Abstract
During many viral infections, antigen-specific CD8+ T cells undergo large-scale expansion. After viral clearance, the vast majority of effector CD8+ T cells undergo apoptosis. Previous studies have implicated reactive oxygen intermediates (ROI) in lymphocyte apoptosis. The purpose of the experiments presented here was to determine the role of ROI in the expansion and contraction of CD8+ T cells in vivo during a physiological response such as viral infection. Mice were infected with lymphocytic choriomeningitis virus (LCMV) and treated with Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), a metalloporphyrin-mimetic compound with superoxide dismutase activity, from days 0 to 8 postinfection. At the peak of CD8+-T-cell response, on day 8 postinfection, the numbers of antigen-specific cells were 10-fold lower in MnTBAP-treated mice than in control mice. From days 8 to 30, a contraction phase ensued where the numbers of antigen-specific CD8+ T cells declined 25-fold in vehicle-treated mice compared to a 3.5-fold decrease in MnTBAP-treated mice. Differences in contraction appeared to be due to greater proliferation in drug-treated mice. By day 38, the numbers of antigen-specific CD8+ memory T cells were equivalent for the two groups. The administration of MnTBAP during secondary viral infection had no effect on the expansion of antigen-specific CD8+ secondary effector T cells. These data suggest that ROI production is critical for the massive expansion and contraction of antigen-specific CD8+ T cells during primary, but not secondary, viral infection.
Apoptosis plays a key role in controlling homeostasis of the immune system. It is now established that viral infections can cause large expansions of CD8+ T cells. During lymphocytic choriomeningitis virus (LCMV) infection of mice, the expansion of CD8+ T cells is particularly dramatic, with up to 50% of CD8+ T cells in the spleen being specific for viral antigens at the peak of the immune response on day 8 postinfection (20). Following viral clearance, a contraction phase ensues where apoptosis of the vast majority of effector cells ensures a return to homeostasis from days 8 to 30. The surviving antigen-specific CD8+ T cells differentiate into memory cells that persist at constant numbers and can provide lifelong protection against disease (17). Determining the mechanisms that cause apoptosis of effector cells is critical not only for understanding antiviral immune responses but also for optimizing cancer vaccines, preventing graft rejection, and developing therapeutics for autoimmunity.
Reactive oxygen intermediates (ROI) have been shown to have key roles in cell proliferation and death (9, 16, 21, 24). ROI are a class of molecules including superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (.OH). In addition to these species, other radicals can be generated through reactions with nitric oxide to produce species such as peroxynitrite. The signaling function of ROI is thought to be through activation of components of signal transduction, such as extracellular signal-related kinases 1 and 2 (1). In addition to signaling in lymphocytes, ROI have been implicated in apoptosis. Double positive thymocytes are highly susceptible to apoptosis induced by chemical agents. When treated with dexamethasone, these cells undergo a rapid cell death that is accompanied by a burst of superoxide and loss of mitochondrial potential (29). The role of ROI in lymphocyte death does not appear to be restricted to developing T cells, as peripheral cells that are induced to undergo expansion and deletion by staphylococcal enterotoxin B superantigen produce increased levels of ROI as well. A major piece of evidence that ROI play a key role in lymphocyte apoptosis comes from studies by Hildeman and colleagues, in which cells that had been expanded in vivo with superantigen were cultured in vitro in the presence of Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), a potent antioxidant. Cells survived to a greater extent in the presence of the antioxidant than with no treatment (14). While these results highlight the role of ROI in CD8+-T-cell apoptosis in vitro, the role of these molecules in apoptosis of cells in vivo after activation by a physiological stimulus such as viral infection is unclear.
Recently, it was documented that during LCMV infection antigen-specific CD8+ effector T cells contain increased levels of superoxide prior to the initiation of the contraction phase (11). To determine the role of ROI in controlling the expansion and contraction of antigen-specific CD8+ T cells in vivo, we treated mice with MnTBAP during the expansion phase of primary and secondary LCMV infection. MnTBAP is a small-molecule mimetic compound with superoxide dismutase-like activity. We adopted a treatment regimen similar to the one used by Mevlov and colleagues to prolong the life span of MnSOD2−/− mice. These mutant mice normally die as neonates due to cardiomyopathy, but treatment with MnTBAP extends the life span to 3 weeks, when the animals succumb to neurological defects (19). We found that treatment with MnTBAP blocked 90% of the increase of superoxide production in activated CD8+ T cells. Early expansion of antigen-specific CD8+ T cells was not affected, but by the peak of the T-cell response, the numbers of virus-specific cells were 10-fold lower in MnTBAP-treated mice. During the contraction phase, the numbers of antigen-specific CD8+ T cells contracted 25-fold, compared to 3.5-fold for drug-treated animals. This difference in contraction was accompanied by an increased proliferation of antigen-specific CD8+ T cells in MnTBAP-treated mice. When the memory phase was reached, the numbers of antigen-specific CD8+ T cells were equivalent for the two groups of mice. Interestingly, the decrease in proliferation after MnTBAP treatment was not observed during secondary infection. These studies demonstrate the important role of ROI in controlling the proliferation and contraction of antigen-specific CD8+ T cells in vivo during primary but not secondary viral infection.
MATERIALS AND METHODS
Mice and viral infection.
Six- to eight-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, Md.). Mice were infected with either 2 × 105 PFU of LCMV Armstrong intraperitoneally or 2 × 106 PFU of LCMV clone 13 intravenously and were used at the indicated time points. LCMV Armstrong and clone 13 stocks were grown and quantitated as described previously (2).
Preparation of MHC class I tetramers.
The construction and purification of DbGP33-41, DbNP396-404, and DbGP276-286 have been described previously (20).
Surface and intracellular staining.
All antibodies were purchased from BD Pharmingen (San Diego, Calif.). Surface staining was performed as described previously (20). Bromodeoxyuridine (BrdU) staining was performed by using a BrdU kit from BD Pharmingen according to the manufacturer's instructions. For analysis of direct ex vivo apoptosis, splenocytes were isolated, surface stained as described above, incubated briefly with Annexin V and 7-amino-actinomycin D (7AAD) at room temperature in the dark, and acquired immediately. Samples were acquired on a FACSCalibur instrument and analyzed with CellQuest software (BD Biosciences, San Diego, Calif.).
Cell isolation.
