Abstract
The mechanism of the divergent expression of the varicella-zoster virus (VZV) ORF 28 and ORF 29 genes from a common intergenic DNA element, the ORF 28/29 promoter, is of interest based on the observation that both genes are expressed during VZV lytic infection but only the ORF 29 gene is expressed in latently infected neurons. In the work presented here, expression driven by the ORF 28/29 intergenic region was examined. We found that the promoter activity towards the ORF 29 direction is more responsive to activation by the major viral transactivator IE62 than that towards the ORF 28 direction in the context of our experimental system. Analysis of the functional DNA elements involved in IE62 activation of the bidirectional ORF 28/29 regulatory element revealed that in both transfected and VZV-superinfected cells it is a fusion of two unidirectional promoters overlapping an essential USF binding site but with distinct TATA elements. A single TATA element directs expression in the ORF 28 direction, whereas the two TATA elements directing ORF 29 gene expression are alternatively and differentially utilized for transcription initiation. We also identified an Sp1 site localized proximal to the ORF 28 gene which functions as an activator element for expression in both directions. These results indicate that the ORF 28 and ORF 29 genes can be expressed either coordinately or independently and that the observed expression of only the ORF 29 gene during VZV latency may involve neuron-specific cellular factors and/or structural aspects of the latent viral genome.
Varicella-zoster virus (VZV) is the causative agent of two human diseases: varicella (chickenpox) and zoster (shingles). Primary infection gives rise to varicella with characteristic skin lesions resulting from lytic infection of the virus in cutaneous epithelial cells. As a member of the neurotropic alphaherpesvirus subfamily, VZV can establish latency in the dorsal root ganglia following primary infection (4). Latent VZV DNA is predominantly localized in the neurons, although some researchers also identified viral sequences in nonneuronal satellite cells (14, 22, 23, 32, 37). Data obtained from human ganglia and animal models indicate that during latent infection, a small subset of lytic viral genes is expressed while most of the VZV genome is transcriptionally quiescent. These latency-expressed genes include open reading frames (ORFs) 63, 62, 29, 21, 4, and 66 (2, 7, 8, 11, 12, 20, 24, 33, 35, 66). In the majority of studies conducted, expression of these genes has been detected at the level of RNA. However, expression of all of these genes at the protein level has also been reported (8, 12, 33, 35, 66), raising the possibility of a role for one or more of them as trans-acting factors during latency. While the majority of the expressed proteins detected appear to be cytoplasmic rather than nuclear, the possibility of the presence of protein below the levels of detection in the nuclei or a rapid shuttling between the nucleus and cytoplasm cannot be discounted. Because several of the latent proteins are VZV gene products involved in the regulation of viral gene expression during productive infection (reviewed in reference 25), the function of these proteins in VZV latency may possibly involve latent gene expression and/or VZV reactivation. Nothing is presently known about the mechanism or mechanisms by which these genes are expressed while the remainder of the genome is silent.
To gain insight into this question, we have examined the cis- and trans-acting factors involved in expression of the VZV ORF 29 gene. The ORF 29 gene encodes the VZV major DNA binding protein, which is the orthologue of the herpes simplex virus type 1 (HSV-1) ICP8 protein (15). The ORF 29 gene product has been shown, as expected, to bind preferentially to single-stranded DNA and is inferred to be required for replication of VZV DNA based on its homology with ICP8 (26, 49). The ORF 29 protein has also been shown to play a promoter- and cell-type-dependent role in IE62-mediated VZV gene expression (6). During lytic infection, ORF 29 is expressed via a 221-bp intergenic regulatory element with its divergently oriented neighboring gene, ORF 28, which encodes the viral DNA polymerase (Fig. 1A). Both genes produce polyadenylated transcripts, with ORF 28 yielding a 4.1-kb message and ORF 29 producing RNA of 4.2 kb and a very low-level, minor transcript of 4.1 kb (39). During latent infection, a different pattern of the expression of these two genes is observed in which only the 4.2-kb transcript of ORF 29 is detected while the ORF 28 transcript is absent (11, 14, 23). Thus, the regulatory element involved in expression of these two genes should contain information required for both lytic and latent viral gene expression.
FIG. 1.
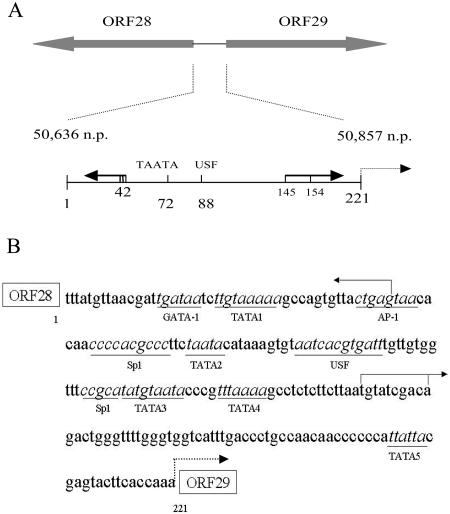
(A) The ORF 28/29 intergenic bidirectional regulatory element. The divergent ORF 28/29 regulatory element is located in the intergenic region between VZV ORFs 28 and 29, which are shown as filled arrows. The element extends from n.p. 50,636 to n.p. 50,857 in the DNA sequence of the VZV Dumas strain (14) and contains 221 bp. The numerical positions (1 to 221) of the nucleotides within the intergenic region are shown in reference to the origin of the coding sequence of the ORF 28 gene. Indicated are functional cis-acting sequences previously mapped by Meier and coworkers (38, 39). Multiple transcription start sites of the ORF28 gene were mapped centered at n.p. 42, as indicated by the arrow towards the ORF 28 direction. The solid arrow towards the ORF 29 direction represents two major ORF 29 gene transcription start sites at n.p. 145 (T) and 154 (A), which were identified by in vitro transcription (39). The differences in n.p. assigned to these two elements in this study compared to those previously designated may be due to the fact that the in vitro transcription experiments were performed using a truncated intergenic region. The dashed arrow represents a minor, equivocal ORF 29 start site. The positions of the previously identified TATA box directing ORF 28 gene expression and the essential USF consensus binding site are also shown. (B) Potential transcription factor binding sites within the full-length intergenic region. The sequence of the 221-bp ORF 28/29 intergenic region was analyzed in silico using the programs MatInspector V2.2 (64) and TESS (57) to predict the potential transcription factor binding sites. Predicted binding sites are underlined. The arrows indicate the transcription start sites.
This regulatory element was first identified and examined in a series of studies by Meier and coworkers (38, 39) and was designated the ORF 28/29 promoter. Expression of both genes was shown to be transactivator dependent, requiring the viral IE62 protein for efficient expression. A consensus binding site (48, 50, 52) for the cellular transcription factor USF is present in a 12-bp palindrome localized near the middle of the intergenic region. The integrity of this site was shown to be essential for IE62-mediated transactivation in both directions. A TATA element directing transcription toward the ORF 28 direction was also identified, whereas expression of the ORF 29 gene was suggested to be directed by a TATA-less mechanism because none of the putative ORF 29 TATA elements examined were shown to be essential for expression. Truncation analyses performed in these studies failed to uncouple the expression of the two genes, and it was proposed that the regulatory element was a single promoter symmetrically activated by the combined actions of USF and IE62. However, the extensive amount of evidence now accumulated for the differential expression of the ORF 28 and 29 genes during VZV latency and the presence of numerous other potential cis-acting elements in the 221-bp sequence (Fig. 1B) led us to hypothesize that there is either asymmetry in the promoter with respect to its activation by viral or cellular factors or it consists of two separate elements allowing differential expression of the ORF 28 and 29 genes under specific circumstances.
In the studies presented here, we have found that, in the context of IE62 activation alone or with VZV superinfection, the bidirectional ORF 28/29 regulatory element is a fusion of two unidirectional promoters which overlap the consensus USF binding site and utilize different TATA elements. In contrast to the single TATA element required for expression of the ORF 28 gene, two atypical TATA motifs are alternatively and differentially utilized to direct ORF 29 gene expression. We have also found that the cellular transcription factor Sp1, while not required for activation, upmodulates the USF-mediated IE62 activation in both directions despite the fact that the functional Sp1 binding site is located in the minimal ORF 28 promoter. Similar data were obtained using a continuous neuroblastoma cell line. These results indicate that the ORF 28 and ORF 29 genes can potentially be expressed either coordinately or independently based on the structure of their intergenic regulatory element. They also suggest that the observed expression of only the ORF 29 gene during VZV latency may involve neuron-specific cellular factors induced by latent infection and/or structural aspects of the latent viral genome.
MATERIALS AND METHODS
Cells and viruses.
MeWo cells, a human melanoma cell line that supports the replication of VZV, were grown in Eagle's minimal essential medium supplemented with 10% fetal bovine serum as previously described (60). VZV strain MSP was propagated in MeWo cell monolayers as described by Lynch et al. (34) and Peng et al. (44). A3.01 cells, a continuous CD4+ T-cell line, was propagated as described by Boucaud et al. (6). SH-SY5Y cells (CRL-2266; American Type Culture Collection [ATCC]), a continuous human neuroblastoma cell line, were provided by J. Feng (University at Buffalo). These cells were grown in ATCC modified Eagle's minimal essential medium formulation and supplemented with 10% fetal bovine serum and 2 mM l-glutamine.
Plasmids.
The cloning of pCMV62 plasmid expressing IE62 under the control of the cytomegalovirus (CMV) immediate-early (IE) promoter was described previously by Perera et al. (45, 46). The following plasmids were constructed in this study: p28CAT, p29CAT, pRFL/pless, pRFL/WT, pRFL/SU, pRFL/SD, pRFL/MB, pRFL/CF, pRFL/TA28m, pRFL/TA29.1m, pRFL/TA29.2m, pRFL/TA29.3m, pRFL/USFm, and pRFL/Sp1m. The p28CAT and p29CAT vectors were derived from pCAT-basic (Promega, Madison, Wis.). The full-length 221-bp ORF 28/29 intergenic region was amplified by PCR using primers containing either KpnI or XhoI sites at the 5′ end. The pVSpe5 cosmid (59) containing a fragment of the VZV(Oka) genome from nucleotide position (n.p.) 21875 to 62008 was used as template. The PCR product was digested and inserted between the KpnI and XhoI sites of the pCAT-basic vector in orientations such that the CAT reporter gene represents either ORF 28 gene expression (p28CAT) or ORF 29 gene expression (p29CAT).
