Abstract
The arenavirus small RING finger Z protein is the main driving force of arenavirus budding. The primary structure of Z is devoid of hydrophobic transmembrane domains, but both lymphocytic choriomeningitis virus (LCMV) and Lassa fever virus Z proteins accumulate near the inner surface of the plasma membrane and are strongly membrane associated. All known arenavirus Z proteins contain a glycine (G) at position 2, which is a potential acceptor site for a myristoyl moiety. Metabolic labeling showed incorporation of [3H]myristic acid by wild-type Z protein but not by the G2A mutant. The mutation G2A eliminated Z-mediated budding. Likewise, treatment with the myristoylation inhibitor 2-hydroxymyristic acid inhibited Z-mediated budding, eliminated formation of virus-like particles, and caused a dramatic reduction in virus production in LCMV-infected cells. Budding activity was restored in G2A mutant Z proteins by the addition of the myristoylation domain of the tyrosine protein kinase Src to their N termini. These findings indicate N-terminal myristoylation of Z plays a key role in arenavirus budding.
Arenaviruses merit significant attention both as tractable experimental models to study acute and persistent infections and as clinically important human pathogens, including hemorrhagic fever (HF) agents such as Lassa fever virus (LFV) and the South American hemorrhagic fever viruses (6). Increased traveling to and from areas in sub-Sahara Africa where these pathogens are endemic has led to the importation of LFV into unexpected areas, including the United States, Europe, Japan, and Canada (16). Due to its severe morbidity and high mortality, lack of immunization or effective treatment, and ease of introduction into a susceptible population and transmissibility human to human, LFV is included in Category A of potential bioterrorism microbial weapons (3, 4); thus, developing novel effective antiviral drugs to combat HF arenaviruses is important. Such a task would be facilitated by a detailed understanding of the arenavirus molecular and cellular biology, for which the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) provides us with an excellent model system.
LCMV is an enveloped virus with a bisegmented, negative-strand (NS) RNA genome. Each RNA segment directs the expression of two gene products, encoded in ambisense orientation and separated by an intergenic region. The large segment (L; 7.2 kb) encodes the RNA-dependent RNA polymerase (L; ca. 200 kDa) and the small RING finger protein Z (ca. 11 kDa), which has no homologue among other known NS RNA viruses. Z is a structural component of the virion (34), and in LCMV-infected cells Z it has been shown to interact with several cellular proteins, including the promyelocytic leukemia protein (2) and the eukaryote translation initiation factor 4E (7, 18). The former has been proposed to contribute to the noncytolytic nature of LCMV infection (2), whereas the latter has been proposed to repress cap-dependent translation (7, 18). Moreover, early studies suggested a role of Z in arenavirus transcriptional regulation (14). However, it has been shown that Z is not required for virus RNA replication and transcription (20, 22); rather, it exhibits a dose-dependent inhibitory effect on RNA synthesis mediated by the arenavirus polymerase (11, 22). The small segment (S; 3.5 kb) encodes the viral nucleoprotein (NP; ca. 63 kDa) and the glycoprotein precursor (GPC; ca. 75 kDa). GPC is processed into GP1 (40 to 46 kDa) and GP2 (35 kDa) by the S1P cellular protease (1, 32). Heterooligomeric structures of GP1 and GP2, noncovalently associated, form the virion surface spikes, and the GP1 portion of the spike is responsible for initial binding to cellular receptor proteins (8).
A reverse genetics system for LCMV has been described previously (21). This system is based on the intracellular coexpression of a LCMV synthetic minigenome (MG) RNA and plasmid-supplied viral proteins that allow for amplification (RNA replication) and expression (transcription) of the MG-encoded chloramphenicol acetyltransferase (CAT) reporter gene. In addition, this system is also competent in assembly and budding of virus-like particles (VLP). Using this system we identified the Z protein as the main driving force of arenavirus budding (30), a process that takes place at the plasma membrane of infected cells (6). Both LCMV and LFV Z proteins exhibit self-budding activity (30, 35) and substituted efficiently for the late domain present in the Gag protein of Rous sarcoma virus (30). These results indicate that Z is competent for both targeting to the plasma membrane and budding activities. Consistent with its key role in arenavirus budding, Z accumulates near the inner surface of the plasma membrane. Strong interaction of Z with cellular membranes has been observed (30, 35). However, the mechanisms by which Z interacts and associates with the plasma membrane of the cell have not been determined.
