Abstract
Increasing numbers of patients are treated with mega-highly active antiretroviral therapy (HAART), or multiple-combination antiretroviral therapy, in an attempt to overcome the viral resistance that has contributed to treatment failure. Studies of human immunodeficiency virus (HIV) viral dynamics are used to quantify the potency of a given regimen. While mega-HAART is expected to provide potent therapy, its potency among heavily experienced HIV-infected children who have failed previous treatment is untested. HIV dynamics studies performed in children have provided minimal information on viral dynamics during mega-HAART. The present study estimates first- and second-phase viral dynamics in six children on mega-HAART, following failure of combination therapy. The first phase of viral decay was rapid, relative to rates reported in previous pediatric studies (median δ = 0.778d−1, range = 0.583 to 1.088, half-life 1 [t11/2] = 0.894d), while the second phase revealed results similar to those of previous studies (median μ = 0.026d−1, range = −0.005 to 0.206, t21/2 = 9.316d). This indicates that mega-HAART can provide potent therapy among heavily experienced pediatric patients.
With the success of highly active antiretroviral therapy (HAART), increasing numbers of human immunodeficiency virus (HIV)-infected children have been treated with antiretroviral therapy for extended periods of time. Children who fail HAART regimens are initially switched to alternative regimens consisting of drugs to which they have not been exposed. However, those failing numerous regimens run out of new drugs and are in need of salvage therapy. Increasing numbers of such patients are treated with mega-HAART, or multiple-combination antiretroviral therapy, in an attempt to overcome the viral resistance that has contributed to treatment failure (10).
The potency of therapeutic regimens is reflected in the rate at which viral load decreases during the first few days after initiation of treatment (phase 1 viral decay) and during subsequent treatment (phase 2 viral decay) (4).
However, studies of viral dynamics in children provide little information concerning the potency of mega-HAART among children who have failed HAART regimens (8, 9). Extrapolation from adult viral dynamics studies to pediatric populations can be problematic because of various factors including the following: (i) greater viral burden in children, (ii) higher CD4 counts and the decrease of these target cells with age in children, (iii) differences in pharmacokinetics, and (iv) differences in viral replication.
The primary objective of the present study was to estimate the HIV decay rates among HAART-experienced children who have failed therapy and who have been changed to mega-HAART regimens. We hypothesized that these regimens would be relatively potent in the initial phase of viral reduction and would continue to reduce viral load during the second phase, despite the viral resistance which would be expected in such a group of subjects.
MATERIALS AND METHODS
Subjects.
Six HIV-infected children who had failed previous HAART due to increased viral load or rapid decrease in CD4+ count were enrolled. Patient 1 was in Centers for Disease Control and Prevention class B2, patients 2 and 5 in class B3, and patients 3, 4, and 6 in class C3 (1). The median age was 11 years (range, 6 to 14 years). Durations of previous antiretroviral regimens were 12 to 32 months (median, 16 months). Previous regimens were stopped at least 14 days prior to starting the new regimen. Table 1 presents previous and current treatment regimens. All patients had genotype resistance testing done before changing to the current therapy. We used the consensus statement of the International AIDS Society-USA Panel to guide our choices (7). In addition, we took into consideration all previously taken drugs and tried to avoid a drug that was previously used, even if the genotype testing suggested that the isolated strain was sensitive to it. These methods resulted in four of the six patients receiving at least two new drugs. In two patients (2 and 5), we had to use seven drugs because we could not identify at least three drugs to which they were sensitive and had not been exposed.
TABLE 1.
