Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV) is the pathogen of SARS, which caused a global panic in 2003. We describe here the screening of Chinese herbal medicine-based, novel small molecules that bind avidly with the surface spike protein of SARS-CoV and thus can interfere with the entry of the virus to its host cells. We achieved this by using a two-step screening method consisting of frontal affinity chromatography-mass spectrometry coupled with a viral infection assay based on a human immunodeficiency virus (HIV)-luc/SARS pseudotyped virus. Two small molecules, tetra-O-galloyl-β-d-glucose (TGG) and luteolin, were identified, whose anti-SARS-CoV activities were confirmed by using a wild-type SARS-CoV infection system. TGG exhibits prominent anti-SARS-CoV activity with a 50% effective concentration of 4.5 μM and a selective index of 240.0. The two-step screening method described here yielded several small molecules that can be used for developing new classes of anti-SARS-CoV drugs and is potentially useful for the high-throughput screening of drugs inhibiting the entry of HIV, hepatitis C virus, and other insidious viruses into their host cells.
Severe acute respiratory syndrome (SARS) is a viral respiratory disease that spread to more than two-dozen countries in Asia, North America, South America, and Europe in the spring of 2003 (36, 49). Approximately 8,400 people worldwide suffered from SARS and more than 900 died according to a report of the World Health Organization executive board on 27 November 2003. A novel coronavirus, SARS-coronavirus (SARS-CoV), was identified as the pathogen that caused SARS (13, 26). SARS-CoV is highly infectious and is transmitted via the respiratory route (13, 15, 26, 36, 49). Although the first outbreak is over, it is possible that even a low-level epidemic or accidental infection, as recently happened in Singapore and Taiwan, could cause a sudden worldwide epidemic. At present, few drugs are available for treating SARS. Ribavirin was initially used, but it proved to be barely effective and had serious side effects (25, 50). Cinatl et al. reported recently that interferon exhibited anti-SARS-CoV potency (8) and that glycyrrhizin had an anti-SARS-CoV activity with a 50% effective concentration (EC50) of 300 mg/liter (364.5 μM) (9).
We have targeted our drug discovery to small molecules that can inhibit SARS-CoV from entering its host cells. Virus entry is an attractive target step for therapy because it can block the propagation of virus at an early stage, thus minimizing the chance for the virus to evolve and acquire drug resistance. Inhibitors of the entry of several important viruses have been developed. For example, a new class of virus entry inhibitors has been developed for the human immunodeficiency virus type 1 (HIV-1) (14, 32), and one of these, T20 (enfuvirtide), is now in clinical usage (14, 20, 23, 32). For the respiratory syncytial virus (RSV), the small molecules RFI 641 (33, 37) and VP-14637 (11) have been developed to inhibit its entry process. For herpes simplex virus, entry inhibitors such as FGF4 signal peptide (4, 5) and n-docosanol (35) are under development.
We have developed a two-step screening method combining frontal affinity chromatography-mass spectrometry (FAC/MS) and pseudotyped virus infection assay to search for drugs that can interfere with the entry of SARS-CoV into host cells. FAC/MS allows for competitive assays of interacting molecules, such as enzymes and ligands, as well as for a quick analysis and direct presentation of the assay (6, 41, 42). Here, in the first step, we use the FAC/MS to perform high-throughput screening of small molecules that can bind with high affinity to the SARS-CoV S2 protein. The other rationale of our study is that some of these S2-binding molecules may interfere with the function of the S protein, thus preventing the SARS-CoV from entering its host cells. To test this, we developed an HIV-luc/SARS pseudotyped virus that can infect Vero E6 cells resulting in the cellular expression of luciferase that can be detected by luminometry. Thus, as a second step, we used HIV-luc/SARS pseudotyped virus to check the function of the small molecules obtained in the first step. This system is highly sensitive, rapid, and reproducible; the materials are safe to handle and can be used for high-throughput screening.
We used the FAC/MS approach to screen small molecule libraries consisting of extracts from 121 Chinese herbs, including Prunella vulgaris and Saussurea lappa Clarks, that exhibited antiviral activities against HIV-1 (7), RSV (30), and hepatitis B virus (27). We identified two small molecules that bind avidly to the SARS S2 protein and can interfere with the entry of SARS-CoV into Vero E6 cells, with potent antiviral activities against wild-type SARS-CoV with EC50 values of 4.5 and 10.6 μM.
MATERIALS AND METHODS
Preparation for the polymeric carrier.
