Abstract
The inhibition of multilineage hematopoiesis which occurs in the severe combined immunodeficiency mouse with transplanted human fetal thymus and liver tissues (SCID-hu Thy/Liv) due to human immunodeficiency virus type 1 (HIV-1) infection is also accompanied by a severe loss of c-Mpl expression on these progenitor cells. Inhibition of colony-forming activity (CFA) of the CD34+ progenitor cells is partially revived to about 40% of mock-infected Thy/Liv implants, following reconstitution of the CD34+ cells that were exposed to HIV-1 infection, in a new Thy/Liv stromal microenvironment of irradiated secondary SCID-hu recipients at 3 weeks post-re-engraftment. In addition, in these reconstituted animals, the proportion of c-Mpl+ CD34+ cells relative to c-Mpl− CD34+ cells increased by about 25%, to 35% of mock-infected implants, suggesting a reacquirement of c-Mpl phenotype by the c-Mpl− CD34+ cells. These results suggest a correlation between c-Mpl expression and multilineage CFA of the human CD34+ progenitor cells that have experienced the effects of HIV-1 infection. Treatment of the secondary-recipient animals with the c-Mpl ligand, thrombopoietin (Tpo), further increased c-Mpl expression and CFA of re-engrafted CD34+ cells previously exposed to virus in the primary implants to about 50 to 70% over that of those re-engrafted CD34+ cells derived from implants of untreated animals. Blocking of c-Mpl with anti-c-Mpl monoclonal antibody in vivo by injecting the SCID-hu animals resulted in the reduction or loss of CFA. Thus, inhibition, absence, or loss of c-Mpl expression as in the c-Mpl− CD34+ subset of cells is the likely cause of CFA inhibition. Further, CFA of the CD34+ cells segregates with their c-Mpl expression. Therefore, c-Mpl may play a role in hematopoietic inhibition during HIV-1 infection, and control of its expression levels may aid in hematopoietic recovery and thereby reduce the incidence of cytopenias occurring in infected individuals.
Human immunodefiency virus (HIV)-infected patients often suffer from multiple hematopoietic abnormalities, which include cytopenias and myelodysplastic or hyperplastic alterations of the bone marrow microenvironment (30, 36, 38, 53). Hematopoietic progenitor cell colony growth and differentiation is inhibited in long-term bone marrow cultures of HIV-positive patients (8, 12, 16, 43). In general, investigators have failed to detect HIV infection in hematopoietic progenitor cells isolated from infected individuals, suggesting that HIV might have an indirect effect on hematopoiesis (38). Moreover, Shen et al. found that bone marrow and peripheral blood-derived CD34+ progenitor cells are not susceptible to HIV type 1 (HIV-1) infection in vitro (47), although infection of CD34+ progenitor cells in vitro has been reported by some investigators (9, 44). Our group and others have found that HIV-1 inhibits multilineage hematopoiesis in vivo without direct infection of the CD34+ progenitor cells and presumably via indirect effects of the infected microenvironment (22, 29). These results suggest that HIV possibly alters the stromal/progenitor cell microenvironment that supports hematopoiesis. Such perturbation causes hematopoietic abnormalities due to altered stem cell differentiation, possibly arising from abnormal lineage-specific expression of certain cellular genes, such as cytokines relevant to hematopoiesis (45).
Transfer of the stem cells exposed to the indirect effects of HIV infection into a fresh stromal microenvironment may help revive and sustain functional progenitor cells differentiating into multiple lineages. In this regard, the cellular proto-oncogene of myeloproliferative leukemia, c-mpl, also known as the thrombopoietin receptor proto-oncogene, is likely to be an important target during HIV infection in regulation of multilineage hematopoiesis (18, 23, 50). The role of c-Mpl in HIV-induced hematopoietic inhibition is not well understood. Pluripotent murine stem cells constitutively expressing c-Mpl exhibited normal hematopoiesis, not limited to a preferential megakaryopoiesis, in response to thrombopoietin (Tpo) treatment (23, 50). c-Mpl has also been shown not to be lineage restricted in mice repopulated with bone marrow cells expressing human c-Mpl followed by Tpo treatment (18, 23). It has been shown that in c-Mpl-expressing human hematopoietic progenitors, Tpo also promotes myelopoiesis and erythropoiesis, in addition to its primary role in lineage-specific megakaryopoiesis (18, 23, 25, 48, 50). Since Tpo also influences erythropoiesis, the synergy of Tpo and erythropoietin (Epo) in erythropoiesis is also of importance. It has also been found that a polypeptide related to Tpo, megakaryocyte growth and development factor, which stimulates megakaryocyte production, also supports retroviral transduction of T-lymphoid progenitor cells capable of contributing to long-term thymopoiesis (4). Thus, c-Mpl-Tpo interactions are involved in multilineage hematopoiesis and thus multiple cytopenias. In addition, individuals with congenital cytopenias revealed mutations in their c-Mpl gene (6, 17).
The presence of c-Mpl on CD34+ progenitor cells and treatment with Tpo contributed to their enhanced engraftment as well as colony-forming activity (CFA) (23, 50). In this regard, it has been reported with respect to murine c-Mpl that c-Mpl+ cells deliver a greater engraftment and colony-forming potential than c-Mpl− cells (50). The CD34+ cellular subsets expressing the Tpo receptor, c-Mpl, showed significantly better engraftment of the hematopoietic progenitor cells in SCID mice (50). Since CD34+ c-Mpl+ cells aid in enhanced engraftment of both murine and human hematopoietic stem cells in SCID and SCID-hu mice, respectively, (50), the role of c-Mpl in HIV-1-mediated hematopoietic inhibition and its alleviation are of significance for investigation. Further, SCID mouse serves as a useful small-animal recipient of human progenitor cells and also allows us to study the differentiation of these cells in vivo. We have investigated herein the role of c-Mpl in hematopoietic inhibition induced by HIV-1, using the SCID-hu thymus and liver (Thy/Liv) model system (33, 39). The chimeric SCID mouse coimplanted with human fetal thymus and liver tissues (2, 7, 31, 33, 39, 52), resulting in a functional human hematopoietic organ (Thy/Liv), allows maintenance and differentiation of human hematopoietic progenitor cells (39) and also recapitulates or mimics the effects of HIV-1 infection in the human thymus. The confounding factors found in HIV-infected patients are absent in this model system. Also, the high virus loads attained following infection of SCID-hu make this model an extremely stringent tool for the susceptibility of the various CD34+ progenitor cells present to HIV-1 infection. Thus, this model allows the causal role of c-Mpl in vivo to be assessed under changing conditions of engraftment of HIV-affected stem cells into a new stromal microenvironment and therapeutic treatments, including cytokines and stem cell growth factors. Thus, the coengrafted Thy/Liv model of SCID-hu provides a means of exposure of the CD34+ c-Mpl+ cells to HIV-1 infection via the thymocytes of Thy/Liv implants (2, 7, 31, 52). We report here that re-engraftment of SCID-hu Thy/Liv implants with CD34+ progenitor cells which are either c-Mpl+ or c-Mpl− into a fresh stromal microenvironment and further, Tpo treatment of the host, may be effective in reduction or reversal of hematopoietic inhibition which occurs during HIV-1 infection.
