacerbations. PVD = peripheral vascular disease.
a Chi-square tests for stroke patients had no COPD, COPDne, and COPDe.
b Chi-square tests between patients with COPDne and patients with COPDe.
Table 4 shows that COPDne was associated with post-stroke epilepsy (OR, 1.27; 95% CI, 1.17–1.38), pneumonia (OR, 1.24; 95% CI, 1.18–1.29), and mortality (OR, 1.14; 95% CI, 1.07–1.22). Stroke patients with pre-stroke COPDe had higher risk of mortality (OR, 1.34; 95% CI, 1.20–1.52), epilepsy (OR, 1.43; 95% CI, 1.22–1.67), and pneumonia (OR, 1.50; 95% CI, 1.39–1.62) after stroke compared with those without COPD. Stroke patients with previous COPDne (p = 0.0048) or COPDe (p<0.0001) had longer hospital stays compared with stroke patients without COPD. Pre-stroke characteristics of COPDe (Table 5) such as experienced intubations (OR, 2.01; 95% CI, 1.89–2.15) and ≥3 visits for inpatient COPD care (OR, 1.66; 95% CI, 1.34–2.05) were significant factors associated with post-stroke adverse events. Similar situation was also found in patients with COPDne that experienced intubations (OR, 2.27; 95% CI, 2.19–2.36) and ≥3 visits for inpatient COPD care (OR, 1.66; 95% CI, 1.37–2.02) were risk factors for post-stroke adverse events.
Table 4. Risks of complications and mortality during stroke hospitalization associated with COPD and COPD exacerbations.
| No COPD | COPDne | COPDe | Risk of outcomes for COPDne | Risk of outcomes for COPDe | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | OR (95% CI)c | OR (95% CI)c | |
| Number | 230553 | 38997 | 7924 | |||||
| Mortality | 7827 | (3.4) | 1373 | (3.5) | 344 | (4.3) | 1.14 (1.07–1.22) | 1.34 (1.20–1.52) |
| Epilepsy | 3786 | (1.6) | 798 | (2.1) | 192 | (2.4) | 1.27 (1.17–1.38) | 1.43 (1.22–1.67) |
| Pneumonia | 12877 | (5.6) | 3353 | (8.6) | 921 | (11.6) | 1.24 (1.18–1.29) | 1.50 (1.39–1.62) |
| Acute myocardial infarction | 1846 | (0.8) | 337 | (0.9) | 79 | (1.0) | 0.90 (0.80–1.02) | 0.92 (0.72–1.16) |
| Gastrointestinal bleeding | 5563 | (2.4) | 1087 | (2.8) | 245 | (3.1) | 0.98 (0.91–1.05) | 0.97 (0.85–1.11) |
| Urinary tract infection | 21071 | (9.1) | 4027 | (10.3) | 849 | (10.7) | 1.02 (0.98–1.06) | 1.06 (0.98–1.14) |
| Adverse eventsa | 22672 | (9.8) | 5051 | (13.0) | 1335 | (16.9) | 1.23 (1.19–1.28) | 1.55 (1.42–1.63) |
| Length of stay, daysb | 8.6±7.0 | 8.5±6.8 | 9.3±7.1 | 0.0048 | <0.0001 | |||
CI = confidence interval; COPD = chronic obstructive pulmonary disease; COPDe = chronic obstructive pulmonary disease exacerbations; OR = odds ratio.
a Adverse events include pneumonia, epilepsy and mortality
b Mean±SD
c Adjusted for all covariates listed in Table 3 except for respiratory medications.
Table 5. Risk of post-stroke adverse events for patients with COPD exacerbations.
| Pre-stroke characteristics of COPD | n | Adverse events after strokea | ||
|---|---|---|---|---|
| Cases | (%) | OR (95% CI)b | ||
| No COPD | 230553 | 22672 | (9.8) | 1.00 (Reference) |
| Stroke patients with COPDne (N = 38997) | ||||
| Had intubations | 3126 | 1310 | (41.9) | 2.27 (2.19–2.36) |
| Had 1 visit for inpatient care | 4030 | 650 | (16.1) | 1.33 (1.22–1.45) |
| Had 2 visits for inpatient care | 885 | 162 | (18.3) | 1.53 (1.29–1.82) |
| Had ≥3 visits for inpatient care | 668 | 130 | (19.5) | 1.66 (1.37–2.02) |
| Stroke patients with COPDe (N = 7924) | ||||
| Had intubations | 1082 | 420 | (38.8) | 2.01 (1.89–2.15) |
| Had 1 visit for inpatient care | 2606 | 392 | (15.0) | 1.20 (1.07–1.34) |
| Had 2 visits for inpatient care | 692 | 134 | (19.4) | 1.61 (1.33–1.96) |
| Had ≥3 visits for inpatient care | 563 | 110 | (19.5) | 1.66 (1.34–2.05) |
CI = confidence interval; COPD = chronic obstructive pulmonary disease; COPDe = chronic obstructive pulmonary disease exacerbations; OR = odds ratio.
