Abstract
Background and Aims Populations of many hemiparasitic plants are fragmented and threatened by inbreeding depression (ID). In addition, they may also be strongly affected by a lack of suitable host species. However, nothing is known about possible interactive effects of inbreeding and host quality for parasitic plants. Poor host quality represents a special type of biotic stress and the magnitude of ID is often expected to be higher in more stressful environments.
Methods We studied the effects of inbreeding and the quality of host species for the declining root hemiparasite Rhinanthus alectorolophus. Selfed and open-pollinated parasites from two natural populations were grown (1) with 13 potential host species and (2) with 15 four-species mixtures.
Key Results ID differed among host species and mixtures. In the first experiment, ID was highest in parasites grown with good hosts and declined with stress intensity. In the second experiment, ID was not influenced by stress intensity, but was highest in mixtures of hosts from only one functional group and lowest in mixtures containing three functional groups. Both parasite performance with individual host species and the damage to these host species differed between parasites from the two study populations.
Conclusions Our results contradict the common assumption that ID is generally higher in more stressful environments. In addition, they support the importance of diverse host communities for hemiparasitic plants. The differences in host quality between the two parasite populations indicate genetic variation in the adaptation to individual hosts and in host-specific virulence. However, inbreeding did not affect specific host–parasite interactions.
Keywords: Rhinanthus alectorolophus, functional diversity, host mixture, inbreeding depression, environmental stress
INTRODUCTION
Root hemiparasites are capable of photosynthesis, but attach themselves to the roots of other plants via specialized root-organs called haustoria (Kuijt, 1969; Rümer et al., 2007) and obtain water, nutrients and assimilates from these hosts (Heide-Jørgensen, 2008; Tĕšitel et al., 2011). Most hemiparasites may use a wide range of host species, but species differ strongly in their quality as hosts for hemiparasites (de Hullu, 1984; Matthies, 1996; Guo and Luo, 2010). The host species thus represent a very important part of the environment of the parasites and their reproductive success depends strongly on their host species. Many hemiparasites are endangered, and it has been emphasized that in addition to the threats that rare species generally face in small populations, parasite conservation has to take into account the requirement for suitable host species (Marvier and Smith, 1997).
One of the problems plants face in small populations is increased inbreeding and its negative effects on plant fitness. Inbreeding depression (ID) can contribute to the extinction of rare species in small and fragmented populations (Gilpin and Soulé, 1986; Hedrick and Kalinowski, 2000; Keller and Waller, 2002; Frankham, 2005). However, little is known about the effects of inbreeding in parasitic plants. In addition to direct effects on parasite fitness, inbreeding can reduce their infection success, as was shown for the holoparasite Tristerix aphylla (Gonzáles et al., 2007). It is not known if inbreeding affects the ability of hemiparasites to grow with hosts of different quality and how inbreeding influences the effect of hemiparasites on the growth of their hosts.
Inbreeding has been proposed to be especially detrimental to parasites. The evolution of new resistant host genotypes may require a rapid counteradaptation by the parasites, which is facilitated by outcrossing (Gemmill et al., 1997; Agrawal and Lively, 2001; Gonzáles et al., 2007). However, for parasitic plants selfing can be a means to ensure reproduction if cross-pollination fails. Reproductive assurance is especially important for short-lived monocarpic species (Barrett, 2002; Charlesworth and Charlesworth, 2010), like most of the hemiparasitic species of Rhinanthus, Odontites, Euphrasia and Melampyrum (Orobanchaceae). All Rhinanthus species are annuals, and some of them are known to be self-compatible and to have a mixed mating system (Kwak, 1979; Oja and Talve, 2012). In such regularly selfing species, at least a part of the genetic load of recessive deleterious alleles is likely to be removed by selection (purging) or to become fixed by genetic drift, which results in reduced ID after enforced selfing in short-lived, self-compatible species compared to long-lived and self-incompatible species (Husband and Schemske, 1996; Angeloni et al., 2011).
During the last two decades, evidence has accumulated that the magnitude of ID may differ among environments (Cheptou and Donohue, 2011). It is often assumed that ID is stronger under more stressful conditions because inbred plants are more sensitive to stress (Dudash, 1990; Frankham et al., 2010; Reed et al., 2012), which has been regarded as an additional threat to small populations if environmental conditions deteriorate. Although in the majority of studies ID has been found to be higher in stressful environments, i.e. in environments that reduce average fitness compared to more benign environments (Armbruster and Reed, 2005; Fox and Reed, 2011), this pattern is not very consistent. Some studies found no effect of stress on ID while others even found higher ID under more favourable conditions (Norman et al., 1995; Armbruster and Reed, 2005; Waller et al., 2008). ID may decrease with stress intensity if offspring resulting from cross pollinations are better able to exploit benign conditions, but offspring from both cross types have similarly low fitness under stressful conditions (Cheptou and Donohue, 2011; Sandner and Matthies, 2016). Alternatively, it has been proposed that differences in ID among environments may not be due to differences in stress intensity, but due to the effects of an environment on phenotypic variation (phenotypic variation hypothesis, Waller et al., 2008). Studies which have compared the effects of more than one stress treatment on ID in the same plant species suggest that there may be differences among stress types. While some types of stress reduce ID, others may increase or not affect ID in the same species (Waller et al., 2008; Walisch et al., 2012; Sandner and Matthies, 2016). For hemiparasitic plants, a special biotic stress that might potentially influence ID is poor quality of the host species. A good host species is an important part of a beneficial environment for parasitic plants, while a poor host species strongly limits parasite growth and represents a stressful environment. We used Rhinanthus alectorolophus (Orobanchaceae) as a model system to study the effects of inbreeding and stress by poor host quality on hemiparasites. The importance of inbreeding for root hemiparasites such as Rhinanthus is increasing, as habitats suitable for them are decreasing due to agricultural intensification and the remaining populations are often small and fragmented (Blažek and Lepš, 2015).