We mixed 4.7 mmol of functional monomer MAA (Acros, Geel, Belgium) and 24 mmol of cross-linker trimethylolpropane trimethacrylate (Sigma-Aldrich, Munich, Germany) and then added with 32 mg of initiator AIBN (Geel). The mixture was degassed and then placed in a 60°C water bath for 24 h. Finally, the mixture was frozen in N2. The rigid polymers were ground in a mortar and passed through a 30-μm-pore-size sieve. The fine particles were removed by decanting them in acetone. The remainders were vacuum dried.
Expression, purification, and activity detection of GST-S2 protein.
The full-length cDNA of the SARS-CoV S gene (strain BJ01, GenBank accession no. AY278488) was a gift provided by Shengli Bi at Institute of Virology, China CDC. We used it as a template to amplify the gene for S2 protein and cloned the PCR products into pGEM-T vector (Promega) and sequenced it. The desired fragments were then subcloned into pGEX-4T-1 vector (Amersham Biosciences). After we screened for the positive clones, the recombinant plasmids were transfected into Escherichia coli JM109 (DE3)-competent cells. The glutathione S-transferase (GST)-S2 fusion fragment was expressed and then purified from the inclusion body. The purified GST-S2 was then refolded with the Protein Refolding kit (Novagen).
The activity of the expressed GST-S2 protein was tested with convalescent SARS patient sera (provided by Beijing Ditan Hospital) by enzyme-linked immunosorbent assay (ELISA). Briefly, we precoated 96-well plates (Maxisorp; Nunc) with 1 μg of GST-S2 protein/well in 50 mM carbonate buffer (pH 9.6) for 2 h at 37°C and blocked them with 3% bovine serum albumin in carbonate buffer. We added 1:800-diluted human sera in phosphate-buffered saline with 0.05% Tween 20 (PBS-T; pH 7.4) to the wells, followed by incubation for 2 h at 37°C. The secondary antibodies conjugated with horseradish peroxidase were diluted 1:2,000 to 1:10,000 in PBS-T with 1% bovine serum albumin. The optical densities at 450 nm were determined with an ELISA plate reader (Bio-Rad model 550).
Immobilization of GST-S2 protein on the carrier.
First, 3.9 ml of a 1.12-mg/ml GST-S2 protein solution (0.05 M NaHCO3 [pH 8.5]) was dialyzed in 200 ml of 0.01 M NaHCO3 buffer (pH 8.0) at 4°C for 5 h, and then 4.8 mg of EDCI (Sigma-Aldrich, Germany) was added into the solution as an activation reagent. Then, 1.137 g of polymeric carrier that had been infiltrated in 3 ml of 0.01 M NaHCO3 (pH 8.0) buffer was added, and the mixture was shaken at 20°C for 4 h. The affinity columns (PEEK tubing, 0.75 by 50 mm) were packed with the GST-S2 protein linked covalently to the polymeric carrier by using a self-pack device (PerSeptive Biosystems). There were in all about 20 μl of wet polymeric particles that had been linked with GST-S2 protein and filled in the column. The chromatographic columns were then equilibrated with 2 mM NH4Ac (pH 6.7) solution and kept at 4°C.
Preparation of the extract samples.
Next, 121 Chinese herbs were extracted by macerating them with 85% ethanol at room temperature for 2 weeks (16, 17, 52). The solvent was evaporated in a vacuum, and then the extract sample was redissolved in dimethyl sulfoxide to a final concentration of 10 mg/ml. Thereafter, individual extracts were pooled in groups of five, and each pool was diluted to a concentration of 50 μg/ml (total concentration) with 2 mM NH4Ac (pH 6.7) before they were loaded onto the chromatographic column.
Screening the crude extracts by using a GST-S2 protein column (FAC/MS).
The frontal affinity chromatographic experiments were carried out at room temperature as preciously described (6, 41, 42). Briefly, the immobilized S2 proteins on the polymeric material were then packed wetly in the affinity columns (PEEK tubing, 0.75 by 50 mm). The diluted extracts were then loaded onto the chromatographic column. The flow rate was 5 μl/min. An extract sample solution containing various kinds of Chinese herbs at a concentration of 10 μg/ml (in 2 mM NH4Ac [pH 6.7]) was then mixed with methanol to allow the mixture to enter the detector of Mariner electrospray ionization time-of-flight mass spectrometer (PE Biosystems). For electrospray ionization, the mass spectrometer was operated in the negative mode under the following conditions: spray tip potential, 5,000 V; nozzle potential, 100 V; detector voltage, 2,250 V; nozzle temperature, 140°C; and quad temperature, 140°C. All of the other parameters were set at the default values.