A set of luciferase reporter plasmids was constructed by using the pGL-2 basic vector (Promega). In the pRFL series of dual-luciferase bidirectional reporter vectors, the renilla luciferase reporter gene (RL) fragment containing the coding sequence of the renilla luciferase gene and the simian virus 40 late polyadenylation signal was amplified from the pRL-null vector (Promega) from n.p. 300 to 1507. The 5′ primer annealed across the translation start site of the renilla luciferase gene contained an NheI site, and the 3′ primer annealed across the polyadenylation end contained a KpnI site. The resulting PCR product was digested and inserted between the KpnI and NheI sites of pGL-2 basic, yielding a promoterless bidirectional dual-luciferase reporter vector designated the pRFL/pless vector. The full-length ORF 28/29 intergenic domain was amplified and inserted between the XhoI and HindIII sites of the pRFL/pless vector to produce the pRFL/WT vector, in which the firefly luciferase reporter gene (FL) represents the ORF 29 gene and the RL represents the ORF 28 gene. All other pRFL vectors contain either truncations or point mutations of the full-length regulatory element. Truncated promoter fragments were inserted into the pRFL/pless vector in the same way as that used for the pRFL/WT vector. These fragments include the short upstream (SU) fragment (n.p. 38 to 105), short downstream (SD) fragment (n.p. 77 to 146), minimal bidirectional (MB) fragment (n.p. 38 to 146), and the central fragment (CF) (n.p. 77 to 105).
The pRFL vectors containing point mutations of known or putative promoter elements were derived by site-directed mutagenesis of the pRFL/WT vector using the Quick Change site-directed mutagenesis kit (Stratagene, Cedar Creek, Tex.) following the manufacturer's directions. These include mutation of the TATA element for ORF 28 gene expression (pRFL/TA28m), mutation of one putative TATA element for ORF 29 gene expression (pRFL/TA29.1m), mutation of a second putative ORF 29-oriented TATA element (pRFL/TA29.2m), and the double mutation of these two TATA elements (pRFL/TA29.3m). The USF binding site was mutated in the pRFL/USFm vector, and a putative Sp1 binding site was mutated in the pRFL/Sp1m vector. The n.p. and the sequences of the wild-type and mutant sites are listed in Table 1. All reporter vectors were sequenced at the Roswell Park Cancer Institute Biopolymer Facility.
TABLE 1.
Wild-type and mutant sequences of the functional elements within the ORF28/29 intergenic regiona
| Promoter element (n.p.) | Wild-type sequence | Mutant sequence |
|---|---|---|
| TA28 (68-72) | TAATA | TCGCA |
| TA29.1 (111-119) | TATGTAATA | GCTGTTGAA |
| TA29.2 (124-130) | TTTAAAA | TTTCGTA |
| USF (83-94) | AATCACGTGATT | AATCACGCTCTT |
| Spl (55-64) | CCCCACGCCC | CCAAACGAAC |
The n.p. of each functional element is numbered as its position in the full-length ORF 28/29 intergenic region from 1 to 221. The target sequences were mutagenized using the Quick Change site-directed mutagenesis kit (Stratagene) as described in Materials and Methods. All base changes are shown in italics.
Transient transfections and reporter gene assays.
MeWo cells were transfected with lipofectamine reagent (Invitrogen, Carlsbad, Calif.) following the manufacturer's instructions. Six microliters of lipofectamine reagent was used per microgram of transfected DNA in each transfection. In transfections performed in 35-mm-diameter petri dishes, 4 × 105 MeWo cells per dish were seeded in 2 ml of complete growth medium. The cells were 80% confluent at the time of transfection. One microgram of a given chloramphenicol acetyltransferase (CAT) reporter vector or 1.5 μg of a given dual-luciferase reporter vector was cotransfected with various amounts of the pCMV62 plasmid and complementary amounts of the pCMV · SPORT · βgal plasmid to equalize the total amount of DNA and CMV IE promoter sequences in each transfection. In the superinfection experiments, melanoma cells in 6-well plates were transfected with reporter plasmids and 24 h later the monolayer was overlayed with 105 VZV-infected cells. SH-SY5Y cells were transfected with lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Four microliters of lipofectamine reagent was used per microgram of transfected DNA in each transfection. Cells were collected 48 h after transfection and were lysed in appropriate buffers by alternating freeze-thaw cycles.
Extracts of cells transfected with CAT reporter vectors were prepared in 100 μl of 0.25 M Tris (pH 7.5) and were subjected to CAT assays and β-galactosidase (β-Gal) assays. CAT assays were performed as previously described (45, 46). β-Gal assays were performed using the β-Gal assay kit (Invitrogen) following the microtiter plate protocol recommended by the manufacturer. Extracts of cells transfected with dual-luciferase reporter vectors were prepared in 200 μl of the passive lysis buffer included in the dual-luciferase reporter assay system (Promega) followed by dual-luciferase and β-Gal assays. Dual-luciferase assays were performed using the dual-luciferase reporter assay system (Promega) with 20 μl of cell extracts, 50 μl of LARII reagent, and 50 μg of Stop & Glo reagent in each assay. All transfections were performed in triplicate in all experiments, and all results were confirmed by independent experiments. In experiments with titrations of pCMV62 plasmid and complementary amounts of pCMV · SPORT · βgal plasmid cotransfected to equalize total DNA in transfections, CAT and luciferase (LUC) activities were standardized to protein concentration. In experiments where a fixed amount of pCMV62 plasmid was transfected, LUC activities were standardized to β-Gal activities by using a constant amount of pCMV · SPORT · βgal plasmid in the transfections. CAT activities were quantified as percent acetylation. LUC activities were represented by fold induction of the measured activity over either background luminescence, the empty reporter vector, or basal activity, with the wild-type reporter vector in the absence of effector vector, as indicated.
Primer extension analysis.
MeWo cells in 6-well plates were cotransfected with 2 μg of either pRFL/WT, pRFL/TA28m, pRFL/TA29.1m, pRFL/TA29.2m, or pRFL/TA29.3m plasmid and 0.2 μg of pCMV62 plasmid. In experiments involving superinfection with VZV, monolayers were transfected with the reporter vectors as described followed by overlay with infected cells. Total RNA was isolated 48 h posttransfection from the transfected cells as well as untransfected control cultures using Trizol reagent (Invitrogen) following the manufacturer's instructions. The isolated RNA from each well was dissolved in diethyl pyrocarbonate (DEPC)-H2O at final concentrations ranging from 2 to 3 μg/μl and were stored at −20°C for later use. Transcription initiation from the ORF 29 promoter was primed with a 26-base oligonucleotide (5′-TTTGGCGTCTTCCATTTTACCAACAG-3′) complementary to a portion of the firefly luciferase reporter gene and located 18 nucleotides downstream of the 3′ end of the ORF 28/29 regulatory element in the reporter constructs. Transcription initiation from the ORF28 promoter was primed with a 26-base oligonucleotide (5′-AACTTTCGAAGTCATGGTGGCTAGGC-3′) complementary to a portion of the RL located 10 nucleotides upstream of the 5′ end of the ORF 28/29 regulatory element. Both oligonucleotides were commercially synthesized (IDT, Coralville, Iowa), polyacrylamide gel electrophoresis (PAGE) purified, and 5′ end-labeled with T4 polynucleotide kinase (PNK) (Promega) and [γ-32P]ATP (6,000 Ci/mmol) (Perkin Elmer, Boston, Mass.). The primers were labeled in a 40-μl reaction mixture containing 4 μl of 10× PNK buffer, 2 μl of primer (10 pmol/μl), 5 μl of [γ-32P]ATP, 2 μl of T4 PNK, and 27 μl of DEPC-H2O. Primer extension was performed following the protocol of the primer extension system (Promega). The control primer and the DNA marker provided by the system were labeled per the manufacturer's instructions. The experimental primer extension reactions were performed in an identical manner, except that 0.1 μl of actinomycin D (10 μg/μl) and 0.5 μl of RNasin were added in the master reverse transcription extension mix. The primer extension products were resolved on a denaturing polyacrylamide gel (8% acrylamide, 7 M urea). The gel was dried and the products were visualized and quantified by PhosphorImage analysis and Quantity One software (Bio-Rad, Hercules, Calif.).
Nuclear extracts and magnetic bead recruitment assays.
Nuclear extracts of MeWo cells and T cells were prepared as previously described (44) and were stored at −70°C for subsequent use. Magnetic bead recruitment assays identifying proteins bound to the ORF 28/29 regulatory element were performed as described previously (34, 44). Briefly, a 221-bp 5′ biotinylated oligonucleotide encoding either the wild-type or mutant ORF 28/29 regulatory elements were generated from the pRFL/WT and pRFL/Sp1m plasmids, respectively, via PCR using biotinylated primers (IDT). Ten picomoles of each DNA fragment was coupled to 50 μl of M-280 streptavidin-conjugated magnetic beads (Dynal, Lake Success, N.Y.) in 200 μl of 1× B&W buffer (10 mM Tris-HCl, 1 mM EDTA, 1 M NaCl). The beads were collected and washed twice with 400 μl of 1× B&W buffer and then twice with TEN buffer (10 mM Tris-HCl, 1 mM EDTA, 0.1 M NaCl). The DNA-coupled beads were then incubated for 1 h at 4°C with aliquots of the nuclear extracts containing 250 μg of total protein. The beads were collected and washed three times with 400 μl of TEN buffer, and the specifically bound proteins were eluted with 10 μl of 2× B&W buffer. The eluted fractions were boiled in 2× sodium dodecyl sulfate (SDS)-PAGE loading buffer for 5 min and were analyzed by SDS-10% PAGE and immunoblotting. Antibodies against AP-1, GATA-1, Sp1, USF, and TBP were all purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
RESULTS
Response of ORF 28/29 regulatory element reporter constructs to IE62 activation in MeWo cells.
