Abstract
The expression of lentivirus-transduced enhanced green fluorescent protein (EGFP) was detectable in rabbit retinal pigment epithelium (RPE) within 3 to 5 days after subretinal injection of the vector. Within 2 to 3 weeks, EGFP-expressing cells were eliminated by rejection. In the current experiments, we monitor serum antibody titers for EGFP before and after transduction and determine whether systemic immunosuppression prevents recognition of EGFP by the immune system. While all control rabbits developed antibodies against EFGP and showed signs of rejection, no such evidence was observed with animals which received immunosuppression. One month of systemic immunosuppression permanently prevented rejection of RPE with EGFP expression. Fluorescence has been maintained for more than a year. If a control eye was injected with the same virus after terminating immunosuppression, both eyes showed signs of rejection. The lack of rejection is not due to tolerance but to a failure of the animals to detect the foreign protein. Detection must depend upon a brief window of time after surgery needed to introduce the vector, perhaps related to a concurrent but transient inflammation. This strategy may be useful in managing other types of rejection in the retina.
Numerous investigators have used viral vectors to transduce retinal cells with enhanced green fluorescent protein (EGFP), with no evidence of rejection of this foreign protein (2, 3, 15, 20, 21). Similar results have been reported with other reporter genes that express foreign proteins in the retina, such as LacZ (6, 8, 14). We have found that with relatively high retinal expression of EGFP, transduced by lentivirus, rejection will occur (7). GFP has been shown to be immunogenic (9) and will be rejected in mice (19). In order to examine this phenomenon more completely, we have determined whether antibodies to EGFP can be detected in rabbits during the course of rejection in the retina. We have also determined whether this rejection can be prevented by immunosuppression. Our results indicate that serum antibodies to EGFP are detectable concurrent with retinal rejection of this fluorescent protein. If the rabbits are immunosuppressed, rejection does not occur. Surprisingly, if immunosuppression is stopped after 1 month, rejection never occurs and antibodies to EGFP are not detectable. These results imply that foreign proteins transduced in the retina are only detectable immunologically during a brief period of time after the surgical procedure needed to introduce the viral vector into the retina.
MATERIALS AND METHODS
Preparation of viral stocks.
The viral stocks were produced as described previously (16) by cotransfection of human kidney-derived 293T cells with three plasmids by the calcium phosphate method (4). The packaging construct designated pCMVΔR8.2 contained the cytomegalovirus (CMV) promoter and the insulin polyadenylation signal to express all of the viral proteins in trans form, except the envelope and Vpu. The second plasmid, pHR′-CMV-GFP, provided a vector with all the cis-acting elements that allow transfer and integration of the viral gene into the target's cell genome. In this transducing vector, an expression cassette with the Rev responsive element and the CMV promoter are used to direct the expression of EGFP. The third plasmid, pMD.G, provides the envelope protein from the vesicular stomatitis virus glycoprotein to enhance viral stability and broaden the range of host cell targets. Cells (1.5 × 106) were plated on a 10-cm-diameter dish and kept overnight, and the medium was changed 2 h prior to transfection. A DNA mixture containing 15 μg of pCMVΔR8.2, 20 μg of pHR′-CMV-GFP, and 5 μg of pMD.G was used for calcium phosphate precipitation. The medium was changed 18 h after transfection. After another 48-h incubation, virus-containing medium was harvested from 40 10-cm dishes. Debris was cleared by low-speed centrifugation. First ultracentrifugation at 50,000 × g for 90 min was performed after filtration of the medium through a 0.45-μm-pore-size filter. The pellet was suspended in 600 μl of virus incubation buffer (50 mM Tris-HCl, pH 7.8, 130 mM NaCl, 10 mM KCl, 10 mM MgCl2, 0.1 mM deoxynucleoside triphosphates [dNTPs], 3 mM spermine, 0.3 mM spermidine) and incubated for 2 h at 37°C. The suspension was diluted with TBS (50 mM Tris-HCl, pH 7.8, 130 mM NaCl, 10 mM KCl, 5 mM MgCl2) followed by a second ultracentrifugation at 50,000 × g for 90 min. The final pellet was suspended in 100 μl of TBS containing 8-μg/ml Polybrene. The lentiviral titers were determined by infection of 293T cells seeded in six-well plates at 105 cells per well on the night before infection. A serial dilution of the concentrated viral stock by using culture medium supplemented with 8-μg/ml Polybrene was exchanged with old medium. After incubation overnight, the culture medium was changed and the cells were incubated for 2 more days. GFP fluorescent cells were identified by fluorescence-activated cell sorter. The wells with approximately 5% GFP-positive cells were used to calculate the titers by dividing the actual percentage by the dilution factor. Titers ranged from 107 to 1010 infectious units/ml.