MATERIALS AND METHODS
Construction and HIV-1 infection of SCID-hu mice.
SCID mice received simultaneous transplants of 1-mm3 pieces of human fetal thymus and liver tissues under the renal capsule, and the resulting conjoint organ of the SCID-hu animal was allowed to grow for 3 to 5 months, as described previously (28). The Thy/Liv implants were then infected by intraimplant injection of HIV-1NL4-3 (100 IU in 50 to 100 μl) which was produced in CEM cells. Control mock-infected animals were injected with the same volume of mock-infected CEM culture medium alone. At specific time intervals postinfection, sequential wedge biopsies (∼25% of each implant) were obtained and were processed and analyzed as described below. Viral infectivity of the thymocytes of the Thy/Liv implants was determined by PCR of the proviral DNA, as described previously (28).
Isolation of CD34+ CD38− cells from Thy/Liv implants.
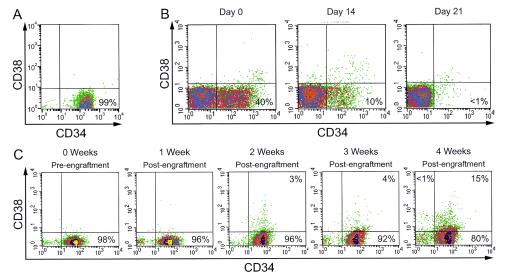
We isolated the CD34+ cells from the mock- and HIV-1-infected Thy/Liv implants by labeling these cells with unlabeled anti-CD34 monoclonal antibody. Total cells from the SCID-hu Thy/Liv implants were labeled with anti-CD34 monoclonal antibody (clone QBEND/10) conjugated to superparamagnetic MicroBeads (Miltenyi Biotec, Auburn, Calif.) and subjected to separation by AutoMacs, using the “possel d” software setting to maximize both recovery and purity of the CD34+ cells. This automated separation system, manufactured by Miltenyi Biotec, is available for use at the UCLA Flow Cytometry Core Facility. The more mature CD34+ CD38+ cells are removed from the CD34+ cells by using AutoMacs by first labeling the CD38+ cells with anti-CD38-phycoerythrin (PE) monoclonal antibody and then conjugating them with anti-PE monoclonal antibody-coupled magnetic microbeads, followed by separation using the “deplete” or “deplete s” program. The negative fraction is collected and then subjected to positive selection for the CD34+ cells as follows. The separated CD34+ CD38− cells are concentrated by centrifugation and relabeled with anti-CD34-allophycocyanin (APC) antibody (clone AC136, which recognizes a class III epitope of CD34 antigen; Miltenyi Biotec) for fluorescence-activated cell sorting (FACS) analysis both for positive selection and to estimate purity. The use of two different anti-CD34 monoclonal antibodies derived from clones QBEND/10 and AC136, as suggested by the manufacturer, is to facilitate binding at different epitopes of the CD34 cell surface marker antigen. The purity of the CD34+ cells was estimated to be >95% by FACS analyses (Fig. 1A).
FIG. 1.
(A) Purity of CD34+ cells separated by AutoMacs. The CD34+ progenitor cells were derived from the Thy/Liv implants of mock- or HIV-1-infected SCID-hu animals and were estimated to be >95% pure by flow cytometric analysis as described in Materials and Methods. (B) Loss of CD34+ cells of the Thy/Liv implants of secondary recipients postirradiation. The endogenous secondary recipient CD34+ CD38− cells were identified by their HLA-B8+ type (in this case), using colabeling with mouse anti-human anti-HLA-B8-FITC, anti-CD34-APC, and anti-CD38-PE monoclonal antibodies. As shown, by 3 weeks postirradiation, the endogenous human CD34+ cells either lose their phenotype or are killed. (C) Engraftment, maintenance, and differentiation of the donor CD34+ CD38− cells in the Thy/Liv implants of irradiated SCID-hu secondary recipients. Beyond 3 weeks postengraftment, the CD34+ CD38− cells begin to acquire the mature CD38 phenotype in vivo (15% CD38+ at 4 weeks), suggesting the onset of multilineage differentiation postreconstitution in vivo. This result, combined with the result that virus-induced hematopoietic inhibition begins by 3 weeks postinfection (28), is the reason for harvesting the CD34+ cells from Thy/Liv implants at 3 weeks post(re)engraftment/postinfection in subsequent experiments. In this case, the engrafted donor CD34+ cells are HLA-B27+, distinguished from the recipient HLA-B8+ cells (see Fig. 2).
Transfer and re-engraftment of CD34+ cells into SCID-hu Thy/Liv implants.
The separated and purified CD34+ cells derived from primary Thy/Liv implants are then transferred by injection (5 × 104 cells in 100 μl) into the Thy/Liv implants of irradiated (300 rads) secondary-recipient SCID-hu mice (1). Irradiation of the secondary-recipient animals is carried out immediately prior to re-engraftment with the purified CD34+ cells from primary recipients. Following reconstitution of the human CD34+ cells derived from primary-recipient implants in the implants of secondary recipients, these different CD34+ cells were distinguished by flow cytometric analysis using anti-HLA monoclonal antibodies (purchased from One Lambda Inc., Canoga Park, Calif.). One of these two different anti-HLA class I monoclonal antibodies is specific for the exogenous re-engrafted donor CD34+ cells previously derived from the primary implants, and the other is specific for the endogenous CD34+ cells of the implants of the irradiated SCID-hu secondary recipients.
Antiretroviral drug treatment of SCID-hu mice.
The secondary SCID-hu recipient animals that were irradiated and re-engrafted received treatment with two antiretroviral drugs to prevent secondary infection that might be carried over from the previously virus-exposed CD34+ cells of the primary implants by any contaminating traces of viral particles and to halt further virus replication. The combination drug treatment used was similar to but at reduced doses from that previously described (28, 57) and included the following: protease inhibitor indinavir sulfate (Merck, Rahway, N.J.) at an approximate dose of 0.45 mg/kg of body weight/day was diluted into drinking water (pH 3) at a concentration of 1.5 mg/ml, which corresponds to a dose of 20 mg/kg/day (28, 57); zidovudine (Aldrich Chemical Co., Milwaukee, Wis.) was delivered in drinking water (0.05 mg/ml) at a calculated dose of 10 mg/kg/day, based on an estimate of consumption of 3 ml of water/day/animal. The drug doses were calculated based on an average weight of 20 g/mouse. The animals received the drugs at these reduced doses in these experiments also to minimize any drug-induced hematopoietic inhibition.