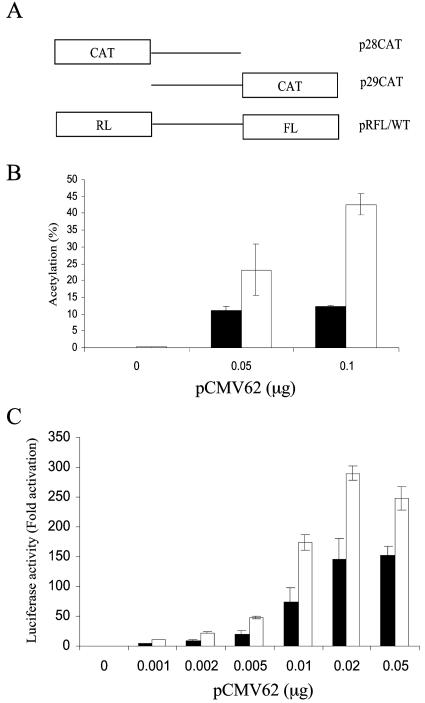
The ORF 29 and ORF 28 genes are differentially expressed during latent VZV infection in neuronal cells, although they appear to share a common intergenic bidirectional regulatory element. To gain insight into the mechanism by which this differential expression is accomplished, the functional architecture of this DNA element was examined. Previous studies have shown that activation of the ORF 28/29 regulatory element is dependent on the presence of the VZV major trans-activating protein, IE62 (38, 39). To examine its responsiveness to IE62 activation in each direction, unidirectional CAT reporter vectors were constructed with the full-length 221-bp regulatory element inserted in opposite orientations in order to direct CAT expression in either the ORF 28 (p28CAT) or the ORF 29 (p29CAT) direction (Fig. 2). The reporter plasmids were transfected into MeWo cells, a human melanoma cell line that supports productive infection of VZV either alone or with various amounts of the pCMV62 plasmid expressing IE62 under the control of the CMV IE promoter. To equalize both the total amount of DNA and the amount of CMV promoter in the transfections, the plasmid pCMV · SPORT · βgal was cotransfected in amounts complementary to that of the pCMV62 plasmid. The CAT assay results presented in Fig. 2B show that the basal expression of both reporters in the absence of IE62 is extremely low under our experimental conditions (0.1 to 0.2% acetylation). Expression in both directions was activated by addition of the IE62-expressing plasmid and was dose dependent on the amount of pCMV62 transfected into the cells. The level of IE62-induced CAT expression from the ORF 29 reporter was consistently higher (2.5- to 4-fold) than that from the ORF 28 reporter at each level of the effector plasmid used. Similar data were obtained by unidirectional FL constructs in this study (data not shown), and Cohrs et al. (12), using unidirectional luciferase reporters in Vero cells, also indicated preferential IE62 activation of expression (∼twofold) of luciferase reporters in the ORF 29 position compared to that of the ORF 28 position. These results suggested that the elements within the 221-bp intergenic region regulating ORF 29 expression are more sensitive to IE62 activation than those regulating ORF 28 expression, thus leaving open the possibility of separable promoter elements for each gene. However, the constructs used contained only a single reporter and, therefore, no choice regarding the directionality of expression was afforded to the transcriptional apparatus in these experiments.
FIG. 2.
Responsiveness of the ORF 28/29 regulatory element to IE62 activation in MeWo cells. (A) The structure of the unidirectional CAT reporter plasmid and the dual-luciferase bidirectional reporter plasmid. MeWo cell cultures in 35-mm-diameter petri dishes were cotransfected with 1 μg of pCAT reporter vectors (B) or 1.0 μg of pRFL/WT vector in 12-well plates (C) and increasing amounts of the IE62-expressing plasmid, pCMV62. Shown here are the results of triplicate experiments for each transfection. The promoter activities are reported as the extent of acetylation of radiolabeled chloramphenicol (B) or as the fold induction of luciferase activities (C) normalized to the basal promoter activities in the absence of pCMV62. The solid bar represents the promoter activity towards the ORF 28 direction, and the open bar represents that towards the ORF 29 direction.
To assess this question and to determine the activity of the regulatory element in the context of a more native genetic structure, a bidirectional, dual-luciferase reporter vector, pRFL/WT, was constructed. In this reporter, the RL was placed upstream of the ORF 28/29 regulatory element, representing the ORF 28 gene, and was designated ORF 28 RL; the FL was placed downstream, representing the ORF 29 gene, and was designated ORF 29 FL. Thus, this reporter vector would give the transcription apparatus the same choice presented to it in the native genetic configuration with regard to driving relative levels of expression of the two reporters and would allow rapid and sensitive quantification of expression from either end of the intergenic regulatory element in samples derived from a single transfection.
The pRFL/WT vector was cotransfected with various amounts of the pCMV62 plasmid into MeWo cells, and expression was analyzed by the dual-luciferase assay. The IE62-activated promoter activity in each direction was assessed as fold induction of luciferase activity over basal levels, which were normalized to 1. As shown in Fig. 2C, titration with levels of pCMV62 ranging from 0.001 to 0.05 μg yielded a linear response with both promoters. The response of ORF 29 FL was consistently some 2- to 2.5-fold higher than that of the ORF 28 RL relative to their basal activities, indicating that the differential level of expression of the two reporters remained constant at all levels of the effector plasmid. These data demonstrate that the bidirectional ORF 28/29 regulatory element is trans-activated by IE62 to a greater extent in the ORF 29 direction than in the ORF 28 direction under our experimental conditions in the context of both unidirectional reporter constructs and reporter constructs designed to mimic the native genetic configuration within the VZV genome. Thus, these combined results strengthen the idea that the basis of the differential regulation of these genes during latency may lie within the sequence of the regulatory element itself.
The bidirectional ORF 28/29 regulatory element is composed of two unidirectional promoters which share a consensus USF binding site.
Numerous examples of bidirectional promoters have been identified in mammals, plants, bacteria, fungi, and viruses, and a recent report indicates that more than 10% of the genes in the human genome may be divergently transcribed from such promoters (9, 17, 19, 27, 36, 42, 51, 53-56, 61-63). These regulatory sequences fall into two general categories. The first type are bidirectional promoters in which common cis elements and assembled transcription factors are used for transcription of both divergently oriented genes flanking the promoter. In the second type, expression in the two directions employs either totally distinct or only partially overlapping cis elements within the intergenic sequence. As a result, expression of the flanking genes can be either coordinately or independently regulated from the promoter. Previous studies demonstrating that the ORF 28 and ORF 29 genes are differentially expressed during VZV latency, coupled with the observations described above that expression of reporters mimicking the positions of these two genes respond differently to IE62 activation in permissive cells, strongly suggested that the ORF 28/29 regulatory element may be physically separable into two parts, with the individual parts driving transcription in only one direction.
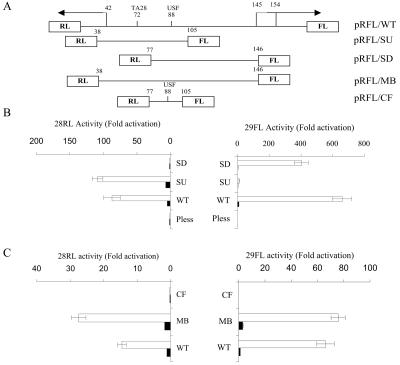
To clarify the anatomy of the ORF 28/29 regulatory element, the 221-bp intergenic region was truncated from both the 5′ and 3′ ends and the resulting fragments were ligated into the bidirectional dual-luciferase reporter plasmid pRFL (Fig. 3). Initially, two truncated fragments were generated in an effort to determine if sequences which drove expression in only one direction could be identified even if both reporter genes were present. The first truncation was designated the SU fragment and contained nucleotides 38 to 105 of the intergenic region. The 5′ end of this fragment had previously been identified (39) as the posttranscriptional start site region of the ORF 28 gene, and the 3′ end lies 11 nucleotides downstream of the essential USF site. The second truncation, designated the SD fragment, contained nucleotides 77 to 146 of the intergenic region. The 5′ end of this fragment lies 5 nucleotides upstream of the essential USF site, and the 3′ end lies one nucleotide beyond one of the two major transcriptional start sites of the ORF 29 gene.
FIG. 3.
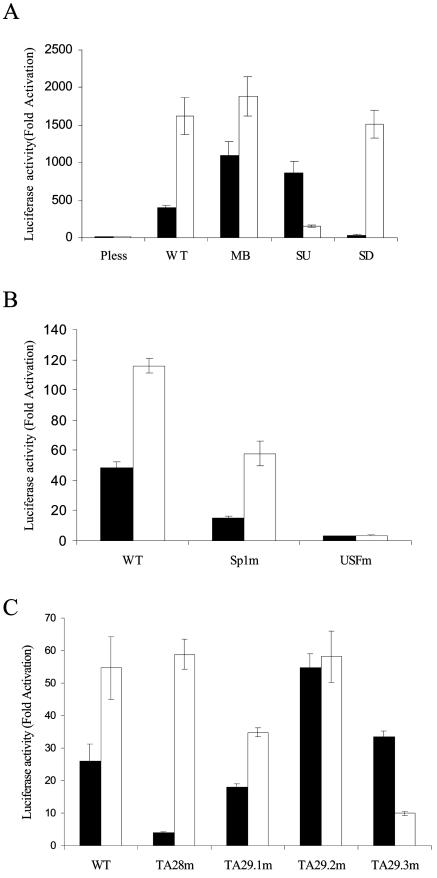
Dissection of the bidirectional ORF 28/29 regulatory element. (A) Schematic representation of the structure of the dual-luciferase vectors containing full-length or truncated intergenic fragments. (B and C) Each pRFL vector (1.5 μg) was transfected into MeWo cells in the absence (solid bar) or presence of 0.05 μg of pCMV62 plasmid (open bar). The promoter activities from triplicate transfections are presented either as fold induction of luciferase activities normalized to the FL and RL luciferase activities expressed from the parental promoterless vector, pRFL/pless, in the absence of pCMV62 (B) or normalized to the luciferase activities expressed from the pRFL/WT vector in the absence of pCMV62 (C).
The resulting reporter plasmids, pRFL/SU and pRFL/SD, as well as wild-type and promoter-less control plasmids (pRFL/WT and pRFL/pless, respectively) were transfected into MeWo cells in the presence or absence of 0.05 μg of pCMV62. When pCMV62 was absent in the transfections, 0.2 μg of pCMV · SPORT · βgal plasmid was cotransfected while 0.15 μg of pCMV · SPORT · βgal plasmid was added to the pCMV62 transfections to equalize both total DNA and the CMV promoter in all the transfections. β-Gal assays were performed along with dual-luciferase assays following transient transfections to normalize the basal and IE62-mediated luciferase activities expressed from each reporter vector.
The results from the dual-luciferase assays using the wild-type full-length intergenic region and the SU and SD fragments are presented in Fig. 3B as fold induction of the luciferase activities compared to that of the promoterless control in the absence of pCMV62, which was normalized to 1. The pRFL/pless control was not inducible by IE62, thus, any IE62-mediated induction in reporters containing the promoter elements under study was due to the presence of those elements. ORF 29 FL expression was induced to higher levels than ORF 28 RL expression by IE62 from the wild-type promoter, in agreement with the results presented in Fig. 2C. The SU promoter fragment supported activation of expression of the ORF 28 RL reporter by IE62 but not the ORF 29 FL reporter. The SD fragment, in contrast, exhibited the opposite activity, supporting IE62-mediated expression of the ORF 29 FL reporter but not the ORF 28 RL reporter to any significant extent (Fig. 3B). These results demonstrate that the SU and SD fragments act as unidirectional promoter elements to direct either ORF 28 and ORF 29 gene expression, respectively. Additionally, the level of expression of the ORF 28 RL driven by the SU fragment was consistently somewhat greater than that observed with the full-length wild-type intergenic region, whereas the level of ORF 29 FL expression from the SD fragment was consistently lower than that from the wild type. The increase in the promoter activity present in the SU fragment could be due to the deletion of inhibitory elements that exist within the deleted regions. Similarly, cis-acting elements that contribute to ORF 29 promoter activity may have been deleted in the generation of the SD fragment.