We grew offspring from selfed and open-pollinated Rhinanthus alectorolophus from two populations autotrophically and with 13 potential host species of different quality. As hemiparasites rarely use single host species in natural populations, we grew the same parasites in a second experiment with 15 different four-species mixtures. We address the following questions: (1) Does R. alectorolophus show ID after self-pollination? (2) Does the magnitude of ID differ among hosts and host mixtures? Specifically, does ID increase or decrease with host quality, or increase with hosts that increase phenotypic variation? (3) Does inbreeding affect the suppression of host growth by the parasite?
MATERIALS AND METHODS
Study species
Rhinanthus alectorolophus (Scop.) Poll. (Orobanchaceae) is an annual root hemiparasite that grows in nutrient-poor to moderately fertile meadows throughout Europe (Hartl, 1974). Rhinanthus alectorolophus is not yet a rare species, but is declining in many parts of Europe due to agricultural intensification (Blažek and Lepš, 2015) and is endangered in some German states (e.g. Garve, 2004). Flowers of Rhinanthus species are pollinated by long-tongued bees (Kwak and Jennersten, 1986), but are self-compatible and the plants thus have a mixed mating system (Oja and Talve, 2012). Outcrossing rates have been studied in the related R. angustifolius with a similar flower morphology and were found to be high (Ducarme and Wesselingh, 2013). Seeds germinate during winter and haustoria are formed when the roots of the hemiparasite come into contact with host roots. Rhinanthus is a facultative parasite that can flower and produce seeds even without a host, but remains much smaller than when grown with a suitable host. Plant species differ strongly in their quality as hosts for Rhinanthus (de Hullu, 1984; Hautier et al., 2010).
Host species in the two experiments
Single plants of the hemiparasite R. alectorolophus were grown in pots together with single host plants of one of 13 different species (experiment 1) or with different combinations of four host plants (experiment 2).
Experiment 1.
We grew R. alectorolophus with 13 species that are known to differ in their quality as hosts for the parasite (Table 1). Parasites grown with poor hosts were assumed to grow in a stressful environment. All chosen host species naturally occur together with R. alectorolophus. Seeds of the host species were obtained from a commercial supplier (Appels Wilde Samen, Darmstadt, Germany). In the following, we will refer to the host species only by their genus name.
Table 1.
Host species used in the two experiments and their expected quality as hosts for Rhinanthus spp
| Species | Abbrev. | FG* | Quality | Source |
|---|---|---|---|---|
| Anthoxanthum odoratum | Ao | G | Poor | Hautier et al. (2010); D. Matthies, unpubl. data |
| Anthyllis vulneraria | Av | L | Poor | T. Sandner, unpubl. data |
| Dactylis glomerata | Dg | G | Good | Hautier et al. (2010); D. Matthies, unpubl. data |
| Lotus corniculatus | Lc | L | Good | D. Matthies, unpubl. data |
| Lolium perenne | Lp | G | Good | D. Matthies, unpubl. data |
| Leucanthemum vulgare | Lv | F | Poor | Cameron et al. (2006); D. Matthies, unpubl. data |
| Medicago sativa | Ms | L | Good | D. Matthies, unpubl. data |
| Onobrychis viciifolia | Ov | L | Not known | |
| Plantago lanceolata | Pl | F | Poor | Cameron et al. (2006); D. Matthies, unpubl. data |
| Sanguisorba minor | Sm | F | Intermediate | D. Matthies, unpubl. data |
| Trisetum flavescens | Tf | G | Good | Hautier et al. (2010); D. Matthies, unpubl. data |
| Taraxacum officinale | To | F | Intermediate | D. Matthies, unpubl. data |
| Trifolium pratense | Tp | L | Good | D. Matthies unpubl. data |
FG = functional group: F = forb, G = grass, L = legume.
Experiment 2.
We created 15 different mixtures of host species, each consisting of four individuals from different species. The mixtures were compiled from the 13 host species and differed in two features that were likely to influence the quality of the mixture for R. alectorolophus, the number (1–3) of host functional groups (i.e. grasses, legumes, forbs) and the number (0–4) of legumes in the mixture (Supplementary Data Table S1).
Pollination, germination and growth
In June 2012, a total of 60 Rhinanthus alectorolophus plants with flower buds but no open flowers in two populations near Großalmerode, Germany, were covered with nylon mesh bags to prevent insect pollination of the flowers. Population Rösberg was a large, continuous meadow population consisting of several thousand individuals of R. alectorolophus at high density, while population Eisenberg was a smaller population at lower density growing along a field lane. The distance between the two populations was 2 km. Both populations were similar in management regime (grazed or mown once a year) and species composition. They consisted of Arrhenatheretum grasslands including all of the studied host species in different frequencies, although the grasses Anthoxanthum odoratum and Lolium perenne occurred only in population Rösberg and the legume Anthyllis vulneraria occurred only in population Eisenberg. Seeds from the 28 remaining bagged plants (14 in each population) and from 53 open-pollinated plants nearby (31 in the Rösberg population and 22 in the Eisenberg population) were collected in July. The seeds from each plant were counted and weighed, and mean seed mass was calculated to estimate inbreeding effects on seed mass. For germination, parasite seeds were pooled per population and pollination treatment. In February 2013, seeds were incubated in Petri dishes for 3 d at room temperature and kept for 8 weeks at 5 °C for cold stratification. They were then kept at fluctuating temperatures (20/10 °C, 12 h of light) until cotyledons emerged. Germination speed did not vary consistently among treatments, but because of variation in germination speed within treatments and populations, germinated parasite seedlings were transferred to a fridge and kept at 4 °C (12 h of light) to synchronize development until a sufficient number of seedlings had developed.