Cytokine and anti-c-Mpl antibody treatment of SCID-hu mice.
The cytokines, Tpo (200 ng/mouse), Epo (20 U/mouse), and Tpo plus Epo, were administered intraperitoneally (54) to each of the mock-infected and HIV-1-infected SCID-hu animals, thrice a week. The cytokines were purchased from Stem Cell Technologies, Vancouver, Canada. For the c-Mpl blocking experiments, the animals were injected with the anti-c-Mpl or anti-Epo receptor (anti-EpoR) monoclonal antibody (see Table 2 for antibody concentrations). Mock- and HIV-1-infected SCID-hu Thy/Liv implants were injected with various concentrations of these monoclonal antibodies thrice a week. The anti-c-Mpl monoclonal antibody was purchased from BD Pharmingen (San Diego, Calif.). Anti-EpoR antibody was purchased from Upstate USA (Lake Placid, N.Y.).
TABLE 2.
Blocking of c-Mpl with anti-c-Mpl monoclonal antibody inhibits CFAa
| Engrafted CD34+ subset | Anti-c-mpl antibody treatment (ng/20 g mouse) | CFA
|
|||||
|---|---|---|---|---|---|---|---|
| Myeloid
|
Erythroid
|
Megakaryocytoid
|
|||||
| Mock | HIV-1 | Mock | HIV-1 | Mock | HIV-1 | ||
| c-mpl+ | 0 | 85.13 ± 6.47 | 39.63 ± 3.20 | 82.88 ± 7.04 | 38.13 ± 3.09 | 92.25 ± 5.52 | 43.00 ± 2.93 |
| c-mpl+ | 25 | 42.63 ± 3.20 | 13.38 ± 1.06 | 41.25 ± 3.49 | 13.00 ± 1.31 | 46.00 ± 2.93 | 14.38 ± 1.06 |
| c-mpl+ | 50 | 15.75 ± 1.04 | 1.13 ± 1.13 | 15.13 ± 1.46 | 1.38 ± 1.51 | 16.50 ± 1.20 | 0.50 ± 0.53 |
| c-mpl+ | 100 | 2.75 ± 1.49 | 0.88 ± 0.83 | 2.63 ± 1.51 | 1.13 ± 1.13 | 3.50 ± 1.20 | 0.43 ± 0.53 |
| c-mpl− | 0 | 18.75 ± 1.83 | 0.88 ± 0.83 | 18.00 ± 2.39 | 0.75 ± 0.71 | 20.75 ± 2.49 | 0.38 ± 0.52 |
| c-mpl+ | 100b | 67.63 ± 3.20 | 27.63 ± 3.20 | 20.50 ± 2.00 | 8.75 ± 1.28 | 71.00 ± 2.93 | 40.00 ± 2.62 |
Increasing anti-c-Mpl antibody concentration decrease multilineage CFA. Engrafted c-Mpl− CD34+ cells showed a partial reacquirement of c-Mpl expression and CFA. However, the c-Mpl− CD34+ cells exhibit background CFA levels prior to re-engraftment (data not shown). P values for statistical significance between comparisons of CFA from mean values for four animals each when different antibody concentrations (ng/20 g of mouse) were used are as follows: 0 versus 25, 0 versus 50, 0 versus 100, 25 versus 50, and 25 versus 100, P < 0.00001; 50 versus 100 for mock-infected animals, P < 0.00001; 50 versus 100 for HIV-1-infected animals, P = 0.6 to 0.8 (not significant). Determination of statistical significance for comparisons of myeloid, erythroid, and megakaryocytoid CFA between mock- and HIV-1-infected animals yielded P values of <0.00001 for antibody concentrations of 0, 25, and 50 ng/20 g of mouse, but at a 100 ng/20 g of mouse concentration, P values were < 0.01 for myeloid, < 0.05 for erythroid, and < 0.0001 for megakaryocytoid CFA. Determination of statistical significance for comparison between c-Mpl+ and c-Mpl− subset engraftment and when no antibody was used yielded P values of < 0.00001. Unlike the multilineage CFA inhibition with injection of anti-c-Mpl antibody, injection with the anti-Epo receptor monoclonal antibody preferentially inhibits erythroid colony formation. For comparisons of CFA between CD34+ cells of mock- versus HIV-1-infected implants and also between myeloid and erythroid and megakaryocytoid and erythroid lineages, P values were < 0.00001.
Anti-EpoR antibody was used.
Hematopoietic colony assays.
As described previously, myeloid and erythroid CFA of the cells derived from Thy/Liv implants was determined by plating 5 million cells in methylcellulose in the presence of 2 U of erythropoietin per ml and 100 ng of each of the growth factors, interleukin 3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor, and SCF (28), per ml. Megakaryocytoid colonies were counted using the megacult-C culture medium as recommended by the manufacturer. Methylcellulose and megacult-C are purchased from Stem Cell Technologies, Vancouver, Canada. These assays were performed in duplicate, and the colonies were counted by viewing them under the microscope.
Flow cytometry.
As reported earlier (28), FACS analyses were performed with the appropriately labeled monoclonal antibodies as described in the text or figure legends. Briefly, live cells were gated as determined by forward versus side scatter and analyzed using the CellQuest program (Becton Dickinson).
Statistical analyses.
Statistical tests between groups were conducted by utilizing the Wilcoxon rank sum test, a nonparametric test appropriate for this type of data, which is a nonparametric test that is powerful with small sample sizes (27). Cell counts were segregated by treatment and time points. Since replicated data can deflate the standard errors, we averaged the replicates for each mouse so that the data would retain their independence.
RESULTS
We have separated the CD34+ progenitor cells from the other cell types of mock- and HIV-1-infected Thy/Liv implants to study further the mechanisms and alleviation of virus-induced hematopoietic inhibition, following reconstitution of these CD34+ cells in a new Thy/Liv stromal microenvironment of irradiated SCID-hu secondary recipients. We have determined the phenotypic and consequent functional changes as assessed ex vivo by c-Mpl expression and multilineage CFA, respectively, of CD34+ cells, during and in the absence of HIV-1 infection in vivo.