Baseline characteristics and phase 1/2 viral kineticsa
| Treatment
|
Baseline
|
Phase 1 decay
|
Phase 2 decay
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PID | Prestudy | Study | Sex | Age (yr) | CD4 count | Log10 RNA (copies/ml) | d1 (d−1) | t11/2 (d)c | d2 (d−1)c | t21/2 (d) |
| 1 | D4T/3TC/DDI/HU | APV/RTV+LPV/EFV | M | 11 | 530 | 5.510 | 0.631 | 1.099 | 0.045 | 15.270 |
| 2 | DDI/D4T/EFV/HU | ZDV/DDI/3TC/EFV/NFV/APV/RTV+LPV | M | 7 | 25 | 5.955 | 0.731 | 0.948 | 0.206b | 3.361 |
| 3 | D4T/RTV/APV | ZDV/3TC/IDV/RTV | M | 14 | 29 | 4.809 | 1.088 | 0.637 | −0.005 | Infinitee |
| 4 | RTV/D4T | RTV+LPV/ZDV/3TC/ABC | F | 12 | 1,148 | 4.986 | 0.826 | 0.839 | —d | — |
| 5 | APV/RTV/D4T/DDI | ZDV/3TC/DDI/EFV/APV/RTV+LPV/SQV | F | 11 | 534 | 5.311 | 0.583 | 1.189 | 0.007 | 103.000 |
| 6 | 3TC/APV/RTV+LPV | D4T/EFV/APV/RTV+LPV | F | 6 | 8 | 5.162 | 0.916 | 0.756 | — | — |
| Median | 11 | 279.5 | 5.236 | 0.778 | 0.894 | 0.026 | 9.316 | |||
D4T, stavudine; 3TC, lamivudine; DDI, didanoside; APV, amprenavir; RTV, ritonavir; LPV, lopinavir; EFV, efavirenz, NFV, nelfinavir; ZDV, zidovudine; IDV, indinavir; ABC, abacavir; SQV, saquinavir; HU, hydroxyurea; M, male; F, female.
Data from day 84 not available.
The half-life of the two phase decays was calculated as H1 = ln(2)/d1 and H2 = ln(2)/d2.
—, d2 and t21/2 not meaningful, since data only extended through day 6.
not interpretable due to viral rebound. Treated as longest half-life estimate in calculating median.
Quantification of plasma HIV-RNA copy number by RT-PCR.
HIV-1 RNA was quantified in 200 μl of plasma (stored at −70C within 6 to 18 h after phlebotomy) by reverse transcription-PCR (RT-PCR) (Amplicor; Roche), according to the manufacturer's specifications. The lower detection limit of the assay is 400 copies of HIV-1 RNA per ml of plasma. All assays were performed in the Children's Memorial Special Infectious Diseases Laboratory, which participates in an ongoing quality assurance program of the Centers for Disease Control and Prevention.
Statistical analysis.
Preliminary data analysis consisted of graphic displays of the data, based upon the basis spline smoothing method (5). The primary analysis of plasma RNA data utilized the biphasic viral dynamics model proposed by Wu and Ding (13).
The basis spline smoothing method is a nonparametric technique used to examine data, with respect to pattern and distribution, without prior distribution assumptions. The graphic display of patterns in the data is informative for subsequent model fitting and parameter estimation (5).
The statistical model used to estimate viral decay rates can be written as follows: V(t) = P1 e−d1t + P2 e−d2t + e(t), where d1 and d2 are the first- and second-phase decay rates, P1 and P2 are macroparameters, representing baseline viral levels, such that P1 plus P2 is equal to the model estimate of the baseline viral load, and e(t) is measurement error.
This model does not include parameter estimates for the so-called “shoulder effect, ” representing the delay between the onset of therapy and the time at which viral load begins to drop (6, 12, 13). Moreover, this method does not require the assumption of pretreatment steady state.
The nonlinear mixed-effects (NLME) model approach was used to fit this model (14). Comparisons of this method with other methods can be found in two recent papers by Ding and Wu (2, 3). The nonlinear mixed-effects model approach uses all data points together to get population estimates of the decay rates (fixed effects); it then uses individual data to estimate parameters accounting for patient variability in decay rates (random effects); finally, it combines them to get empirical Bayes estimates of each patient's first- and second-phase viral decay, denoted as d1 and d2.
The half-life of the two phase decays can be calculated as follows: H1 = ln(2)/d1 and H2 = ln(2)/d2.
Written informed consent for the Children's Memorial Hospital Institutional Review Board was obtained. The children were admitted to the Children's Memorial Hospital Clinical Research Center before the new mega-HAART therapy was started. Blood was drawn (for baseline determination) just before the first dose of the new regimen and every 6 h for the first 3 days, every 12 h on days 4 and 5, and every 24 h on days 6 and 7. Blood samples were also taken on days 14, 28, and 84. At each time point, HIV RNA copies per milliliter was measured by the RT-PCR Roche Amplicor assay.