In vivo transduction.
Dutch belted rabbits were used following the guidelines of the Association for Research in Vision and Ophthalmology (1a). Each rabbit was anesthetized with ketamine (20 mg/kg) and xylazine (10 mg/kg) intramuscularly. The pupil of one eye was dilated with 2% (wt/vol) cyclopentolate and 2.5% (wt/vol) phenylephrine hydrochloride (Neo-Synephrine). A conjunctival flap and a sclerotomy were made about 3 mm behind the limbus. A contact lens was placed over the cornea cushioned with hyaluronic acid (Healon) in order to facilitate viewing the retina. A glass micropipette with a tip outer diameter of 50 μm containing the viral suspension was introduced into the vitreal cavity through the sclerotomy and brought to the retinal surface with the aid of a surgical microscope. The tip of the pipette was gently pressed on the retinal surface while the viral suspension was injected through the neural retina into the subretinal space. This produced a rapid circular bleb detachment of the neural retina of about 2 mm in diameter, which contained the viral suspension. The pipette was then withdrawn from the eye, and the sclera and conjunctiva were sutured with 9-0 nylon.
Experiments were performed on 11 eyes of 9 rabbits. All rabbits received a subretinal injection of the viral suspension in one eye. Four of these rabbits were immunosuppressed starting on the day of surgery, receiving intramuscular injections of methylprednisolone sodium succinate (Solu-Medrol; 6.25 mg), azathioprine (8 mg), and oral administration of cyclosporin (Neoral solution; 50 mg) daily for 1 month. After one month the immunosuppression was terminated. Two of the rabbits being immunosuppressed and two not being suppressed had blood samples obtained every 3 days from an ear vein for enzyme-linked immunosorbent assays (ELISAs) for antibodies to GFP. Two rabbits that received immunosuppression and one that did not had a second subretinal injection of the viral suspension to the other eye. The surgery took place several months after the previous operation at a time when they were not being immunosuppressed.
ELISA.
MaxiSorp 96-well plates (Nalge Nunc International) were coated with 1 μg of rEGFP protein (Clontech) per 100 μl in 50 mM sodium carbonate buffer (pH 9.6). After washing the plate with phosphate-buffered saline (PBS) containing 0.05% Tween 20, 3% bovine serum albumin in PBS was used for blocking. Rabbit serum was diluted with blocking solution, 100 μl of which was applied to each well. After 90 min of incubation at 37°C, the plate was washed and reacted with 100 μl of secondary antibody containing 0.1-μg/ml horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) in peroxidase stabilizing buffer (Sigma). HRP was detected with a 3,3′, 5,5′-tetramethylbenzidine liquid substrate system for ELISA (Sigma) followed by colorimetric analysis with a Multiskan RC plate reader (Labsystems).
Retinal examination.
The retina of each rabbit was examined mainly with a scanning laser ophthalmoscope (SLO) (Rodenstock); biomicroscopy with a contact lens and a surgical microscope was used to examine the retina during the initial surgical procedure. The retinal examination by SLO used four different wavelengths of infrared diode (780 nm), helium-neon red (633 nm), argon green (514 nm), and blue (488 nm) lasers. The latter was used with and without a barrier filter for monitoring EGFP fluorescence. We used a subjective grading scale to evaluate the amount of EGFP-expressing cells (7). If there were more than 50 spots of fluorescence within the injected area, the grade was 3; if there were between 10 and 50, the grade was 2; if there were less than 10, the grade was 1; and the absence of fluorescence was graded as 0.
Histology.