The volume of the small molecules that bind to S2 protein (V), the volume of the nonbinding small molecules (V0), and the concentration of the bound small molecules (C) were recorded. Using these data, we calculated the binding affinity of the small molecules (Kd) to S2 protein according to the intercept in the following liner equation (22):
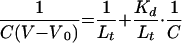 |
HIV-luc/SARS pseudotyped virus entry inhibition assays.
To produce HIV-luc/SARS pseudotyped virus, 10 μg of pNL4-3E-R-Luc (HIV-luc) and 10 μg of humanized SARS-CoV spike protein expression plasmids pcTSh were mixed and transfected into 293T cells with calcium phosphate as described previously (21, 55). The pseudotyped virus was harvested after 24 h of incubation, filtered through a 0.45-μm-pore-size Millipore membrane and normalized by p24 ELISA by using a Vironostika HIV-1 Antigen MicroELISA kit (Biomerieux bv, Boxtel, The Netherlands).
For the serum testing, the samples were serially twofold diluted from 1:100 and mixed with 5 ng of pseudotyped virus (p24). After incubation for 30 min at 37°C, the mixture was added onto the target cells. After overnight incubation, the medium was replaced, and the sample was incubated for an additional 36 h. The cells were then measured for luciferase activities by a Wallac Microbeta 1420 Counter (Perkin-Elmer, Inc.) by using the Luciferase Assay System (Promega, Inc.).
For the small-molecule testing, the supernatant containing 5 ng of pseudotyped virus (p24) was incubated with different concentrations of small molecules at 37°C for 30 min, and then the mixture was transferred into 96-well plates seeded with Vero E6 cells (3 × 103 cells/well), each concentration was repeated eight times. After overnight incubation, the medium was replaced, and the sample was incubated for an additional 36 h. The cells were then measured for luciferase activities as described above.
Wild-type SARS-CoV infection inhibition assay.
SARS-CoV Wild-type virus BJ01 strain was a gift of the Beijing Genomics Institute. We coincubated 200 50% tissue culture infective doses of wild-type SARS-CoV with 50-μl aliquots of small molecules at different concentrations at 37°C for 30 min. The mixture was then transferred to 96-well plates, with eight wells for each dilution. After incubation for 60 h, an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide; Sigma-Aldrich] assay was performed as described previously (19). Briefly, 10 μl of 5 mg of MTT/ml was added to each well, followed by incubation for a further 4 h. The medium in each well was then replaced with 100 μl of dimethyl sulfoxide, and then the media were left for 10 min at room temperature for color development before being read by Bio-Rad model 550 ELISA reader (at a test wavelength of 570 nm and a reference wavelength of 630 nm). The cytotoxicity of each small molecule was also determined by MTT assay without the addition of the virus.
RESULTS
Screening of small molecules by FAC/MS.
For the FAC/MS approach, we generated a fusion protein (GST-S2) in which an S2 protein fragment corresponded to the sequence between Asn733 to Gln1190 of the SARS-CoV S protein and then used the fusion protein to screen the small molecules pooled from the extracts of more than 121 Chinese herbs. The GST-S2 fusion protein was expressed in E. coli, purified from the inclusion body, and refolded. The purity and activity of the purified GST-S2 were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and ELISA (Fig. 1a and b). The results showed that the renatured recombinant GST-S2 could specifically bind to the anti-S protein antibodies present in the sera of convalescent SARS patients.
FIG. 1.
Identification of Galla chinensis components that have a high affinity to the SARS S2 protein by MS coupled with frontal affinity chromatography. (a) Purity of the GST-S fusion protein as shown by SDS-15% PAGE. (b) The specific binding of the GST-S2 protein with sera of three convalescent SARS patients (P1, P2, and P3) as shown by ELISA. Two normal sera (N1 and N2) and GST were used as controls. (c) The mass spectra of the crude extract of Galla chinensis (the main components are numbered 1 to 10; also see Table 1). (d) Frontal affinity chromatographic traces (selected ion chromatogram from the mass spectra, Fig. 1c) of the 10 main components in Galla chinensis. V0 indicates the volume of the nonbinding small molecules. Note the high retention time (t1/2 = 85 min) of component 10, indicating an exceptionally strong binding affinity to the SARS S2 protein.
