Abstract
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 are complex retroviruses that persist in the host, eventually causing leukemia and neurological disease in a small percentage of infected individuals. In addition to structural and enzymatic proteins, HTLV encodes regulatory (Tax and Rex) and accessory (open reading frame I and II) proteins. The viral Tax and Rex proteins positively regulate virus production. Tax activates viral and cellular transcription to promote T-cell growth and, ultimately, malignant transformation. Rex acts posttranscriptionally to facilitate cytoplasmic expression of viral mRNAs that encode the structural and enzymatic gene products, thus positively controlling virion expression. Here, we report that both HTLV-1 and HTLV-2 have evolved accessory genes to encode proteins that act as negative regulators of both Tax and Rex. HTLV-1 p30II and the related HTLV-2 p28II inhibit virion production by binding to and retaining tax/rex mRNA in the nucleus. Reduction of viral replication in a cell carrying the provirus may allow escape from immune recognition in an infected individual. These data are consistent with the critical role of these proteins in viral persistence and pathogenesis in animal models of HTLV-1 and HTLV-2 infection.
Human T-cell leukemia virus type 1 (HTLV-1) and type 2 HTLV-2 are distinct complex oncogenic retroviruses that persist in the infected individual despite a robust virus-specific host immune response (17). HTLV-1 is the causative agent of adult T-cell leukemia, a malignancy of CD4+ T lymphocytes, and of a chronic neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (15, 20, 34, 35). The association between HTLV-2 infection and disease is less clear in that only a few cases of variant hairy cell leukemia (CD8+ T-cell origin) and several cases of neurological disease have been reported (21, 38, 39).
In addition to structural and enzymatic proteins, Gag, Pol, and Env, HTLV encodes the Tax and Rex trans-regulatory gene products that are essential for efficient viral replication and cellular transformation. Tax increases the rate of transcription from the viral long terminal repeat (LTR) (4, 12, 22) and modulates the transcription or activity of numerous cellular genes involved in cell growth and differentiation, cell cycle control, and DNA repair (29, 30, 36, 41, 42). In addition, Tax is highly immunogenic in vivo (16, 23). Rex acts posttranscriptionally by preferentially binding, stabilizing, and selectively exporting the unspliced and incompletely spliced viral mRNAs from the nucleus to the cytoplasm, thus controlling the expression of the structural and enzymatic proteins (1, 28, 31).
Proteins encoded by open reading frame (ORF) I and ORF II near the 3′ end of the viral genome (3, 7, 27) promote viral persistence in vivo (Fig. 1A) (13, 19, 26). These proteins are dispensable for replication and immortalization of primary T lymphocytes in vitro (11, 18, 37). However, ORF II has been shown to be important for viral persistence in vivo in a rabbit model of infection (2, 9, 10, 43). The HTLV-1 ORF II protein, p30II, localizes to the nucleolus and nucleus (27) and has the capacity to modulate viral gene expression by interacting with the coactivator p300 and destabilizing the Tax-CREB interaction (46, 47). The HTLV-2 ORF II protein, p28II, also localizes to the nucleus, and its N-terminal 49 amino acids share 77.5% identity with the C-terminal portion of HTLV-1 p30II, suggesting that the two proteins might have a similar function (6). The mechanism of action for these proteins in viral replication and survival in vivo remains unclear.
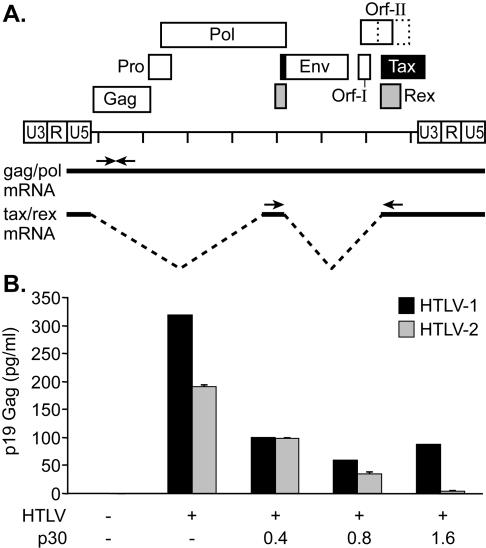
FIG. 1.
HTLV-1 p30II inhibits viral replication. (A) Schematic representation of a generic HTLV genome. Protein ORFs are indicated. ORF-II, depicted as overlapping solid and dotted boxes, shows the locations of the HTLV-1 p30II and HTLV-2 p28II, respectively. The full-length gag/pol and doubly spliced tax/rex mRNAs are depicted below the genome. Arrows indicate the locations of primers used to specifically detect viral mRNAs by PCR. (B) Increasing the concentration of p30II cotransfected with the HTLV-1 or HTLV-2 proviral clone causes a dose-dependent reduction of p19 Gag as measured by ELISA. Error bars indicate standard deviations.