Rabbits were sacrificed after ophthalmoscopic signs of rejection appeared: i.e., loss of GFP fluorescence and retinal pigment epithelial disruption. Immunosuppressed rabbits, which continued to exhibit EGFP fluorescence, were sacrificed at later times. One rabbit expressing GFP at 1 year after surgery is still alive. The eyes of rabbits showing evidence of rejection were fixed by immersion in 3%(wt/vol) glutaraldehyde in PBS after first penetrating the eye with an 18-gauge needle at three sites around the limbus in order to facilitate penetration of the fixative. These eyes were kept in fixative at 4°C for 1 to 7 days before being processed for histological examination. The eyes were then washed in a balanced salt solution, and the anterior half was removed with the lens and vitreous. The posterior eye cup was examined with a surgical microscope, and the treated area was identified and cut out as a rectangular segment about 0.5 by 1 cm in dimension. This segment was washed, dehydrated, and embedded in Epon for sectioning for light and, in selected cases, electron microscopy. The eyes of rabbits that had been immunosuppressed and had no rejection were fixed in 4% (wt/vol) paraformaldehyde in PBS, dissected into a similar segment, immersed in OCT compound, and frozen by dry ice. Cryosectioning was performed on a Leica 1850 cryotome (Leica Instruments). Sections were mounted on gelatinized glass slides with Fluoromount-G and examined for fluorescence with a Zeis Axiovert S100 epifluorescent microscope.
RESULTS
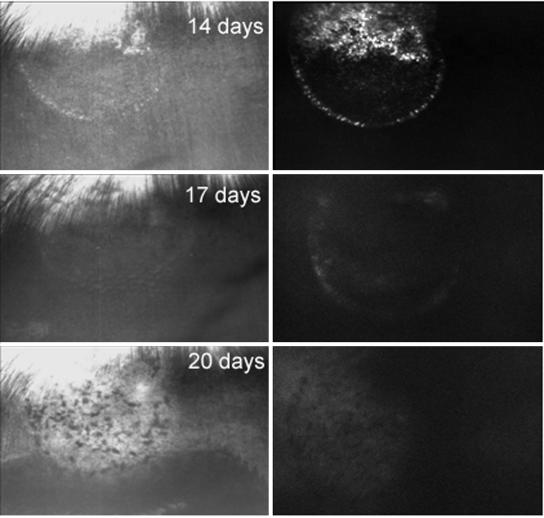
Figure 1 shows SLO images of rabbit retina in reflected blue light (left) and fluorescence (right) at different times after surgery necessary to introduce the viral suspension subretinally. At 6 and 14 days after surgery, the appearance of the retina in the treated area appeared relatively normal, covered above by highly reflecting myelinated nerve fibers entering the optic nerve head; there is a small white spot marking the point where the retina was entered in order to produce the transient retinal detachment and the injection of the viral suspension (Fig. 1, left). GFP fluorescence (Fig. 1, right) was apparent in the superior aspect of this circular area as well as along its edges. At 17 days after surgery, it became difficult to see the treated area because of slight edema, and EGFP fluorescence disappeared. At 20 days after surgery, there was disruption of the retinal pigment epithelium (RPE) layer, which we have found characteristic of rejection (7). The dark areas are due to piling up of these migrating, activated, retinal pigment epithelial cells. All five rabbits that were transduced but not immunosuppressed showed signs of rejection in a similar fashion within 2 to 3 weeks after surgery.
FIG. 1.
SLO photographs of rabbit retina in which expression and rejection of EGFP occurs. On the left are photographs in blue light; on the right are photographs of EGFP fluorescence. At 6 and 14 days after surgery, the retina appears normal and EGFP fluorescence is seen. At 17 days, the view of the retina is poor because of suspected edema and fluorescence has disappeared. At 20 days and 2 months, there is disruption of the RPE layer and EGFP fluorescence remains absent. The approximate diameter of the transduced area visible by a circular demarcation line is about 1 mm.