Based on these findings, a putative MB promoter element, containing nucleotides 38 to 146, was generated by incorporating the sequences falling between the 5′ and 3′ ends of the SU and SD fragments. A second promoter element, designated CF, was generated by PCR. CF contained nucleotides 77 to 105 of the intergenic region and represented the region of the two unidirectional promoters which overlapped. This 29-bp fragment contained the consensus USF binding site with minimal flanking sequences.
These DNA fragments were inserted into the dual-luciferase reporter vector, and their activities were compared to that of the full-length wild-type intergenic region. Results from transient transfection assays using the MB fragment and CF are shown in Fig. 3C and are presented as fold induction of the luciferase activity compared to that expressed from the pRFL/WT in the absence of pCMV62. The MB fragment was highly responsive to IE62 trans-activation in both directions, indicating that this region contains virtually all cis-acting elements required for divergent IE62 activation of flanking genes. The CF showed no responsiveness to IE62 trans-activation in either direction, indicating that the essential USF binding motif is not sufficient for mediating trans-activation by IE62. This result regarding expression of the ORF 28 RL reporter was anticipated, because the CF lacks the TATA element previously identified as being required for IE62-mediated expression in the ORF 28 direction (39). The lack of observed expression of the ORF 29 FL reporter with both CF and the SU fragment indicates that sequences downstream of the USF site are required for IE62-mediated expression in the ORF 29 direction.
Two atypical TATA elements are differentially utilized to direct ORF 29 gene expression in MeWo cells.
In previous work, Meier and Straus (39) were unable to identify a TATA element essential for the expression of reporter genes placed in the position of the ORF 29 gene. Expression of ORF 29 from the bidirectional regulatory element was postulated to occur via a TATA-less mechanism, although the existence of a TATA-less initiator element for ORF 29 remained to be proven. In the same study and using the same mutational strategy, it was shown that expression of the ORF 28 gene required the presence of a single, specific TATA element (nucleotides 68 to 72) in the intergenic region. The concept of a TATA-less expression mechanism for the ORF 29 gene is intriguing in that it could account for the differential regulation of gene expression from the ORF 28/29 promoter. To clarify the mechanism of transcription initiation of the ORF 29 gene, the positions of transcription factor binding sites, including TATA elements, within the intergenic region were predicted using the programs MatInspector V2.2 and TESS (57, 64). A summary of the predictions is shown in Fig. 1B and indicates that there are five putative TATA elements within the 221-bp regulatory element.
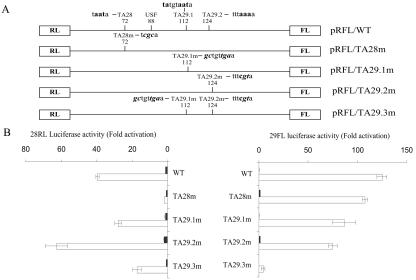
TATA1 lies outside the MB promoter and is downstream of the ORF 28 transcriptional start and is, thus, unlikely to figure in expression from the regulatory element. TATA2 is the TATA element identified previously as being required for expression in the ORF 28 direction. TATA3 and TATA5 are the two elements that were assessed by Meier and Straus (39) by site-specific mutagenesis as potential TATA boxes involved in ORF 29 gene expression. TATA5, like TATA1, lies outside the MB promoter. The computer analysis performed in this study identified an additional potential, although atypical, TATA element, TATA4, downstream of TATA3. TATA3 and TATA4 are both present within the minimal ORF 29 promoter element. Based on this we considered them to be the best ORF 29 TATA element candidates. TATA2, TATA3, and TATA4 were renamed TA28, TA29.1, and TA29.2, respectively, for the purposes of this study.
Site-directed mutations of the TA28, TA29.1, and TA29.2 sequences were created within the full-length intergenic region and were inserted into the dual-luciferase reporter vector (Fig. 4A). The sequences of the wild-type and mutagenized TATA elements are shown in Table 1. The mutation generated in the TA28 element was identical to that created by Meier and Straus (39). As shown in Fig. 4B, mutation of TA28 abolished ORF 28 RL expression but had little effect on ORF 29 FL expression. Individual mutation of either the TA29.1 or TA29.2 elements failed to substantially reduce ORF 29 FL expression. However, the double mutation of both TA29.1 and TA29.2 present in the pRFL/TA29.3m vector dramatically reduced the ORF 29 FL activity in response to IE62 transactivation compared to wild-type levels. This result suggests that these two TATA elements are alternatively or simultaneously utilized in the ORF 28/29 regulatory element to drive transcription in the ORF 29 direction.
FIG. 4.
Identification of the TATA elements that direct transcription of the ORF29 gene. (A) Schematic representation of the structure and sequence of the known and predicted TATA motifs in the wild-type and mutant pRFL vectors evaluated in this study. (B) Each pRFL vector (1.5 μg) was transfected into MeWo cells in the absence (solid bar) or presence of 0.05 μg of pCMV62 plasmid (open bar). The promoter activities are presented as fold induction of luciferase activities normalized to the luciferase activities expressed from the pRFL/WT vector in the absence of pCMV62. All values represent the averages from triplicate experiments.
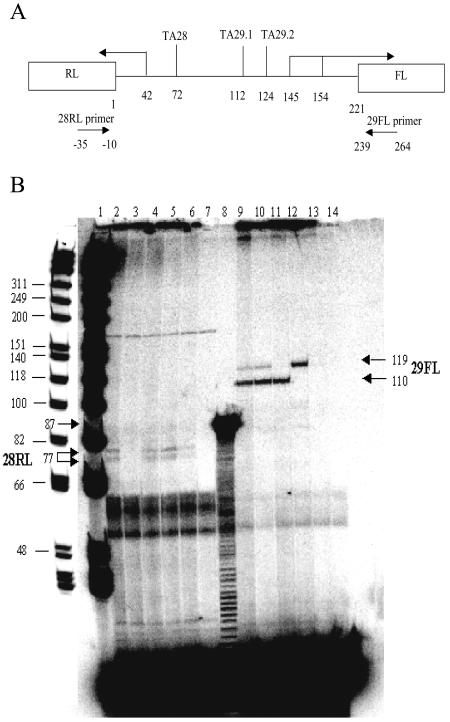
To test this hypothesis, primer extension experiments were performed to assess the role of the two TATA elements in transcription initiation from the ORF 29 promoter. The pRFL/WT, pRFL/TA28m, pRFL/TA29.1m, pRFL/TA29.2m, and pRFL/TA29.3m plasmids were cotransfected with pCMV62 plasmid into MeWo cells. The RNA products were isolated from transfected cells, and the relative levels of transcription of the ORF 28 RL and ORF 29 FL reporters was quantified by primer extension. As diagramed in Fig. 5A, the predicted size of the ORF 28 RL extension product is 77 bp, and those of the ORF 29 FL extension products are 110 and 119 bp based on the positions of the transcription start sites previously mapped for ORF 28 and ORF 29. Ten micrograms of total RNA was used for both ORF 28 RL and ORF 29 FL extension. The extended products were resolved on denaturing polyacrylamide gels and visualized by autoradiography.
FIG. 5.
Determination of individual TA29 TATA box usage by primer extension analysis. (A) The position of individual TATA elements within the ORF 28/29 regulatory element. The transcription start sites of ORF 28 and ORF 29 genes are indicated by arrows. The relative position and orientation of each primer in reference to that of the promoter is shown by the horizontal arrows below the intergenic region schematic. (B) The primer extension products were resolved on polyacrylamide gel followed by autoradiography. The arrows indicate the positions of the extension products. The extension products derived from the ORF 28 RL primer were loaded in lanes 2 to 7 in the following order: wild type (WT), TA28m, TA29.1m, TA29.2m, TA29.3m, and untransfected MeWo control. The extension products derived from the ORF 29 FL primer were loaded in lanes 9 to 14 in the same order. An extension product of 87 bases from a control reaction mixture provided in the primer extension kit (Promega) was loaded in lane 8. A radiolabeled DNA marker ladder was loaded in lane 1, and the positions of the individual marker fragments are indicated based on a lighter exposure of the gel, which is shown aligned to the left of lane 1 in this panel.
The results are shown in Fig. 5B. The yield of extension products was low, most likely due to the rareness of the target RNAs in the total RNA sample. The gel was overexposed relative to the size standards (lane 1) and the positive control (lane 8) to visualize the reaction products. The positions of the ORF 28 RL and ORF 29 FL extension products were readily distinguished from the nonspecific signals resulting from the use of RNA isolated from uninfected MeWo cells (lanes 7 and 14). Two closely spaced bands of equal intensity were observed with extension of the ORF 28 RL primer using RNA derived from cells transfected with pRFL/WT (lane 2), whereas two well-separated bands of unequal intensity were observed with extension of the ORF 29 FL primer (lane 9). Based on their positions in the gel, the slower migrating, less intense band corresponds to the size expected for extension to the ORF 29 transcriptional start site at position 145 in the ORF 28/29 regulatory element, whereas the faster migrating, more intense band resulted from extension to the start site at 154, indicating that the latter start is favored by IE62 activation.