Lymphocytes were isolated from the spleen and lymph nodes as described previously (12, 20). For isolation of nonlymphoid tissues, mice were euthanized, the abdomen was opened, the hepatic vein was cut, and 5 ml of ice-cold PBS was injected directly into the hepatic artery to perfuse the liver. The gall bladder was removed, and the entire liver was excised. The liver tissue was homogenized with a wire screen and incubated in 0.25 mg of collagenase B/ml (Boehringer Mannheim, Mannheim, Germany) and 1 U of DNase (Sigma, St. Louis, Mo.)/ml at 37°C for 45 min. Digested liver was centrifuged, and the pellet was resuspended in 5 to 10 ml of 44% Percoll (Sigma). This solution was underlaid with 56% Percoll and spun at 2,000 rpm for 20 min at 20°C. The intrahepatic lymphocyte populations were harvested from the interface, and red blood cells were lysed with 0.83% ammonium chloride, washed, and counted. Lung lymphocytes were isolated in a similar manner.
Intracellular cytokine staining.
Intracellular cytokine staining was performed by using a Cytofix/Cytoperm Plus kit (with GolgiPlug) as described previously (20).
In vitro CTL assay.
Two days prior to use in the cytotoxic T lymphocyte (CTL) assay, MC57G cells were infected at a 1:1 multiplicity of infection with LCMV clone 13 to serve as infected targets. On the day of the assay, uninfected and infected targets were labeled with [51Cr]sodium chromate for 1 h. Peptide-coated cells were prepared by coincubation with 0.1 μg of peptide (GP33-41 or NP396-404)/ml and [51Cr]sodium chromate. After labeling, cells were washed three times in complete medium. For the infected target CTL assay, 2 × 104 target cells were incubated with effector cells in 96-well round-bottom plates and after 5 h, 100 μl of the supernatant was removed and counted in a Packard Auto-Gamma 5650 counter (Perkin-Elmer, Wellesley, Mass.). Specific lysis was calculated as (experimental value − spontaneous value)/(maximum value − spontaneous value) × 100%. Spontaneous values were between 5 and 10%. Maximum values were generated by incubation with 1% Triton X-100.
To determine per-cell lytic activity, splenocytes were stained with anti-CD8α and either DbGP33-41 or DbNP396-404 MHC class I tetramer to enumerate antigen-specific cells. Effector samples were then mixed at a 1:1 ratio of tetramer-positive cells to peptide-coated cells. In each assay, 104 antigen-specific cells were incubated with 104 peptide-coated cells. A total of 5 × 105 splenocytes were added, and sample numbers were normalized by the addition of naïve splenocytes, similar to an approach described by Harrington and colleagues (13). Specific lysis was calculated as described above. Relative lysis was calculated by dividing the average for each group (vehicle- or MnTBAP-treated) by the value obtained for the vehicle group.
Superoxide detection.
To determine the levels of superoxide, 106 splenocytes were incubated for 30 min at 37°C in 5 μM dihydroethidium (HE) (Molecular Probes, Eugene, Oreg.) diluted in RPMI medium plus 10% fetal calf serum. Cells were then washed three times in ice-cold fluorescence-activated cell sorter buffer and then surface stained and acquired immediately.
MnTBAP treatment.
MnTBAP was purchased from CalBioChem (La Jolla, Calif.) and dissolved in 0.1 N NaOH as a 6 mg/ml stock. Naïve C57BL/6 mice were weighed, given an intraperitoneal dose of MnTBAP (5 mg/kg of body weight) diluted in sterile PBS, and infected with LCMV 4 h later. A maintenance dose was administered to the mice every 24 h for the duration of treatment.
Statistical analysis.
Data from vehicle- and MnTBAP-treated mice were analyzed by using two-tailed Student's t test, and a P value of ≤ 0.05 was considered significant.
RESULTS
Administration of MnTBAP reduces ROI levels in activated T cells.
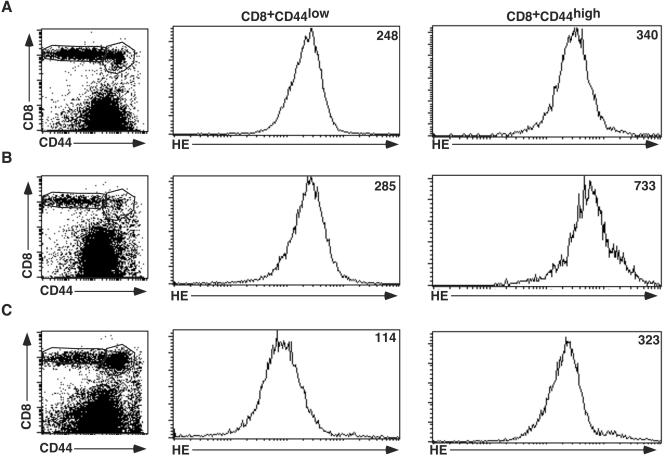
To determine the effect of decreasing ROI levels on antigen-specific CD8+-T-cell responses during viral infection, we administered MnTBAP, a potent antioxidant, to mice infected with LCMV. We assessed production of superoxide in CD8+ T cells by using the fluorescent dye dihydroethidium. Figure 1A shows that naïve, CD8+CD44low T cells contained a basal level of superoxide, with a mean fluorescence intensity (MFI) of 248. Activated/memory phenotype (CD8+CD44high) cells of undefined specificity contained slightly higher levels of superoxide, with an MFI of 340. After LCMV infection, there was massive expansion of antigen-specific CD8+ T cells. Five days postinfection, superoxide levels in CD8+CD44low T cells from vehicle-treated mice remained low, at 285, while those in CD44high cells had risen to 733 (panel B). In MnTBAP-treated mice (panel C), the situation was different. The superoxide levels in CD8+CD44low cells had declined to 114, while those in the CD44high cells were at 340. Thus, treatment with MnTBAP reduced the levels of superoxide in both naïve and activated/memory phenotype cells. Since naïve (CD44low) T cells become CD44high after activation, treatment with MnTBAP prevented 90% of the increase observed upon T-cell activation (MFIs were 323 for MnTBAP-treated cells, 733 for vehicle-treated cells, and 248 for naïve cells).
FIG. 1.
Treatment with MnTBAP reduces superoxide levels in CD8+ T cells. Naïve spleen cells (A) and spleen cells from mice that were infected with LCMV Armstrong 5 days earlier and treated with either vehicle (B) or 5 mg of MnTBAP/kg (C) were incubated with HE and then stained with anti-CD8α and CD44 antibodies. The HE levels are plotted in histogram formats, with the MFIs indicated in the upper right corners of the plots. Three to six mice were examined under each condition, and results from a representative mouse are presented in each histogram.
Treatment with MnTBAP results in increased levels of virus in the spleen during acute infection.