We used the FAC/MS method to identify the small herbal molecules that had a relatively strong binding affinity to the GST-S2 protein. Extracts of 121 Chinese herbs were separately applied to the FAC column that was packed with purified GST-S2 protein (GST was used as control [data not shown]). The binding affinity of each main component of an extract to the GST-S2 protein was monitored by its elution front that could be deduced from its FAC/MS spectra. For example, among the frontal affinity chromatographic traces (for a typical ion chromatogram from the mass spectra, see Fig. 1c) of the 10 main components of Galla chinensis, the frontal volume of the tenth component (432.5 μl) was much higher than those of the other components (Table 1); correspondingly, this component also displayed the longest retention time (t1/2 = 85 min [Fig. 1d]). These results demonstrated that the tenth component exhibited the strongest affinity to S2 protein. Structural analysis established that this component is tetra-O-galloyl-β-d-glucose (TGG). This method allowed us to identify ∼130 small molecules with Kd values of <10 μM for further analyses.
TABLE 1.
Frontal volumes of the 10 main components in Galla chinensis extract
| Component | Frontal vol (μl) |
|---|---|
| 1 | 74 |
| 2 | 101.5 |
| 3 | 183 |
| 4 | 243 |
| 5 | 49 |
| 6 | 233.5 |
| 7 | 345.5 |
| 8 | 180 |
| 9 | 340.5 |
| 10 | 432.5 |
Inhibition of entry of HIV-luc/SARS pseudotyped virus into host cells.
We then used HIV-luc/SARS pseudotyped virus to investigate the antiviral activity of the 130 small molecule candidates. To produce the HIV-luc/SARS pseudotyped virus, we cotransfected a humanized S protein expression plasmid pcDSh with pNL4-3E-R-Luc (HIV-luc), an HIV-1 vector containing luciferase gene as a reporter into 293T cells. The pseudotyped viruses were then collected and used to infect Vero E6 cells, which are permissive to infection by wild-type SARS-CoV. To evaluate the relevance of our pseudovirus assay, we first tested the inhibition ability of normal sera and the sera of SARS patients. This infection could be blocked by the sera of SARS patients and appeared to be SARS specific because the same sera did not neutralize the vesicular stomatitis virus (VSV) G glycoprotein pseudotyped virus (Fig. 2a).
FIG. 2.
The inhibitory activities of SARS patients' sera and selected small molecules against the HIV-luc/SARS pseudotyped virus to enter Vero E6 cells. (a) Detection of inhibitory activities of sera of SARS patients. Note the ability of the SARS patient sera to block the infectivity of HIV-luc/SARS (SARS) versus the absence of such blocking activity in normal serum; note also the lack of neutralizing activity of the SARS sera against the pseudotyped virus bearing the G protein of VSV (VSV), which could infect the target cells at a similar level to that of HIV-luc/SARS. The serum dilution are indicated. (b) Structures and inhibitory activities of TGG and luteolin. Note the high inhibitory activity of TGG (EC50 = 2.86 μM), which is consistent with its high affinity to the SARS S2 protein, as our data demonstrated (Fig. 1c and d and Table 1). (c) Specificities of TGG and luteolin inhibitory activities. HIV-luc/VSV (VSV) pseudotyped viruses were used as a control. Note the inhibition by TGG and luteolin of the entry of HIV-luc/SARS virus but not the HIV-luc/VSV pseudotyped virus. Values are averages of triplicate determinations. The bars indicate the standard deviations.
To test the anti-HIV-luc/SARS activity, we added different concentrations of the small molecules to the infection mixture. Of the 130 small molecules, two were found to have potent antiviral activities against the HIV-luc/SARS pseudotyped virus, with EC50 values of 2.86 and 9.02 μM. Structural analysis revealed that the two small molecules were TGG and luteolin (Fig. 2b).
Specificity of small molecules.
To investigate the specificity of the small molecules, we tested their antiviral activities against HIV-luc/VSV pseudotyped virus, another pseudotyped virus enveloped with the G protein of VSV. Instead of the S protein of SARS-CoV, infection of the HIV-luc/VSV pseudotyped virus was also determined by the luciferase activity in the infected cells. Both TGG and luteolin showed little anti-VSV activity at the same concentration levels that can effectively inhibit the entry of HIV-luc/SARS pseudotyped virus to its host cells (Fig. 2c). HIV-luc/SARS pseudotyped virus and HIV-luc/VSV pseudotyped virus share the same genome and the genome replication apparatus; the only difference between them is that one is coated with SARS-CoV S protein, whereas the other is coated with VSV G protein. These studies indicate that the two small molecules, especially TGG, were highly specific against SARS-CoV. Since we isolated TGG and luteolin through analysis of their binding to the S2 protein of SARS-CoV, these small molecules most likely work through their ability to block the entry of HIV-luc/SARS pseudotyped virus to its host cells.