Host seeds were germinated in Petri dishes at room temperature and seedlings were kept in the fridge at 4 °C (12 h light) until seeds of all species had germinated. Seedlings were planted in 0·9-L pots filled with sand and loam (1:1) and kept in a greenhouse. They were fertilized with 10 mL of 8 g L−1 solution of a commercial fertilizer (N:P:K = 14:7:14 %; Hakaphos Gartenprofi, Compo, Wien, Austria) and watered sparely from above to stimulate root growth close to the surface.
When the hosts were 2 weeks old (June 2013), one parasite seedling each was planted at 1 cm distance to single hosts or, for mixtures, in the centre of the four host individuals, each 1 cm from the parasite. If parasites died during the first 2 weeks after planting they were replaced. The first experiment consisted of 15 replicates for each of the 56 combinations of host, population and pollination treatment (840 overall), plus 15 control pots per host species grown without parasites (195 overall). The second experiment was smaller, with five replicates per mixture × population × pollination treatment (300), plus eight control pots per host mixture grown without parasites (120).
Pots from both experiments were transferred from the greenhouse to flower beds in an experimental garden of the University of Marburg after 2 weeks, when the parasite seedlings had established themselves. Plants were watered when necessary, and their positions in the flower beds were randomized regularly. During the growth period, pots were fertilized twice with 20 mL of 8 g L−1 commercial fertilizer. Parasites and host plants where harvested above ground after 9 weeks, when most of the parasites had finished flowering. Parasites and host plants where dried separately for 24 h at 80 °C and weighed.
Data analysis
The effects of population and pollination type on mean seed mass were analysed with analysis of variance (ANOVA) using the 81 open- or self-pollinated plants as replicates.
Experiment 1.
ANOVAs with Type I sums of squares were used to study the effects of population, pollination treatment and host species on measures of plant performance. Population was regarded as a fixed effect as seeds from only two parasite populations were used, which differed in size and density. Data for biomass, height and number of flowers were log-transformed prior to the analysis to achieve homoscedasticity and normally distributed residuals. The binary variables early and late mortality were analysed by generalized linear models with a logit link and binomial errors, using the same model structure as in the ANOVA models (analysis of deviance, Quinn and Keough, 2002).
Experiment 2.
The effects of population, pollination treatment and host mixture on parasite performance were studied by ANOVAs. However, the population of origin had no effect on any of the studied variables, and population was removed from the analysis. In the ANOVAs, the effect of host mixture was split into the linear contrasts number of legume species (0–4) and number of functional groups (1–3), and a rest. This requires additivity of sums of squares, which is why type I sums of squares were used. These contrasts are not completely independent, as all mixtures of four legumes consisted of only one functional group and mixtures containing three functional groups could not contain more than two legumes. However, the number of functional groups and of legumes in the mixtures had been designed to be as independent as possible, and the order of the two contrasts did not qualitatively change any of the results.
To analyse the effects of the intensity of stress on ID, stress intensity was calculated as one minus the biomass of the open-pollinated parasites per host species, relative to the biomass of the open-pollinated plants grown with the best host (Fox and Reed, 2011). It thus is equal to 1 – host quality. This was done separately for each parasite population, as the host quality differed between the populations (see Results). Biomass was chosen as a fitness measure instead of survival, because it was assumed to better reflect host quality. Stress intensity is thus a quantitative variable with one value per combination of population and host species. It was used as a linear contrast in ANOVAs and explains a part of the population × host interaction.
Mean values of log-transformed biomass data were back-transformed before calculating stress intensity and ID. ID was calculated for every combination of population of origin and host species as 1 minus the relative fitness of the inbred vs. that of the presumably outbred (open-pollinated) individuals: δ = 1 – (wi/wo). When inbred plants performed better than open-pollinated plants, ID was calculated as δ = (wo/wi) – 1 to keep all values between 1 and −1 (Ågren and Schemske, 1993).
To analyse the effects of phenotypic variation on ID, the opportunity for selection (CV²) was calculated as the squared coefficient of variation for biomass of selfed and open-pollinated individuals for each combination of population and host species (experiment 1) or for each host mixture (experiment 2). The separate CV²-values for selfed and open-pollinated offspring were then averaged within each combination of parasite population and host species (experiment 1) or within each mixture (experiment 2; Waller et al., 2008; Reed et al., 2012). The effect of average CV² on ID was determined by linear regressions for each experiment.
The effect of the parasite population and parasite inbreeding on host biomass (exp. 1) and host mixture biomass (exp. 2) was analysed with ANOVAs. All statistical analyses, if not stated otherwise, were performed with the software IBM SPSS Statistics for Windows, Version 21.0. (Armonk, NY, USA).
RESULTS
The mean seed mass of the sampled R. alectorolophus plants was 4·77 ± 0·12 mg and did not differ significantly between the populations (F1,77 = 2·67, P > 0·1) nor between pollination treatments (F1,77 = 3·59, P = 0·062), with a tendency of selfed seeds (5·01 mg) to be larger than seeds from open pollinations (4·53 mg). The populations did not differ in their response to self-pollination (F1,77 = 1·14, P = 0·29). After 8 weeks of cold stratification and 67 d of alternating temperatures, 63 % of selfed seeds and 48 % of open-pollinated seeds had germinated.
Experiment 1: parasite performance with single host species
Of the planted seedlings, 12 % died in the first weeks and had to be replaced. This early mortality differed among populations of origin, pollination treatments and host species (Table 2). More seedlings died from the Rösberg population (14·8 %) than from the Eisenberg population (8·8 %), and among Rösberg plants, more selfed seedlings died than seedlings resulting from open pollination (19·0 vs. 10·5 %).