Only a subset of HTLV-infected cells actively expresses viral RNA in vivo (14), leading to the hypothesis that a negative regulator(s) of HTLV gene expression is required for the survival of the virus in the infected host. Indeed, the p30II protein of HTLV-1 recently was shown to act as a negative regulator of viral gene expression (33). Since HTLV-2 is genetically related to HTLV-1, we investigated whether the HTLV-1 p30II also may function reciprocally as a negative regulator of HTLV-2 expression. Our data demonstrate not only that p30II blocks HTLV-1 and HTLV-2 replication but that HTLV-2 encodes a functionally related protein, p28II, which inhibits HTLV-2 as well as HTLV-1 replication. Both p30II and p28II inhibit Tax-1 and Tax-2 but only when Tax is expressed from a full-length proviral clone. Similarly, p30II and p28II inhibit Rex-1 and Rex-2. Since Tax and Rex are expressed from the same doubly spliced mRNA, we hypothesized that this inhibitory effect may occur at the RNA level. We show that p28II, like p30II, binds to and retains tax/rex RNA of HTLV-2 in the nucleus, thereby reducing its level in the cytoplasm. By repressing Tax and Rex functions, both p30II and p28II down-modulate viral expression and, in turn, promote viral persistence. This phenomenon provides an example of the evolutionary conservation of a common regulatory pathway by two distinct retroviruses.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml).
The HTLV-1 proviral clone ACH (25) and HTLV-2 proviral clone, pH6neo (5), were used in this study. pME-p30-HA (a kind gift from B. Michael, Ohio State University) was generated from ORF II of the ACH proviral clone, tagged with hemagglutinin (HA) at the C terminus, and cloned into the expression vector pME-18S at the EcoRI and NotI sites. The protein was detected by Western blotting with anti-HA monoclonal antibody (Covance). Tax and Rex were expressed from a vector encoding the respective cDNA under the control of the cytomegalovirus immediate-early gene promoter that has been described previously (45). An HTLV-2 p28II expression vector (p28-AU1) was generated from ORF II of the pH6neo proviral clone, tagged with AU1 (DTYRYI) at the C terminus, and cloned into the cytomegalovirus-based expression vector BC12 at the HindIII and KpnI sites. The protein was detected by immunoprecipitation with anti-AU1 monoclonal antibody (Covance). p28II-GFP (with green fluorescent protein [GFP] fused to the amino terminus) was constructed by inserting the HindIII-EcoRI p28II cDNA fragment into the EGFP-N3 vector (Promega). The LTR-luciferase Tax reporter plasmid (40), pcTat, and the Rex-1 (pCgag-RxRE-I) or Rex-2 (pCgag-RxRE-II) reporter plasmid were previously described (8, 44). Thymidine kinase-Renilla luciferase plasmid was used to control for transfection efficiency.
Transfection, luciferase assay, and p19 and p24 ELISA.
To measure Tax function, 1.5 × 105 293T cells were transfected by using Lipofectamine (Invitrogen) according to the manufacturer's recommendations. The total amount of DNA was kept constant and was composed of 0.1 μg of LTR-luciferase reporter along with 0.4 μg of an empty plasmid, Tax cDNA expression plasmid, or HTLV proviral clone. Increasing amounts (0.4 to 1.6 μg) of p30II or p28II expression plasmid were cotransfected to test the effect of p30II or p28II on Tax activity. After 48 h, cells were pelleted and the cell supernatants were used for p19 enzyme-linked immunosorbent assay (ELISA) (Zeptometrix) according to manufacturer's recommendations. The cell pellets were lysed in passive lysis buffer (Promega), and Tax activity was measured in light units as described previously (8, 44). The Rex functional assay was performed as described previously (8, 44). Briefly, 0.4 μg of an empty plasmid, Rex cDNA expression plasmid, or HTLV proviral clone was cotransfected with 0.1 μg of pcTat and 0.3 μg of the Rex reporter plasmid pCgag-RxRE, which contains the human immunodeficiency virus type 1 (HIV-1) LTR promoter and gag gene linked to the Rex response element (RxRE). Cell lysates were prepared in passive lysis buffer at 48 h posttransfection, and luciferase activity was determined to control for transfection efficiency. HIV-1 p24 Gag levels in the cell lysates were determined by ELISA (Beckman-Coulter). All transfection experiments were performed in triplicate and normalized for transfection efficiency by using Renilla luciferase.
RNA preparation, radiolabeled reverse transcriptase PCR (RT-PCR), and real-time RT-PCR.
Transfected 293T cells were lysed in hypotonic lysis buffer (10 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, and 0.5 mM dithiothreitol) for 10 min on ice. The cytoplasmic and nuclear fractions were separated by centrifugation at 700 × g for 8 min. The supernatant (cytoplasmic fraction) was further cleared by centrifugation at 3,300 × g for 5 min. The pellet served as the nuclear fraction. The RNA was extracted by using Tri-reagent (Molecular Research Center), and samples were treated three times with RNase-free DNase.
Semiquantitative RT-PCR was performed as previously described (28), using primers LA79 (5085CCGGTGGATCCCGTGGCGAT5104) and LA78 (7234GTCCAAATCCTGGGAAATGG7214) to detect tax-2/rex-2 and primers 20 (1314AGCCCCCAGTTCATGCAGACC1334) and 21 (1412GAGGGAGGAGCATAGGTACTG1392 to detect gag-2/pol-2. Briefly, the antisense primer from each set was end labeled for 1 h with γ-[32P]ATP by using T4 polynucleotide kinase (New England Biolabs). The reverse transcription reaction was performed with the labeled antisense primer at 65°C for 10 min, followed by 30 cycles of amplification. The radiolabeled products were separated on a 6% acrylamide gel and quantified by using Image Quant NT (Molecular Dynamics).