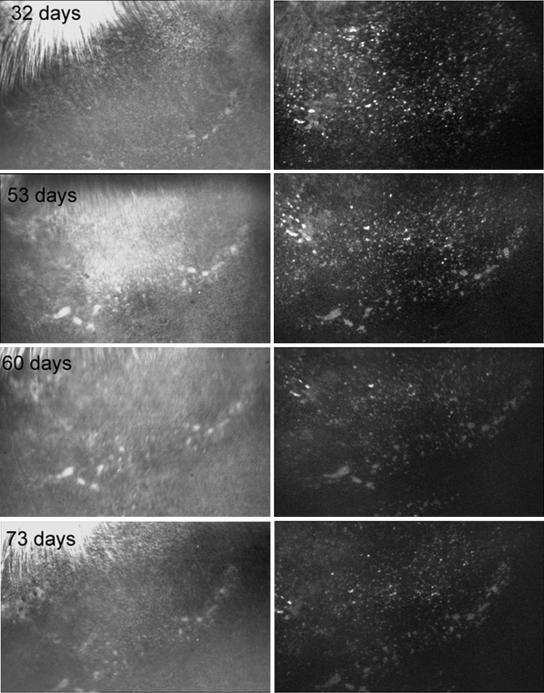
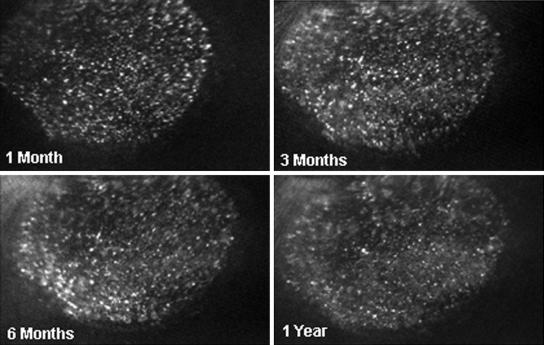
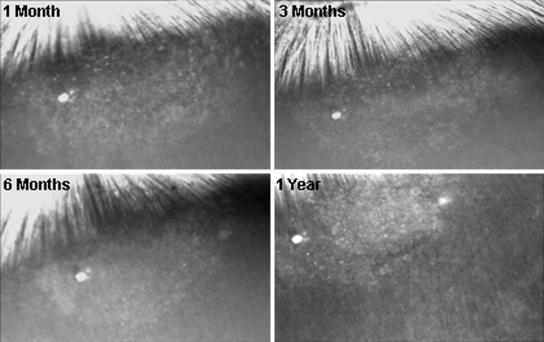
On the other hand, all four rabbits that were immunosuppressed showed no signs of rejection and continued to express EGFP for as long as we monitored them. Figure 2 illustrates the retina from such immunosuppressed rabbits at 32, 53, 60, and 73 days after surgery. The blue light image of the retina (left) has a relatively normal appearance, and EGFP fluorescence continued over this time (right). Figures 3 and 4 show the fundus of another rabbit, which was also immunosuppressed for 1 month after transduction. There was no evidence of rejection (Fig. 3) and strong EGFP fluorescence continued for 1 year after surgery (Fig. 4). The small fluorescent spots, seen in these retinas, have the dimensions of single retinal epithelial cells, as we have noted previously (7). It is possible to follow these single fluorescent cells and cell clusters over relatively long periods of time. In general, most of these fluorescing structures remain stable over time but some changes can be seen, perhaps due to variations in GFP expression or cell migration.
FIG. 2.
SLO photographs of continued EGFP fluorescence in a rabbit that had been immunosuppressed for 1 month after surgery. There is relatively little change in EGFP fluorescence for 32 to 73 days after surgery.
FIG. 3.
SLO photographs of another rabbit that continues to show relatively unchanged EGFP fluorescence for 1 year after surgery and was also immunosuppressed for 1 month.
FIG. 4.
SLO photographs of blue light images of the same retina as shown in Fig. 3. There is no evidence of any disruption of the RPE layer characteristic of rejection.
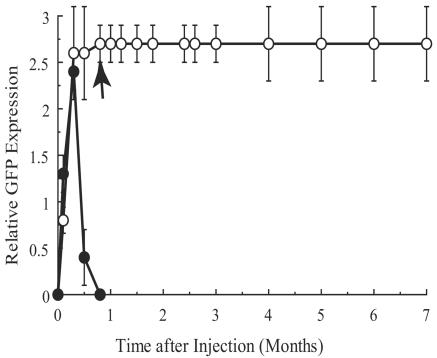
Figure 5 shows the relative EGFP fluorescence graded in the retinas of immunosuppressed and nonimmunosuppressed rabbits. All of the rabbits that were not immunosuppressed showed strong GFP expression during the first 1 to 2 weeks after subretinal administration but completely lost the fluorescence after this period. All of these rabbits developed the characteristic funduscopic appearance of rejection with disruption of the retinal epithelial layer shortly after GFP fluorescence was lost. All four of the rabbits that were immunosuppressed for 1 month continued to express EGFP at the same levels for months after transduction.
FIG. 5.