Transfer and re-engraftment of CD34+ progenitor cells into a new stromal microenvironment in vivo. It is found that the endogenous human CD34+ cells derived from the Thy/Liv implants of irradiated animals are rendered functionally inactive as assessed by their CFA, which is reduced to background levels (data not shown). The irradiated animals steadily lose the preexisting “endogenous” CD34+ cells of their Thy/Liv implants, as shown by flow cytometric analyses, using anti-CD38-fluorescein isothiocyanate (FITC) and anti-CD34-PE monoclonal antibodies (Fig. 1B). It is likely that postirradiation, the loss of the CD34 phenotype is due to changes in differentiation leading to gradual killing of the CD34+ cells. Eventual death of the CD34− cells beyond 3 weeks postirradiation has not been tested, since these CD34− CD38− or CD34− CD38+ cells do not exhibit CFA, and hence is not relevant to our studies. Further, these coexpressing CD38+ cells are also selected out in any harvest or purification of the CD34+ cells. The Thy/Liv implants of these animals are (re)engrafted with purified CD34+ CD38− cells (Fig. 1A) derived from the Thy/Liv implants of the primary recipients, following irradiation of the animals to be used as secondary recipients.
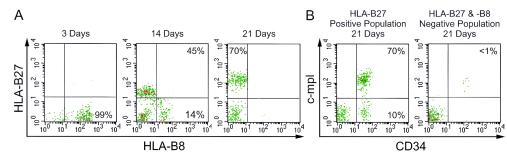
Following reconstitution of the human CD34+ cells derived from primary-recipient Thy/Liv implants after their transfer into the implants of secondary recipients (Fig. 1C), these engrafted and exogenous CD34+ cells were distinguished from the endogenous CD34+ cells by flow cytometric analysis. This FACS analysis was performed on the CD34+ cells derived from secondary recipients by using anti-HLA-B8 monoclonal antibody to detect endogenous cells and anti-HLA-B27 monoclonal antibody to detect re-engrafted exogenous donor cells originally derived and transferred from the implants of primary recipients (Fig. 2A). However, in the implants of irradiated secondary-recipient animals, endogenous CD34+ cells (HLA-B8+) were present only at background levels, in addition to their loss of hematopoietic potential (CFA) following reconstitution at 3 weeks post-re-engraftment. Figure 2A illustrates using anti-HLA-B27-PE and anti-HLA-B8-FITC monoclonal antibodies for FACS analyses, the repopulation of the donor HLA-B27+ CD34+ cells in the Thy/Liv implants of the irradiated secondary recipients, which gradually are depleted of their endogenous HLA-B8+ cells. These cells are also colabeled with anti-c-Mpl-biotin-streptavidin red 613 (two step) (21) and anti-CD34-APC antibodies for further backgating analyses. Figure 2B shows by backgating on the repopulated HLA-B27+ cells of Fig. 2A that at 21 days post-re-engraftment, the reconstituted HLA-B27+ CD34+ cells are about 70 to 80% c-Mpl+ CD34+. It is also shown in Fig. 2B, by backgating on an HLA-B27 and HLA-B8 double-negative population (Fig. 2A, 21 days, lower left quadrant), that their CD34+ cell population is present only at background levels, if at all (Fig. 2B), due to loss following irradiation. Thus, these data confirm our observation of depletion of preexisting HLA-B8+ CD34+ cells following irradiation (Fig. 1B and 2) and also allow the reconstitution of re-engrafted HLA-B27+ CD34+ cells (Fig. 2).
FIG. 2.
Repopulation of donor CD34+ cells post-re-engraftment in the Thy/Liv implants of irradiated secondary SCID-hu recipients. (A) Reconstitution of exogenous HLA-B27+ CD34+ cells derived from the Thy/Liv implants of primary recipient SCID-hu animals following re-engraftment into the implants of irradiated secondary recipients endogenous for HLA-B8+ cells. (B) Backgating analysis of the HLA-B27+ and HLA-B27−/HLA-B8− cell populations for c-Mpl and CD34 expression at 3 weeks post-re-engraftment. Both phenotypic and functional reconstitution following re-engraftment into irradiated secondary recipients is about 70 to 80%, as assessed by the proportion of c-Mpl+ CD34+ subset cells shown in this figure, and overall multilineage CFA (shown in Fig. 3), respectively, at 3 weeks post-re-engraftment.
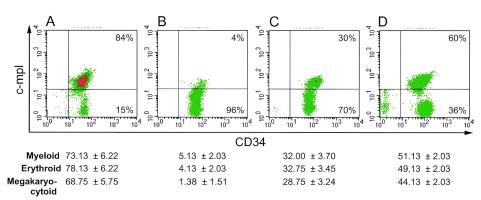
Decrease of c-Mpl expression and CFA by the CD34+ cells derived from the Thy/Liv implants of HIV-1-infected SCID-hu animals and their revival in a new stromal microenvironment. Our data indicate that HIV-1NL4-3 infection of the SCID-hu animals results in the loss or decrease of c-Mpl expression of the CD34+ cells derived from their Thy/Liv implants (Fig. 3B). Such a decrease of c-Mpl expression is also accompanied by a decrease in hematopoietic CFA of these cells (also shown as part of Fig. 3 below the FACS profiles). Following re-engraftment of these CD34+ cells that suffered the loss of c-Mpl due to the exposure and indirect effects of HIV-1 infection of the thymocytes of the Thy/Liv implants, a partial reacquirement of c-Mpl expression occurs (Fig. 3C), again accompanied by a partial resurgence of CFA, at 3 weeks post-re-engraftment. Both reacquirement of c-Mpl expression and CFA revival are considerably enhanced by treatment of the engrafted SCID-hu animals with the c-Mpl ligand, Tpo (Fig. 3D). These results suggest that CFA correlates with c-Mpl expression and that with transfer of CD34+ cells that suffered the effects of HIV-1, the effects are reversible, both in phenotype and function, in a new stromal microenvironment.
FIG. 3.