The results obtained with the reporter plasmids carrying the TATA element mutations gave insights into their usage and yielded surprising and novel information concerning expression in the ORF 29 direction. Mutation of TAxxx28 resulted in a loss of the ORF 28 RL doublet while showing no effect on either the presence or relative intensity of the ORF 29 FL products (lanes 3 and 10). These results confirm that TAxxx28 is necessary and sufficient for expression in the ORF 28 direction in this experimental system and, as previously found (39), that there appears to be a cluster of closely spaced start sites for ORF 28 transcription. In contrast, mutation of TAxxx29.1 resulted in no change in the ORF 28 RL doublet but showed a loss of the slower migrating, less intense ORF 29 FL product (lanes 4 and 11), indicating that TAxxx29.1 is responsible for expression from the ORF 29 start site at position 145. Mutation of TAxxx29.2 again showed no obvious change in the ORF 28 RL products but resulted in a loss of the faster migrating ORF 29 FL band. Moreover, and most surprisingly, the intensity of the remaining ORF 29 FL extension product showed a significant increase. Finally, mutation of both TAxxx29.1 and -29.2 eliminated the ORF 29 FL extension product and apparently decreased the level of the ORF 28 RL product (lanes 6 and 13). These results were confirmed by quantification of data from three separate experiments. As shown in Table 2, the faster migrating ORF 29 FL extension product is approximately eight times as abundant as the slower migrating product. Mutation of either ORF 29 TATA element very significantly decreases the amount of one but not both of the products and, more importantly, shifts the total amount of ORF 29 FL expression, which remains stable, to the other TATA element and start site. Thus, these data indicate that, as hypothesized, the two ORF 29 TATA boxes are used differentially and simultaneously; moreover, elimination of one results in alternative usage of the other in order to keep expression in the ORF 29 direction at a constant level. These data parallel the luciferase data presented in Fig. 4. In addition, consistent with the results observed in the reporter assays (Fig. 4B), mutation of the TAxxx29.2 element resulted in a measurable increase in the amount of ORF 28 RL extension product, whereas mutation of both of the 29 TATA elements resulted in a decrease (40 to 50%) in expression of ORF 28 RL message. These last data imply that although the two promoters within the ORF 28/29 regulatory element are separable, the level and nature of transcriptional activity being directed by one can influence that of the other.
TABLE 2.
Quantification of primer extension productsa
| FL or RL | Amt. of product
|
||||
|---|---|---|---|---|---|
| WT | TA28m | TA29.1m | TA29.2m | TA29.3m | |
| 29FL upper | 12.78 ± 2.67 | 12.68 ± 3.89 | 2.92 ± 2.03 | 107.79 ± 21.6 | 2.12 ± 1.33 |
| 29FL lower | 100 | 108.28 ± 11.88 | 120.09 ± 6.77 | 1.88 ± 1.58 | 1.33 ± 0.77 |
| 28RL | 100 | 1.10 ± 0.78 | 98.22 ± 25.47 | 149.27 ± 36.93 | 60.14 ± 13.91 |
The primer extension products from MeWo cells transfected with pRFL/WT, pRFL/TA28m, pRFL/TA29.1m,pRFL/TA29.2m, and pRFL/TA29.3m were quantified by phosphorimage analysis. The upper and lower bands of 29 FL extension products were analyzed in the same group for comparison. In the case of the 29 FL extension products, the level of expression observed with the lower (110-base) product in the wild-type (WT) promoter was normalized to 100%. In the case of the 28 RL reporter, the total level of expression present in both bands was normalized to 100%. Shown here are the averages and standard deviations derived from three independent experiments.
Sp1 is involved in the IE62-mediated activation of the ORF 28/29 regulatory element.
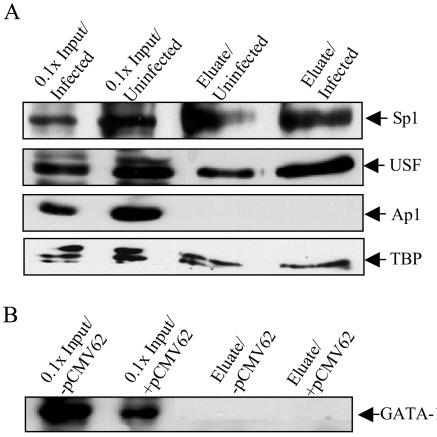
The analysis of the ORF 28/29 regulatory element shown in Fig. 1B predicted the presence of several potential binding sites for cellular transcription factors in addition to USF and TBP/TFIID. These factors included AP-1, Sp1, and GATA-1, the last of which is T-cell specific (40). The binding of transcription factors to the ORF 28/29 regulatory element was examined by magnetic bead recruitment assays. In these assays, biotinylated oligonucleotides encompassing the sequence of the full-length wild-type intergenic region were conjugated to streptavidin-coupled magnetic beads and then were incubated with nuclear extracts derived from uninfected and VZV-infected MeWo cells or untransfected and pCMV62-transfected T cells (in the case of GATA-1). Stably bound proteins were eluted from the beads with high salt, resolved by SDS-PAGE, and analyzed by immunoblotting. As expected, USF and TBP both bound to the ORF 28/29 intergenic region. Readily detectable and reproducible binding of Sp1 binding was also observed, whereas binding of AP-1 and GATA-1 was not (Fig. 6).
FIG. 6.
Binding of cellular transcription factors to the ORF 28/29 intergenic sequence in magnetic bead recruitment assays. (A) A 5′-biotinylated double-stranded DNA fragment containing the entire 221-bp intergenic region was coupled to streptavidin-conjugated magnetic beads and incubated with nuclear extracts derived from uninfected MeWo cells and VZV-infected MeWo cells. Bound proteins were eluted with 1 M KCl, and the presence or absence of Sp1, USF, Ap1, and TBP was determined by immunoblotting with antibodies specific for each factor. (B) Results of magnetic bead recruitment assays performed with nuclear extracts of untransfected T cells and pCMV62-transfected T cells. Binding of the lymphoid tissue specific factor GATA-1 to the promoter was detected by immunoblot.
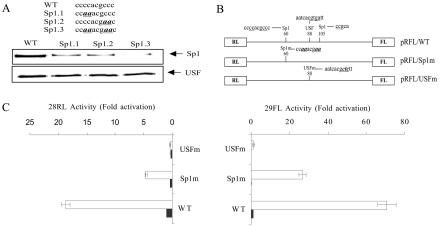
To precisely localize the Sp1 binding site or sites within the promoter and to determine the functional significance of the observed binding, three mutations in the extensive putative Sp1 binding site centered at n.p. 60 were created (Fig. 7). Sp1.1 and Sp1.2 each contained 2-bp C-to-A substitutions, and Sp1.3 contained both 2-bp substitutions. Sp1 binding to the wild-type and mutant promoter sequences was then compared in recruitment assays. Both of the 2-bp substitutions of the Sp1 binding site, Sp1.1 and Sp1.2, substantially decreased Sp1 binding to the promoter, and the double mutation Sp1.3 essentially eliminated Sp1 binding within the limits of detection. None of these mutations, however, had any effect on USF binding in control experiments (Fig. 7A). Mutation of a second putative Sp1 binding site centered at nucleotide 105 had no effect on Sp1 binding, indicating that the GC-rich element at nucleotide 60 is the genuine Sp1 binding site within the ORF 28/29 regulatory element (data not shown).
FIG. 7.
Functional analysis of a putative Sp1 binding site within the ORF 28/29 intergenic element. (A) Results of magnetic bead recruitment assays. Two hundred-fifty micrograms of uninfected MeWo cell nuclear extract was incubated with 10 pmol of immobilized 5′-biotinylated full-length wild-type (WT) or mutant (Sp1.1, Sp1.2, Sp1.3) intergenic regions. The sequences of the Sp1 binding site within the promoters are listed with the mutagenized nucleotides underlined and in bold. Following washing and elution, the binding of Sp1 and USF to the various DNA fragments were assessed by SDS-PAGE and immunoblotting of the eluates. (B) Schematic representation of the structure and target sequences of the WT and mutant dual-luciferase vectors containing mutagenized Sp1 (pRFL/Sp1m) and USF (pRFL/USFm) binding site used in transient transfection assays. (C) Results of transfection assays. Each pRFL vector (1.5 μg) was transfected into MeWo cells in the absence (solid bar) or presence of 0.05 μg of pCMV62 plasmid (open bar). The results are presented as described in the legend to Fig. 3C.
To assess the functional significance of Sp1 binding on the ORF 28 and 29 promoter activities, the Sp1.3 mutation was inserted into the dual-luciferase vector to generate the pRFL/Sp1m reporter plasmid (Fig. 7B). As a control, the essential USF site was mutated exactly as described by Meier et al. (38) to generate the pRFL/USFm reporter. The levels of expression from the wild-type and mutant reporter constructs in the presence and absence of pCMV62 are shown in Fig. 7C. As anticipated, the USF binding site mutation eliminated promoter responsiveness to IE62 activation in both directions. Surprisingly, mutation of the Sp1 binding site significantly reduced the expression of both ORF 28 RL and ORF 29 FL despite the fact that this site is located within the minimal ORF 28 promoter element. Thus, its presence in the wild-type sequence results in upmodulation of IE62/USF-mediated expression in both directions. In contrast, when the predicted Sp1 binding site at nucleotide 105 was mutated and the consequences of the mutation were tested in transfection assays, no effect on expression of either reporter was observed, further confirming that this site is nonfunctional in the context of the ORF 28/29 regulatory element (data not shown). These results, in conjunction with those obtained with the TATA-box mutations, indicate that cis elements within each of the unidirectional promoter elements can influence the transcriptional regulation of the other to facilitate coordination of expression from the divergent promoters in cooperation with the shared, essential USF site.
Assessment of expression from promoter constructs in superinfected cells.
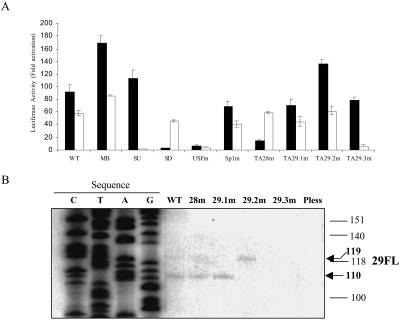
The analysis of the ORF 28/29 regulatory element constructs described above had been performed in the context of activation by IE62 in the absence of other viral proteins. While previous studies as well as the data presented in Fig. 2 to 4 and 7 indicated that IE62 is required for significant activation above basal levels, several other viral proteins, including the products of VZV ORFs 4, 10, 61, and 63, have been shown to activate viral promoters in the absence of IE62 in transient transfections (reviewed in reference 25). Moreover, cellular factors altered by VZV infection could also influence expression from this regulatory element. To gain insight as to whether or not IE62 activation via the cis elements thus far identified is the primary underlying mechanism in activation of the ORF 28/29 regulatory element, expression from all of the bidirectional luciferase reporter constructs was evaluated in the context of VZV superinfection.