Correlation between relative EGFP fluorescence and time after subretinal injection of viral suspension. The data represent means ± standard deviations. Open circles represent data from immunosuppressed rabbits (n = 4). Data from rabbits that were not immunosuppressed are shown by filled circles (n = 5). An arrow designates the time point at which immunosuppression was terminated.
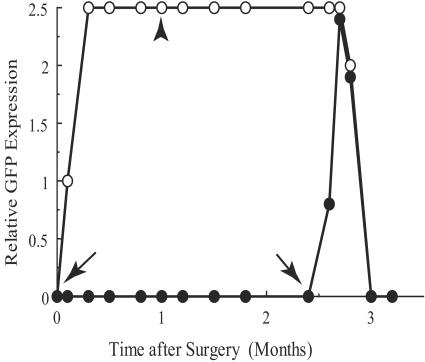
One of these immunosuppressed rabbits was given a second injection in the other eye without immunosuppression. EGFP expression developed to a similar degree in this eye, but then fluorescence in both eyes disappeared within 2 weeks (Fig. 6), and those retinas developed the characteristic features of rejection.
FIG. 6.
Correlation between the relative EGFP fluorescence and the time after surgery. This rabbit was immunosuppressed for 1 month after injection to one eye (oculus sinister [left eye; OS]; open circles). Between 2 and 3 months after surgery, the retina in the other eye (oculus dexter [right eye; OD]; filled circles) was injected with a viral suspension producing EGFP fluorescence. Rejection in both eyes was observed thereafter. Two arrows represent the points when injections were made to OS and OD eyes. An arrowhead represents the point when immunosuppression was terminated.
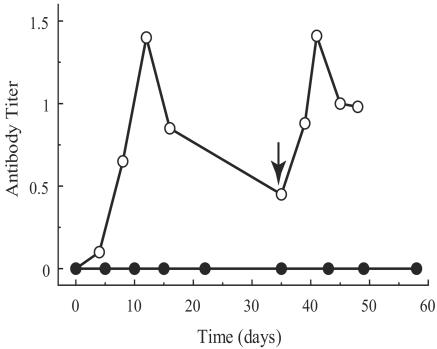
Figure 7 shows the results of ELISAs for EGFP antibodies in the serum of immunosuppressed and nonimmunosuppressed rabbits. Antibodies to EGFP were not detectable in immunosuppressed rabbits. In the nonimmunosuppressed rabbit, antibodies against EGFP appeared at about 5 days after injection and increased to a maximum at 12 days at which time rejection was seen in the retina. After the rejection, serum antibodies decreased until the other eye was transduced, whereupon the antibody titer increased again, concurrently with rejection.
FIG. 7.
ELISA-determined titer of systemic antibodies against EGFP at different time points after surgery. Open circles represent data obtained from a rabbit that was not immunosuppressed. An arrow shows the point of the second injection. Filled circles represent data obtained from two rabbits with immunosuppression. All rabbits received their first injection at time zero.
Histology of the retinas in which rejection had occurred was similar to what we have already described (7). There were areas of retinal pigment epithelial disruption, migration, and piling up of these cells in the subretinal space. Areas of inflammatory cells were present in the choroid adjacent to disrupted epithelial layer. The retinas of immunosuppressed rabbits that continued to express EGFP revealed fluorescence exclusively in the retinal epithelial layer.
DISCUSSION
The results show that transgenic expression of EGFP in rabbit RPE is a target for rejection by the immune system, even though the retina is considered an immunologically privileged site (10). The presence of antibodies to EGFP and their concentration peaking at the time of overt rejection indicate that EGFP has been detected by the immune system, and this immune response occurs concurrently with rejection. Whether EGFP is the sole target of the immune system leading to rejection is not certain. Other foreign proteins, perhaps attributable to the viral vector, could also be playing a role in this immune response, although injecting the viral vector without any transgene does not lead to rejection (7). Subretinal administration of lentiviral vectors has been shown to produce a humoral immune response detectable by IgG2b and IgGl isotypes but no cellular reaction (2).
The immunosuppression completely prevents tissue damage and loss of EGFP expression, indicating that immune rejection is responsible for these changes. The results imply that other foreign proteins transduced in the RPE could lead to rejection and consequently limit the potential of gene therapy in this layer of retinal cells. Why other investigators using EGFP as well as other foreign proteins, such as LacZ, to examine gene expression in the retina have not observed rejection is interesting. It could be due to species differences or to weaker expression of the transgenic proteins. We have observed that weak expression of EGFP in rabbit RPE will also avoid rejection (7). Another difference is the tendency for adeno-associated virus transduction to start the expression of GFP more slowly than lentivirus (2). This may minimize both the amount of antigen exposed within the time window for detection and thereby avoid rejection.