a Adverse events include post-stroke pneumonia, epilepsy, and mortality
b Adjusted for all covariates listed in Table 3 except for respiratory medications.
Discussion
We conducted study 1 based on the claims data of Taiwan’s National Health Insurance system to analyze long-term risk of stroke in patients with COPDe during the follow-up period. Our Study 1 showed that COPDe is associated with significantly increased risk of stroke, while COPDne is not. The Study 2 showed that pre-stroke COPDe increased mortality and adverse events after stroke. Increased incidence of endotracheal intubation and hospitalization for COPD are significantly associated with post-stroke adverse events. This may be the first report verifying the impact of COPDe on risk of stroke and outcomes after stroke. The nationwide representative population, large sample size, cohort study design, and multivariate adjustment strengthen these two studies’ analytic power. In addition, our findings were similar with previous that COPD was more associated with hemorrhagic stroke than ischemic stroke [9,10].
Age, gender, and socioeconomic status were considered as potential confounding factors associated with COPD and stroke [3,15–18]. Considering the impact of COPDe on outcomes after stroke in the study 2, these characteristics need to be adjusted under the multivariate regression models. The present study showed approximately doubled stroke risk in patients with COPDe aged from 50 to 69, but not in patients aged 70 and higher. It is possible that older people have more medical illnesses, which may dilute the contribution of COPDe to risk of stroke. Another possible reason is that small size of stroke events occurred in patients with COPDe aged 40–49 years and ≥70 years lead to insignificances of statistical value.
Comorbidities including hypertension, mental disorder, diabetes, liver cirrhosis, hyperlipidemia, congestive heart failure, traumatic brain injury, anemia, atrial fibrillation, peripheral vascular disease, renal dialysis, epilepsy, and obesity were known factors associated with stroke [8,12,13,19–22]. These medical conditions also commonly coexisted in patients with COPD [1,23,24,25]. However, the previous study was limited by inadequate control of coexisting medical conditions, and this might cause confounding bias when estimating the risk of stroke in patients with COPDe [9,10]. To reduce these confounding effects, we used multivariate regression models to adjust coexisting medical conditions and calculate the risk and outcomes of stroke in patients with previous COPDe and patients with COPDne.
Stroke and COPDe have risk factors in common, such as smoking and low socioeconomic status. However, we suggest that increased inflammation is a plausible explanation for increased stroke risk in patients with COPDe. Most patients with COPDe were initially triggered by airway infection followed by systemic inflammation [26]. Previous studies have shown increased concentrations of fibrinogen and C-reactive protein during COPDe [27,28]. Elevated fibrinogen levels have been recognized as a predictor of stroke and marker of atherosclerosis [29]. C-reactive protein levels indicate underlying systemic inflammation and a novel plasma marker of atherothrombotic disease [30–32]. Elevated plasma C-reactive protein levels significantly predict the risk of ischemic stroke and transient ischemic attack [33]. Another possible explanation is that patients with COPDe are markedly inactive in daily life [34]. Physical activity lowers blood pressure and improves lipid profiles [35]. In addition, physical activity can also play an antithrombotic role by reducing blood viscosity [36], fibrinogen levels [37], and platelet aggregability [38], all of above might reduce stroke risk. Skeletal muscle dysfunction and wasting, which is commonly found in patients with COPDe, might play a role in reducing physical activity and therefore increase stroke risk [39,40]. An additional factor could be increased use of anticholinergics and β-agonists by the patient with COPDe. The former is believed to suppress parasympathetic control, whereas the latter is believed to stimulate sympathetic control, both causing an increased risk of tachyarrhythmias, stroke, and death [41,42]. Indeed, the effects of inflammation, inactivity, and tachyarrhythmias might be synergistic, while the underlined mechanisms need further investigation.