For real-time PCR, first-strand cDNA was generated by using SuperScript II reverse transcriptase (Invitrogen) and oligo(dT) primers. Then, 10% of the cDNA was mixed with SybrGreen master mix (Stratagene) and a 0.5 μM concentration of primers RT-tax2s (5143GAACTCGCCGAGCACGCC5160) and RT-tax2as (7320GGAACATAGACCACCTGA7303) to amplify tax-2/rex-2 or primers 20 and 21 to amplify gag-2/pol-2. The real-time PCR was performed with the Roche LightCycler system (Roche). Calibration curves were generated by using serial dilutions of linearized plasmid DNA. The expected size of the amplified fragments was confirmed by agarose gel electrophoresis.
In vivo RNA binding.
Detection of RNA bound to p28II was performed as described previously (32) with some modifications. Briefly, transfected 293T cells were lysed in NP-40 lysis buffer (50 mM KCl, 10 mM Tris [pH 8.0], 5 mM MgCl2, 0.65% NP-40, 2 mM phenylmethylsulfonyl fluoride, and 100 U of RNasin) for 30 min on ice. Lysates were cleared by incubation with 50 μl of protein A-Sepharose beads (Amersham) for 2 h at 4°C. Then, 10% of the cleared lysate was used as input RNA, and the rest was immunoprecipitated with either anti-AU1 antibody to capture p28II or anti-HA (nonspecific antibody). The immune complexes were washed three times with lysis buffer, and the RNA was extracted by using Tri-reagent and subjected to radiolabeled RT-PCR as described above.
Immunofluorescence.
HeLa cells were electroporated with 5 μg of p28-GFP or p28-TA (HA tagged). For p28-GFP detection, cells were plated and visualized by using a Zeiss LSM 510 microscope. For p28-TA detection, plated cells were fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton X-100, and incubated with anti-HA monoclonal antibody (1:100). The cells then were washed, and incubated with anti-mouse immunoglobulin G conjugated with Cy3 at 1:1,000 (Jackson Laboratories), and visualized by using a Zeiss LSM 510 microscope.
RESULTS
HTLV-1 p30II represses HTLV-2 replication.
Recently, it was demonstrated that the HTLV-1 p30II protein encoded by ORF II suppresses HTLV-1 replication (33). Tax-1 and Tax-2 activate transcription, although at different levels, through the HTLV-1 and HTLV-2 promoters. Similarly, the Rex-1 and Rex-2 proteins bind to RxRE-1 and RxRE-2 and transport the unspliced and singly spliced viral mRNA from the nucleus to the cytoplasm, thereby positively regulating structural and enzymatic protein expression and virion production (44). To assess whether p30II also was able to inhibit HTLV-2 replication, we coexpressed increasing concentrations of p30II protein with the replication-competent HTLV-1 proviral clone ACH, as well as with the HTLV-2 proviral clone pH6neo. The addition of 30II resulted in a significant reduction of p19 Gag production in the supernatant of transfected cells, indicating that p30II represses HTLV-1 expression as expected (33), but also reduced HTLV-2 expression, indicating significant inhibition of virus replication (Fig. 1B).
p30II inhibits Tax-1, Tax-2, Rex-1, and Rex-2 at a posttranscriptional level.
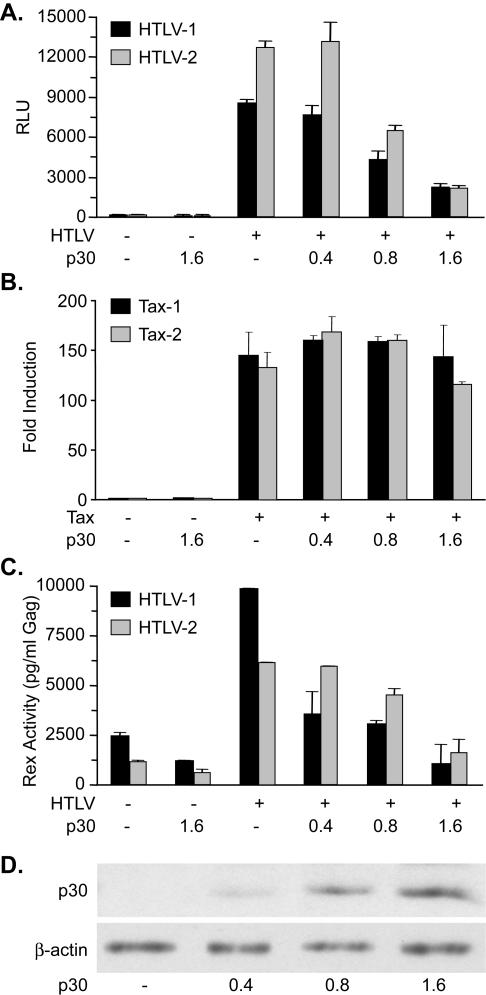
Since Tax-1 and Tax-2 are the key transactivators of transcription from the viral promoter, and since p19 Gag and other viral gene expression are highly dependent on functional Tax, we investigated whether the repressive effect of p30II could be due to inhibition of Tax transcriptional activity. Cotransfection of either the HTLV-1 or HTLV-2 proviral clone as the source for Tax-1 or Tax-2, respectively, and the LTR-Luc reporter with increasing concentrations of p30II (0.4 to 1.6 μg) resulted in a dose-dependent inhibition of both Tax-1 and Tax-2 function (Fig. 2A). We then ruled out the possibility that the repressive effect of p30II is a direct result of the inhibition of Tax-mediated transcription from the LTR. Coexpression of p30II with LTR-Luc in the absence of Tax did not result in any inhibition of LTR-mediated transcription at the doses used (data not shown) (33). Also, the p30II repressive effect was not observed if either Tax-1 or Tax-2 was expressed from a cDNA expression vector (Fig. 2B), ruling out a more downstream block involving the Tax protein and its function. Our data indicate that p30II does not affect the basal level of transcription mediated by Tax-1 and Tax-2 or directly disrupt the protein itself, thus suggesting that p30II inhibits Tax-1 and Tax-2 by a posttranscriptional mechanism.