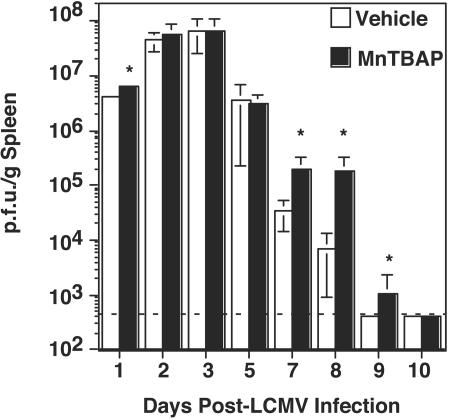
To determine the effect of reduced superoxide on viral clearance, we examined virus titers of the spleens of treated and untreated mice. Figure 2 shows that early during infection, on days 1 to 3, virus levels were roughly equivalent for vehicle- and MnTBAP-treated mice. On day 1 postinfection, virus levels were slightly higher for MnTBAP-treated mice (6.4 × 106, versus 4.2 × 106 for vehicle-treated mice), but by day 2 postinfection, the two groups had similar virus levels. In both groups, virus levels peaked at ∼6 × 107 PFU/g on day 3 postinfection and were reduced to ∼4 × 106 PFU/g by day 5. However, for the vehicle-treated mice, there was a substantial reduction in virus titer to ∼4 × 104 PFU/g by day 7 postinfection. This result contrasts with that for MnTBAP-treated mice, which had reduced virus titers to only ∼2 × 105 PFU/g. The virus level continued to drop in vehicle-treated mice such that the virus was cleared by day 9 postinfection. The levels in MnTBAP-treated mice remained elevated on day 8 postinfection, but started to drop by day 9 and were below detection by day 10. Thus, treatment with MnTBAP results in increased viral load and delayed clearance compared to results with vehicle-treated mice.
FIG. 2.
Treatment with MnTBAP results in increased levels of virus in the spleen during acute infection. C57BL/6 mice were treated with either vehicle or 5 mg of MnTBAP/kg and then infected with LCMV Armstrong. A maintenance dose was administered every 24 h from days 0 to 8. At the given time points, mice were sacrificed, and viral levels in the spleen were determined by plaque assays. The means and standard deviations were calculated and are shown for each time point. Four to six mice were used in each group at each time point. *, significant difference between vehicle- and MnTBAP-treated mice; P ≤ 0.05.
The expansion of antigen-specific T cells is reduced in MnTBAP-treated mice but the memory set point is equivalent.
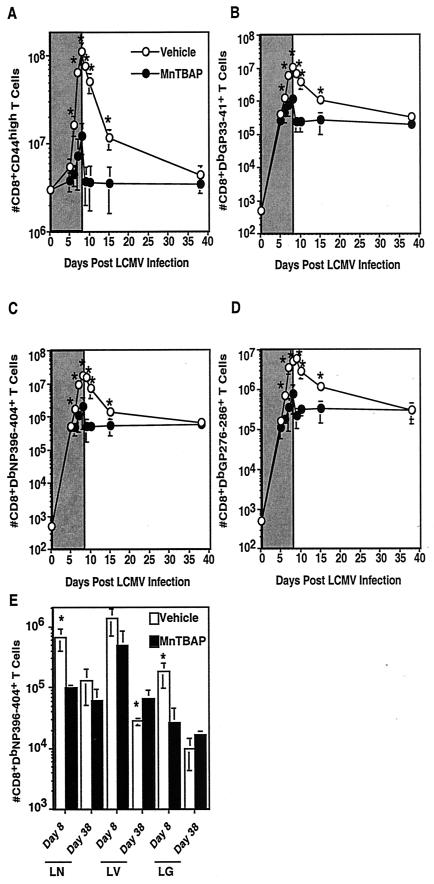
LCMV is known to induce very large expansions of CD8+ and CD4+ T cells (6, 15, 20, 27). Vehicle-treated mice had a significant expansion of CD44high activated/memory phenotype CD8+ T cells, such that on day 8 postinfection there were ∼108 cells (Fig. 3A). This result contrasts with that from MnTBAP-treated mice. Early during infection, on day 5, the mice had comparable numbers of activated cells (5 × 106 for vehicle-treated mice versus 3 × 106 for MnTBAP-treated mice), but by day 8, the numbers of activated cells were severely reduced (108 for vehicle-treated mice versus 107 for MnTBAP-treated mice). By day 9 postinfection, both groups of mice had started to undergo contraction in the numbers of activated/memory CD8+ T cells, and by day 38, the numbers of cells were equivalent for vehicle- and MnTBAP-treated mice. When antigen-specific CD8+ T cells were enumerated by MHC class I tetramer staining (panels B, C, and D), similar trends were observed. During early infection, the numbers for the two groups were indistinguishable but by day 8 there was a significant reduction in the numbers of antigen-specific CD8+ T cells in MnTBAP-treated mice. For each of the three epitopes examined (GP33-41, NP396-404, and GP276-286), there were approximately 10-fold fewer cells in MnTBAP versus vehicle-treated mice. There was no difference in the expression of CD44, CD62L, CD69, and 1B11 on antigen-specific CD8+ T cells for vehicle- and drug-treated mice, suggesting that the T cells were fully activated (data not shown). By the time the memory phase was analyzed, on day 38 postinfection, the numbers of antigen-specific CD8+ T cells for the two groups were equivalent. Upon rechallenge, MnTBAP-treated immune mice cleared the virus and underwent a similar expansion of secondary effector cells as vehicle-treated immune mice (data not shown). In addition to examining cell numbers in the spleen, we examined other lymphoid and nonlymphoid tissues. Figure 3E shows the results for DbNP396-404-specific CD8+ T cells, although similar trends were observed for other epitopes. In the lymph nodes, there was a decrease of NP396-404 specific CD8+ T cells in MnTBAP compared to vehicle-treated mice on day 8 postinfection. By day 38, the numbers of cells were similar for vehicle- and MnTBAP-treated mice. In the nonlymphoid tissues, a difference between results for the liver and lung was observed. In the lung, the trends were similar to those for the lymph node: reduced expansion in MnTBAP-treated mice on day 8 postinfection but comparable numbers of cells by the time memory was established. In the liver, the trend was different. At day 8 postinfection, the numbers of NP396-404-specific CD8+ T cells from MnTBAP-treated mice were reduced but were not significantly different from those for the vehicle-treated group. When the memory phase was established, the numbers of cells in the MnTBAP-treated mice were higher than in the vehicle-treated group. Thus, treatment with MnTBAP reduces the numbers of antigen-specific CD8+ T cells at the peak of the effector response in the spleen, lymph node, and lung, while having less of an effect in the liver. Correspondingly, the memory set points for spleen, lymph node, and lung are the same, but numbers are higher for the livers of MnTBAP-treated mice.
FIG.3.