Loss of c-Mpl expression and CFA by the CD34+ CD38− cells, due to HIV-1 infection, and their revival post-re-engraftment and post-Tpo treatment. The CD34+ cells that suffered the deleterious consequences of HIV-1-mediated effects in the primary recipient Thy/Liv implants were re-engrafted in equal numbers of 5 × 104 as described in Materials and Methods into the implants of irradiated secondary recipients (FACS profiles B, C, and D). The revival of c-Mpl expression and CFA post-re-engraftment was further enhanced by Tpo treatment of the animals. The c-Mpl expression is shown by the flow cytometric profiles, and the corresponding CFA levels for 300 input cells are shown directly below their FACS profiles. (A) CD34+ cells derived from implants of mock-infected primary recipients, pre-re-engraftment. (B) CD34+ cells derived from implants of HIV-1-infected primary recipients, pre-re-engraftment. (C) CD34+ cells derived from implants of irradiated secondary recipients 3 weeks post-re-engraftment with CD34+ cells derived from implants of HIV-1-infected primary recipients. (D) CD34+ cells derived from implants of irradiated secondary recipients 3 weeks post-re-engraftment with CD34+ cells derived from implants of HIV-1-infected primary recipients and Tpo treatment. Statistical analyses of the CFA comparisons using four animals each were performed and obtained as follows: statistical significance was seen in the comparisons of CFA between erythroid and megakaryocytoid lineages, with P values ranging from 0.0002 to 0.03. Also showing significance were the P values for myeloid versus megakaryocytoid CFA, P < 0.001. Among each of the categories of CFA of myeloid, erythroid, and megakaryocytoid lineages, comparisons between mock (pre-engraftment) and HIV-1 (pre-engraftment), mock (pre-engraftment) and HIV-1 (postengraftment), HIV-1 (postengraftment) and HIV-1 (postengraftment and Tpo treatment), and HIV-1 (pre-engraftment) and HIV-1 (postengraftment) were all statistically significant, with P values of <0.0001.
Reacquirement of c-Mpl expression and revival of CFA following re-engraftment of CD34+ cells from primary into secondary SCID-hu recipients.
Following re-engraftment into secondary recipients, the levels of CD34+ cells positive for c-Mpl expression, which were decreased due to exposure to an HIV-1-infected microenvironment in the SCID-hu Thy/Liv implants of the primary recipients, were restored or increased by about 50%, to 70% of levels for the mock-infected implants (Fig. 2). It is suggested that the c-Mpl phenotype lost by CD34+ cells due to exposure to an infected microenvironment in the primary recipient SCID-hu Thy/Liv implants was reacquired (Fig. 3C and D) due to self-renewal of the CD34+ CD38− cells (Fig. 1C) when reconstituted in the new stromal microenvironment of the secondary recipients. Since c-Mpl is activated by its ligand, Tpo, we administered the cytokines Tpo, Epo, or Tpo plus Epo to separate mock- and HIV-1-infected SCID-hu animals (see Materials and Methods). There was a partial recovery (∼60%) of CFA by the re-engrafted CD34+ cells upon Tpo treatment following reconstitution in a new stromal microenvironment compared to results for mock-infected implants (Fig. 3D). Cytokine treatment increased the CFA of these reconstituted CD34+ cells from these Thy/Liv implants compared to those of untreated animals by 45 to 75% in secondary recipients (Table 1).
TABLE 1.
Revival of multilineage CFA of CD34+ cells derived from primary and re-engrafted secondary-recipient SCID-hu Thy/Liv implantsa
| Infection or exposure | Cytokine treatment | No. of colonies from Thy/Liv implants of SCID-hu recipientsa
|
|||||
|---|---|---|---|---|---|---|---|
| Myeloid
|
Erythroid
|
Megakaryocytoid
|
|||||
| Primary | Secondary | Primary | Secondary | Primary | Secondary | ||
| Mock | None | 77.38 ± 5.83 | 85.00 ± 5.71 | 84.00 ± 6.19 | 84.88 ± 5.74 | 73.88 ± 4.73 | 81.88 ± 5.49 |
| Tpo | 81.88 ± 5.74 | 87.00 ± 5.71 | 78.88 ± 5.25 | 88.88 ± 6.29 | 84.88 ± 5.62 | 90.00 ± 5.71 | |
| Epo | 74.88 ± 4.73 | 81.00 ± 5.71 | 86.00 ± 5.71 | 91.00 ± 5.71 | 81.00 ± 5.50 | 74.88 ± 6.64 | |
| Tpo + Epo | 84.00 ± 5.71 | 91.00 ± 5.71 | 88.88 ± 4.73 | 97.00 ± 5.71 | 86.00 ± 5.71 | 84.00 ± 6.09 | |
| HIV-1 (NL4-3) | None | 9.13 ± 2.03 | 39.13 ± 2.03 | 7.13 ± 2.03 | 37.13 ± 2.03 | 2.00 ± 1.60 | 36.13 ± 2.03 |
| Tpo | 14.00 ± 1.85 | 52.13 ± 2.03 | 12.00 ± 1.85 | 55.13 ± 2.03 | 13.00 ± 1.85 | 61.13 ± 2.03 | |
| Epo | 15.00 ± 3.21 | 37.00 ± 1.85 | 15.00 ± 3.38 | 58.13 ± 2.03 | 1.00 ± 1.41 | 28.00 ± 3.70 | |
| Tpo + Epo | 19.00 ± 3.21 | 55.13 ± 2.03 | 16.00 ± 3.38 | 64.13 ± 2.90 | 5.00 ± 1.60 | 65.13 ± 2.90 | |
Secondary recipients were reconstituted with CD34+ cells from implants of mock or infected primary recipients and were not further infected postreconstitution. The myeloid and erythroid colonies were enumerated in methyl cellulose as described earlier. The megakaryocytoid colonies were enumerated separately in megacult-C according to the protocol provided by the manufacturer (Stem Cell Technologies, Vancouver, Canada). Three hundred input CD34+ cells were seeded for colony formation. The average values (± standard deviations) for four animals, each enumerated in duplicate for each category, are treated as described in the Methods section for statistical analysis. All the comparisons between CFA values of mock- and HIV-1-infected data for each of myeloid, erythroid, and megakaryocytoid colony formation show statistical significance, with P values of < 0.00001. The comparison of CFA values for HIV-1-infected or exposed implants between primary and secondary recipients for each of the myeloid, erythroid, and megakaryocytoid lineages yields statistical significance with P values of < 0.0001.
Effect of blocking c-Mpl on multilineage CFA.
We thus established a correlation between c-Mpl expression and multilineage CFA of CD34+ cells (Fig. 3). In addition, we investigated whether blocking of c-Mpl in vivo by injecting the SCID-hu animals with anti-c-Mpl monoclonal antibody would result in the reduction or loss of CFA compared to levels for untreated animals. These results indicate that blocking of c-Mpl inhibits CFA in an antibody concentration-dependent manner and that inhibition, absence, or loss of c-Mpl expression, as in c-Mpl−CD34+ subset cells, is the likely cause of CFA inhibition (Table 2). This anti-c-Mpl antibody inhibits multilineage CFA, thus confirming the reports of the role of c-Mpl in multilineage hematopoiesis (4, 6, 17, 18, 23, 25, 48, 50). However, used as a control, the anti-Epo receptor (EpoR) monoclonal antibody preferentially inhibits erythropoiesis to a considerable extent (∼25%) but not myelopoiesis and megakaryopoiesis (Table 2).
