Abstract
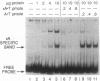
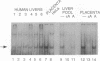
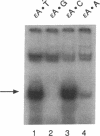
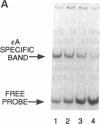
A human DNA binding protein has been characterized from cell-free extracts of liver, placenta, and cultured cells. This protein, apparent molecular mass approximately 35 kDa, to our knowledge, does not resemble other proteins reported to bind to carcinogen-modified DNA. The probe used for characterization was a 25-base oligonucleotide containing a single site-specifically placed 1,N6-ethenoadenine (epsilon A), a product of vinyl chloride metabolism. When annealed to form an epsilon A.T or epsilon A.C pair, a strong affinity to the protein was observed, with a binding constant of approximately 1 x 10(9) M-1. In contrast, very little binding was found with an epsilon A.A pair and none was found with an epsilon A.G pair. This suggests protein recognition of a specific structural alteration. Other defined probes with alkyl adducts did not bind. In addition, the human cell extracts and a rat liver extract were found to nick specifically at the 5' side of the epsilon A adduct, which could indicate a possible associated repair activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Donahue B. A., Augot M., Bellon S. F., Treiber D. K., Toney J. H., Lippard S. J., Essigmann J. M. Characterization of a DNA damage-recognition protein from mammalian cells that binds specifically to intrastrand d(GpG) and d(ApG) DNA adducts of the anticancer drug cisplatin. Biochemistry. 1990 Jun 19;29(24):5872–5880. doi: 10.1021/bi00476a032. [DOI] [PubMed] [Google Scholar]
- Eberle G., Barbin A., Laib R. J., Ciroussel F., Thomale J., Bartsch H., Rajewsky M. F. 1,N6-etheno-2'-deoxyadenosine and 3,N4-etheno-2'-deoxycytidine detected by monoclonal antibodies in lung and liver DNA of rats exposed to vinyl chloride. Carcinogenesis. 1989 Jan;10(1):209–212. doi: 10.1093/carcin/10.1.209. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Lucas J. L., Dannenberg A. A damage-specific DNA binding protein. Large scale purification from human placenta and characterization. J Biol Chem. 1982 Jun 10;257(11):6394–6401. [PubMed] [Google Scholar]
- Guengerich F. P., Geiger L. E., Hogy L. L., Wright P. L. In vitro metabolism of acrylonitrile to 2-cyanoethylene oxide, reaction with glutathione, and irreversible binding to proteins and nucleic acids. Cancer Res. 1981 Dec;41(12 Pt 1):4925–4933. [PubMed] [Google Scholar]
- Jiricny J., Hughes M., Corman N., Rudkin B. B. A human 200-kDa protein binds selectively to DNA fragments containing G.T mismatches. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8860–8864. doi: 10.1073/pnas.85.23.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchakdjian M., Eisenberg M., Yarema K., Basu A., Essigmann J., Patel D. J. NMR studies of the exocyclic 1,N6-ethenodeoxyadenosine adduct (epsilon dA) opposite thymidine in a DNA duplex. Nonplanar alignment of epsilon dA(anti) and dT(anti) at the lesion site. Biochemistry. 1991 Feb 19;30(7):1820–1828. doi: 10.1021/bi00221a014. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U. Comparison of apurinic DNA-binding protein from an ataxia telangiectasia and a HeLa cell line. Evidence for an altered processing of apurinic/apyrimidinic endonuclease. J Biol Chem. 1985 Dec 5;260(28):14918–14924. [PubMed] [Google Scholar]
- Leithauser M. T., Liem A., Stewart B. C., Miller E. C., Miller J. A. 1,N6-ethenoadenosine formation, mutagenicity and murine tumor induction as indicators of the generation of an electrophilic epoxide metabolite of the closely related carcinogens ethyl carbamate (urethane) and vinyl carbamate. Carcinogenesis. 1990 Mar;11(3):463–473. doi: 10.1093/carcin/11.3.463. [DOI] [PubMed] [Google Scholar]
- Lenz J., Okenquist S. A., LoSardo J. E., Hamilton K. K., Doetsch P. W. Identification of a mammalian nuclear factor and human cDNA-encoded proteins that recognize DNA containing apurinic sites. Proc Natl Acad Sci U S A. 1990 May;87(9):3396–3400. doi: 10.1073/pnas.87.9.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard N. J. Etheno-substituted nucleotides and coenzymes: fluorescence and biological activity. CRC Crit Rev Biochem. 1984;15(2):125–199. doi: 10.3109/10409238409102299. [DOI] [PubMed] [Google Scholar]
- Ludlum D. B. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990 Nov-Dec;233(1-2):117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Moranelli F., Lieberman M. W. Recognition of chemical carcinogen-modified DNA by a DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3201–3205. doi: 10.1073/pnas.77.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Bodell W. J. Detection of 3'-hydroxy-1,N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabeling of DNA reacted with p-benzoquinone. Chem Res Toxicol. 1991 Mar-Apr;4(2):199–202. doi: 10.1021/tx00020a012. [DOI] [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Revich G. G., Beattie K. L. Utilization of 1,N6-etheno-2'-deoxyadenosine 5'-triphosphate during DNA synthesis on natural templates, catalyzed by DNA polymerase I of Escherichia coli. Carcinogenesis. 1986 Sep;7(9):1569–1576. doi: 10.1093/carcin/7.9.1569. [DOI] [PubMed] [Google Scholar]
- Singer B., Abbott L. G., Spengler S. J. Assessment of mutagenic efficiency of two carcinogen-modified nucleosides, 1,N6-ethenodeoxyadenosine and O4-methyldeoxythymidine, using polymerases of varying fidelity. Carcinogenesis. 1984 Sep;5(9):1165–1171. doi: 10.1093/carcin/5.9.1165. [DOI] [PubMed] [Google Scholar]
- Stephenson C., Karran P. Selective binding to DNA base pair mismatches by proteins from human cells. J Biol Chem. 1989 Dec 15;264(35):21177–21182. [PubMed] [Google Scholar]
- Tsang S. S., Kuhnlein U. DNA-binding protein from HeLa cells that binds preferentially to supercoiled DNA damaged by ultraviolet light or N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1982 May 31;697(2):202–212. doi: 10.1016/0167-4781(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Vollberg T. M., Siegler K. M., Cool B. L., Sirover M. A. Isolation and characterization of the human uracil DNA glycosylase gene. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8693–8697. doi: 10.1073/pnas.86.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdela F., Croisy A., Barbin A., Malaveille C., Tomatis L., Bartsch H. Carcinogenicity of chloroethylene oxide, an ultimate reactive metabolite of vinyl chloride, and bis(chloromethyl)ether after subcutaneous administration and in initiation-promotion experiments in mice. Cancer Res. 1980 Feb;40(2):352–356. [PubMed] [Google Scholar]
- de los Santos C., Kouchakdjian M., Yarema K., Basu A., Essigmann J., Patel D. J. NMR studies of the exocyclic 1,N6-ethenodeoxyadenosine adduct (epsilon dA) opposite deoxyguanosine in a DNA duplex. Epsilon dA(syn).dG(anti) pairing at the lesion site. Biochemistry. 1991 Feb 19;30(7):1828–1835. doi: 10.1021/bi00221a015. [DOI] [PubMed] [Google Scholar]