Table 2.
Results of analyses of deviance and variance of the effects of population of origin, pollination type (selfed vs. open) and host species on early mortality of seedlings, mortality until harvest, and biomass at harvest of the parasite R. alectorolophus
| Source of variation | Early mortality |
Mortality |
Biomass |
||||
|---|---|---|---|---|---|---|---|
| d.f. | Mdev | qF | Mdev | qF | MS | F | |
| Population | 1 | 7·16 | 10·77** | 7·00 | 5·95* | 1·56 | 7·90** |
| Pollination type | 1 | 1·98 | 2·98+ | 1·64 | 1·40 | 1·72 | 8·75** |
| Host species | 13 | 1·33 | 2·00* | 2·20 | 1·87* | 5·46 | 27·72*** |
| Population × pollination | 1 | 5·05 | 7·60** | 2·52 | 2·14 | 2·80 | 14·19*** |
| Population × host species | 13 | 1·63 | 2·45** | 0·88 | 0·75 | 0·34 | 1·71+ |
| Pollination × host species | 13 | 1·50 | 2·25** | 0·93 | 0·79 | 0·17 | 0·85 |
| Pop. × host species × pollination | 13 | 1·27 | 1·91* | 1·99 | 1·69+ | 0·43 | 2·20** |
| Stress intensity × poll. | 1 | 3·84 | 5·78* | 6·96 | 5·92* | 3·23 | 16·37*** |
| Rest | 12 | 1·06 | 1·59+ | 1·57 | 1·34 | 0·20 | 1·02 |
| Residual | 539–783 | 0·66 | 1·18 | 0·20 | |||
The three-way interaction was partitioned into the linear contrast stress intensity (i.e. the proportional reduction in the biomass of open-pollinated parasites from each population grown with a certain host in comparison with the performance with the best host) and the residual variation (‘rest’).
P < 0·001; **P < 0·01; *P < 0·05; +P < 0·10.
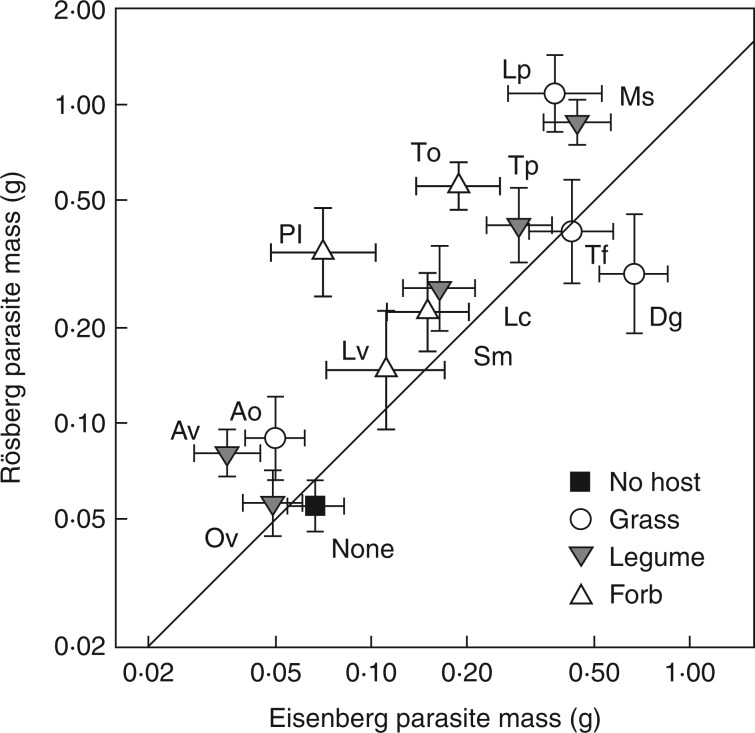
Of the established parasites, 28 % died before harvest. Mortality was higher for seedlings from the Rösberg population (33·1 %) than for those from the Eisenberg population (24·8 %), but did not depend on pollination type (Table 2). At harvest, parasite biomass was strongly correlated with parasite height (r² = 0·91) and number of flowers (r² = 0·85). Parasites from the Rösberg population were larger than those from the Eisenberg population (0·185 vs. 0·152 g, Table 2). In addition, parasite biomass differed strongly depending on host species (Table 2). Grown with the best host, the grass Lolium, parasites were eight times as large as parasites without a host, whereas grown with the poorest host, Onobrychis, parasites were even smaller than without a host. However, the effect of the hosts on the parasite depended also on parasite population of origin (population × host interaction in Table 2). Plants of R. alectorolophus from the Rösberg population were more than twice as large as plants from the Eisenberg population when grown with Anthyllis, Plantago, Taraxacum and Lolium, while parasites from the Eisenberg population grew larger with Dactylis (Fig. 1).
Fig. 1.
Effects of host and population of origin on the size of parasites resulting from open pollination. Each point represents the biomass of parasites from the two populations grown with one host species; for full host names see Table 1. Symbols indicate host functional groups. If parasites from both populations produced the same biomass with a host, points would fall on the diagonal line. Bars indicate + 1 s.e. Note log-scale of the axes.