Medical complications are frequent after stroke. Pre-existing medical conditions, advanced age, and pre-stroke disability can affect an individual’s risk of developing post-stroke complications [43]. The current study showed that previous COPD exacerbations increased post-stroke mortality, pneumonia, and epilepsy, as well as medical expenditure, length of hospital stay, and ICU care. The increased risk of pneumonia in COPD patients might be explained by the lung changes associated with COPD that lower resistance to lung infections [44]. Although we were unable to demonstrate why COPDe increased the risk of epilepsy in stroke patients, associated nocturnal oxygen desaturation is a plausible explanation [45,46].
Wang et al conducted a nested case-control study and reported that inhaled ipratropium bromide was associated with stroke risk in people with COPD [47]. Lin and his colleagues’ retrospective cohort study reported that pharmacotherapy was associated with stroke risk in patients with COPD [48]. Based on the Taiwan’s National Health Insurance Research Database, the purposes of both these two previous studies were to investigate the influence of COPD medications on stroke risk [47,48]. Different from the previous two studies, our present study evaluated the impacts of COPDne and COPDe on the stroke risk and on the stroke outcomes. The inadequate adjustment for potential confounding factors and the immortal time bias were the main study limitations of the previous study [48]. Besides, the case-control study design could not provide causal inferences for the association between inhaled ipratropium bromide and stroke risk [47]. In addition, short-term follow-up period (3 years) may underestimate the stroke incidence in COPD patients [47]. Finally, mental disorder and hyperlipidemia may be potential confounders for the association between COPD and stroke that were not considered into adjustment in their analysis [47,48].
This study has some limitations. First, we used insurance claims data that lacked information on detailed sociodemographics, lifestyle (such as smoking and alcohol drinking), nutritional level, pulmonary function tests, body mass index, and biomedical measures. Second, though the accuracy of major diagnosis codes from the research database in studies based on these has been accepted by peer reviewers for prominent scientific journals worldwide [11–13,47,48], validity of COPD, stroke, and other codes indicating comorbidities and complications might still be a limitation of this study. Third, our study based on the insurance claims of National Health Insurance program that lack of the information of clinical risk scores for COPD and stroke, such as pneumonia severity index, CURB65, BAP65, or National Institutes of Health Stroke Scale. Therefore, we could not evaluate the impact of these risk score on the risk and adverse events for stroke. In addition, physicians followed standard instructions with using spirometry to diagnose and identify COPD patients in the clinical settings in Taiwan. However, the results of spirometry of COPD patients were not available because the limitation of Taiwan’s National Health Insurance Research Database. The reimbursement claims reported from hospitals and clinics were reviewed strictly by the Ministry of Health and Welfare. Any errors and incorrect information in the insurance reimbursement claims were be withdrawn with punishment and fine. We considered that this limitation may not cause fatal bias to the results of this study. Finally, although we used multivariate adjustment to control several confounders, residual confounding is always possible.
Conclusion
In conclusion, COPD exacerbation is an important independent risk factor of stroke and post-stroke adverse events. History of COPDe may alert clinicians to these anticipated risks and can affect early decisions about surveillance. Further studies are needed to develop specific strategies to decrease stroke risks and post-stroke adverse outcomes for this patient population.
Acknowledgments
This study is based in part on data obtained from the National Health Insurance Research Database provided by the Bureau of National Health Insurance of the Taiwan Department of Health and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, the Taiwan Department of Health, or the National Health Research Institutes.
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- OR
odds ratio
Data Availability
The data are owned by the National Health Insurance Research Database (NHIRD) Committee and the contact information is available as email: nhird@nhri.org.tw. We confirm that no special agreement existed between our research team and the NHIRD, and that all interested researchers will be able to access data in the same manner in which we did.