FIG. 2.
HTLV-1 p30II inhibits Tax and Rex function when both are expressed from HTLV proviral clones. (A) p30II dose-dependent inhibition of Tax expressed from the HTLV-1 or HTLV-2 proviral clone. Tax function was measured as firefly luciferase activity from LTR-Luc normalized to Renilla luciferase activity. RLU, relative light units. Error bars indicate standard deviations. (B) p30II does not inhibit Tax activity, expressed as fold activation over the basal level, if Tax-1 and Tax-2 are expressed from cDNA expression vectors. (C) p30II dose-dependent inhibition of Rex expressed from HTLV-1 or HTLV-2 proviral clones. Rex functional activity was determined by using the HIV-1 p24 Rex reporter assay as described in Materials and Methods. (D) Western blot analysis to confirm increasing concentrations of p30II-HA used in panels A and B. β-Actin levels were assessed as a loading control.
Since Tax and Rex are expressed from the same viral RNA in both HTLV-1 and HTLV-2 (Fig. 1A), we hypothesized that p30II also may inhibit Rex function, confirming that the effect of p30II is at the RNA level. Rex-1 or Rex-2 was cotransfected into 293T cells with either a cDNA plasmid or full-length proviral clone (HTLV-1 or HTLV-2), with increasing concentrations of p30II (0.4 to 1.6 μg). Consistent with the inhibition of p19 Gag production and Tax function, p30II expression resulted in a dose-dependent inhibition of both Rex-1 and Rex-2 (Fig. 2C). As with Tax, p30II repression was not observed if either Rex-1 or Rex-2 was produced from a cDNA expression vector (data not shown). Western blot analysis confirmed that the amount of p30II protein expressed correlated directly with the amount of plasmid DNA transfected, whereas a control cellular protein, β-actin, remained unchanged (Fig. 2D). It is important to note for these experiments that although Tax and Rex activity expressed from 0.4 μg of transfected proviral clone can be quantitatively measured by using a sensitive reporter assay, the level of protein expressed is below the limit of detection by Western blotting.
The functional homologue of p30II in HTLV-2 is p28II.
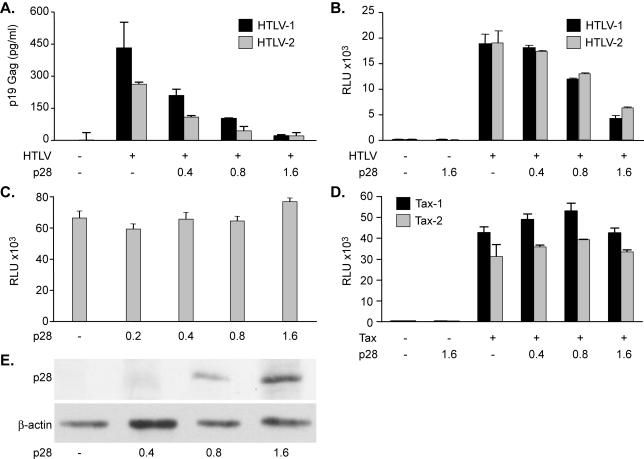
Since the HTLV-1 p30II was able to inhibit the Tax and Rex functional activities of both HTLV-1 and HTLV-2, we hypothesized that HTLV-2 must have evolved a similar function. The 3′ end of the HTLV-2 genome encodes a protein of 28 kDa (p28II) with unknown function (6). Since the N-terminal 49 amino acids of p28II and the C-terminal region of p30II have 77.5% identity, we hypothesized that p28II may be the functional homologue of p30II. Indeed, when cotransfected with HTLV-1 and HTLV-2 molecular clones, p28II expression decreased p19 Gag production in a dose-dependent manner (Fig. 3A). Furthermore, like p30II, p28II repressed both Tax-1 and Tax-2 functions when Tax was expressed from HTLV-1 or HTLV-2 proviral clones (Fig. 3B). Next, we determined that the inhibitory effects of p28II were due neither to inhibition of basal-level transcription (Fig. 3C) nor to Tax-mediated transcription (Fig. 3D) from the viral LTR when Tax-1 or Tax-2 was expressed from cDNA expression plasmids. Immunoprecipitation of p28II from transfected cells confirmed an increase in p28II protein production as a function of increased plasmid DNA transfected, whereas a control cellular protein, β-actin, remained unchanged (Fig. 3E).