The most remarkable result is that if these rabbits are immunosuppressed for only 1 month, rejection never occurs subsequently. This indicates that there is a limited time period within which this foreign protein can elicit a response from the immune system. The time must begin either with the surgical event and/or the expression of EGFP. The complete suppression of immune rejection in the central nervous system by transient immunosuppression is reminiscent of lymphocytic choriomeningitis virus-induced central nervous system disease (5). In this case, an immune response to a virus infecting brain tissue is prevented by administering a single dose of an immunosuppressive agent, cyclophosphamide. Drug-administered mice never develop brain pathology, while littermates that are not immunosuppressed suffer severe brain damage. This is another example of a window in time within which transient suppression of the immune system leads to permanent absence of rejection. Something makes the immune system much more able to respond during a short period of time after the antigenic substance is introduced into the tissue. This suggests that the surgical procedure required to introduce the viral suspension triggers an immune response and the ability to trigger this process diminishes with time as the effects of the surgery wear off. Inflammation invariably occurs during such surgery, which could attract cells capable of incorporating and presenting antigen in the subretinal space. Even the RPE cell is capable of presenting antigen (13, 17; E. M. E. Dafgard-Kopp, A. M. Winter-Vernersson, and P. Algvere, Abstr., Investig. Ophthalmol. Vis. Sci. 38:S396, 1997; J. E. Silbert, E. K. Gao, X. H. Yu, and H. K. Kaplan, Abstr. Investig. Ophthalmol. Vis. Sci. 35:S1261, 1994), especially after being activated by inflammatory cytokines. There are also many dendritic cells in the choroid, which could be recruited into the subretinal space by inflammation (11). In other parts of the central nervous system, inflammation leads to the recruitment of dendritic cells into this immunologically privileged tissue (18). These cells could linger in this region after surgery and set the time period within which detection occurs.
These results suggest further experiments are needed to determine more precisely the time period during which the immune system is able to detect a foreign protein being expressed in the RPE and whether all of the immunosuppressant substances we used are required to prevent rejection. It is possible that only suppression of the inflammatory response is necessary. It would also be interesting to know whether a similar strategy of transient immunosuppression can thwart the suspected rejection of RPE allografts in the subretinal space (1, 12, 22). Brief immunosuppression might be a strategy to employ in gene therapy of the RPE if rejection of a foreign transgenic protein is suspected.
An incidental observation is also of considerable interest to gene therapy in the retina. It has been reported that the introduction of lentiviral vector subretinally in rat (15) and mouse (21) retina transduces cells not only around the injection area but covering whole surface of the retina, suggesting that the virus particles diffuse throughout the subretinal space. This does not appear to be the case in rabbit retina, where there is a precise zone of expression confined to the area of the detachment—invariably a circular area that is easy to identify. Therefore, there must be either species differences, variations in surgical technique, or perhaps mechanical differences in the way the retina detaches and reattaches in these different species after the virus is introduced into the subretinal space. This is of considerable importance if one wants to express a particular gene over large areas of the retina or conversely wants to restrict the transduction to specific areas.
Acknowledgments
We thank Research to Prevent Blindness, Inc., for their support.
REFERENCES
- 1.Algvere, P. V., P. Gouras, and E. Dafgard Kopp. 1999. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur. J. Ophthalmol. 9:217-230. [DOI] [PubMed] [Google Scholar]
- 1a.Association for Research in Vision and Ophthalmology. 2002. ARVO resolution for the use of animals in research. Association for Research in Vision and Ophthalmology, Rockville, Md. http://www.arvo.org/AboutArvo/animalst.asp.