Thus, our results on the role of Tpo/c-Mpl in the regulation of multilineage hematopoiesis and its revival will allow us to further understand the mechanisms of HIV-1-induced hematopoietic inhibition and help develop new treatment strategies for HIV-infected individuals suffering from cytopenias. Thus, the combined therapeutic strategy of CD34+ cell re-engraftment and cytokine treatment is promising for alleviation of HIV-1-induced hematopoietic inhibition and hence multiple cytopenias.
DISCUSSION
To enable sustained multilineage differentiation of the CD34+ progenitor cells and cessation of the hematopoietic inhibition induced by the indirect effects of HIV-1 infection in vivo, it is important to minimize the loss of CFA of CD34+ cells as assessed ex vivo from the SCID-hu model. We have established that CD34+ cells are not infected by HIV-1, yet are functionally impaired to the extent of not being able to differentiate into myeloid, erythroid and megakaryocytoid colonies, when these progenitors are exposed to the indirect effects of HIV-1 within their cellular microenvironment. Our data herein indicate the following: (i) re-engraftment of CD34+ cells into a fresh stromal microenvironment enhances c-Mpl expression and CFA (Fig. 3C and D); (ii) treatment of HIV-1-infected SCID-hu mice with Tpo further enhances multilineage CFA of CD34+ progenitor cells when re-engrafted into secondary recipients (Fig. 3D and Table 1); (iii) HIV-1 infection results in decreased numbers of CD34+ c-Mpl+ cells (Fig. 3B); and (iv) blocking of c-Mpl with anti-c-Mpl antibody inhibits CFA in vivo (Table 2). Together these results suggest that CFA generally segregates with c-Mpl expression, and thus, c-Mpl is likely involved in HIV-1-mediated effects on the differentiation of these progenitor cells. Though c-Mpl is known preferentially for its megakaryocytic lineage-directed differentiation, c-Mpl is also known to be involved in multilineage hematopoiesis and therefore may be responsible for the induction of multiple cytopenias in HIV-infected patients. Epo and Tpo were found to be synergistic on CFA of erythroid lineage, while Tpo also promotes myelopoiesis (23, 24, 50). Therefore, changes in c-Mpl expression levels are likely to play an important role not only in the induction or development of thrombocytopenia involving changes in megakaryopoiesis but for cytopenias in general in HIV-infected individuals and those receiving highly active antiretroviral therapy (HAART). In addition to perturbation of cytokine expression (3, 5, 10, 13, 14, 24, 26, 27, 32, 38, 41, 45, 46, 51, 56, 58), levels of expression of cytokine or growth factor receptors may also be altered due to HIV infection, resulting in changes in the binding capacity of the appropriate cytokine ligands to their receptors, affecting hematopoiesis. Thus, we have established a correlation between c-Mpl expression and multilineage CFA of CD34+ cells in vivo.
Therefore, possible mechanisms for HIV-induced hematopietic inhibition in HIV-infected individuals most likely include the involvement of c-Mpl, since this cytokine receptor is known to play a role in stem cell differentiation, through its activation of signaling pathways (37). We will therefore continue such investigation, which is significant due to the evident role of c-Mpl in HIV-1-induced multilineage hematopoietic inhibition and ensuing cytopenias, since they result in immune suppression or contribute to other disorders, such as cardiac dysfunction, in patients suffering from thrombocytopenia (11, 20, 40, 55). Our earlier studies revealed that the resurgence of CFA following HIV-1-induced hematopoietic inhibition due to treatment with combination antiretroviral drugs is only transient (28), and other investigators have found that these drugs induced cytopenias in HIV-infected individuals (34, 35). Therefore, a treatment that combines the use of cytokines (Tpo, Epo, other) and drugs, such as ritonavir, with a dual effect of being a protease inhibitor and having antiapoptotic activity (49), together with engraftment of c-Mpl+ CD34+ cells, might stabilize hematopoiesis for extended periods of time. Consequently, autologous bone marrow transplantation for HIV-infected patients with lentivirus-transduced CD34+ cells expressing human c-Mpl may provide further relief from hematopoietic abnormalities to these individuals.
Thrombocytopenia is more persistent than the other cytopenias (15, 19, 42), and it contributes to cardiac dysfunction (11, 20, 35, 40, 55). Cardiac patients undergoing coronary surgery also develop thrombocytopenia, and for HIV-infected individuals receiving HAART and who also require cardiovascular surgery, it can present a greater risk (55). Therefore, this work deals with the preservation or enhancement of c-Mpl-mediated CD34+-progenitor-cell differentiation to achieve sustained multilineage hematopoiesis during HIV-1 infection and hence reduce the occurrence of different cytopenias, including thrombocytopenia.
Acknowledgments
We thank Vizarath Ali and Shehma Khan for technical assistance, Greg Bristol for manipulations with the SCID-hu animals, Michael Gulrajani for assistance with flow cytometric analyses, and Donna Crandall for graphics and illustrations.
This work was supported by grants from the University of California Universitywide AIDS Research Program (ID01-LA-082) and the National Institutes of Health (HL79846) to P.S.K. and from the National Institutes of Health (HL71776) to S.T.R.