RESULTS
Data used for the NLME analysis.
Since the primary purpose of the study was to assess viral decay rates, rather than overall patient success on the treatment regimens, data reflecting periods in which the drugs were not taken were eliminated. Patient 2 had Pseudomonas aeruginosa sepsis, which resulted in a drug interruption during the time of the 84-day RNA measure. Treatment with mega-HAART was reinitiated 10 days later. Patients 4 and 6 developed toxicity rash and intolerance to the mega-HAART therapy after days 9 and 6, respectively. Thus, the last data point for patient 2 is at day 28 and the last data point for patients 4 and 6 is at day 6. For the remaining patients, all data available through day 84 were used.
Results from the NLME analysis.
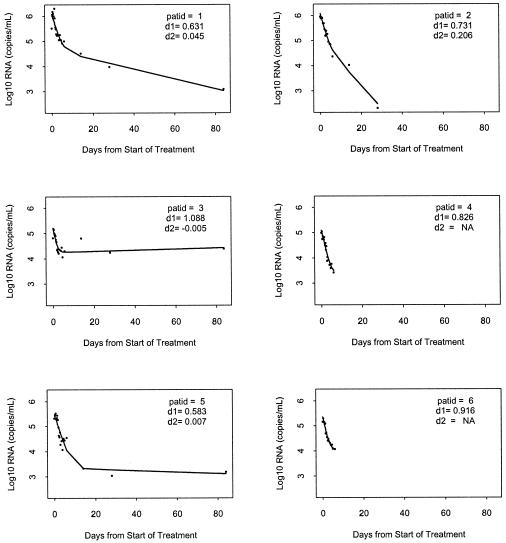
The results of modeling RNA change among the six study subjects are shown in Fig. 1. Note that the model fits each patient's data quite well. The first- and second-phase estimates of viral decay rates are presented in Table 1. The first phase estimates are relatively steep (median δ = 0.778d−1, range = 0.583 to 1.088, t11/2 = 0.894d) in comparison with those found in a previous study which included experienced children (3) (median δ = 0.43d−1, range 0.18 to 0.77, t11/2 = 1.6d) and compare favorably with those reported from adult studies which have used standard HAART therapy (10-12). The second-phase viral decay estimates (median μ = 0.026d−1, range = −0.005 to 0.206, t21/2 = 9.316d), while similar to those observed in previous pediatric studies, should be interpreted with caution, since only three patients had complete data for the phase 2 time periods.
FIG. 1.
HIV-RNA from the six patients (dots) and the fitted curves based on the basis spline smoothing method. Right panels, HIV-RNA from the six patients (dots) and the corresponding fitted trajectories using NLME modeling.
The statistical model used in the present study did not exclude data from the first few hours after initiation of mega-HAART therapy, which could have been influenced by the shoulder effect. This effect consists of a delay in initial viral reduction, due to virions produced before the pharmacologic effect of therapy, which are still infectious and capable of producing viral particles that would be detected by the RNA assay. Exclusion of these data would have resulted in even steeper phase 1 estimates of viral decay. This suggests that the estimates from the present study are conservative, relative to those from studies which have not used data taken during the first few hours after the introduction of study treatment (13).
DISCUSSION
The relatively steep phase 1 decay rates found in the children studied here indicate that a large component of their baseline virus was sensitive to the mega-HAART regimens (e.g., wild-type and/or partially resistant virus). The fact that their first-phase rates of elimination were more rapid than those of experienced pediatric patients in previous, non-mega-HAART studies suggests that the clearance rate of sensitive HIV strains may increase as the number of potent antiretroviral drugs increases.
The first-phase elimination of the sensitive component of the virus leaves a residue of resistant virus and/or virus from latently infected cells. Change in this residual component of the virus is estimated by the phase 2 decay rates. In the present study, these rates were comparable to those reported in pediatric studies involving children who were less experienced and were treated less aggressively (8, 9). Thus, although the heavily experienced patients in the present study may have had greater viral resistance than subjects in previous pediatric studies, the quantity of medication administered in the mega-HAART regimens might have been sufficient to overcome resistance and/or prevent its emergence. However, the findings presented here may also reflect a complex effect in which therapeutic activity against virus from latently infected cells and/or moderately resistant virus outweighs the replication of highly resistant virus. Our interpretation of the results may be limited by the small sample size of only six patients and further studies are needed.