Inhibition of wild-type SARS-CoV infection.
To further confirm the antiviral activities of the small molecules identified above, we analyzed their inhibitory effects against infection by a wild-type SARS-CoV by using a MTT assay. In our experiments, we compared the antiviral activity of TGG and luteolin with the previously described glycyrrhizin and ribavirin. Our results (Table 2) showed that (i) TGG and luteolin could inhibit, in a dose-dependent fashion, SARS-CoV infection with EC50 values of 4.5 and 10.6 μM, respectively; (ii) the EC50 of glycyrrhizin in our experiments was >607.6 μM, which was relatively consistent with a previous report (9); and (iii) ribavirin, as reported earlier, showed little, if any, anti-SARS-CoV effects (9).
TABLE 2.
Inhibitory effects of TGG and luteolin against infection by wild-type SARS-CoVa
| Small molecule | MW | EC50 (μM) | SI | CC50 (mM) | LD50 (mg/kg) |
|---|---|---|---|---|---|
| TGG | 788.3 | 4.5 (1.96-5.8) | 240 | 1.08 | 232.2 |
| Luteolin | 286.3 | 10.6 (9.2-12.2) | 14.62 | 0.155 | 456 |
| Glycyrrhizin | 822.94 | >607.6 | ND | ND | ND |
| Ribavirin | 244.2 | No effect | ND | ND | ND |
Experiments were repeated three times, and the data in the table show the average EC50 values. MW, molecular weight; SI, selective index; ND, not determined.
Cytotoxicity and toxicity of the small molecules.
To test the biosafety of these small molecules, we examined their cytotoxicity against the Vero E6 cells by using the MTT assay. The CC50s (i.e., the concentrations of drug that caused 50% cell death) of TGG and luteolin were 1.08 and 0.155 mM, respectively (Table 2). Therefore, the selective index (i.e., the ratio of CC50 to EC50) values of TGG and luteolin were 240.0 and 14.62, respectively (Table 2). These results suggest that TGG and luteolin can be used at high concentrations to inhibit SARS-CoV without substantial cytotoxic effects. We then assessed the acute toxicity of the small molecules on the ICR mice by administrating TGG and luteolin intraperitoneally. The 50% lethal doses (i.e., the concentration of drug that causes 50% of animal death) of TGG and luteolin were ∼456 and 232.2 mg/kg, respectively, suggesting that these molecules can be used in mice at relatively high concentrations.
Antiviral activity of an analog of luteolin.
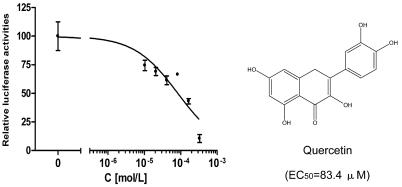
Since quercetin, which is structurally related to luteolin, is an ingredient of antioxidant and antiallergy medicines that had been approved by the U.S. Food and Drug Administration (FDA; the national drug code numbers of the medicines are 65448-3085, 65448-3005), we sought to determine whether quercetin could also antagonize SARS-CoV entry. Assays with the HIV-luc/SARS pseudotyped virus showed that quercetin also had antiviral activity against HIV-luc/SARS, with an EC50 of 83.4 μM (Fig. 3). The cytotoxicity of quercetin was very low, with a CC50 of 3.32 mM (data not shown). As an FDA-approved drug ingredient, quercetin offers great promise as a potential drug in the clinical treatment of SARS.
FIG. 3.
Structure and inhibitory activities of quercetin against the entry of HIV-luc/SARS pseudotyped virus into Vero E6 cells. Similar results were obtained in three independent experiments.
DISCUSSION
Using a two-step screening method, we screened a library containing extracts of 121 Chinese herbs and identified two small molecules, TGG and luteolin, that displayed inhibitory activity for the entry process of SARS-CoV into host cells. The combined use of FAC/MS and an infection assay utilizing the HIV-luc/SARS pseudotyped virus for the screening of antiviral drugs offers several advantages. (i) FAC/MS has been developed recently for high-throughput screening of enzymatic ligands (6) and for determining the strength of ligand and enzyme binding (dissociation constant) (53, 54). (ii) Pseudotyped viruses have proven to be a powerful method for studying the entry of viruses to their host cells. For example, studies utilizing HIV pseudotyped virus has led to the discovery of CCR5, the coreceptor of HIV-1 (10), and recently it was used to identify TRIM5α (a component of cytoplasmic bodies), which can inhibit HIV-1 infection in Old World monkeys (46). Pseudotyped hepatitis C viruses (HCVs) are being used to search for HCV receptors (2, 18). Pseudotyped viruses provide particularly useful assays for neutralizing antibodies (3), cell tropism (29, 51), and the identification of drugs that inhibit the entry of viruses into host cells (12, 38, 39, 44, 48). In the present study, we have utilized the SARS pseudotyped virus to screen for the small molecules that can antagonize SARS-CoV entry. We showed that FAC/MS in conjunction with the HIV-luc/SARS pseudotyped virus entry assay led to the rapid identification of two small inhibitory molecules. A similar strategy should be applicable for the search of drugs that antagonize other enveloped viruses such as HIV-1, HCV, and RSV.