REFERENCES
- 1.Akkina, R., J. D. Rosenblatt, A. G. Campbell, I. S. Y. Chen, and J. A. Zack. 1994. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood 84:1393-1398. [PubMed] [Google Scholar]
- 2.Aldrovandi, G. M., G. Feuer, L. Gao, M. Kristeva, I. S. Y. Chen, B. D. Jamieson, and J. A. Zack. 1993. HIV-1 infection of the SCID-hu mouse: an animal model for virus pathogenesis. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, W. 1998. Cytokines in hematopoiesis. Int. Rev. Immunol. 16:651-682. [DOI] [PubMed] [Google Scholar]
- 4.Amado, R. G., G. Symonds, B. D. Jamieson, G. Zhao, J. D. Rosenblatt, and J. A. Zack. 1998. Effects of megakaryocyte growth and development factor on survival and retroviral transduction of T lymphoid progenitor cells. Hum. Gene Ther. 9:173-183. [DOI] [PubMed] [Google Scholar]
- 5.Bailer, R. T., A. Holloway, J. Sun, J. B. Margolick, M. Martin, J. Kostman, and L. J. Montaner. 1999. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J. Immunol. 162:7534-7542. [PubMed] [Google Scholar]
- 6.Ballmaier, M., M. Germeshausen, H. Schulze, K. Cherkaoui, S. Lang, A. Gaudig, S. Krukemeier, M. Eilers, G. Straul, and K. Welte. 2001. C-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood 97:139-146. [DOI] [PubMed] [Google Scholar]
- 7.M. L. Bonyhadi, L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 8.Calenda, V., and J. C. Chermann. 1992. The effects of HIV on hematopoiesis. Eur. J. Hematol. 48:181-186. [DOI] [PubMed] [Google Scholar]
- 9.Chelucci, C., I. Casella, M. Federico, U. Testa, G. Macioce, E. Pelosi, R. Guerriero, G. Mariani, A. Giampaolo, H. J. Hassan, and C. Peschle. 1999. Lineage-specific expression of human immunodeficiency virus (HIV) receptor/coreceptors in differentiating hematopoietic precursors: correlation with susceptibility to T- and M-tropic HIV and chemokine-mediated HIV resistance. Blood 94:1590-1600. [PubMed] [Google Scholar]
- 10.Clerici, M., D. Trabattoni, S. Piconi, M. L. Fusi, S. Ruzzante, C. Clerici, and M. L. Villa. 1997. A possible role for the cortisole/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinology 22:S27-S31. [DOI] [PubMed] [Google Scholar]
- 11.Daszynski, J., and T. Ciszewski. 1989. Blood component therapy in open heart surgery. Master Med. Pol. 21:207-211. [PubMed] [Google Scholar]
- 12.Davis, B. R., and G. Zauli. 1995. Effect of human immunodeficiency virus infection on hematopoiesis. Bailliers Clin. Hematol. 8:113-130. [DOI] [PubMed] [Google Scholar]
- 13.Esser, R., W. Glienke, R. Andreesen, R. E. Unger, M. Kreutz, H. Rubsamen-Waigmann, and H. von Briesen. 1998. Individual cell analysis of the cytokine repertoire in human immunodeficiency virus-1-infected monocytes/macrophages by a combination of immunocytochemistry and in situ hybridization. Blood 91:4752-4760. [PubMed] [Google Scholar]
- 14.Estcourt, C., Y. Rousseau, H. M. Sadeghi, N. Thieblemont, M. P. Carreno, L. Weiss, and N. Haeffner-Cavaillon. 1997. Flow-cytometric assessment of in vivo cytokine-producing monocytes in HIV-infected patients. Clin. Immunol. Immunopathol. 83:60-67. [DOI] [PubMed] [Google Scholar]
- 15.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 16.Geissler, R. G., O. G. Ottmann, K. Kleiner, I. U. Mentze, A. Bickelhaupt, D. Hoelzer, and A. Ganser. 1993. Decreased hematopoietic colony growth in long-term bone marrow cultures of HIV-positive patients. Res. Virol. 144:69-73. [DOI] [PubMed] [Google Scholar]
- 17.Germeshausen, M., M. Ballmaier, and K. Welte. 2001. Implications of mutations in hematopoietic growth factor receptor genesin congenital cytopenias. Ann. N. Y. Acad. Sci. 938:305-321. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves, F., C. Lacout, J. L. Villeval, F. Wendling, W. Vainchenker, and D. Dumenil. 1997. Thrombopoietin does not induce lineage-restricted commitment of Mpl-R expressing pluripotent progenitors but permits their complete erythroid and megakaryocytic differentiation. Blood 89:3544-3553. [PubMed] [Google Scholar]
- 19.Harbol, A. W., J. L. Liesveld, P. J. Simpson-Haidaris, and C. N. Abboud. 1994. Mechanisms of cytopenia in human immunodeficiency virus infection. Blood Rev. 8:241-251. [DOI] [PubMed] [Google Scholar]
- 20.Jakobs, D. 2001. Heparin induced thrombocytopenia, left ventricular thrombus and cerebral embolism during an acute myocardial infarction. Med. Klin. 96:101-104. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson, B. D., and J. A. Zack. 1998. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J. Virol. 72:6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins, M., M. B. Hanley, M. B. Moreno, E. Wieder, and J. M. McCune. 1998. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood 91:2672-2678. [PubMed] [Google Scholar]
- 23.Kaushansky, K. 1998. Thrombopoietin and the hematopoietic stem cell. Blood 92:1-3. [PubMed] [Google Scholar]
- 24.Kaushansky, K., V. C. Broudy, A. Grossmann, J. Humes, N. Lin, H. P. Ren, M. C. Bailey, T. Papayannopoulou, J. W. Forstrom, and K. H. Sprugel. 1995. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. J. Clin. Investig. 96:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushansky, K., N. Lin, A. Grossman, J. Humes, K. H. Sprugel, and V. C. Broudy. 1996. Thrombopoietin expands erythroid, granulocyte-macrophage, and megakaryocytic progenitor cells in normal and myelosuppressed mice. Exp. Hematol. 24:265-269. [PubMed] [Google Scholar]
- 26.Klein, S. A., J. M. Dobmeyer, T. S. Dobmeyer, M. Pape, O. G. Ottmann, E. B. Helm, D. Hoelzer, and R. Rossol. 1997. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS 11:1111-1118. [DOI] [PubMed] [Google Scholar]
- 27.Koka, P. S., D. G. Brooks, A. Razai, C. M. Kitchen, and J. A. Zack. 2003. HIV type 1 infection alters cytokine mRNA expression in thymus. AIDS Res. Hum. Retrovir. 19:1-12. [DOI] [PubMed] [Google Scholar]
- 28.Koka, P. S., J. K. Fraser, Y. Bryson, G. C. Bristol, G. M. Aldrovandi, E. S. Daar, and J. A. Zack. 1998. Human immunodeficiency virus type 1 inhibits multi-lineage hematopoiesis in vivo. J. Virol. 172:5121-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koka, P. S., B. D. Jamieson, D. G. Brooks, and J. A. Zack. 1999. Human immunodeficiency virus type-1 induced hematopoietic inhibition is independent of productive infection of progenitor cells in vivo. J. Virol. 73:9089-9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koka, P. S., and S. T. Reddy. 2004. Cytopenias in HIV infection: mechanisms and alleviation of hematopoietic inhibition. Curr. HIV Res. 2:275-282. [DOI] [PubMed] [Google Scholar]