In these experiments, the specific reporter construct was transfected into melanoma cells and, 24 h later, the monolayer was superinfected by overlaying with infected cells. The cells were then harvested 24 h later, and expression from the reporter vectors was analyzed. As shown in Fig. 8A, the wild-type full-length intergenic element and the MB element were both capable of supporting expression in both directions. The relative ratio of ORF 28 RL and ORF 29 FL was reversed in the infected cells compared to that in cells transfected with pCMV62 alone, indicating that other factors present in VZV-infected cells are involved in modulation of the levels of expression driven by this element. However, relative to the levels of expression from the full-length element, all of the other reporter constructs behaved in a manner very similar, if not identical, to that observed in cells where only IE62 was expressed. Thus, expression from the MB fragment was higher than that observed for the wild type, and the SU fragment exhibited expression of only ORF 28 RL, whereas the SD fragment exhibited expression of only ORF 29 FL. The USF site mutation resulted in a nearly complete loss of expression in both directions, while the reporter containing the Sp1 mutation showed a more modest decrease in expression of both reporters. Mutation of TA28 resulted in a significant decrease in ORF 28 RL expression but no change in ORF 29 FL repression, and only mutation of both ORF 29 TATA boxes resulted in a loss of ORF 29 FL expression. Thus, these data strongly suggest that all of the cis-acting elements identified are important for expression and that IE62 is the viral protein whose presence and action is fundamental to the mechanism of expression above basal levels.
FIG. 8.
Expression driven by the intergenic region in the context of VZV superinfection. (A) Expression of ORF 28 RL and ORF 29 FL in melanoma cells which were superinfected with VZV following transfection of the indicated bidirectional reporter plasmids. The open bar indicates ORF 29 FL expression, and the solid bar indicates ORF 28 RL expression. All data were normalized against the expression levels obtained in uninfected cells. (B) Results of primer extension assays of the ORF 29 FL reporter using wild-type (WT) and mutant intergenic regions in the context of VZV superinfection. The primers used were the same as those described for the experiment shown in Fig. 5. The arrows indicate the positions of the longer and shorter primer extension products; the first four lanes contain products of a sequencing reaction performed with the wild-type reporter vector. The positions of size markers are indicated on the right.
We also wished to determine if the differential level of expression from the two ORF 29 start sites was retained in the presence of VZV superinfection. RNA was harvested from melanoma cells which had been transfected with the bidirectional reporter plasmid and then superinfected, and primer extension was performed as described above. The results are presented in Fig. 8B and show that the relative ratios of the two primer extension products are very similar to those observed in cells transfected with the pCMV62 plasmid. Moreover, the level of the signal derived from the 29.1 TATA element increased dramatically when TAxxx29.2 was mutated. Thus, the differential levels of expression and alternate use of the two ORF 29 TATA elements also appear to be dependent primarily on the activity of the IE62 protein even when other viral transcriptional regulatory proteins are present.
Expression from promoter constructs in a neuroblastoma cell line.
We next wished to examine expression from the wild-type and mutant bidirectional reporter plasmids in the context of a neuronal cell line to determine if the pattern of expression shifted to one resembling that observed in latently infected ganglia. The cell line chosen was the SH-SY5Y neuroblastoma cell line. This cell line displays differences between replication of neurovirulent and attenuated poliovirus strains and has been used as a model system for studying biosynthesis of neuropeptides (3, 29). IE62 is required for significant expression of our reporters in this cell line above basal levels, which were extremely low (data not shown). The expression patterns from the various mutant and truncated promoter constructs in the presence of IE62 (Fig. 9) were similar to those in melanoma cells, including the higher levels of induction of ORF 29 FL compared to those of ORF 28 RL, indicating that under these experimental conditions this effect is cell type independent. These results indicate that, within the limits of using this cell line as a model neuronal culture, there appear to be no significant effects due to the presence of neuronal factors or differences in the levels of ubiquitous cell factors such as Sp1 and USF.
FIG. 9.
Expression driven by the intergenic region in a neuroblastoma cell line. (A) Expression from the wild-type (WT), MB, SU, and SD elements in SH-SY5Y cells. The results are normalized to the levels observed with promoterless constructs. Solid bars indicate ORF 28 RL, and open bars indicate ORF 29 FL. (B) Expression from promoter elements carrying site-specific mutations in the USF and Sp1 consensus binding sequences. The results are normalized to the levels observed with the wild-type element in the absence of the IE62 expression plasmid. (C) Expression from promoter elements carrying site-specific mutations in the three functional TATA elements. The results are normalized to the levels observed with the wild-type element in the absence of the IE62 expression plasmid. The amount of effector plasmid used in all experiments was 0.05 μg.
DISCUSSION
During latent VZV infection in ganglia, a small subset of lytic-phase genes are expressed at the level of both transcript and protein. The most frequently and consistently detected VZV gene products in latently infected ganglia from humans and in animal models include the products of VZV ORFs 63, 29, and 62 (22, 41). The function of these gene products either in the establishment, maintenance, or reactivation of VZV latency remains unknown. Similarly, the mechanism or mechanisms by which only this small set of genes is expressed while the remainder of the genome remains transcriptionally silent is unknown. To gain insight into this latter question, we have analyzed the functional anatomy of the regulatory element responsible for expression of the ORF 28 and ORF 29 genes. Our rationale for choosing this sequence for analysis lay in the fact that while both genes are expressed during lytic infection, only one (ORF 29) has been reported to be expressed during latency. There appear to be two major possibilities regarding the ORF 28/29 regulatory element which could explain these observations. The first possibility holds that this region is composed of two separable unidirectional promoters which can be differentially utilized during VZV infection (both lytic and latent) despite sharing some common elements. Alternatively, the promoter is truly bidirectional in permissive cells and, despite having both unique and shared cis elements, expression of both genes is concordantly regulated by IE62 and cannot be separated; however, it contains sequences which are specifically recognized by viral or cellular factors during latency resulting in expression of only ORF 29.
The results of our experiments using the divergent dual-luciferase bidirectional reporter constructs clearly indicate that this intergenic region is made up of two overlapping unidirectional promoters (Fig. 10), one controlling expression of the ORF 28 gene and the other controlling expression of the ORF 29 gene, both of which require the VZV IE62 protein for significant activation. Furthermore, fusion of theses two unidirectional elements restores bidirectional expression. Both of these promoters contain the USF site previously shown to be required by Meier et al. (38) for IE62-mediated activation. This USF site alone, however, is not sufficient for IE62 activation as shown in the results obtained with the minimal CF (Fig. 3) and also requires the presence of TATA elements specific to each of the unidirectional promoters. This promoter arrangement suggests the possibility that the two genes are alternatively transcribed during lytic infection, because studies have shown that USF appears to be capable of interaction with only a single general transcription complex at one time (1).
FIG. 10.
Anatomy of the ORF 28/29 regulatory element in the context of IE62 activation. The two overlapping minimal unidirectional promoters are shown as striped arrows. The difference in thickness reflects the consistently higher levels of IE62-mediated activation above basal levels observed in the ORF 29 direction with the full-length and MB regulatory elements. The positions of the three TATA elements are shown, and the relative strengths of the ORF 29 TATA elements and their relationships to the two transcriptional start sites is indicated by the relative thicknesses of the vertical bars. The fact that the USF site and the Sp1 site are required for or modulate the levels of IE62-mediated activation of expression in both directions, respectively, is indicated by the italicized lettering.
Three TATA elements were shown to be required for bidirectional expression, one for expression in the ORF 28 direction, and, surprisingly, two for expression in the ORF 29 direction. The TATA element required for expression of the ORF 28 RL reporter in our analysis is the same TATA element identified by Meier and Straus (38, 39) as being required for expression of a luciferase reporter placed in the position of the ORF 28 gene. The results generated in the mutational analysis of potential ORF 29 TATA elements showed that two TATA elements (TAxxx29.1 and -29.2) could be utilized both simultaneously and alternatively for equivalent expression of the ORF 29 FL reporter and that only combined mutation of both resulted in a significant decrease of activation in the ORF 29 direction. Why this redundancy is built into expression of the ORF 29 gene is presently not known. However, the ability of the promoter to support expression in the ORF 29 direction when one of the two TATA elements is rendered transcriptionally inactive, either by engineered mutation as was the case here or possibly by cellular processes which occlude it, may figure into a mechanism by which the ORF 29 gene is expressed during latency while the ORF 28 gene is not.
Several other novel aspects regarding the ORF 29 TATA elements are also important to note. The two transcripts derived from the ORF 29 FL reporter are some eightfold different in abundance, and each has its own distinct TATA element and transcriptional start site. Surprisingly, the TATA element involved in expression of the more abundant transcript is noncanonical, whereas the TATA element involved in expression of the less abundant transcript is similar to the TATA consensus sequence TATA(A/T)AA(G/A) (58). Further, the results were obtained under conditions where there was a choice between two closely spaced TATA boxes, suggesting that noncanonical or less canonical sequences may be favored and, thus, ensuring effective expression of viral genes whose promoters contain such elements (15, 21). Second, clearly in the case of mutation of TAxxx29.2 the level of transcription driven by the remaining intact TATA element did not remain the same but rather increased to compensate for the loss of function in the other. A slight but similar shift also occurs upon mutation of TAxxx29.1, although this is somewhat obscured by the fact that the shorter of the two transcripts, driven by TAxxx29.2, is already the dominant transcript in the wild-type situation. Thus, IE62 and the cellular transcription apparatus can and do shift within the promoter to maintain a constant level of expression in the ORF 29 direction under these experimental conditions. Because the ORF 29 protein has been implicated in regulation of viral gene expression as well as DNA replication (6), such a mechanism would ensure that high levels of ORF 29 expression can be maintained during lytic infection.
Analysis of potential regulatory sequences in addition to the TATA elements and the USF binding site revealed that while the Sp1 site upstream of the USF site is dispensable, it is nonetheless important for full activation of the promoter in the presence of IE62. This effect appears to be roughly equivalent for activation in both directions, suggesting the Sp1 bound to this site can further activate or facilitate expression which is initiated by a mechanism centered at the USF site. Recent work has shown that there is a direct physical interaction between both of these ubiquitous cellular transcription factors and IE62 (44, 48). Sp1 can interact directly with TBP and TAF 110 and has been shown to be capable of recruiting TFIID to specific promoters (18, 43). The presence of USF has been shown to stabilize binding of TBP/TFIID to promoter elements and to facilitate the formation of preinitiation complexes (47, 52, 65). Thus, the binding of USF and Sp1 in close proximity could result in both increased recruitment of IE62 to the promoter and a higher local concentration of TFIID than would occur in the absence of Sp1, allowing a more efficient initiation of transcription via the USF site. Sp1 could perhaps act as a holding site for IE62, which could then be transferred to USF bound to its site. Alternatively, Sp1 could aid in activating transcription via its glutamine-rich activation domains (43), and this effect would be added to that observed with USF alone, as has been postulated for the human transcobamalin II promoter, which also contains an essential USF consensus site and an Sp1 site whose presence is necessary for full activation of the promoter (30).