A wide range of viruses express proteins that are myristoylated (reviewed in reference 24). Myristoylation of N-terminal glycine residues of cellular and viral proteins changes the lipophilicity of these proteins and facilitates their interactions with membranes and hydrophobic protein domains. N-terminal sequence motifs that direct incorporation of fatty acids into proteins are necessary for targeting of these proteins to membranes and also have been shown to be required for the acquisition of the viral envelope by several RNA and DNA viruses (24). The nonreversible covalent attachment of myristate, a 14-carbon saturated fatty acid, to the N-terminal glycine of myristoylated proteins is catalyzed by the myristoyl-coenzyme A:protein N-myristoyltransferase (NMT) (33) and occurs usually cotranslationally following removal of the initiator methionine by a methionine aminopeptidase (13). Data from studies on both sequence of known myristoylated proteins and sequence requirements for NMT activity indicate that the N-terminal acceptor amino acid is always a G residue (23). Myristoylation alone does not meet the requirements for protein membrane targeting. Additional factors, such as the presence of basic amino acid residues that facilitate electrostatic interactions with membrane lipids, contribute to membrane association of myristoylated proteins (13). Notably, comparison of 17 arenavirus Z protein sequences, including LCMV Z and those of HF arenaviruses, revealed the presence of a conserved glycine (G) residue on position 2 in the context of a consensus myristoylation signal (13). In addition, clusters of basic amino acid residues are found within the N termini of arenavirus Z proteins (Fig. 1A).
FIG. 1.
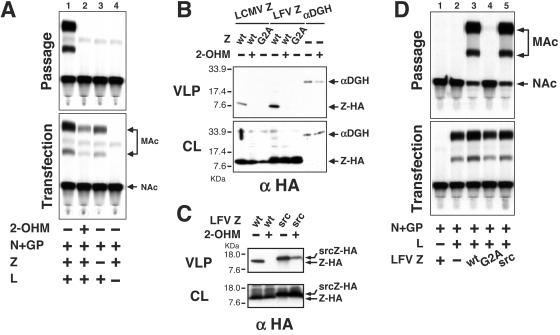
Myristoylation of arenavirus Z protein is required for VLP formation. (A) Alignment of the N termini of LCMV and LFV Z protein sequences. The arrow indicates the conserved G2 residue. Basic amino acid residues are underlined. (B) Expression levels of wt and mutant G2A Z proteins. 293T cells were transfected with pCAGGS plasmids coding for the indicated protein. Cell lysates were prepared 48 h after transfection and were analyzed by Western blot using an anti-HA antibody. (C) Mutation G2A eliminates VLP formation. 293T cells were transfected with the indicated combination of plasmids. Forty-eight hours later, SP were saved and cell lysates were prepared for CAT assay. An aliquot of each SP was used to infect a fresh monolayer of BHK-21 cells, which were subsequently infected with LCMV (MOI = 3). Seventy-two hours postinfection cell lysates were prepared for CAT assay. Aliquots from lysates of 293T (transfection) and BHK-21 (passage) cells were assayed for CAT activity. NAc, nonacetylated chloramphenicol; MAc, monoacetylated chloramphenicol. (D) wt, but not G2A, Z protein is myristoylated. COS-1 cells were transfected with plasmid expressing wt or G2A mutant Z proteins, and 44 h later they were metabolically labeled with either [35S]methionine-cysteine (35S) or [9,10 (n)-3H]myristic acid (3H) for 4 h. Z proteins were immunoprecipitated from cell extracts by using an anti-HA antibody. Immunoprecipitates were resolved by SDS-PAGE. The gel was then subjected to fluorography, dried, and exposed.