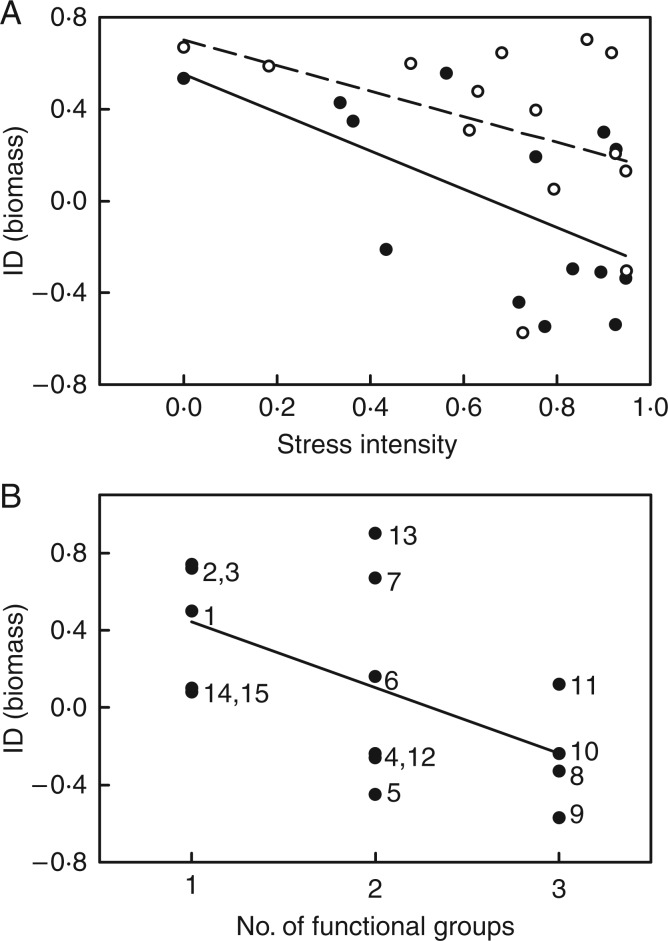
The mean biomass of parasites resulting from self and open pollinations grown with the same host plant was strongly correlated (r² = 0·88), but offspring from selfed plants were smaller than offspring from open-pollinated plants. However, this ID depended on the population of origin (Table 2), with 39 % ID for Rösberg plants and no ID (−3 %) for parasites from the Eisenberg population. In addition, ID decreased with stress intensity for biomass (Fig. 2A), early and late survival (Table 2). Stress intensity (i.e. 1 – host quality) was calculated per population × host species combination, because host quality differed among populations (see above). When included as a linear contrast in the ANOVA, the interaction of stress intensity × pollination type explained most of the three-way interaction population × pollination type × host species (Table 2, Supplementary Data Fig. S1). In contrast, differences in the average opportunity for selection (CV²) did not explain differences in ID (r² = 0·024, P > 0·4). Phenotypic variation in parasite biomass (CV²) was not correlated with stress intensity (r² = 0·008, P > 0·6), but increased with the early mortality per host (r² = 0·117, P = 0·075).
Fig. 2.
Effects on inbreeding depression of biomass in the hemiparasite R. alectorolophus. (A) Effects of stress by poor host quality (i.e. 1 – relative biomass of crossed parasites per host) in experiment 1. Open symbols and dashed line: population Rösberg; filled symbols and continuous line: population Eisenberg. (B) Effects of the number of functional groups per mixture in experiment 2. Numbers refer to the mixtures in Table S1.
Experiment 2: parasite performance with mixtures of host species
Of the seedlings planted, 32 % died in the first 2 weeks and were replaced. This early mortality was not influenced by host mixture (quasi-F14,270 = 1·12, P > 0·1) or by pollination treatment (quasi-F1,270 = 0·21, P > 0·5). Of the established parasites, 44 % died before harvest. Mortality was higher for offspring from selfed plants (52 %) than for offspring from open-pollinated plants (37 %, quasi-F1,270 = 5·50, P = 0·02), but did not differ among host mixtures. In contrast, parasite biomass differed strongly among mixtures (Table 3).When grown with the best mixture, parasites were 25·9 times as large as parasites grown with the poorest mixture.
Table 3.
ANOVA of the effects of pollination type (selfed vs. open) and host species mixture on the biomass of R. alectorolophus
| Source of variation | d.f. | Parasite biomass |
|
|---|---|---|---|
| MS | F | ||
| Pollination | 1 | 0·114 | 0·53 |
| Mixture | 14 | 1·421 | 6·61*** |
| Pollination × mixture | 14 | 0·335 | 1·56+ |
| Pollination × number of legumes | 1 | 0·110 | 0·513 |
| Pollination × number of functional groups | 1 | 1·072 | 4·991* |
| Rest | 12 | 0·292 | 1·359 |
| Residual | 136 | 0·215 | |
For a better understanding of the pollination × mixture interaction, the effect of mixture was partitioned into the three linear contrasts number of legumes in the mixture, number of functional groups and the residual variation among mixtures.
P < 0·001; *P < 0·05; +P < 0·10.
The effect of pollination type on parasite biomass depended on the host mixture (Table 3). ID decreased with the number of functional groups in a mixture (Fig. 2B, see linear contrast in Table 3). These differences in ID were due to the reduced size of selfed parasites in mixtures with only one or two functional groups, whereas the biomass of open-pollinated parasites did not change with the number of host functional groups. The magnitude of ID did not depend on stress intensity (r² = 0·014, P > 0·6). Moreover, differences in the average opportunity for selection (CV²) could also not explain differences in ID (r² = 0·002, P > 0·8).
Effects on host biomass
In both experiments, host biomass was reduced by the parasites. When a parasite was present, the effect on the biomass of the host species differed between parasites from the two populations (population × host interaction, Table 4). This interaction remained significant when log parasite biomass was included as a covariate (F12,506 = 1·85, P = 0·038). However, the pollination treatment of the parasites did not influence host biomass in experiment 1 (P > 0·20, Table 4), nor the biomass of host mixtures in experiment 2 (F1,137 = 0·01, P > 0·9), suggesting no inbreeding effects on parasite virulence.
Table 4.