FIG. 3.
HTLV-2 p28II inhibits viral replication and Tax function. (A) Increasing the concentration of p28II cotransfected with the HTLV-1 or HTLV-2 proviral clone causes dose-dependent reduction of p19 Gag. Error bars indicate standard deviations. (B) p28II dose-dependent inhibition of Tax expressed from the HTLV-1 or HTLV-2 proviral clones. RLU, relative light units. (C) Basal-level transcription from the viral LTR is not inhibited by p28II. (D) p28II does not inhibit Tax activity if Tax-1 and Tax-2 are expressed from cDNA expression vectors. (E) Immunoprecipitation of radiolabeled p28II-AU1 to confirm increasing concentrations of p28II in panels A to D. β-Actin levels were assessed as a loading control.
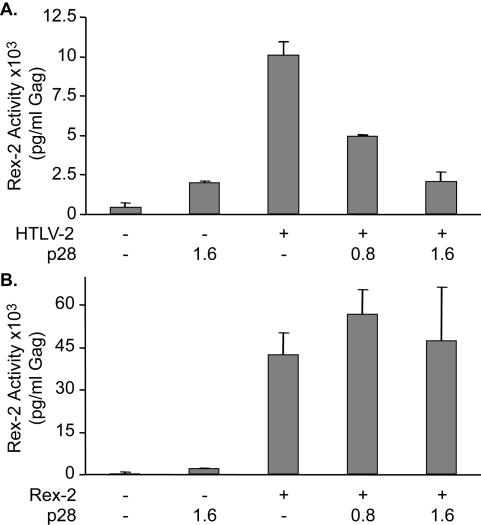
We next evaluated the effect of p28II on Rex-2 function. Cotransfection of increasing concentrations of p28II (0.4 to 1.6 μg) with the HTLV-2 proviral clone (pH6neo), as the source for Rex-2, and the RxRE linked to a HIV Gag reporter resulted in a dose-dependent inhibition of Rex-2 function (Fig. 4A). Like p30II, p28II had no effect on Rex-2 when it was expressed from a cDNA expression plasmid (Fig. 4B). This provides the first report of a functional activity for HTLV-2 p28II and supports the overall conclusion that the p30II and p28II homologues exert their inhibitory effect at a posttranscriptional level.
FIG. 4.
p28II inhibits Rex function when Rex is expressed from an HTLV proviral clone. (A) p28II dose-dependent inhibition of Rex expressed from the HTLV-1 or HTLV-2 proviral clone. Rex activity is a measure of p24 Gag expression from the pcGagRxRE-II reporter plasmid. Error bars indicate standard deviations. (B) Same as panel A, but Rex-2 is expressed from a cDNA expression plasmid.
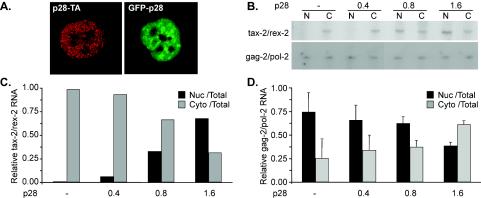
The nuclear p28II binds to and retains the doubly spliced tax/rex mRNA in the nucleus.
To investigate the mechanism of p28II suppression of HTLV-2 gene expression, we assessed the cellular localization of p28II. Both p28-TA and p28-GFP localized to the nucleus as expected, showing that the addition of either tag to the protein does not affect its localization (Fig. 5A). Since we ruled out a transcriptional effect of p28II, we investigated whether the p28II suppressive effects could be exerted at a posttranscriptional level. Therefore, we studied the distribution of selected viral mRNA species in HTLV-2-transfected 293T cells in the presence or absence of exogenous p28II. Semiquantitative RT-PCR was conducted on nuclear and cytoplasmic RNA fractions by using a primer pair that spans exons 2 and 3 of tax/rex mRNA, as well as a specific primer pair that detects the unspliced gag/pol mRNA (Fig. 1A). p28II resulted in an increase of tax/rex mRNA in the nucleus and a consistent reduction of this mRNA in the cytoplasm (Fig. 5B). The nuclear retention of tax/rex mRNA was specific, because the distribution of gag/pol mRNA was not affected by p28II (Fig. 5B). In order to get a better quantitative measure of the nuclear retention of tax/rex mRNA by p28II, nuclear and cytoplasmic RNA fractions were subjected to real-time RT-PCR. As shown in Fig. 5C, expression of p28II lead to dose-dependent retention of tax/rex mRNA in the nuclear fraction with a concomitant reduction of this mRNA species in the cytoplasm. Confirming the RT-PCR results, gag/pol mRNA was not significantly affected by p28II (Fig. 5D).