- 2.Auricchio, A., G. Kobinger, V. Anand, M. Hildinger, E. O'Connor, A. M. Maguire, J. M. Wilson, and J. Bennett. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10:3075-3081. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, J., A. M. Maguire, A. V. Cideciyan, M. Schnell, E. Glover, V. Anand, T. S. Aleman, N. Chirmule, A. R. Gupta, Y. Huang, G. P. Gao, W. C. Nyberg, J. Tazelaar, J. Hughes, J. M. Wilson, and S. G. Jacobson. 1999. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc. Natl. Acad. Sci. USA 96:9920-9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, G. A., N. Nathanson, and R. A. Prendergast. 1972. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature 238:335-337. [DOI] [PubMed] [Google Scholar]
- 6.Derksen, T. A., S. L. Sauter, and B. L. Davidson. 2002. Feline immunodeficiency virus vectors. Gene transfer to mouse retina following intravitreal injection. J. Gene Med. 4:463-469. [DOI] [PubMed] [Google Scholar]
- 7.Doi, K., J. Hargitai, J. Kong, S. H. Tsang, M. Wheatley, S. Chang, S. Goff, and P. Gouras. 2002. Lentiviral transduction of green fluorescent protein in retinal epithelium: evidence of rejection. Vision Res. 42:551-558. [DOI] [PubMed] [Google Scholar]
- 8.Galileo, D. S., K. Hunter, and S. B. Smith. 1999. Stable and efficient gene transfer into the mutant retinal pigment epithelial cells of the Mitf(vit) mouse using a lentiviral vector. Curr. Eye Res. 18:135-142. [DOI] [PubMed] [Google Scholar]
- 9.Gambotto, A., G. Dworacki, V. Cicinnati, T. Kenniston, J. Steitz, T. Tuting, P. D. Robbins, and A. B. DeLeo. 2000. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: identification of an H2-Kd-restricted CTL epitope. Gene Ther. 7:2036-2040. [DOI] [PubMed] [Google Scholar]
- 10.Gregerson, D. S., and J. Yang. 2003. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Investig. Ophthalmol. Vis. Sci. 44:3083-3093. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, H. R., L. Lumsden, and J. V. Forrester. 1999. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Investig. Ophthalmol. Vis. Sci. 40:3177-3185. [PubMed] [Google Scholar]
- 12.Jiang, L. Q., M. Jorquera, and J. W. Streilein. 1994. Immunologic consequences of intraocular implantation of retinal pigment epithelial allografts. Exp. Eye Res. 58:719-728. [DOI] [PubMed] [Google Scholar]
- 13.Liversidge, J., and J. V. Forrester. 1992. Antigen processing and presentation in the eye: a review. Curr. Eye Res. 11(Suppl.):49-58. [DOI] [PubMed] [Google Scholar]
- 14.Lotery, A. J., T. A. Derksen, S. R. Russell, R. F. Mullins, S. Sauter, L. M. Affatigato, E. M. Stone, and B. L. Davidson. 2002. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum. Gene Ther. 13:689-696. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi, H., M. Takahashi, F. H. Gage, and I. M. Verma. 1997. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc. Natl. Acad. Sci. USA 94:10319-10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naldini, L., U. Blömer, F. H. Gage, D. Trono, and I. M. Verma. 1996. Efficient transfer, integration, and sustained long-term expression of the trangene in adult rat brains injected with lentiviral vector. Proc. Natl. Acad. Sci. USA 93:11382-11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezai, K. A., R. T. Semnani, S. C. Patel, J. T. Ernest, and G. A. van Seventer. 1997. The immunogenic potential of human fetal retinal pigment epithelium and its relation to transplantation. Investig. Ophthalmol. Vis. Sci. 38:2662-2671. [PubMed] [Google Scholar]
- 18.Serafini, B., S. Columba-Cabezas, F. Di Rosa, and F. Aloisi. 2000. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am. J. Pathol. 157:1991-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stripecke, R., M. Carmen Villacres, D. Skelton, N. Satake, S. Halene, and D. Kohn. 1999. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 6:1305-1312. [DOI] [PubMed] [Google Scholar]
- 20.Surace, E. M., A. Auricchio, S. J. Reich, T. Rex, E. Glover, S. Pineles, W. Tang, E. O'Connor, A. Lyubarsky, A. Savchenko, E. N. Pugh, Jr., A. M. Maguire, J. M. Wilson, and J. Bennett. 2003. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J. Virol. 77:7957-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi, M., H. Miyoshi, I. M. Verma, and F. H. Gage. 1999. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J. Virol. 73:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye, J., H. M. Wang, T. E. Ogden, and S. J. Ryan. 1993. Allotransplantation of rabbit retinal pigment epithelial cells double-labelled with 5-bromodeoxyuridine (BrdU) and natural pigment. Curr. Eye Res. 12:629-639. [DOI] [PubMed] [Google Scholar]