ANOVA of the effects of host species, the population of origin of the parasite R. alectorolophus and parasite pollination type (selfed vs. open) on the biomass of 13 different host species
| d.f. | Host biomass |
||
|---|---|---|---|
| MS | F | ||
| Host species | 12 | 1·402 | 38·41*** |
| Parasite population | 1 | 0·067 | 1·84 |
| Pollination type | 1 | 0·056 | 1·52 |
| Host species × parasite population | 12 | 0·070 | 1·91* |
| Host species × pollination type | 12 | 0·024 | 0·67 |
| Population × pollination type | 1 | 0·020 | 0·54 |
| Host × population × pollination type | 12 | 0·022 | 0·59 |
| Residual | 508 | 0·037 | |
Only pots with parasites were included in the analysis.
P < 0·001; *P < 0·05.
DISCUSSION
Inbreeding depression in Rhinanthus alectorolophus
Overall, Rhinanthus alectorolophus expressed only little ID. The early traits seed mass and germination, but also mortality until flowering showed no ID at all. This corresponds to the expectation for frequently selfing angiosperms, which on average show 0–5 % ID in early traits and juvenile survival, but 21 % ID in growth and reproduction, and is attributed to a history of purging strongly deleterious, early acting mutations (Husband and Schemske, 1996). In addition, ID in R. alectorolophus may have been underestimated because inbred offspring were compared with offspring from open pollinations, which may have partly resulted from geitonogamous self-pollination (de Jong et al., 1993). The fact that with some hosts ID in early traits and ID in biomass tended even to be negative is probably the result of a maternal effect of resource reallocation to a lower number of pollinated flowers in the bagged plants (Zimmerman and Pyke, 1988; Knight et al., 2006), which is indicated by the slightly larger seeds and better germination after self- than open pollination.
The two sampled parasite populations differed in their size. In parasites from the smaller population (Eisenberg), ID averaged across all host species was absent even in final biomass, whereas in parasites from the larger and more dense population ID was higher. Plants from small populations often show less ID than plants from large populations (Angeloni et al., 2011), which can be due to purging in small populations (but see Byers and Waller, 1999; Glémin, 2003) or due to an increased genetic load as a consequence of genetic drift (Keller and Waller, 2002; Angeloni et al., 2011). Plants from the Eisenberg population were on average smaller than plants from the Rösberg population, which lends support to a higher genetic load in the Eisenberg population.
Parasitic plants can considerably reduce the growth of their hosts (Matthies, 1996; Westbury, 2004; Ameloot et al., 2005), but the effect of parasite inbreeding on host suppression has not been studied. In R. alectorolophus inbreeding did not influence the negative effect of the parasite on the host in spite of inbreeding effects on parasite biomass.
Inbreeding depression and stress by poor host quality
The magnitude of ID differed among parasites grown with different host species (exp. 1) and among parasites grown with different mixtures (exp. 2). In experiment 1, ID increased with the quality of a species as host for R. alectorolophus. As poor hosts can be regarded as stressful environments for the parasite, ID thus decreased with stress intensity. In contrast, ID was not affected by host quality in experiment 2, but decreased with the functional diversity of a mixture.
It has been proposed to test the phenotypic variation hypothesis as a null-model before discussing the effects of stress intensity on ID (Waller et al., 2008). This hypothesis posits that an environment may increase ID not because it is stressful, but because it increases the amount of phenotypic variation among individuals. Mixed support for the hypothesis is provided by studies on inbreeding in animals, in which ID often increased with both CV² and stress intensity (Reed et al., 2012; Long et al., 2013). In a study of Silene vulgaris under eight different stress treatments, ID in biomass increased with CV² but decreased slightly with stress intensity (Sandner and Matthies, 2016). In contrast to these studies, the phenotypic variation hypothesis did not explain any differences in ID in R. alectorolophus among host species or mixtures, and the CV² of parasite biomass was not correlated with stress intensity.
In experiment 1, ID was highest when parasites were grown with good hosts and lowest for parasites grown with poor hosts or autotrophically. This is in contrast to the prevalent expectation that ID increases with stress and supports the alternative hypothesis that crossed plants can use favourable conditions better than selfed plants (capable crossed hypothesis, Cheptou and Donohue, 2011; Sandner and Matthies, 2016). This pattern has been found in other plants in response to different nutrient levels (Norman et al., 1995; Kéry et al., 2000; Walisch et al., 2012; Sandner and Matthies, 2016) and suggests that under conditions of strong resource limitation, the performance of inbred and crossed offspring is similarly poor, whereas ID increases under good conditions because crossed plants have a higher phenotypic plasticity in their growth rates.
When grown with mixtures of host species, ID in R. alectorolophus decreased with the number of functional groups in a mixture. Host species from the three different functional groups differ in physiological and morphological traits and may differ in the quality and quantity of compounds they deliver to the parasites. The hemiparasite Odontites verna received mostly carbohydrates from the grass Hordeum vulgare, but nitrogenous compounds from the legume Trifolium repens (Govier et al., 1967). In addition, functional groups may differ in their defence mechanisms (Rümer et al., 2007). For instance, the grasses Phleum bertolonii and Hordeum vulgare showed some lignification of their roots in response to parasite haustoria, while the forb Leucanthemum vulgare successfully blocked haustoria by suberization of the cell walls and Plantago lanceolata by local cell death (Rümer et al., 2007). The buffering of ID by functional diversity of hosts suggests that the deficits of inbred R. alectorolophus may be better compensated for if hosts from different functional groups are available. This supports the view that for hemiparasites such as R. alectorolophus the diversity of communities is of particular importance (Marvier and Smith, 1997; Joshi et al., 2000).