FIG. 5.
p28II retains HTLV-2 tax/rex mRNA in the nucleus. (A) Nuclear localization of p28II-TA and the GFP-p28 fusion protein. (B) p28II affects the distribution of tax-2/rex-2 doubly spliced mRNA. Nuclear (N) and cytoplasmic (C) mRNAs were extracted from 293T cells cotransfected with the HTLV-2 proviral clone in the presence or absence of exogenous p28II. RT-PCR was used to amplify virus-specific mRNAs by using 32P-labeled primers. (C and D) Equal amounts of mRNA from panel B were subjected to real-time RT-PCR to determine the ratio of nuclear (Nuc) to total RNA and of cytoplasmic (Cyto) to total RNA by using primers specific for tax/rex (C) or gag/pol (D) mRNA. Error bars indicate standard deviations.
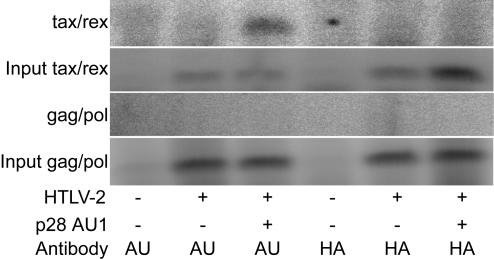
We evaluated whether p28II and tax/rex mRNA can associate with each other by using an in vivo RNA binding assay. We cotransfected 293T cells with an HTLV-2 proviral clone and p28II or an empty plasmid expression vector. Following immunoprecipitation of p28II, the RNA bound to the p28II immune complex was extracted and subjected to RT-PCR. Our data indicate that p28II can specifically associate with tax/rex mRNA but not gag/pol mRNA in vivo (Fig. 6). An antibody to an HA-tagged epitope not contained in the p28II could not capture tax/rex mRNA, confirming that the specificity of binding is dependent on the presence of p28II and its specific antibody. We conclude that p28II binds either directly or indirectly to the tax/rex mRNA. Collectively our data support the conclusion that p28II and p30II accessory proteins decrease viral replication by forming a protein-RNA complex that is retained in the nucleus.
FIG. 6.
In vivo binding of p28II to tax/rex mRNA. 293T cells cotransfected with HTLV-2 and p28II were lysed in NP-40 buffer, and lysates were immunoprecipitated with anti-AU1 or anti-HA monoclonal antibody. The specific mRNAs bound to the immune complex were identified by RT-PCR. Input bands represent 10% of the total RNA before immunoprecipitation.
DISCUSSION
Efficient expression of the HTLV-1 and HTLV-2 structural and enzymatic proteins from a provirus is dependent on the regulatory proteins Tax and Rex. Tax increases overall transcription, whereas the posttranscriptional regulator Rex is essential for nuclear export of unspliced and partially spliced RNAs to the cytoplasm (24, 28). Inhibiting the activity of either regulatory protein has drastic effects on virus replication. On the other hand, an infected host cell that expresses high levels of foreign proteins could be eliminated by immune surveillance. Thus, in order for the virus to persist in the host, it would be advantageous to suppress, at least partially, the positive regulatory proteins, leading to a state referred to as viral latency. It is not fully understood whether in vivo HTLV-1 and HTLV-2 achieve complete latency at the molecular level. Some insight came from experiments in which the region encoding the accessory proteins was shown to be dispensable for viral replication and transformation of activated primary T-lymphocytes in vitro (11, 18) but not in vivo in rabbits with a competent immune system (2, 9, 10). Based on these observations, we hypothesized that the accessory proteins played a role in dampening the function of Tax and Rex and overall viral expression and contributed to viral persistence in vivo. In the present study, we showed that HTLV-1 p30II suppresses both HTLV-1 and -2 replication, and we uncovered the function of HTLV-2 p28II. Our data suggest that both proteins may play a very important role in viral persistence by posttranscriptionally inhibiting Tax and Rex gene expression and ultimately repressing viral replication.
Our data show that the coexpression of either HTLV-1 p30II or HTLV-2 p28II with replication-competent HTLV-1 or HTLV-2 proviral clones results in a dose-dependent inhibition of both Tax and Rex functions. This repression was not observed when Tax and Rex were expressed from cDNA expression vectors, suggesting that p30II and p28II do not affect Tax and Rex at the protein level. In addition, neither p30II nor p28II inhibited basal-level or Tax-1- and Tax-2-mediated transcription from the LTR. Collectively, these data indicate that the inhibitory effects of p30II and p28II are posttranscriptional. Thus, we examined the effect of p28II on the distribution of tax/rex doubly spliced mRNA expressed from a proviral clone. We provided evidence that p28II retains tax/rex mRNA in the nucleus, with a concomitant reduction of this RNA in the cytoplasm. The implication is that this effect would lead to less protein production, which could allow the infected cell to have a lower profile and escape the immune system. Since Tax and Rex protein levels expressed from transfected proviral clones are below the limit of detection, we cannot directly quantitate Tax and Rex protein levels by immunoprecipitation or Western blotting. However, since HTLV Gag production is highly dependent on both functional Tax and Rex, we can indirectly correlate p19 Gag levels with Tax and Rex functional activities. Indeed, coexpression of p30II and p28II with the full-length HTLV proviral clones caused significant reduction in the viral p19 Gag production. Finally, our in vivo RNA binding analysis revealed that p28II has the ability to specifically associate with the doubly spliced tax/rex mRNA but not the unspliced gag/pol mRNA. Whether this interaction is direct or through another adaptor protein remains to be tested.