Treatment with MnTBAP results in decreased expansion and contraction of CD8+ T cells during primary viral infection. C57BL/6 mice were treated with either the vehicle or 5 mg of MnTBAP/kg and then infected with LCMV Armstrong. A maintenance dose was administered every 24 h. At the indicated time points, mice were sacrificed, the spleen was removed, and cells were stained with anti-CD8α and either anti-CD44 (A), DbGP33-41 (B), DbNP396-404(C), or DbGP276-286 (D). The numbers of activated (A) and antigen-specific CD8+ T cells (B-D) were quantitated, and the averages and standard deviations are shown. To determine the effect of MnTBAP administration on lymphocyte numbers in other tissues, mice were sacrificed on days 8 and 38 postinfection and lymphocytes were isolated from the lung, liver and lymph nodes and stained with anti-CD8α and DbNP396-404 (E). The numbers of NP396-404-specific CD8+ T cells were quantitated, and the average and standard deviation are shown. Six to 10 mice were analyzed at each time point. The gray areas in panels A through D indicate the windows of treatment. *, significant difference between vehicle- and MnTBAP-treated mice; P ≤ 0.05.
Transient decrease in cytokine production at the peak of the effector response.
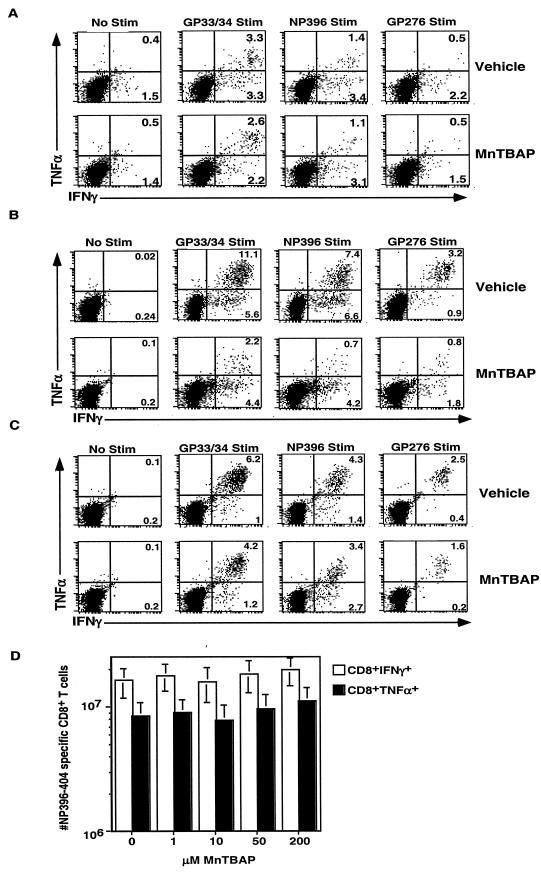
The ability to produce cytokines is critical for T cell function and control of virus. As shown in Fig. 4, we compared the ability of CD8+ T cells from days 5, 8, and 38 postinfection to produce gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Previous studies have demonstrated that at the peak of the CD8+-T-cell response to LCMV, a portion of the antigen-specific CD8+ T cells will produce IFN-γ and some will produce both IFN-γ and TNF-α when stimulated with viral peptides in vitro (23). In vehicle-treated mice 5 days postinfection (Fig. 4A), we observed that after GP33/34 peptide stimulation, 3.3% of CD8+ T cells produced both cytokines, while 3.3% produced only IFN-γ. Background staining results for no-peptide samples were 0.4% for IFN-γ+ TNF-α+ cells and 1.5% for IFN-γ+ cells. MnTBAP-treated mice showed a similar trend; 2.6% produced both cytokines, and 2.2% produced only IFN-γ. Background staining results for MnTBAP-treated mice were similar to those for vehicle-treated mice. When vehicle-treated splenocytes were stimulated with NP396 peptide, 1.4% made both cytokines while 3.4% made only IFN-γ. After stimulation, MnTBAP-treated mice had 1.1% double positive cells while 3.1% made IFN-γ. Similar trends were observed for GP276 stimulation. Thus, at an early time point, similar percentages of functional cells were observed with MnTBAP- and vehicle-treated mice.
FIG.4.
Decreased production of TNF-α in effector but not memory cells from mice treated with MnTBAP during acute viral infection. C57BL/6 mice were treated with either the vehicle or 5 mg of MnTBAP/kg and then infected with LCMV Armstrong. A maintenance dose was administered every 24 h from days 0 to 8. Mice were sacrificed on days 5 (A), 8 (B), or 38 (C) postinfection, and splenocytes were stimulated with the indicated LCMV epitope. Cells were then stained with anti-CD8α, IFN-γ, and TNF-α. The dot plots are gated on CD8+ T cells, and the number in the plot indicates the percentage of CD8+ T cells that are present in that region. Six to 10 mice were analyzed at each time point, and results for a representative mouse are shown. To determine the effect of MnTBAP on cytokine production (D), splenocytes were isolated from a mouse 8 days postinfection with LCMV Armstrong and were incubated with NP396-404 and increasing concentrations of MnTBAP. The numbers of cytokine producing CD8+ T cells were quantitated, and the average and standard deviation are shown. Six mice were analyzed in two independent experiments.
At the peak of the effector response, on day 8, the percentage of functional cells was dramatically reduced in MnTBAP-treated mice (Fig. 4B). In vehicle-treated mice, we observed that after GP33/34 peptide stimulation, 11.1% of CD8+ T cells produced both cytokines, while 5.6% produced only IFN-γ. The background for both vehicle- and MnTBAP-treated mice was 0.24% or lower. With MnTBAP-treated mice, we observed that after GP33/34 peptide stimulation 2.2% produced both cytokines, and 4.4% produced only IFN-γ. When vehicle-treated splenocytes were stimulated with NP396 peptide, 7.4% made both cytokines while 6.6% made only IFN-γ. MnTBAP-treated mice had significant reductions in the numbers of double positive cells, such that only 0.7% of CD8+ T cells made both cytokines while 4.2% made IFN-γ. Similar trends were observed for GP276 stimulation.
When the memory phase was analyzed on day 38 (Fig. 4C), the situation was very different. In the vehicle-treated mice, almost all of the CD8+ T cells that responded to peptide stimulation made both IFN-γ and TNF-α. In the MnTBAP-treated mice, similar trends were observed although the percentage that made both cytokines was slightly reduced. In both groups, background staining was below 0.2%. Thus, during the early portion of the effector phase, function was comparable for vehicle- and MnTBAP-treated mice, but at the peak of the effector response, on day 8, there was a significant loss of function in drug-treated mice. This loss of function was transient, however, as memory cells from both groups of mice express IFN-γ and TNF-α.