Population differences in host quality
A range of mechanical and chemical defence mechanisms against parasitization have been observed in different host species (Cameron et al., 2006; Rümer et al., 2007; Heide-Jørgensen, 2008). However, the effects of host species on the growth of R. alectorolophus were not influenced by pollination type, suggesting that inbreeding did not influence the ability of the parasites to overcome specific resistance mechanisms. In contrast, in the holoparasite Tristerix aphylla, which grows endophytically in South American cacti, inbreeding reduced many early fitness traits, including infection success (Gonzáles et al., 2007). It has been suggested that outbreeding is especially important for parasites, because they have to overcome defences evolving in different host genotypes (Agrawal and Lively, 2001; Gonzáles et al., 2007). However, little is known about the genetics of host–parasite coevolution in Rhinanthus. A reciprocal transplant experiment found no local adaptation between Rhinanthus and its host Agrostis capillaris from different populations, but parasites from different populations differed in biomass and in their effect on the host plant (Mutikainen et al., 2000). In our study the quality of the individual species as hosts for parasites from the two studied populations differed. In addition, the suppression of host biomass by the parasite differed between the two parasite populations, which suggests different adaptations in the two parasite populations, although they are only 2 km apart from each other. Similarly, Rhinanthus from two different populations differed in their growth with identical genotypes of Hordeum vulgare (Rowntree et al., 2011). There is thus substantial evidence for genetic variation in the response of hemiparasites to the same host species. This finding stresses the importance of conserving many and large populations of hemiparasites to ensure sufficient genetic variation in the response to different host species.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: composition of the 15 different mixtures of hosts in experiment 2. Figure S1: effects of host species and pollination type on the biomass of R. alectorolophus from two different populations, Rösberg and Eisenberg.
ACKNOWLEDGEMENTS
We thank Renate Wesselingh and an anonymous reviewer for helpful comments that improved the manuscript.
LITERATURE CITED
- Agrawal AF, Lively CM. 2001. Parasites and the evolution of self-fertilization. Evolution 55: 869–879. [DOI] [PubMed] [Google Scholar]
- Ågren J, Schemske DW. 1993. Outcrossing rate and inbreeding depression in two annual monoecious herbs, Begonia hirsuta and B. semiovata. Evolution 47: 125–135. [DOI] [PubMed] [Google Scholar]
- Ameloot E, Verheyen K, Hermy M. 2005. Meta-analysis of standing crop reduction by Rhinanthus spp. and its effect on vegetation structure. Folia Geobotanica 40: 289–310. [Google Scholar]
- Angeloni F, Ouborg NJ, Leimu R. 2011. Meta-analysis on the association of population size and life history with inbreeding depression in plants. Biological Conservation 144: 35–43. [Google Scholar]
- Armbruster P, Reed DH. 2005. Inbreeding depression in benign and stressful environments. Heredity 95: 235–242. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2002. The evolution of plant sexual diversity. Nature Reviews Genetics 3: 274–284. [DOI] [PubMed] [Google Scholar]
- Blažek P, Lepš J. 2015. Victims of agricultural intensification: mowing date affects Rhinanthus spp. regeneration and fruit ripening. Agriculture, Ecosystems & Environment 211: 10–16. [Google Scholar]
- Byers DL, Waller DM. 1999. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annual Review of Ecology, Evolution, and Systematics 30: 479–513. [Google Scholar]
- Cameron DD, Coats AM, Seel WE. 2006. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany 98: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. 2010. The evolution of breeding systems, sex ratios, and life histories In: Charlesworth B, Charlesworth D, eds. Elements of evolutionary genetics. Greenwood Village, CO: Roberts, 444–520. [Google Scholar]
- Cheptou P-O, Donohue K. 2011. Environment-dependent inbreeding depression: its ecological and evolutionary significance. New Phytologist 189: 395–407. [DOI] [PubMed] [Google Scholar]
- de Hullu E. 1984. The distribution of Rhinanthusangustifolius in relation to host plant species In: C Parker, ed. Third International Symposium on Parasitic Weeds. Aleppo, Syria, 43–52.
- de Jong TJ, Waser NM, Klinkhamer PGL. 1993. Geitonogamy: the neglected side of selfing. Trends in Ecology and Evolution 8: 321–325. [DOI] [PubMed] [Google Scholar]
- Ducarme V, Wesselingh RA. 2013. Outcrossing rates in two self-compatible, hybridising Rhinanthus species: implications for hybrid formation. Plant Biology 15: 541–547. [DOI] [PubMed] [Google Scholar]
- Dudash MR. 1990. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution 44: 1129–1139. [DOI] [PubMed] [Google Scholar]
- Fox CW, Reed DH. 2011. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65: 246–258. [DOI] [PubMed] [Google Scholar]
- Frankham R. 2005. Genetics and extinction. Biological Conservation 126: 131–140. [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. 2010. Introduction to conservation genetics, 2nd edn. Cambridge: Cambridge University Press. [Google Scholar]
- Garve E. 2004. Rote Liste und Florenliste der Farn- und Blütenpflanzen in Niedersachsen und Bremen. Informationsdienst Naturschutz Niedersachsen 1: 1–76. [Google Scholar]
- Gemmill AW, Viney ME, Read AF. 1997. Host immune status determines sexuality in a parasitic nematode. Evolution 51: 393–401. [DOI] [PubMed] [Google Scholar]