There are two ways to obtain small molecules that can be used as virus entry inhibitors. These are, first, through chemical design and synthesis, such as SCH-C and TAK-779 for HIV-1 (1, 47) and as entry inhibitors for measles virus (34). The second is through isolation from natural products, e.g., different plant varieties. Chinese herbs are a great source of small molecules, leading to clinically used drugs; we identified here two small molecules from extracts of Chinese herbs, i.e., TGG and luteolin, that appear to be highly effective in inhibiting the entry of both wild-typed SARS-CoV and HIV-luc/SARS pseudotyped virus into Vero E6 cells (Fig. 2 and Table 2). Their anti-SARS-CoV potency is much greater than that of glycyrrhizin, a small molecule that has recently been reported to have anti-SARS-CoV activity (9). The detailed mechanism by which TGG and luteolin exert anti-SARS-CoV activity has not yet been established. The entry process of enveloped viruses usually involves three steps: attachment, receptor binding, and virus-cell fusion, which are mediated by viral envelope proteins. For SARS-CoV, it is presumed that its S protein participates in the viral entry process (24, 31, 40, 45) and that the transmembrane subunit of S protein, the S2 subunit, plays a crucial role in the virus-cell fusion process (24, 45). Our FAC/MS results identified TGG and luteolin as having the highest affinity, among all of the Chinese herbal components that we have studied thus far with the S2 protein. These data raise the possibility that TGG and luteolin may achieve their antiviral activity by interfering with the virus-cell fusion process. Additional studies are needed to test this idea.
TGG and luteolin offer excellent opportunities for further optimization and potential clinical use as anti-SARS drugs. TGG is a component of Galla chinensis that has been used in traditional Chinese medicine for treating chronic coughing. Luteolin has been identified in extracts of many Chinese herbs such as Veronica lina riifolia Pall by MS (28). We found this was a component of Rhodiola kirilowii, which has been used in Chinese medicine for treating hepatitis and tuberculosis (43). These two compounds and the luteolin-related, FDA-approved quecertin, have potential for use in the clinical treatment of SARS.
Acknowledgments
This research was supported by National Nature Science Foundation of China grant 30340027, Ministry of Science and Technology grant 2003CB514116, National Nature Science Foundation of China for Outstanding Young Scientist award 30125022, National Natural Science Foundation of China and German Research Association grant GZ237(202/10), and 6th Framework Program of European Commission grant 511063 to H.D., Ministry of Science and Technology grant G1999011904 to M.D., and National Nature Science Foundation grant NSFC20375002 to X.X. This research also was supported by the PKU Biomed-X Center.
We thank Tung-Tien Sun, Hui Zhang, Matthew Sleeman, and Zhaoyi Wang for critical reading of the manuscript. We also acknowledge Liying Du, Lijun Zhang, Aihua Zheng, Jianju Hu, Peigang Wang, and Jing Wang for technical assistance.