To determine whether MnTBAP can directly inhibit the production of IFN-γ or TNF-α, splenocytes from untreated animals were incubated with increasing concentrations of MnTBAP on day 8 post infection and the numbers of cytokine-producing CD8+ T cells were determined after NP396-404 peptide stimulation (Fig. 4D). In the absence of MnTBAP, ∼1.5 × 107 CD8+ T cells made IFN-γ, while ∼8 × 106 CD8+ T cells made TNF-α. As the concentration of MnTBAP was increased to 200 μM (a level sufficient in vitro to block the death of superantigen activated cells [14]), no significant differences in the numbers of cytokine-producing cells were observed. Thus, short-term treatment with MnTBAP does not directly inhibit the production of cytokines.
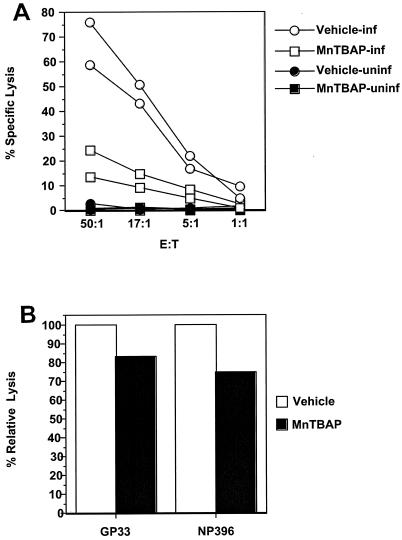
Reduced cytotoxicity in vitro is not due to major defects in per-cell cytolysis.
Previous studies have demonstrated that perforin containing CD8+ T cells is necessary for clearance of LCMV (25). We performed cytotoxicity assays to compare the lytic functions for vehicle- and MnTBAP-treated mice. Figure 5A shows that vehicle-treated mice had vigorous CTL activity, with 60 to 80% specific lysis at an effector-to-target ratio of 50:1. This compares with 15 to 25% observed for MnTBAP-treated mice. Because the numbers of antigen-specific CD8+ T cells were reduced in MnTBAP-treated mice, it was important to determine if cytolytic activity was altered on a per-cell basis. To determine if the cytolytic activity was different, we performed CTL assays with the same numbers of antigen-specific CD8+ T cells in the wells. This number was determined by staining samples with major histocompatibility complex (MHC) class I tetramer (DbGP33-41 or DbNP396-404) and then normalizing samples with naïve splenocytes. The samples were then incubated with the appropriate peptide-coated targets, and specific lysis was determined. In Fig. 5B, we show that the lytic activity levels on a per-cell basis for peptide-coated targets in antigen-specific CD8+ T cells from MnTBAP-treated mice were 83% (for GP33) and 75% (for NP396) of those for vehicle-treated mice. This result suggests that reduced cytotoxicity in vitro is due to decreased cell numbers, not a major defect in cytolysis on a per-cell basis.
FIG. 5.
Reduced in vitro cytotoxicity of MnTBAP-treated mice is not due to a major defect in per-cell cytolysis. Splenocytes from vehicle- and MnTBAP-treated mice 8 days after infection with LCMV Armstrong were harvested and incubated directly ex vivo (A) at the indicated effector-to-target ratio (E:T) with 51Cr-labeled H-2b targets that were untreated (closed symbols) or infected (open symbols) with LCMV clone 13 in a 5-h 51Cr-release assay. Each sample was assayed in triplicate. To determine per-cell lytic activity (B), splenocytes were stained with anti-CD8α and either DbGP33-41 or DbNP396-404 MHC class I tetramer. The numbers of antigen-specific CD8+ T cells were determined, and 104 cells were incubated with 104 appropriately coated peptide targets. Cell numbers were normalized by the addition of naïve splenocytes. Specific lysis was calculated for each mouse, and the average for each treatment was calculated. The relative lysis was determined by dividing the average for each group by the average for the vehicle group. Three to six mice were used in two independent experiments, and each sample was assayed in triplicate.
Reduced contraction phase in MnTBAP-treated mice is due to increased proliferation.
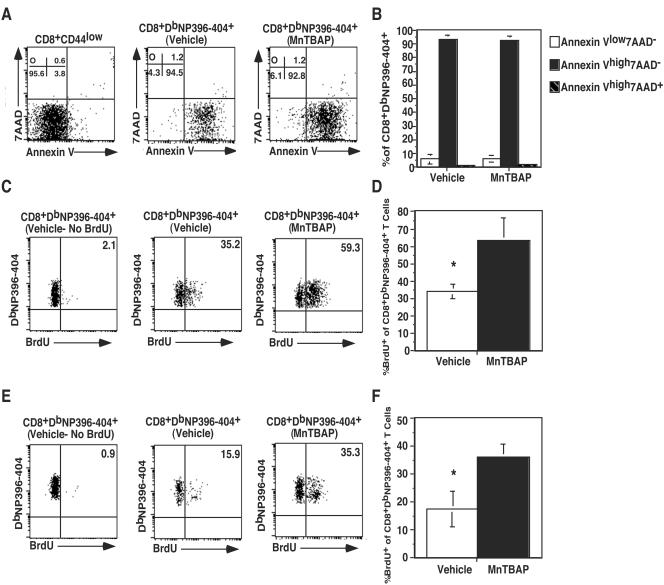
The number of antigen-specific CD8+ T cells at any given time in viral infection represents a balance between proliferation and apoptosis. While we observed decreased cell numbers at the peak of the effector response in MnTBAP-treated mice, there was also a reduction in the total amount of contraction the response underwent (∼25-fold for vehicle-treated mice versus 3.5-fold for MnTBAP-treated mice). To determine the contribution of apoptosis versus that of proliferation, we performed Annexin V and BrdU staining. Cells that are in the early stages of apoptosis are Annexin Vhigh7AAD−, while those that are in the late stages of apoptosis or dead are Annexin Vhigh7AAD+. In Fig. 6A, we examined CD8+DbNP396-404+ T cells and determined the portions of cells in the various stages of apoptosis. CD8+CD44low cells are viable and serve as a control. The majority of these cells (95%) are Annexin Vlow7AAD−. In both the vehicle- and MnTBAP-treated mice, ∼93% of the CD8+DbNP396-404+ T cells were AnnexinVhigh7AAD− and ∼1% of the cells were AnnexinVhigh7AAD+. This result indicates that most of the cells are in the early stages of apoptosis. Data from several mice were compared, and no significant difference in staining between vehicle- and MnTBAP-treated mice was observed (Fig. 6B). To assess proliferation during the contraction phase, we added BrdU to the drinking water of mice from days 8 to 15 and determined the level of BrdU incorporation on day 15. Background staining for mice that had not received BrdU was 1 to 2%. In the vehicle-treated mice (panel C), approximately 35.2% of CD8+DbNP396-404+ T cells were BrdU+, compared to 59.3% for the MnTBAP-treated mice. When data from multiple mice were compared (panel D), there were a significant increases (65% for MnTBAP-treated mice versus 35% for vehicle-treated mice) in the percentages of CD8+DbNP396-404+BrdU+ T cells in MnTBAP-treated mice compared to vehicle-treated mice.