The results presented here reflect data gathered in the first 84 days following initiation of mega-HAART treatment. While not part of the present study, clinical follow-up information was available for four of the study patients. In patient 3 therapy was discontinued within 2 weeks of study completion, due to a potential interaction between the mega-HAART regimen and initiation of therapy for atypical mycobacteria infection. Patient 5 maintained viral load suppression for 6 months but then broke through due to major problems with compliance. In two patients (1 and 2), the viral suppression achieved by day 84 was maintained for 26 and 25 months, respectively.
These results suggest that mega-HAART, or multiple-combination antiretroviral therapy, can dramatically increase the first-phase viral decay rates in experienced children and provide further benefit during the second phase of viral decay. For patients who tolerated the mega-HAART, it continued to be effective during prolonged follow-up. Thus, mega-HAART is an attractive form of salvage therapy for experienced patients who have failed previous HAART regimens.
Acknowledgments
We thank the patients for participation.
This work was supported in part by grant no. RR-00048 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Centers for Disease Control and Prevention. 1994. Revised classification system for HIV-infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 43:1-10. [Google Scholar]
- 2.Ding, A. A., and H. Wu. 1999. Relationships between antiviral treatment effects and biphasic viral decay rates in modeling HIV dynamics. Math. Biosci. 160:63-82. [DOI] [PubMed] [Google Scholar]
- 3.Ding, A. A., and H. Wu. 2000. A comparison study of models and fitting procedures for biphasic viral dynamics in HIV-1 infected patients treated with antiviral therapies. Biometrics 56:16-23. [DOI] [PubMed] [Google Scholar]
- 4.Finzi, D., and R. F. Siliciano. 1998. Viral dynamics in HIV-1 infection. Cell 93:665-671. [DOI] [PubMed] [Google Scholar]
- 5.Green, P. J., and B. W. Silverman. 1994. Nonparametric regression and generalized linear models: a roughness penalty approach. Chapman and Hall, London, United Kingdom.
- 6.Herz, A. V. M., S. Bonhoeffer, R. M. Anderson, R. M. May, and M. A. Nowak. 1996. Viral dynamics in vivo: limitations on estimates of intracellular delay and virus decay. Proc. Natl. Acad. Sci. USA 93:7247-7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch, M. S, B. Conway, R. T. D'Aquila, V. A. Johnson, F. Brun-Vezinet, B. Clotet, L. M. Demeter, S. M. Hammer, D. M. Jacobsen, D. R. Kuritzkes, C. Loveday, J. W. Mellors, S. Vella, D. D. Richman, for the International AIDS Society-USA Panel. 1998. Antiretroviral drug resistance testing in adults with HIV infection. JAMA 279:1984-1991. [DOI] [PubMed] [Google Scholar]
- 8.Luzuriaga, K., H. Wu, M. McManus, et al. 1999. Dynamics of human immunodeficiency virus type 1 replication in vertically infected infants. J. Virol. 73:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melvin, A. J., A. G. Rodrigo, K. M. Mohan, et al. 1999. HIV-1 dynamics in children. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:468-473. [DOI] [PubMed] [Google Scholar]
- 10.Montaner, J. S. F., P. R. Harigan, J. Jahnke, et al. 2001. Multiple drug rescue therapy for HIV-infected individuals with prior virologic failure to multiple regimens. AIDS 12:61-69. [DOI] [PubMed] [Google Scholar]
- 11.Perelson, A. S., P. Essunger, Y. Cao, et al. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 12.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 13.Wu, H., and A. Ding. 1999. Population HIV-1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics 55:410-418. [DOI] [PubMed] [Google Scholar]
- 14.Wu, H., R. D. R. Kuritzkes, M. M. Lederman, et. al. 1999. Characterization of viral dynamics in human immunodeficiency virus type 1-infected patients treated with combination antiretroviral therapy. J. Infect. Dis. 179:799-807. [DOI] [PubMed] [Google Scholar]