REFERENCES
- 1.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosch, B., J. Bukh, J. C. Meunie, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:14199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bultmann, H., and C. R. Brandt. 2002. Peptides containing membrane-transiting motifs inhibit virus entry. J. Biol. Chem. 277:36018-36023. [DOI] [PubMed] [Google Scholar]
- 5.Bultmann, H., J. S. Busse, and C. R. Brandt. 2001. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J. Virol. 75:2634-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, N. W., D. F. Lewis, P. J. Rosner, M. A. Kelly, and D. C. Schriemer. 2003. Frontal affinity chromatography-mass spectrometry assay technology for multiple stages of drug discovery: applications of a chromatographic biosensor. Anal. Biochem. 319:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Chang, R. S., and H. W. Yeung. 1988. Inhibition of growth of human immunodeficiency virus by crude extracts of Chinese medicinal herbs. Antivir. Res. 9:163-176. [DOI] [PubMed] [Google Scholar]
- 8.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, and H. Rabenau. 2003. Treatment of SARS with human interferons. Lancet 362:293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, and H. Rabenau. 2003. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 361:2045-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major coreceptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 11.Douglas, J. L., M. L. Panis, E. Ho, K. Y. Lin, S. H. Krawczyk, D. M. Grant, R. Cai, S. Swaminathan, and T. Cihlar. 2003. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J. Virol. 77:5054-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. USA 97:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, H. Vieth, D. S. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 14.Este, J. A. 2003. Virus entry as a target for anti-HIV intervention. Curr. Med. Chem. 10:1617-1632. [DOI] [PubMed] [Google Scholar]
- 15.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. W. Wong, K. L. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. [DOI] [PubMed] [Google Scholar]
- 16.Hamburger, M., D. Baumann, and S. Adler. 2004. Supercritical carbon dioxide extraction of selected medicinal plants: effects of high pressure and added ethanol on yield of extracted substances. Phytochem. Anal. 15:46-54. [DOI] [PubMed] [Google Scholar]
- 17.Hou, T., Y. An, B. Ru, R. Bi, and X. Xu. 2000. Cysteine-independent polymerization of metallothioneins in solutions and in crystals. Protein Sci. 9:2302-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain, R. F., A. M. E. Nouri, and R. T. D. Oliver. 1993. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods 160:89-96. [DOI] [PubMed] [Google Scholar]
- 20.Imai, M., N. Okda, and H. Okada. 2000. Inhibition of HIV-1 infection by an intramolecular antisense peptide to T20 in gp160. Microbiol. Immunol. 44:205-212. [DOI] [PubMed] [Google Scholar]
- 21.Jordan, M., A. Schallhorn, and F. M. Wurm. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasai, K. I., and Y. Oda. 1986. Frontal affinity chromatography: theory for its application to studies on specific interactions of biomolecules. J. Chromatogr. 376:33-47. [DOI] [PubMed] [Google Scholar]
- 23.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 24.Kliger, Y., and E. Y. Levanon. 2003. Cloaked similarity between HIV-1 and SARS-CoV suggests an anti-SARS strategy. BMC Microbiol. 3:20. [Online.] http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=14499001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles, S. R., E. J. Phillips, L. Dresser, and L. Matukas. 2003. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin. Infect. Dis. 37:1139-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, et al. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 27.Liu, J. P., H. McIntosh, and H. Lin. 2001. Chinese medicinal herbs for asymptomatic carriers of hepatitis B virus infection. Cochrane Database Syst. Rev. 2 CD002231. [Online.] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11406038. [DOI] [PMC free article] [PubMed]
- 28.Ma, C. Y., K. X. Zhu, D. M. Yang, J. S. Yang, and D. Q. Yu. 1991. Studies on chemical constituents of Veronica lina riifolia Pall. ex Link. sub. dilatata (Nakai et Kitagawa) Hong. Yao Xu Xue Bao 26:203-208. [PubMed] [Google Scholar]
- 29.Ma, M., D. B. Kersten, K. I. Kamrud, R. J. Wool-Lewis, C. Schmaljohn, and F. Gonzalez-Scarano. 1999. Murine leukemia virus pseudotypes of La Crosse and Hantaan bunyaviruses: a system for analysis of cell tropism. Virus Res. 64:23-32. [DOI] [PubMed] [Google Scholar]
- 30.Ma, S. C., J. Du, P. P. But, X. L. Deng, Y. W. Zhang, V. E. Ooi, H. X. Xu, S. H. Lee, and S. F. Lee. 2002. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 79:205-211. [DOI] [PubMed] [Google Scholar]
- 31.Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. [DOI] [PubMed] [Google Scholar]