- Gilpin ME, Soulé ME. 1986. Minimum viable populations: processes of species extinction In: ME Soulé, ed. Conservation biology: the science of scarcity and diversity. Sunderland, MA: Sinauer, 19–34. [Google Scholar]
- Glémin S. 2003. How are deleterious mutations purged? Drift versus nonrandom mating. Evolution 57: 2678–2687. [DOI] [PubMed] [Google Scholar]
- Gonzáles WL, Suárez LH, Medel R. 2007. Outcrossing increases infection success in the holoparasitic mistletoe Tristerix aphyllus (Loranthaceae). Evolutionary Ecology 21: 173–183. [Google Scholar]
- Govier RN, Nelson MD, Pate JS. 1967. The transfer of organic compounds from host to Odontites verna (Bell.) Dum. (Scrophulariaceae). New Phytologist 66: 285–297. [Google Scholar]
- Guo Q-S, Luo F-L. 2010. Comparative studies on the growth, chlorophyll, amino acids and minerals of Thesium chinense (Santalaceae) in association with different hosts. Nordic Journal of Botany 28: 632–640. [Google Scholar]
- Hartl D. 1974. Scrophulariaceae; Rhinanthus In: G Hegi, ed. Illustrierte Flora von Mitteleuropa 6/1. München: Carl Hanser Verlag, 374–403. [Google Scholar]
- Hautier Y, Hector A, Vojtech E, Purves D, Turnbull LA. 2010. Modelling the growth of parasitic plants. Journal of Ecology 98: 857–866. [Google Scholar]
- Hedrick PW, Kalinowski ST. 2000. Inbreeding depression in conservation biology. Annual Review of Ecology, Evolution, and Systematics 31: 139–62. [Google Scholar]
- Heide-Jørgensen H. 2008. Parasitic flowering plants. Leiden, Netherlands: Brill. [Google Scholar]
- Husband BC, Schemske DW. 1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54–70. [DOI] [PubMed] [Google Scholar]
- Joshi J, Matthies D, Schmid B. 2000. Root hemiparasites and plant diversity in experimental grassland communities. Journal of Ecology 88: 634–644. [Google Scholar]
- Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends in Ecology and Evolution 17: 230–241. [Google Scholar]
- Kéry M, Matthies D, Spillmann H-H. 2000. Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. Journal of Ecology 88: 17–30. [Google Scholar]
- Knight TM, Steets JA, Ashman T-L. 2006. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. American Journal of Botany 93: 271–277. [DOI] [PubMed] [Google Scholar]
- Kuijt J. 1969. The biology of parasitic flowering plants. Berkeley, CA: University of California Press. [Google Scholar]
- Kwak MM. 1979. Effects of bumblebee visits on the seed set of Pedicularis, Rhinanthus and Melampyrum (Scrophulariaceae) in the Netherlands. Acta Botanica Neerlandica 28: 177–195. [Google Scholar]
- Kwak MM, Jennersten O. 1986. The significance of pollination time and frequency and of purity of pollen loads for seed set in Rhinanthus angustifolius (Scrophulariaceae) and Viscaria vulgaris (Caryophyllaceae). Oecologia 70: 502–507. [DOI] [PubMed] [Google Scholar]
- Long TAF, Rowe L, Agrawal AA. 2013. The effects of selective history and environmental heterogeneity on inbreeding depression in experimental populations of Drosophila melanogaster. American Naturalist 181: 532–544. [DOI] [PubMed] [Google Scholar]
- Marvier MA, Smith DL. 1997. Conservation implications of host use for rare parasitic plants. Conservation Biology 11: 839–848. [Google Scholar]
- Matthies D. 1996. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos 75: 118–124. [Google Scholar]
- Mutikainen P, Salonen V, Puustinen S, Koskela T. 2000. Local adaptation, resistance, and virulence in a hemiparasitic plant–host plant interaction. Evolution 54: 433–440. [DOI] [PubMed] [Google Scholar]
- Norman JK, Sakai AK, Weller SG, Dawson TE. 1995. Inbreeding depression in morphological and physiological traits of Schiedea lydgatei (Caryophyllaceae) in two environments. Evolution 49: 297–306. [DOI] [PubMed] [Google Scholar]
- Oja T, Talve T. 2012. Genetic diversity and differentiation in six species of the genus Rhinanthus (Orobanchaceae). Plant Systematics and Evolution 298: 901–911. [Google Scholar]
- Quinn G, Keough M. 2002. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press. [Google Scholar]
- Reed DH, Fox CW, Enders LS, Kristensen TN. 2012. Inbreeding-stress interactions: evolutionary and conservation consequences. Annals of the New York Academy of Sciences 1256: 33–48. [DOI] [PubMed] [Google Scholar]
- Rowntree JK, Cameron DD, Preziosi RF. 2011. Genetic variation changes the interactions between the parasitic plant–ecosystem engineer Rhinanthus and its hosts. Philosophical Transactions of the Royal Society B 366: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümer S, Cameron DD, Wacker R, Hartung W, Jiang F. 2007. An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora 202: 194–200. [Google Scholar]
- Sandner TM, Matthies D. 2016. The effects of stress type and stress intensity on inbreeding depression in Silene vulgaris. Evolution 70: 1225–1238. [DOI] [PubMed] [Google Scholar]
- Tĕšitel J, Lepš J, Vráblová M, Cameron DD. 2011. The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: a physiological perspective. New Phytologist 192: 188–199. [DOI] [PubMed] [Google Scholar]
- Walisch TJ, Colling G, Poncelet M, Matthies D. 2012. Effects of inbreeding and interpopulation crosses on performance and plasticity of two generations of offspring of a declining grassland plant. American Journal of Botany 99: 1300–1313. [DOI] [PubMed] [Google Scholar]
- Waller DM, Dole J, Bersch AJ. 2008. Effects of stress and phenotypic variation on inbreeding depression in Brassica rapa. Evolution 62: 917–931. [DOI] [PubMed] [Google Scholar]
- Westbury DB. 2004. Rhinanthus minor L. Journal of Ecology 92: 906–927. [Google Scholar]
- Zimmerman M, Pyke GH. 1988. Reproduction in Polemonium: assessing the factors limiting seed set. American Naturalist 131: 723–738. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.