FIG. 6.
Reduced contraction in MnTBAP-treated mice is due to increased proliferation. C57BL/6 mice were treated with either vehicle or 5 mg of MnTBAP/kg and then infected with LCMV Armstrong. A maintenance dose was administered every 24 h. Direct ex vivo apoptosis on day 8 postinfection (A) was assessed by surface staining splenocytes as described above, followed by a brief incubation with Annexin V and 7AAD and immediate acquisition. Naïve (CD8+CD44low) cells were included as a viability control. The value in each quadrant is the percentage of CD8+DbNP396-404+ cells that fall in the quadrant. The averages and standard deviations for each quadrant from the analysis of vehicle- and MnTBAP-treated mice are plotted (B). Proliferation during the contraction phase (days 8 to 15) was assessed (C) by giving the mice BrdU (0.8 mg/ml) in their drinking water from days 8 to 15 after infection. Splenocytes were isolated on day 15 postinfection and stained with anti-CD8α, DbNP396-404, and anti-BrdU antibody. The plots are gated on CD8+DbNP396-404+ cells, and the numbers on the dot plots indicate the percentages of antigen-specific cells that are BrdU+. The average percentage and standard deviation of CD8+DbNP396-404+BrdU+ cells from vehicle- or MnTBAP-treated mice are plotted (D). Proliferation during the memory phase (days 31 to 38) was assessed (E) by giving the mice BrdU (0.8 mg/ml) in their drinking water from days 31 to 38 after infection. Splenocytes were isolated on day 38 postinfection and stained with anti-CD8α, DbNP396-404, and anti-BrdU antibody. The plots are gated on CD8+DbNP396-404+ cells, and the numbers on the dot plots indicate the percentages of antigen-specific cells that are BrdU+. The average percentage and standard deviation of CD8+DbNP396-404+BrdU+ cells from vehicle- or MnTBAP-treated mice are plotted (F). Three to six mice were examined at each time point, and data from a representative mouse is presented in each dot plot. *, significant difference between vehicle- and MnTBAP-treated mice; P ≤ 0.05.
In addition to measuring proliferation during the contraction phase, we examined proliferation of antigen-specific CD8+ T cells from day 31 to day 38, when memory cells should begin to undergo homeostatic proliferation. Background staining for mice that had not received BrdU was 0.9%. In the vehicle-treated mice (Fig. 6E), approximately 15.9% of CD8+DbNP396-404+ T cells were BrdU+, compared to 35.3% for the MnTBAP-treated mice. When data from multiple mice were compared (panel F), there was a significant increase in the percentages of CD8+DbNP396-404+BrdU+ T cells in MnTBAP-treated mice (36.1%) compared to vehicle-treated mice (17.5%). These results indicate that antigen-specific CD8+ T cells in MnTBAP-treated cells underwent greater proliferation during both the contraction phase and the beginning of the memory phase.
Secondary expansion is not affected by MnTBAP treatment.
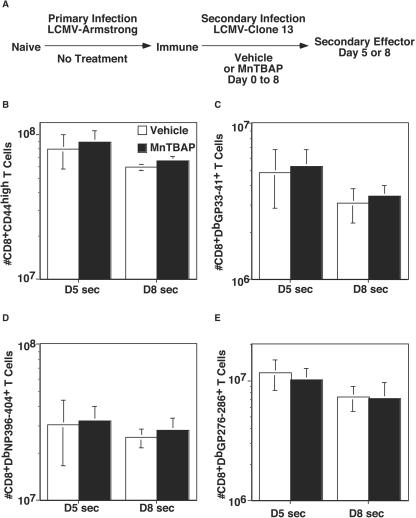
Although primary infection is useful in understanding the differentiation of naïve to effector to memory cells, understanding secondary infection is critical to optimizing vaccines so that the response after immunization can be vigorous and protective from disease. To determine the effect of antioxidant administration during secondary infection, we used C57BL/6 mice that were infected with LCMV Armstrong and had not been manipulated with vehicle or drug. After a period of greater than 60 days, these mice were rechallenged with LCMV clone 13, and either vehicle or MnTBAP was administered (Fig. 7A). By day 5 postinfection, both groups of mice had cleared the virus from the spleen (data not shown). When total activated/memory phenotype CD8+ T cells were enumerated (panel B), there was a large expansion of cells; to 8 × 107 in the vehicle-treated group and 9 × 107 in MnTBAP-treated mice. Because we did not observe major differences in cell numbers in the primary infection at day 5, we examined mice that were treated until day 8 of the secondary infection. By day 8, numbers for both groups had edged down slightly (6.0 × 107 for vehicle-treated mice versus 6.5 × 107 for MnTBAP-treated mice). When the numbers of antigen-specific CD8+ T cells were determined by MHC class I tetramer staining (panels C to E), similar trends were observed; thus, no differences between results for vehicle- and MnTBAP-treated mice were observed. At both time points, there was no effect on cytokine production (data not shown).
FIG. 7.
The number of antigen-specific CD8+ T cells during secondary LCMV infection is unaffected by MnTBAP treatment. C57BL/6 LCMV Armstrong immune mice were treated with either vehicle or 5 mg of MnTBAP/kg and then infected with LCMV clone 13 (A). A maintenance dose was administered every 24 h. At the indicated time points, mice were sacrificed, the spleen was removed, and cells were stained with anti-CD8α and either anti-CD44 (B), DbGP33-41 (C), DbNP396-404 (D), or DbGP276-286 (E). The numbers of activated (B) and antigen-specific CD8+ T cells (C-E) were quantitated, and the averages and standard deviations are shown. Six to eight mice were analyzed at each time point. *, significant difference between vehicle- and MnTBAP-treated mice; P ≤ 0.05.
DISCUSSION
In this study, we have examined the effects of an antioxidant on the proliferation and contraction of antigen-specific CD8+ T cells in vivo during viral infection. We found that the antioxidant MnTBAP reduced both expansion and contraction of antigen-specific CD8+-T-cell responses. Although the numbers of effector cells were reduced in MnTBAP-treated mice, the numbers of memory cells were similar for both groups. Additionally, the effects of antioxidant treatment were observed in the primary but not the secondary antiviral T-cell response.