- 32.Moore, J. P., and R. W. Doms. 2003. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. USA 100:10598-10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikitenko, Y. E. A. A. Raifeld, and T. Z. Wang. 2001. The discovery of RFI-641 as a potent and selective inhibitor of the respiratory syncytial virus. Bioorg. Med. Chem. Lett. 11:1041-1044. [DOI] [PubMed] [Google Scholar]
- 34.Plemper, R. K., K. J. Erlandson, A. S. Lakdawala, A. Sun, A. Prussia, J. Boonsombat, E. Aki-Sener, I. Yalcin, I. Yildiz, O. Temiz-Arpaci, B. Tekiner, D. C. Liotta, J. P. Snyder, and R. W. Compans. 2004. A target site for template-based design of measles virus entry inhibitors. Proc. Natl. Acad. Sci. USA 101:5628-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope, L. E., J. F. Marcelletti, L. R. Katz, J. Y. Lin, D. H. Katz, M. L. Parish, and P. G. Spear. 1998. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir. Res. 40:85-94. [DOI] [PubMed] [Google Scholar]
- 36.Poutanen, S. M., D. E. Low, B. Henry, S. Finkelstein, D. Rose, K. Green, R. Tellier, R. Draker, D. Adachi, M. Ayers, A. K. Chan, D. M. Skowronski, I. Salit, A. E. Simor, A. S. Slutsky, P. W. Doyle, M. Krajden, M. Petric, R. C. Brunham, A. J. McGeer, et al. 2003. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 348:1995-2005. [DOI] [PubMed] [Google Scholar]
- 37.Razinkov, V., A. Gazumyan, A. Nikitenko, G. Ellestad, and G. Krishnamurthy. 2001. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 8:645-659. [DOI] [PubMed] [Google Scholar]
- 38.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Root, M. J., M. S. Kay, and P. S. Kim. 2001. Protein design of an HIV-1 entry inhibitor. Science 291:884-888. [DOI] [PubMed] [Google Scholar]
- 40.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. [DOI] [PubMed] [Google Scholar]
- 41.Schriemer, D. C., D. R. Bundle, L. Li, and O. Hindsgaul. 1998. Micro-scale frontal affinity chromatography with mass spectrometric detection: a new method for the screening of small molecule libraries. Angew. Chem. Int. Eng. Ed. 37:3383-3387. [DOI] [PubMed] [Google Scholar]
- 42.Schriemer, D. C., and O. Hindsgaul. 1998. Deconvolution approaches in screening small molecule mixtures. Comb. Chem. High Throughput Screen. 1:155-170. [PubMed] [Google Scholar]
- 43.Shang, X. Y., J. G. Shi, Y. C. Yang, X. Liu, C. Li, and C. Z. Zhang. 2003. Alkaloids from a Tibetan medicine Meconopsis quintuplinervia Regel. Yao Xue Xue Bao 38:276-278. [PubMed] [Google Scholar]
- 44.Si, Z., N. Madani, J. M. Cox, J. J. Chruma, J. C. Klein, A. Schon, N. Phan, L. Wang, A. C. Biorn, S. Cocklin, I. Chaiken, E. Freire, A. B. Smith III, and J. G. Sodroski. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc. Natl. Acad. Sci. USA 101:5036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiga, O., A. Bernini, A. Ciutti, S. Chiellini, N. Menciassi, F. Finetti, V. Causarono, F. Anselmi, F. Prischi, and N. Niccolai. 2003. Molecular modeling of S1 and S2 subunits of SARS coronavirus spike glycoprotein. Biochem. Biophys. Res. Commun. 310:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 47.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsamis, F. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 77:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsang, K. W., P. L. Ho, G. C. Ooi, W. K. Yee, T. Wang, M. Chan-Yeung, W. K. Lam, W. H. Seto, L. Y. Yam, T. M. Cheung, P. C. Wong, B. Lam, M. S. Ip, J. Chan, K. Y. Yuen, and K. N. Lai. 2003. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348:1977-1985. [DOI] [PubMed] [Google Scholar]
- 50.van Vonderen, M. G., J. C. Bos, J. M. Prins, P. Wertheim-van Dillen, and P. Speelman. 2003. Ribavirin in the treatment of severe acute respiratory syndrome (SARS). Nether. J. Med. 61:238-241. [PubMed] [Google Scholar]
- 51.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie, J., L. Zhu, H. Luo, C. L. Zhou, and X. Li, Xu. 2001. Direct extraction of specific pharmacophoric flavonoids from gingko leaves using a molecularly imprinted polymer for quercetin. J. Chromatogr. A 934:1-11. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, B., M. M. Palcic, H. Mo, I. J. Goldstein, and O. Hindsgaul. 2001. Rapid determination of the binding affinity and specificity of the mushroom Polyporus squamosus lectin using frontal affinity chromatography coupled to electrospray mass spectrometry. Glycobiology 11:141-147. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, B., M. M. Palcic, D. C. Schriemer, G. Alvarez-Manilla, M. Pierce, and O. Hindsgaul. 2001. Frontal affinity chromatography coupled to mass spectrometry for screening mixtures of enzyme inhibitors. Anal. Biochem. 299:173-182. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, H., W. Wang, J. Li, Y. Nie, X. Shi, G. Lian, W. Wang, X. Yin, Y. Zhao, X. Qu, M. Ding, and H. Deng. 2004. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J. Virol. 78:6938-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]