The mechanism of action of p30II and p28II is a posttranscriptional regulation of viral mRNA trafficking. In contrast to Rex, which binds to and facilitates the nucleocytoplasmic export of unspliced and singly spliced viral RNA, p28II and p30II specifically bind and retain the doubly spliced tax/rex mRNA in the nucleus. The exact mechanism of RNA retention is still unclear. One possibility consistent with the data is that p30II and p28II bind to the exon-exon junction that is unique to tax/rex mRNA, preventing the recruitment of factors required for efficient release of the mRNA from the nuclear pore, essentially blocking mRNA export.
The fact that the inhibitory function of p28II and p30II is conserved in both HTLV-1 and HTLV-2 emphasizes its importance and suggests a common pathway for modulation of gene expression by these two distinct but related viruses. However, it is important to note some differences between these two proteins. Unlike p30II, which localizes to the nucleolus (33), we showed that p28II is primarily nuclear and excluded from the nucleolus. This difference may reflect the previously reported ability of p30II to have general transcriptional effects (46, 47). In our assays, p28II did not cause similar effects on the TRE-mediated transcription. Additional comparative experiments will be required to identify the protein domains responsible for these differences and may provide insight into other distinct functional activities, leading to a better understanding of the pathological differences between HTLV-1 and HTLV-2. Understanding the exact mechanism of action of p30II and p28II ultimately could provide a means for therapeutic targeting of these proteins to eradicate HTLV persistence in the host.
Acknowledgments
We thank Matthew Anderson, Kate Hayes, Jianxin Ye, and Li Xie for critical comments on the manuscript and Tim Vojt for preparation of the figures. We also thank Christoph Nicot for sharing unpublished data.
This work is supported by grants from National Institutes of Health (CA77556 and CA92009) and The Ohio State University Glenn Barber Fund.
REFERENCES
- 1.Ballaun, C., G. R. Farrington, M. Dobrovnik, J. Rusche, J. Hauber, and E. Bohnlein. 1991. Functional analysis of human T-cell leukemia virus type I Rex-response element: direct RNA binding of Rex protein correlates with in vivo binding activity. J. Virol. 65:4408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoe, J. T., B. Albrecht, N. D. Collins, M. D. Robek, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 74:1094-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berneman, Z. N., R. B. Gartenhaus, M. S. Reitz, W. A. Blattner, A. Manns, B. Hanchard, O. Ikehara, R. C. Gallo, and M. E. Klotman. 1992. Expression of alternately spliced human T-lymphotropic virus type I pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. USA 89:3005-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cann, A. J., J. D. Rosenblatt, W. Wachsman, N. P. Shah, and I. S. Y. Chen. 1985. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature 318:571-574. [DOI] [PubMed] [Google Scholar]
- 5.Chen, I. Y., J. McLaughlin, J. C. Gasson, S. C. Clark, and D. W. Golde. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305:502-505. [DOI] [PubMed] [Google Scholar]
- 6.Ciminale, V., D. M. D'Agostino, L. Zotti, G. Franchini, B. K. Felber, and L. Chieco-Bianchi. 1995. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology 209:445-456. [DOI] [PubMed] [Google Scholar]
- 7.Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. K. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 66:1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciminale, V., L. Zotti, D. M. D'agostino, and L. Chieco-Bianchi. 1997. Inhibition of human T-cell leukemia virus type 2 Rex function by truncated forms of Rex encoded in alternately spliced mRNAs. J. Virol. 71:2810-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockerell, G. L., J. Rovank, P. L. Green, and I. S. Y. Chen. 1996. A deletion in the proximal untranslated pX region of human T-cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood 87:1030-1035. [PubMed] [Google Scholar]
- 10.Collins, N. D., G. C. Newbound, B. Albrecht, J. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 11.Derse, D., J. Mikovits, and F. Ruscetti. 1997. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology 237:123-128. [DOI] [PubMed] [Google Scholar]
- 12.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 13.Franchini, G., C. Nicot, and J. M. Johnson. 2003. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv. Cancer Res. 89:69-132. [DOI] [PubMed] [Google Scholar]
- 14.Gessain, A., A. Louie, O. Gout, R. C. Gallo, and G. Franchini. 1991. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-I-associated myelopathy. J. Virol. 65:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessain, A., J. C. Vernant, L. Maurs, F. Barin, O. Gout, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis. Lancet ii:407-409. [DOI] [PubMed] [Google Scholar]
- 16.Goon, P. K., E. Hanon, T. Igakura, Y. Tanaka, J. N. Weber, G. P. Taylor, and C. R. Bangham. 2002. High frequencies of Th1-type CD4(+) T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood 99:3335-3341. [DOI] [PubMed] [Google Scholar]