- 31.T. R. Kollmann, A. Kim, M. Peettoello-Mantovani, M. Hachamovitch, A. Rubinstein, M. M. Goldstein, and H. Goldstein. 1995. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J. Immunol. 154:908-921. [PubMed] [Google Scholar]
- 32.Lowry, P. A. 1995. Hematopoietic stem cell cytokine response. J. Cell. Biochem. 58:410-415. [DOI] [PubMed] [Google Scholar]
- 33.McCune, J. M., R. Namikawa, H. Kaneshima, L. D. Schultz, M. Lieberman, and L. Weissman. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632-1639. [DOI] [PubMed] [Google Scholar]
- 34.Miles, S. A., S. Lee, L. Hutlin, K. M. Zsebo, and R. T. Mitsuyasu. 1991. Potential use of human stem cell factor as adjunctive therapy for human immunodeficiency virus-related cytopenias. Blood 78:3200-3208. [PubMed] [Google Scholar]
- 35.Miles, S. A., R. T. Mitsuyasu, J. Moreno, G. Baldwin, N. K. Alton, L. Souza, and J. A. Glaspy. 1991. Combined therapy with recombinant granulocyte colony-stimulating factor and erythropoietin decreases hematologic toxicity from zidovudine. Blood 77:2109-2117. [PubMed] [Google Scholar]
- 36.Mir, N., C. Costello, J. Luckit, and R. Lindley. 1989. HIV-disease and bone marrow changes: a study of 60 cases. Eur. J. Hematol. 42:339-343. [DOI] [PubMed] [Google Scholar]
- 37.Miyakawa, Y., J. G. Drachman, B. Gallis, A. Kaushansky, and K. Kaushansky. 2000. A structure-function analysis of serine/threonine phosphorylation of the thrombopoietin receptor, c-Mpl. J. Biol. Chem. 275:32214-32219. [DOI] [PubMed] [Google Scholar]
- 38.Moses, A., J. Nelson, and G. C. Bagby, Jr. 1998. The influence of human immunodeficiency virus-1 of hematopoiesis. Blood 91:1479-1495. [PubMed] [Google Scholar]
- 39.Namikawa, R., K. N. Weilbaecher, H. Kaneshima, E. J. Yee, and J. M. McCune. 1990. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 172:1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podolsky, S. H., A. Zembowicz, F. J. Schoen, R. J. Benjamin, and L. A. Sonna. 1999. Massive myocardial necrosis in thrombotic thrombocytopenic purpura: a case report and review of the literature. Arch. Pathol. Lab. Med. 123:937-940. [DOI] [PubMed] [Google Scholar]
- 41.Rafii, S., R. Mohle, F. Shapiro, B. M. Frey, and M. A. Moore. 1997. Regulation of hematopoiesis by microvascular endothelium. Leuk. Lymphoma 27:375-386. [DOI] [PubMed] [Google Scholar]
- 42.Ratner, L. 1989. Human immunodeficiency virus-associated autoimmune thrombocytopenic purpura: a review. Am. J. Med. 86:194-198. [DOI] [PubMed] [Google Scholar]
- 43.Re, M. C., G. Zauli, G. Furlini, S. Rarieri, P. Monari, E. Ramazzotti, and M. LaPlaca. 1993. The impaired number of circulating granulocyte/macrophage progenitors (CFU-GM) in human immunodeficiency virus type 1 infected subjects correlates with an active HIV-1 replication. Arch. Virol. 129:53-64. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz, M. E., C. Ciala, J. Arthos, A. Kinter, A. T. Catanzaro, J. Adelsberger, K. L. Holmes, O. J. Cohen, and A. S. Fauci. 1998. Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J. Immunol. 161:4169-4176. [PubMed] [Google Scholar]
- 45.Sachs, L. 1996. Molecular control of development in normal and leukemia myeloid cells by cytokines, tumor suppressor and oncogenes. Curr. Top. Microbiol. Immunol. 211:3-5. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzmeier, J. D. 1996. The role of cytokines in haematopoiesis. Eur. J. Hematol. 57:69-74. [DOI] [PubMed] [Google Scholar]
- 47.Shen, H., T. Cheng, F. I. Preffer, D. Dombkowski, M. H. Tomasson, D. E. Golan, O. Yang, W. Hofmann, J. G. Sodroski, A. D. Luster, and D. T. Scadden. 1999. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J. Virol. 73:728-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvestris, F., P. Cafforio, M. Tucci, and F. Dammacco. 2002. Negative regulation of erythroblast maturation by Fas-L+/TRAIL+ highly malignant plasma cells: a major pathogenic mechanism of anemia in multiple myeloma. Blood 99:1305-1313. [DOI] [PubMed] [Google Scholar]
- 49.Soland, E. M., J. Maciejewski, P. Kumar, S. Kim, A. Chaudhuri, and N. Young. 2000. Protease inhibitors stimulate hematopoiesis and decrease apoptosis and ICE expression in CD34+ cells. Blood 96:2735-2739. [PubMed] [Google Scholar]
- 50.Solar, G. P., W. G. Kerr, F. C. Zeigler, D. Hess, C. Donahue, F. J. de Sauvage, and D. L. Eaton. 1998. Role of c-mpl in early hematopoiesis. Blood 92:4-10. [PubMed] [Google Scholar]
- 51.Speth, C., and M. P. Dierich. 1999. Modulation of cell surface protein expression by infection with HIV-1. Leukemia 13:99-105. [DOI] [PubMed] [Google Scholar]
- 52.Stanley, S. K., J. M. McCune, H. Kaneshima, J. S. Justement, M. Sullivan, E. Boone, M. Baseler, J. Adelsberger, M. Bonyhadi, J. Orenstein, C. H. Fox, and A. S. Fauci. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun, N. C. J., P. Shapshak, N. A. Lachant, M. Hsu, L. Sieger, P. Schmid, G. Beall, and D. T. Imagawa. 1989. Bone marrow examination in patients with AIDS and AIDS-related complex (ARC). Am. J. Clin. Pathol. 92:589-594. [DOI] [PubMed] [Google Scholar]
- 54.Uittenbogaart, C. H., W. J. Boscardin, D. J. Anisman-Posner, P. S. Koka, G. Bristol, and J. A. Zack. 2000. Effect of cytokines on HIV-induced depletion of thymocytes in vivo. AIDS 14:1317-1325. [DOI] [PubMed] [Google Scholar]
- 55.Utley, J. R. 1990. Pathophysiology of cardiopulmonary bypass: current issues. J. Card. Surg. 5:177-189. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J., A. Harada, S. Matsushita, S. Matsumi, Y. Zhang, T. Shioda, Y. Nagaai, and K. Matsushima. 1998. IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+ T lymphocytes and enhance HIV-1 replication. J. Leuk. Biol. 64:642-649. [DOI] [PubMed] [Google Scholar]
- 57.Withers-Ward, E. S., R. G. Amado, P. S. Koka, B. D. Jamieson, A. H. Kaplan, I. S. Y. Chen, and J. A. Zack. 1997. Transient renewal of thymopoiesis in HIV infected human thymic implants following antiviral therapy. Nat. Med. 3:1102-1109. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi, H., E. Ishii, K. Tashiro, and S. Miyazaki. 1998. Role of umbilical vein endothelial cells in hematopoiesis. Leuk. Lymphoma 31:61-69. [DOI] [PubMed] [Google Scholar]