Funding Statement
This research was supported in part by Shuang Ho Hospital, Taipei Medical University (104TMU-SHH-23), Taiwan’s Ministry of Science and Technology (MOST105-2629-B-038-001; MOST105-2314-B-038-025; MOST105-2221-E-038-014; MOST104-2314-B-038-027-MY2; MOST104-2221-E-038-015; NSC102-2314-B-038-021-MY3), and Taiwan’s Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Decramer M, Janssens W, Miravitlles M. (2012) Chronic obstructive pulmonary disease. Lancet 379: 1341–1351. 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349: 1498–1504. 10.1016/S0140-6736(96)07492-2 [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Buist AS. (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370: 765–773. 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2013 Mortality and Causes of Death Collaborators. (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013 Lancet 385: 117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. (2015) Total and state-specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged ≥18 years in the United States for 2010 and projections through 2020. Chest 147: 31–45. 10.1378/chest.14-0972 [DOI] [PubMed] [Google Scholar]
- 7.Towfighi A, Saver JL. (2011) Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 42: 2351–2355. 10.1161/STROKEAHA.111.621904 [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries [the INTERSTROKE study]: a case-control study. Lancet 376: 112–123. 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 9.Söderholm M, Inghammar M, Hedblad B, Egesten A, Engström G. (2016) Incidence of stroke and stroke subtypes in chronic obstructive pulmonary disease. Eur J Epidemiol 31: 159–168. 10.1007/s10654-015-0113-7 [DOI] [PubMed] [Google Scholar]
- 10.Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, et al. (2016) chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med 193: 251–258. 10.1164/rccm.201505-0962OC [DOI] [PubMed] [Google Scholar]
- 11.Liao CC, Chang PY, Yeh CC, Hu CJ, Wu CH, Chen TL. (2014) Outcomes after surgery in patients with previous stroke. Br J Surg 101: 1616–1622. 10.1002/bjs.9639 [DOI] [PubMed] [Google Scholar]
- 12.Liao CC, Chou YC, Yeh CC, Hu CJ, Chiu WT, Chen TL. (2014) Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc 89: 163–172. 10.1016/j.mayocp.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 13.Liao CC, Su TC, Sung FC, Chou WH, Chen TL. (2012) Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One 7: e31527 10.1371/journal.pone.0031527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burge S, Wedzicha JA. (2003) COPD exacerbations: definitions and classifications. Eur Respir J Suppl 41: 46s–53s. [DOI] [PubMed] [Google Scholar]
- 15.Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. (2010) Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 65: 480–485. 10.1136/thx.2009.122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazargani YT, de Boer A, Leufkens HG, Mantel-Teeuwisse AK. (2014) Essential medicines for COPD and asthma in low- and middle-income countries. Thorax 69: 1149–1151. 10.1136/thoraxjnl-2014-205249 [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. (1997) American Heart Association Prevention Conference IV. Prevention and Rehabilitation of Stroke. Risk Factors. Stroke 28: 1507–1517. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. (2000) Early growth, adult income, and risk of stroke. Stroke 31: 869–874. [DOI] [PubMed] [Google Scholar]
- 19.Kesarwani M, Perez A, Lopez VA, Wong ND, Franklin SS. (2009) Cardiovascular comorbidities and blood pressure control in stroke survivors. J Hypertens 27: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 20.Fischer U, Arnold M, Nedeltchev K, Schoenenberger RA, Kappeler L, Höllinger P, et al. (2006) Impact of comorbidity on ischemic stroke outcome. Acta Neurol Scand 113: 108–113. 10.1111/j.1600-0404.2005.00551.x [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Zhou T, Li Y, Chen P, Chen L. (2016) Anemia increases the mortality risk in patients with stroke: a meta-analysis of cohort studies. Sci Rep 6: 26636 10.1038/srep26636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CS, Liao CH, Lin CC, Lane HY, Sung FC, Kao CH. (2014) Patients with epilepsy are at an increased risk of subsequent stroke: a population-based cohort study. Seizure 23: 377–381. 10.1016/j.seizure.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. (2006) Mortality in COPD: Role of comorbidities. Eur Respir J 28: 1245–1257. 10.1183/09031936.00133805 [DOI] [PubMed] [Google Scholar]
- 24.Wheaton AG, Ford ES, Cunningham TJ, Croft JB. (2015) Chronic obstructive pulmonary disease, hospital visits, and comorbidities: National Survey of Residential Care Facilities, 2010. J Aging Health 27:480–499. 10.1177/0898264314552419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. (2012) Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 155–161. 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 26.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, et al. (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618–1623. 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 27.Wedzicha JA, Seemungal TA, MacCallum PK, Paul EA, Donaldson GC, Bhowmik A, et al. (2000) Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 84: 210–215. [PubMed] [Google Scholar]