What are the roles of reactive oxygen intermediates in T-cell responses? These molecules have been implicated in both T-cell proliferation and apoptosis. T-cell proliferation can be divided into antigen-driven and homeostatic proliferation. In this study, we addressed the role of ROI in antigen-driven proliferation through antioxidant administration in vivo. Early proliferation appears to be independent of ROI production, as reduction of ROI by 90% has little effect at 5 days, while cell numbers at the peak of the response (day 8) were reduced 10-fold in the presence of the drug. Because previous studies have shown significant numbers of effector CD8+ T cells in multiple lymphoid and nonlymphoid tissues, we determined whether the effects of MnTBAP were restricted to the spleen (18). Similar results were obtained for all tissues except the liver, where the difference in the effector phase was less exaggerated. This result may be due to the fact the liver is a major site of drug catabolism (5), and the local concentration of drug may be lower there than in other organs. This result notwithstanding, our data show that the administration of the antioxidant reduces the numbers of effector T cells at the peaks of the responses in both vehicle- and MnTBAP-treated mice. These data are consistent with earlier data from in vitro experiments using antioxidants, such as radical scavengers, iron chelators, and aminothiols, which blocked the proliferation of T cells induced by alloantigen (7). In vivo antioxidants have been shown to block the development of autoimmune diabetes. Piganelli and colleagues showed that treatment with AEOL-10113, a superoxide dismutase-mimetic compound, prevented, or delayed disease caused by the transfer of diabetogenic T cells (22). They also observed reduced proliferation of the diabetogenic T cells in vitro and decreased cytokine production. Our results extend these findings to an infectious disease model and show that in addition to self-responses, T-cell expansion to foreign antigens can also be inhibited by antioxidants. Why is the expansion of virus-specific CD8+ T cells reduced in MnTBAP-treated mice? Two potential explanations are that MnTBAP treatment results in either aberrant activation of CTL or reduced proliferation from the beginning of infection. Neither of these seems to be the case, as cell numbers and function are comparable for vehicle- and MnTBAP-treated mice on day 5 postinfection. Additionally, the greatest differences in cell numbers come from days 5 to 8 of MnTBAP treatment. It is important to note that MnTBAP is a small molecule, and as such, it could be affecting other cells in addition to T cells. A recent study by Devadas and colleagues demonstrated that the addition of antioxidants to T-cell lines or human T-cell blasts could inhibit the production of superoxide, suggesting that T cells themselves are capable of producing ROI and that antioxidants can inhibit this production in an autonomous manner (8). Further experiments are needed to determine whether T cells are the only cell type affected by MnTBAP treatment in vivo.
In addition to decreased cell numbers during MnTBAP treatment, we also observed a transient decrease in cytokine production. Why is cytokine production inhibited during treatment of primary but not secondary viral infection with MnTBAP? It is important to note that by surface marker expression, the antigen-specific CD8+ T cells from MnTBAP-treated mice are similar to those from vehicle-treated mice, suggesting that MnTBAP did not inhibit the primary activation of these cells. The cytokine production data for day 5 also lend credence to this idea, as similar IFN-γ and TNF-α expression profiles are observed with both groups. Only at the peak of the response on day 8 is cytokine production impaired. By the time the memory phase is established, all of the antigen-specific CD8+ T cells are able to produce both cytokines. Previous studies of chronic LCMV infection have documented the loss of cytokine production during viral persistence (10, 28). In primary LCMV infection, the virus is cleared from the spleen within 7 to 10 days. In the drug-treated mice, virus levels are 10- to 15-fold higher on day 8 postinfection, but by day 10, both vehicle- and drug-treated animals have reduced virus below the limit of detection in the spleen. In secondary infection, where virus is cleared from the spleen within 2 days (J. M. Grayson, unpublished observations), no loss of function was observed even after 8 days of drug treatment. Additionally, treatment of splenocytes with high levels of MnTBAP (200 μM) does not directly inhibit cytokine production in short-term in vitro assays. Taken together, these data suggest that the loss of function observed with the primary infection could be due to the protracted virus load and may not be a direct effect of the drug.
Why is viral clearance delayed in MnTBAP-treated mice? Clearance of LCMV requires perforin-dependent killing by CD8+ T cells (25). From our in vitro CTL assay performed on infected targets, we observed reduced cytotoxicity for MnTBAP-treated compared to vehicle-treated mice. After normalization to the same numbers of antigen-specific CD8+ cells, we determined that cytolysis was only reduced ∼20% in MnTBAP-treated mice on a per-cell basis. This finding suggests that reduced numbers of CTL, not a cytolysis defect, may be the main reason that viral clearance is delayed. It is also important to note that for CD8+ T cells to kill, they must be in contact with infected cells. It is possible that treatment with MnTBAP not only reduces the expansion of antigen-specific CD8+ T cells, but also reduces their ability to migrate to areas of infection, which results in infected cells being able to continue to produce virus. This may explain why virus titers drop significantly by day 9 after MnTBAP treatment is stopped on day 8.
After viral clearance, cell numbers decline by a process that has been termed contraction, which has been previously shown to be a programmed event that occurs regardless of the bacterial or viral dose administered (3). In that study, Badovinac et al. showed that lower bacterial doses generated fewer effector cells than higher doses of bacteria. However, regardless of expansion, the contraction (n-fold) was the same. Thus, the memory set point was proportional to the bacterial dose. We now show that although both vehicle- and MnTBAP-treated effector populations peak on day 8 postinfection, the vehicle-treated mice undergo a 25-fold contraction from days 8 to 38, while the MnTBAP-treated mice undergo a 3.5-fold contraction from days 8 to 10, with numbers remaining stable from days 10 to 38. Thus, we have made the novel observation that treatment with MnTBAP can uncouple expansion and the degree of contraction, such that a smaller expansion can now result in a smaller degree of contraction. Contraction in cell numbers must be a combination of cell proliferation and death. We found that ∼35% of CD8+DbNP396-404+ T cells had incorporated BrdU in vehicle-treated mice, compared to ∼65% of those in MnTBAP-treated mice. The percentage of BrdU+ cells in vehicle-treated mice was comparable to that observed by Blattman and colleagues (43%) (4), who examined proliferation of NP396-404-specific cells during the contraction phase by using BrdU labeling. Since treatment stopped on day 8 of LCMV infection, the increased proliferation occurs after drug administration. It is unlikely that the drug persists at high levels for extended periods. Thus, the proliferation that occurs is due to changes that occurred during the treatment. When proliferation was examined from days 31 to 38, we found that ∼17% of CD8+DbNP396-404+ T cells had incorporated BrdU in vehicle-treated mice, compared to ∼36% of those in MnTBAP-treated mice. This result suggests that homeostatic proliferation of antigen-specific CD8+ memory T cells is increased in MnTBAP-treated mice. While proliferation is increased, we observe that constant numbers of antigen-specific CD8+ T cells are still maintained. This result suggests that although antioxidant treatment increases proliferation, it does not block the apoptotic mechanisms that keep cell numbers constant during the memory phase. It will be critical to determine whether the increased homeostatic proliferation is autonomous to the T cells or a general function of the splenic environment. In addition to proliferation, contraction is highly influenced by apoptosis of T cells. Based on Annexin V and 7AAD staining, the vast majority of effector cells from both groups are AnnexinVhigh7AAD−, indicating that these cells are in the early stages of apoptosis. This finding confirms previous reports demonstrating that antigen-specific effector T cells at the peak of LCMV infection are Annexin V+ (11, 26). Further studies are needed to determine whether the apoptotic program is completed at similar rates for vehicle- and MnTBAP-treated mice.