The fact that the presence of the Sp1 site located in the minimal ORF 28 promoter affects expression in both directions adds another layer of complexity involving the influences of the individual cis-acting elements on expression of both genes in the context of the native genetic configuration. This is also seen in the effects of the mutations of the ORF 29 TATA elements. While mutation of TAxxx28 did not result in significant changes in the levels of expression in the ORF 29 direction, mutation of TAxxx29.2 resulted in a measurable increase in ORF 28 RL expression in both the level of reporter activity and reporter transcript, and simultaneous mutation of both TAxxx29.1 and -29.2 resulted in a decrease in expression of the ORF 28 RL at both levels (Fig. 4 and Table 2). Thus, the capacity for expression either in a specific direction or via a specific cis-acting element in one of the minimal promoters affects expression of the divergently oriented gene. The most obvious possibility involving such coordinated regulation would be during viral DNA replication, when both the DNA polymerase and major DNA-binding protein would be required.
The results obtained with the transfection and VZV superinfection experiments indicate that other factors in VZV-infected cells modulate IE62-mediated activation of the ORF 28/29 regulatory element as evidenced by the inversion of the relative ratios of the ORF 28 RL and ORF 29 FL activities. However, most importantly, relative to expression from the full-length wild-type regulatory element, all of the constructs show expression patterns which are essentially identical to those observed with IE62 alone. Further, the primer extensions performed using RNA from the superinfection experiments showed the same pattern of differential use of the ORF 29 transcription start sites and TATA elements. Thus, the mechanism of activation of the two promoters in the context of all the cis-acting elements identified in this study is IE62 based, and other viral or cellular factors do not substitute for the fundamental functional role played by IE62 in permissive cells.
During latency the processes involved in expression of the two genes would, by necessity, be decoupled in order to account for the observation that only the ORF 29 gene is transcribed. Several potential mechanisms for this decoupling are possible. In the first, one or more of the VZV proteins expressed during latency, in the absence of the full spectrum of gene products expressed during productive infection, alter the expression from this element and result only in transcription of the ORF 29 gene. Thus, perhaps IE63 or ORF 29 (the two most frequently detected genes in latently infected cells) in combination with IE62 might direct such expression. Several arguments can be made against this possibility.
Thus far, when the VZV latency proteins have been detected in neurons, they have been detected in the cytoplasm rather than the nuclei of these cells (12, 33). Furthermore, although a mechanism in which small amounts of these proteins, which are undetectable with presently available reagents, are shuttled in and out of the nucleus cannot be formally ruled out. Cohrs et al. (13) showed in Vero cells that the ORF 29 protein had a slightly positive effect on IE62-mediated expression of unidirectional FL reporters positioned at either end of the full-length ORF 28/29 regulatory element and that the ORF 21 protein, which is also expressed during latency, had no effect on IE62 activation but did suppress the observed ORF 29 boost. The results obtained with the neuroblastoma cells indicate that, within the limits of this cell line as a model for neuronal cells, the regulatory element was IE62 responsive in both directions and all of the identified cis-acting elements play roles similar to those observed in melanoma cells. Additional work performed in the course of this study showed: (i) results similar to those of Cohrs et al. (13) with both MeWo cells and SH-SY5Y cells for the ORF 29 protein, and (ii) the IE63 protein downregulated both basal level and IE62-mediated expression of an ORF 28 reporter construct (in agreement with Bontems et al. [5]) and an ORF 29 reporter construct in MeWo cells (data not shown). Thus, there is no evidence available at this time to suggest that any of the known VZV latency associated genes are responsible for the unidirectional expression observed from this control element during latent infection.
In a second possible mechanism, a neuronal cell factor either present in primary neurons or induced by VZV infection binds to elements of the ORF 28 minimal promoter, suppressing expression in the 28 direction but not the 29 direction. This would seem highly unlikely, because it suggests that either this same repressor would be involved in suppression of all other nonexpressed lytic genes or each of those genes has its own unique repressor. Furthermore, it would require an activator for ORF 29 gene expression that may or may not require USF for expression because IE62 would not be present.
A third and more plausible possibility would involve a neuronal factor that recognizes and utilizes sequences within the ORF 29 unidirectional promoter to drive expression of ORF 29 but not ORF 28. In this case the mechanism by which the majority of the genome is transcriptionally quiescent would be a general one. The most obvious mechanism for the general silencing of the VZV genome would be via the DNA adopting a chromatin-like structure during latency. The VZV genome has, like the HSV genome, been shown to be episomal during latency and is, therefore, likely to be bound by histones (10, 16, 22, 41). Thus, during latency the structure of the viral DNA could be such that the ORF 29 promoter and the ORF 29 gene could be euchromatic and nonrepressed. Precedence for this possibility comes from a recent report by Kubat et al. (28), which showed that histone hypermethylation is a determinant of HSV-1 latent gene expression. Perhaps even more relevant are the findings of Schuttengruber et al. (53), who showed that alternate activation of the mouse thymidine kinase and kynurenin formamidase genes, which are divergently transcribed from a bidirectional regulatory element, is also linked to histone methylation levels. This would imply that the regions of the VZV genome encoding ORFs 62, 63, 21, 4, and 66 as well as 29 are all at least sometimes in a euchromatic structure, whereas the remainder of the genome is not.
Other major questions raised by this model would include the following. (i) Are there sequences unique to the ORF 29 promoter (and, by extension, to the promoters of the other latency genes) that are recognized by novel neuronal factors induced by latent VZV infection of neurons (a possibility not excluded by the results with the SH-SY5Y transfection experiments)? (ii) Do ubiquitous cellular transcription factors assemble at the transcriptionally active regions and use the existing cis-elements to transcribe these genes at low levels in the absence of nuclear IE62? In silico inspection of the known or putative promoter elements of the other VZV latency genes performed during the course of this work failed to identify any common elements, including the atypical TATA29.2 element. (iii) Is there a cryptic regulatory element within the ORF 29 coding sequence which directs expression of ORF 29 in latently infected cells? The answers to these questions await further experimentation in VZV latency models which are either extant or under development (7, 8, 24, 31, 66).
Acknowledgments
This work was supported by grant AI18449 from the National Institute of Allergy and Infectious Diseases (W.T.R. and J.H.).
REFERENCES
- 1.Adami, G., and L. E. Babiss. 1992. Evidence that USF can interact with only a single general transcription complex at one time. Mol. Cell. Biol. 12:1630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annunziato, P., P. La Russa, P. Lee, S. Steinberg, O. Lungu, A. A. Gershon, and S. Silverstein. 1998. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J. Infect. Dis. 178(Suppl. 1):S48-S51. [DOI] [PubMed] [Google Scholar]
- 3.Arun, P., C. N. Madhavarao, J. R. Hershfield, J. R. Moffett, and M. A. Namboodiri. 2004. SH-SY5Y neuroblastoma cells: a model system for studying the biosynthesis of NAAG. Neuroreport 15:1167-1170. [DOI] [PubMed] [Google Scholar]
- 4.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 5.Bontems, S., E. Di Valentin, L. Baudox, B. Rentier, C. Sadzot-Delvaux, and J. Piette. 2002. Phosphorylation of varicella-zoster virus IE63 protein by casein kinases influences its cellular localization and gene regulation activity. J. Biol. Chem. 277:21050-21060. [DOI] [PubMed] [Google Scholar]
- 6.Boucaud, D., H. Yoshitake, J. Hay, and W. T. Ruyechan. 1998. The VZV ORF 29 protein acts as a modulator of a late VZV gene promoter. J. Infect. Dis. 178(Suppl. 1):34-38. [DOI] [PubMed] [Google Scholar]
- 7.Brunell, P. A., L. C. Ren, J. I. Cohen, and S. E. Straus. 1999. Viral gene expression in rat trigeminal ganglia following neonatal rat infection with varicella zoster virus. J. Med. Virol. 58:286-290. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. J., A. A. Gershon, Z. S. Li, O. Lungu, and M. D. Gershon. 2003. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J. Med. Virol. 70:S71-S78. [DOI] [PubMed] [Google Scholar]
- 9.Christofferson, C. A., T. J. Brickman, I. Hook-Barnard, and M. A. McIntosh. 2001. Regulatory architecture of the iron regulated fepD-ybdA bidirectional promoter region in Escherichia coli. J. Bacteriol. 183:2059-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, P., T. Beer, R. Cohrs, and D. H. Gilden. 1995. Configuration of latent Varicella-Zoster virus DNA. J. Virol. 69:8151-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohrs, R. J., M. B. Barbour, and D. H. Gilden. 1996. Varicella-Zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohrs, R. J., D. H. Gilden, P. R. Kinchington, E. Grinfeld, and P. G. Kennedy. 2003. Varicella-Zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohrs, R. J., J. Wischer, C. Essman, and D. H. Gilden. 2002. Characterization of Varicella-Zoster virus gene 21 and 29 proteins in infected cells. J. Virol. 76:7228-7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croen, K. D., J. M. Ostrove, L. J. Dragovic, and S. E. Straus. 1988. Patterns of gene expression and sites of latency are different for varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. USA 85:9773-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison, A. J., and J. Scott. 1986. The complete sequence of varicella-zoster virus. J. Gen. Virol. 67:1715-1816. [DOI] [PubMed] [Google Scholar]
- 16.Efstathiou, S., A. C. Minson, H. J. Field, J. R. Anderson, and P. Wildy. 1986. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J. Virol. 57:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavalas, G., and H. Zalkin. 1995. Analysis of the chicken GPAT/AIRC bi-directional promoter for de novo purine nucleotide synthesis. J. Biol. Chem. 270:2403-2410. [DOI] [PubMed] [Google Scholar]
- 18.Gill, G., E. Pascal, Z. H. Tseng, and R. Tjian. 1994. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc. Natl. Acad. Sci. USA 91:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen, J. J., P. Bross, M. Westergaard, M. N. Nielsen, H. Eiberg, A. D. Borglum, J. Mogensen, K. Kristiansen, L. Bolund, and N. Gregersen. 2003. Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localized head to head on chromosome 2 separated by a bidirectional promoter. Hum. Genet. 112:71-77. [DOI] [PubMed] [Google Scholar]
- 20.Hay, J., and W. T. Ruyechan. 1994. Varicella zoster virus—a different kind of herpesvirus latency? Semin. Virol. 5:241-247. [Google Scholar]
- 21.Kantakamalakul, W., W. T. Ruyechan, and J. Hay. 1995. Analysis of varicella-zoster virus promoter sequences. Neurology 45:S28-S29. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy, P. G. E. 2002. Varicella zoster virus in human ganglia. Rev. Med. Virol. 12:327-334. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, P. G. E., E. Grinfeld, and J. E. Bell. 2000. Varicella-Zoster virus gene expression in latently infected human trigeminal ganglia. J. Virol. 74:11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy, P. G. E., E. Grinfeld, S. Bontems, and C. Sadzot-Delvaux. 2001. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology 289:218-223. [DOI] [PubMed] [Google Scholar]
- 25.Kinchington, P. R., and J. I. Cohen. 2000. Viral proteins, p. 74-104. In A. M. Arvin and A. A. Gershon (ed.), Varicella zoster virus virology and clinical management. Cambridge University Press, Cambridge, Mass.