- 28.Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, et al. (2006) Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 867–874. 10.1164/rccm.200604-506OC [DOI] [PubMed] [Google Scholar]
- 29.Kofoed SC, Wittrup HH, Sillesen H, Nordestgaard BG. (2003) Fibrinogen predicts ischaemic stroke and advanced atherosclerosis but not echolucent, rupture-prone carotid plaques: the Copenhagen City Heart Study. Eur Heart J 24: 567–576. [DOI] [PubMed] [Google Scholar]
- 30.Gabay C, Kushner I. (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 31.Danesh J, Collins R, Appleby P, Peto R. (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease. JAMA 279: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM. (1999) Evaluating novel cardiovascular risk factors: can we better predict heart attacks? Ann Intern Med 130: 933–937. [DOI] [PubMed] [Google Scholar]
- 33.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. (2001) Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 32: 2575–2579. [DOI] [PubMed] [Google Scholar]
- 34.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. (2005) Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 972–977. 10.1164/rccm.200407-855OC [DOI] [PubMed] [Google Scholar]
- 35.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. (1988) Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med 319: 1173–1179. 10.1056/NEJM198811033191801 [DOI] [PubMed] [Google Scholar]
- 36.Koenig W, Sund M, Döring A, Ernst E. (1997) Leisure-time physical activity but not work-related physical activity is associated with decreased plasma viscosity: results from a large population sample. Circulation 95: 335–341. [DOI] [PubMed] [Google Scholar]
- 37.Ernst E. (1993) Regular exercise reduces fibrinogen levels: a review of longitudinal studies. Br J Sports Med 27: 175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauramaa R, Salonen JT, Seppänen K, Salonen R, Venäläinen JM, Ihanainen M, et al. (1986) Inhibition of platelet aggregability by moderate-intensity physical exercise: a randomized clinical trial in overweight men. Circulation 74: 939–944. [DOI] [PubMed] [Google Scholar]
- 39.Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R. (1998) Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 629–634. 10.1164/ajrccm.158.2.9711023 [DOI] [PubMed] [Google Scholar]
- 40.Sin DD, Man SF. (2006) Skeletal muscle weakness, reduced exercise tolerance, and COPD: is systemic inflammation the missing link? Thorax 61: 1–3. 10.1136/thx.2005.044941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R. (2013) Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 173: 1175–1185. 10.1001/jamainternmed.2013.1016 [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Loke YK, Enright P, Furberg CD. (2013) Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax 68: 114–116. 10.1136/thoraxjnl-2011-201275 [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Selim MH, Caplan LR. (2010) Medical complications after stroke. Lancet Neurol 9: 105–118. 10.1016/S1474-4422(09)70266-2 [DOI] [PubMed] [Google Scholar]
- 44.Ryan M, Suaya JA, Chapman JD, Stason WB, Shepard DS, Thomas CP. (2013) Incidence and cost of pneumonia in older adults with COPD in the United States. PLoS One 8: e75887 10.1371/journal.pone.0075887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Reuck J, Proot P, Van Maele G. (2007) Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol 14: 989–992. 10.1111/j.1468-1331.2007.01829.x [DOI] [PubMed] [Google Scholar]
- 46.Oliveira AJ, Zamagni M, Dolso P, Bassetti MA, Gigli GL. (2000) Respiratory disorders during sleep in patients with epilepsy: effect of ventilatory therapy on EEG interictal epileptiform discharges. Clin Neurophysiol 111(Suppl 2): S141–S145. [DOI] [PubMed] [Google Scholar]
- 47.Wang MT, Tsai CL, Lo YW, Liou JT, Lee WJ, Lai IC. (2012) Risk of stroke associated with inhaled ipratropium bromide in chronic obstructive pulmonary disease: a population-based nested case-control study. Int J Cardiol 158: 279–284. 10.1016/j.ijcard.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 48.Lin HW, Chung CL, Lin YS, Yu CM, Lee CN, Bien MY. (2015) Inhaled pharmacotherapy and stroke risk in patients with chronic obstructive pulmonary disease: a nationwide population based study using two-stage approach. PLoS One 10: e0130102 10.1371/journal.pone.0130102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are owned by the National Health Insurance Research Database (NHIRD) Committee and the contact information is available as email: nhird@nhri.org.tw. We confirm that no special agreement existed between our research team and the NHIRD, and that all interested researchers will be able to access data in the same manner in which we did.