In conclusion, we demonstrate that treatment with an antioxidant during viral infection results in decreased expansion and contraction of antigen-specific CD8+ T cells during primary infection. Although the numbers of effector cells are reduced in MnTBAP-treated mice compared to those in vehicle-treated mice, the clearance of virus was not dramatically delayed. Importantly, the numbers and in vivo function of memory cells are unaffected by this treatment. We also have shown that during secondary infection, the numbers of antigen-specific CD8+ T cells are unaffected by treatment with MnTBAP. This study demonstrates the key roles ROI have in CD8+-T-cell responses in vivo; controlling expansion and contraction. Manipulation of these functions by antioxidant treatment may allow for more rational design of vaccines and immunotherapies.
Acknowledgments
We thank Doug Lyles, Beth Hiltbold, Martha Alexander-Miller, Peter Gray, and Steven Mizel for critically reading this paper and for helpful input. We would also like to thank Rafi Ahmed and Allan Zajac for providing us with initial stocks of LCMV Armstrong and clone 13.
This research was supported by American Cancer Research Society Research Scholar Grant RSG-04-066-01-MBC to Jason Grayson.
REFERENCES
- 1.Abe, M. K., S. Kartha, A. Y. Karpova, J. Li, P. T. Liu, W. L. Kuo, and M. B. Hershenson. 1998. Hydrogen peroxide activates extracellular signal-regulated kinase via protein kinase C, Raf-1, and MEK1. Am. J. Respir. Cell Mol. Biol. 18:562-569. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 3:619-626. [DOI] [PubMed] [Google Scholar]
- 4.Blattman, J. N., J. M. Grayson, E. J. Wherry, S. M. Kaech, K. A. Smith, and R. Ahmed. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9:540-547. [DOI] [PubMed] [Google Scholar]
- 5.Buratti, S., and J. E. Lavine. 2002. Drugs and the liver: advances in metabolism, toxicity, and therapeutics. Curr. Opin. Pediatr. 14:601-607. [DOI] [PubMed] [Google Scholar]
- 6.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhri, G., I. A. Clark, N. H. Hunt, W. B. Cowden, and R. Ceredig. 1986. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. J. Immunol. 137:2646-2652. [PubMed] [Google Scholar]
- 8.Devadas, S., L. Zaritskaya, S. G. Rhee, L. Oberley, and M. S. Williams. 2002. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman, H. J., M. Torres, and J. Fukuto. 2002. Redox signaling. Mol. Cell. Biochem. 234-235:49-62. [PubMed] [Google Scholar]
- 10.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 11.Grayson, J. M., N. G. Laniewski, J. G. Lanier, and R. Ahmed. 2003. Mitochondrial potential and reactive oxygen intermediates in antigen-specific CD8+ T cells during viral infection. J. Immunol. 170:4745-4751. [DOI] [PubMed] [Google Scholar]
- 12.Grayson, J. M., A. J. Zajac, J. D. Altman, and R. Ahmed. 2000. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164:3950-3954. [DOI] [PubMed] [Google Scholar]
- 13.Harrington, L. E., M. Galvan, L. G. Baum, J. D. Altman, and R. Ahmed. 2000. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 191:1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildeman, D. A., T. Mitchell, T. K. Teague, P. Henson, B. J. Day, J. Kappler, and P. C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10:735-744. [DOI] [PubMed] [Google Scholar]
- 15.Homann, D., L. Teyton, and M. B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, M. J., S. Papa, J. Bolanos, R. Bruckdorfer, H. Carlsen, R. M. Elliott, J. Flier, H. R. Griffiths, S. Heales, B. Holst, M. Lorusso, E. Lund, J. Oivind Moskaug, U. Moser, M. Di Paola, M. C. Polidori, A. Signorile, W. Stahl, J. Vina-Ribes, and S. B. Astley. 2002. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Asp. Med. 23:209-285. [DOI] [PubMed] [Google Scholar]
- 17.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 18.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 19.Melov, S., J. A. Schneider, B. J. Day, D. Hinerfeld, P. Coskun, S. S. Mirra, J. D. Crapo, and D. C. Wallace. 1998. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 18:159-163. [DOI] [PubMed] [Google Scholar]
- 20.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 21.Perl, A., P. Gergely, Jr., F. Puskas, and K. Banki. 2002. Metabolic switches of T-cell activation and apoptosis. Antioxid. Redox Signal. 4:427-443. [DOI] [PubMed] [Google Scholar]
- 22.Piganelli, J. D., S. C. Flores, C. Cruz, J. Koepp, I. Batinic-Haberle, J. Crapo, B. Day, R. Kachadourian, R. Young, B. Bradley, and K. Haskins. 2002. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 51:347-355. [DOI] [PubMed] [Google Scholar]
- 23.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 24.Ueda, S., H. Masutani, H. Nakamura, T. Tanaka, M. Ueno, and J. Yodoi. 2002. Redox control of cell death. Antioxid. Redox Signal. 4:405-414. [DOI] [PubMed] [Google Scholar]
- 25.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, X. Z., S. E. Stepp, M. A. Brehm, H. D. Chen, L. K. Selin, and R. M. Welsh. 2003. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity 18:631-642. [DOI] [PubMed] [Google Scholar]
- 27.Whitmire, J. K., M. S. Asano, K. Murali-Krishna, M. Suresh, and R. Ahmed. 1998. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72:8281-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamzami, N., P. Marchetti, M. Castedo, D. Decaudin, A. Macho, T. Hirsch, S. A. Susin, P. X. Petit, B. Mignotte, and G. Kroemer. 1995. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 182:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]