- 17.Green, P. L., and I. S. Y. Chen. 2001. Human T-cell leukemia virus types 1 and 2, p. 1941-1969. In D. M. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, and S. Straus (ed.), Virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 18.Green, P. L., T. M. Ross, I. S. Chen, and S. Pettiford. 1995. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J. Virol. 69:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidaka, M., J. Inoue, M. Yoshida, and M. Seiki. 1988. Post transcriptional regulator (rex) of HTLV-I initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 7:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K.-I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjelle, B., O. Appenzeller, R. Mills, S. Alexander, N. Torrez-Martinez, R. Jahnke, and G. Ross. 1992. Chronic neurodegenerative disease associated with HTLV-II infection. Lancet 339:645-646. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, J. I., M. Yoshida, and M. Seiki. 1987. Transcriptional (p40x) and post-transcriptional (p27xIII) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc. Natl. Acad. Sci. USA 84:3653-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. H., P. A. Kaufman, S. M. Hanly, L. T. Rimsky, and W. C. Greene. 1991. Rex transregulation of human T-cell leukemia virus type II gene expression. J. Virol. 65:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimata, J. T., F. H. Wong, J. J. Wang, and L. Ratner. 1994. Construction and characterization of infectious human T-cell leukemia virus type I molecular clones. Virology 204:656-664. [DOI] [PubMed] [Google Scholar]
- 26.Kiyokawa, T., M. Seiki, S. Iwashita, K. Imagawa, F. Shimizu, and M. Yoshida. 1985. p27xIII and p21xIII proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 82:8359-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koralnik, I. J., A. Gessain, M. E. Klotman, A. Lo Monico, Z. N. Berneman, and G. Franchini. 1992. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 89:8813-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusuhara, K., M. Anderson, S. M. Pettiford, and P. L. Green. 1999. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J. Virol. 73:8112-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung, K., and G. J. Nabel. 1988. HTLV-I transactivator induces interleukin-2 receptor expression through an NFκB-like factor. Nature 333:776-778. [DOI] [PubMed] [Google Scholar]
- 30.Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J. Fullen, M. V. Lorenzi, A. Cara, C. Nicot, C.-Z. Giam, and G. Franchini. 1998. Human T-lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 72:8852-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayan, M., K. Kusuhara, and P. L. Green. 2001. Phosphorylation of two serine residues regulates human T-cell leukemia virus type 2 Rex function. J. Virol. 75:8440-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayan, M., I. Younis, D. M. D'Agostino, and P. L. Green. 2003. Functional domain structure of human T-cell leukemia virus type 2 Rex. J. Virol. 77:12829-12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicot, C., J. M. Dundr, J. R. Johnson, J. R. Fullen, N. Alonzo, R. Fukumoto, G. L. Princler, D. Derse, T. Misteli, and G. Franchini. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 10:197-201. [DOI] [PubMed] [Google Scholar]
- 34.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 35.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ressler, S., G. F. Morris, and S. J. Marriott. 1997. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 71:1181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robek, M., F. Wong, and L. Ratner. 1998. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 72:4458-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenblatt, J. D., J. V. Giorgi, D. W. Golde, J. B. Ezra, A. Wu, C. D. Winberg, J. Glaspy, W. Wachsman, and I. S. Chen. 1988. Integrated human T-cell leukemia virus II genome in CD8 + T cells from a patient with “atypica” hairy cell leukemia: evidence for distinct T and B cell lymphoproliferative disorders. Blood 71:363-369. [PubMed] [Google Scholar]
- 39.Rosenblatt, J. D., D. W. Golde, W. Wachsman, A. Jacobs, G. Schmidt, S. Quan, J. C. Gasson, and I. S. Y. Chen. 1986. A second HTLV-II isolate associated with atypical hairy-cell leukemia. N. Engl. J. Med. 315:372-377. [DOI] [PubMed] [Google Scholar]
- 40.Ross, T. M., A. C. Minella, Z. Y. Fang, S. M. Pettiford, and P. L. Green. 1997. Mutational analysis of human T-cell leukemia virus type 2 Tax. J. Virol. 71:8912-8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1 to S phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siekevitz, M., M. B. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. USA 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman, L. R., A. J. Phipps, A. Montgomery, L. Ratner, and M. D. Lairmore. 2004. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion. J. Virol. 78:3837-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye, J., L. Sileverman, M. D. Lairmore, and P. L. Green. 2003. HTLV-1 Rex is required for viral spread and persistence in vivo but is dispensable for cellular immortalization in vitro. Blood 102:3963-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye, J., L. Xie, and P. L. Green. 2003. Tax and overlapping Rex sequences do not confer the distinct transformation tropisms of human T-cell leukemia virus types 1 and 2. J. Virol. 77:7728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, W., J. W. Nisbet, B. Albrecht, W. Ding, F. Kashanchi, J. T. Bartoe, and M. D. Lairmore. 2001. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J. Virol. 75:9885-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, W., J. W. Nisbet, J. T. Bartoe, W. Ding, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 p30(II) functions as a transcription factor and differentially modulates CREB-responsive promoters. J. Virol. 74:11270-11277. [DOI] [PMC free article] [PubMed] [Google Scholar]