- 26.Kinchington, P. R., G. Inchuaspe, J. H. Subak-Sharpe, F. Robey, J. Hay, and W. T. Ruyechan. 1988. Identification and characterization of a Varicella-Zoster virus DNA-binding protein using antisera directed against a predicted synthetic oligopeptide. J. Virol. 62:802-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus, R. J., L. Shadley, and J. E. Mertz. 2001. Nuclear factor 1 family members mediate expression of the BK virus late promoter. Virology 287:89-104. [DOI] [PubMed] [Google Scholar]
- 28.Kubat, N. J., R. Tran, P. McAnany, and D. C. Bloom. 2004. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J. Virol. 78:1139-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Monica, N., and V. R. Racaniello. 1989. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J. Virol. 63:2357-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, N., and B. Seetharam. 1998. A 69-base pair fragment derived from human transcobalamin II promoter is sufficient for high bidirectional activity in the absence of a TATA box and an intiator element in transfected cells. J. Biol. Chem. 273:28170-28177. [DOI] [PubMed] [Google Scholar]
- 31.Lowry, P. W., C. Sabella, C. M. Koropchak, B. N. Watson, H. M. Thackray, G. M. Abruzzi, and A. M. Arvin. 1993. Investigation of the pathogenesis of varicella-zoster virus infection in guinea pigs using polymerase chain reaction. J. Infect. Dis. 167:78-83. [DOI] [PubMed] [Google Scholar]
- 32.Lungu, O., P. Annunziato, A. Gershon, S. M. Staugaitis, D. Josefson, P. LaRussa, and S. J. Silverstein. 1995. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc. Natl. Acad. Sci. USA 92:10980-10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lungu, O., C. A. Pagiotidis, P. W. Annunziato, A. A. Gershon, and S. J. Silverstein. 1998. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. USA 95:7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch, J. M., T. K. Kenyon, C. Grose, J. Hay, and W. T. Ruyechan. 2002. Physical and functional interaction between the varicella zoster virus IE63 and IE62 proteins. Virology 302:71-82. [DOI] [PubMed] [Google Scholar]
- 35.Mahalingam, R. M. Wellish, R. Cohrs, S. Debrus, J. Piette, B. Rentier, and D. H. Gilden. 1996. Expression of protein coded by varicella zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. USA 93:2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin, J. F. 2000. Molecular control of expression of penicillin biosynthesis genes in fungi: regulatory proteins interact with a bidirectional promoter region. J. Bacteriol. 182:2355-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier, J. L., J. P. Holman, K. D. Croen, J. E. Smialek, and S. E. Straus. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193-200. [DOI] [PubMed] [Google Scholar]
- 38.Meier, J. L., X. Luo, M. Sawadogo, and S. E. Straus. 1994. The cellular transcription factor USF cooperates with varicella-zoster virus immediate-early protein 62 to symmetrically activate a bidirectional viral promoter. Mol. Cell. Biol. 14:6896-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier, J. L., and S. E. Straus. 1993. Varicella-Zoster virus DNA polymerase and major DNA-bing protein genes have overlapping divergent promoters. J. Virol. 67:7573-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merika, M., and S. H. Orkin. 1993. DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol. 13:3999-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell, B. M., D. C. Bloom, R. J. Cohrs, D. H. Gilden, and P. G. E. Kennedy. 2003. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J. Neurol. Virol. 9:194-204. [DOI] [PubMed] [Google Scholar]
- 42.Otte, D. M., U. Schwab, and G. H. Luers. 2003. The Pxmp2 and PoleI genes are linked by a bidirectional promoter in an evolutionary conserved fashion. Gene 313:119-126. [DOI] [PubMed] [Google Scholar]
- 43.Pascal, E., and R. Tjian. 1991. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 5:1646-1656. [DOI] [PubMed] [Google Scholar]
- 44.Peng, H., H. He, J. Hay, and W. T. Ruyechan. 2003. Interaction between the varicella zoster virus IE62 major transactivator and the cellular transcription factor Sp1. J. Biol. Chem. 278:38068-38075. [DOI] [PubMed] [Google Scholar]
- 45.Perera, L. P., J. Mosca, W. T. Ruyechan, and J. Hay. 1992. Regulation of Varicella Zoster virus gene expression in human T-lymphocytes. J. Virol. 66:2468-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perera, L. P., J. D. Mosca, M. Zadeghi-Zadeh, W. T. Ruyechan, and J. Hay. 1992. The varicella zoster virus immediate early protein IE62 can positively regulate its cognate promoter. Virology 191:346-354. [DOI] [PubMed] [Google Scholar]
- 47.Qyang, Y., X. Luo, T. Lu, P. M. Ismail, D. Krylov, C. Vinson, and M. Sawadogo. 1999. Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcriptional activation. Mol. Cell. Biol. 19:1508-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahaus, M., N. Desloges, M. Yang, W. T. Ruyechan, and M. H. Wolff. 2003. Transcription factor USF, expressed during the entire phase of varicella-zoster virus infection, interacts physically with the major viral transactivator IE62 and plays a significant role during virus replication. J. Gen Virol. 84:2957-2967. [DOI] [PubMed] [Google Scholar]
- 49.Ruyechan, W. T., and J. Hay. 2000. Varicella zoster virus: DNA replication, p. 51-73. In A. M. Arvin and A. A. Gershon (ed.), Varicella zoster virus: virology and clinical management. Cambridge University Press, Cambridge, Mass.
- 50.Ruyechan, W. T., H. Peng, M. Yang, and J. Hay. 2003. Cellular factors and IE62 activation of VZV promoters. J. Med. Virol. 70:S90-S94. [DOI] [PubMed] [Google Scholar]
- 51.Ryan, M. T., S. M. Herd, G. Sberna, M. M. Samuel, N. J. Hoogenraad, and P. B. Hoj. 1997. The genes encoding mammalian chaperonin 60 and chaperonin 10 are linked head-to-head and share a bidirectional promoter. Gene 196:9-17. [DOI] [PubMed] [Google Scholar]
- 52.Sawadogo, M. 1988. Multiple forms of the human gene specific transcription factor USF. II. DNA-binding properties and transcriptional activities of the purified HeLa USF. J. Biol. Chem. 263:11994-12001. [PubMed] [Google Scholar]
- 53.Schuttengruber, G., A. Doeltzlhoher, K. Kroboth., E. Wintersberger, and C. Seiser. 2003. Alternate activation of two divergently transcribed mouse genes fro a bi-directional promoter is linked to changes in histone modification. J. Biol. Chem. 278:1784-1793. [DOI] [PubMed] [Google Scholar]
- 54.Seki, Y., S. Ikeda, H. Kiyohara, H. Ayabe, T. Seki, and H. Matsui. 2002. Sequencing analysis of a putative human O-sialoglycoprotein endopeptidase gene (OSGEP) and analysis of a bidirectional promoter between the OSGEP and APEX genes. Gene 285:101-108. [DOI] [PubMed] [Google Scholar]
- 55.Shimoda, A., F. Sugata, H. S. Chen, R. H. Miller, and R. H. Purcell. 1998. Evidence for a bidirectional promoter complex within the X gene of woodchuck hepatitis virus. Virus Res. 56:25-39. [DOI] [PubMed] [Google Scholar]
- 56.Shin, R., M. J. Kim, and K. H. Paek. The CaTin1 (Capsicum annuum TMV-induced clone 1) and CaTin1-2 genes are linked head to head and share a bidirectional promoter. Plant Cell Physiol. 44:549-554. [DOI] [PubMed]
- 57.Shug, J., and G. Christian Overton. 1997. TESS: transcription element search software on the WWW. Technical Report CBIL-TR-1997-1001-v0.0. Computational Biology and Informatics Laboratory, School of Medicine, University of Pennsylvania, Philadelphia, Pa.
- 58.Smale, S. T., and J. T. Kadonaga. 2003. The RNA polymerase II core promoter. Annu. Rev. Biochem. 72:449-479. [DOI] [PubMed] [Google Scholar]
- 59.Sommer, M. H., E. Zagha, O. K. Serrano, C. C. Ku, L. Zerboni, A. Baiker, R. Santos, M. Spengler, J. Lynch, C. Grose, W. Ruyechan, J. Hay, and A. M. Arvin. 2001. Mutational analysis of the repeated open reading frames ORFs 63 and 70 and ORFs 64 and 69 of Varicella-Zoster virus. J. Virol. 75:8224-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spengler, M. L., W. T. Ruyechan, and J. Hay. 2000. Physical interaction between two varicella-zoster virus gene regulatory proteins, IE4 and IE62. Virology 272:375-381. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan, V., W. M. Schnitzlein, and D. N. Tripathy. 2003. A consideration of previously uncharacterized fowl poxvirus unidirectional and bidirectional late promoters for inclusion in homologous recombinant vaccines. Avian Dis. 47:286-295. [DOI] [PubMed] [Google Scholar]
- 62.Takacs, M., D. Salamon, S. Myohanen, H. Li, J. Segesdi, D. Ujvari, J. Uhlig, H. H. Niller, H. Wolf, G. Berenesci, and J. Minarovits. 2001. Epigenetics of latent Epstein-Barr virus genomes: high resolution methylation analysis of the bi directional promoter region of latent membrane protein 1 and 2B genes. Biol. Chem. 382:699-705. [DOI] [PubMed] [Google Scholar]
- 63.Trinklein, N. D., S. F. Aldred, S. J. Hartman, D. I. Schroeder, R. P. Otillar, and R. M. Myers. 2004. An abundance of bidirectional reporters in the human genome. Genome Res. 14:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wingender, E., X. Chen, R. Hehl, H. Karas, I. Liebich, V. Matys, T. Meinhardt, M. Prüβ, I. Reuter, and F. Schacherer. 2000. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 28:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Workman, J. L., R. G. Roeder, and R. E. Kingston. 1990. An upstream transcription factor, USF (MLTF) facilitates the formation of preinitiation complexes during in vitro chromatin assembly. EMBO J. 9:1299-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia, D., S. Srinivas, H. Sato, L. Pesnicak, S. E. Straus, and J. I. Cohen. 2003. Varicella-zoster virus open reading frame 21, which is expressed during latency, is essential for virus replication but dispensable for establishment of latency. J. Virol. 77:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]