For all myristoylated proteins examined, the mutation G2A prevents their myristoylation, which underscores the key role played by the G2 residue in protein myristoylation. We therefore sought to define the role of the G2 residue in the function of Z. For that purpose, we generated G2A mutants for both LCMV and LFV Z proteins. To facilitate detection of these proteins, they were tagged at their C termini with a hemagglutinin (HA) epitope. These cDNAs were cloned into plasmid pCAGGS under the control of a pol II promoter (27). We first evaluated the expression levels and stability of the G2A mutant proteins. For this evaluation, we transfected 293T cells with plasmids encoding the different Z proteins by using Lipofectamine (Invitrogen), and 48 h later cell lysates were prepared and protein expression analyzed by Western blot using a polyclonal anti-HA antibody (Santa Cruz). Mutant and wild-type (wt) proteins were expressed to similar levels (Fig. 1B). We next assessed the effect of the G2A mutation on Z-mediated budding by using a VLP formation assay (30). This system also allowed us to study LFV Z, as this protein substituted very efficiently for its LCMV counterpart (30). We transfected 293T cells with plasmids encoding NP, L, GP, T7 RNA polymerase (21), MG#7Δ2G (30), and the different wt and mutant Z proteins. At 48 h posttransfection, supernatants (SP) were saved and cell extracts (CE) were prepared for CAT assay as described previously (31). Aliquots of the SP were used to infect fresh BHK-21 monolayers. After 4 h of adsorption, cells were infected with LCMV strain Armstrong at a multiplicity of infection (MOI) of 3 to supply the LCMV polymerase components, L and NP, to all of the cells and hence allowing for efficient detection of MG passage. CE were prepared 72 h later and were analyzed in a CAT assay. No differences were observed for levels of CAT activity between cells transfected with wt or G2A Z constructs (Fig. 1C Lower). Nevertheless, the mutation G2A completely abolished particle formation directed by either LCMV or LFV Z mutant proteins, as determined by lack of CAT activity in the passage (Fig. 1C, Passage; compare lanes 3 and 4 and lanes 6 and 7).
Previous studies demonstrated that there was a strong inhibitory effect of Z in viral RNA transcription and replication (11) and that the N terminus of Z was not required for this inhibitory activity (10). Consistent with this, wt and G2A mutant Z proteins exhibited the same inhibitory effect on RNA synthesis mediated by the LCMV polymerase (data not shown).
The lack of budding activity observed with the G2A mutant Z proteins indicated that the G in position 2 is strictly required for Z budding activity and suggested a possible role of myristoylation in this process. To obtain biochemical evidence that Z is a myristoylated protein, we conducted radiolabeling experiments. COS-1 cells were transfected in duplicates with plasmids expressing wt or G2A mutant LFV Z proteins. After 44 h, one plate of each pair was labeled with 250 μCi of [35S]methionine-cysteine (Tran 35S-label; ICN) for 4 h to determine the relative levels of Z protein expression or with 250 μCi of [9,10 (n)-3H]myristic acid (Amersham) for 3 h. After labeling, the cells were lysed and nuclei were removed by centrifugation. Z proteins were collected from the lysates by immunoprecipitation with an anti-HA antibody, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 16% gel, and visualized by fluorography. Labeling with [3H]myristic acid showed myristoylation of wt Z protein but not the G2A mutant Z protein (Fig. 1D). We also detected [3H]myristic acid associated with wt Z precipitated from the SP of the transfected cells (data not shown).
These results led us to hypothesize that myristoylation of Z was required to mediate virus budding. This hypothesis would predict an impairment of Z-mediated budding in the presence of myristoylation inhibitors. To test this we examined the effect of dl-2-hydroxymyristic acid (2-OHM) on VLP formation. 2-OHM is an analog of myristic acid that becomes metabolically activated in cells to form 2-hydroxymyristoyl-coenzyme A, a potent inhibitor of NMT (28). Treatment of cells with 2-OHM results in reduced protein myristoylation while protein synthesis remains unaffected (15). Moreover, 2-OHM specifically inhibits myristoylation, whereas other protein acylation events, such as posttranslational palmitoylation, are not altered (28). Production of LCMV VLP in transfected 293T cells was eliminated in the presence of 2-OHM (100 μM) (Fig. 2A, Passage; compare lanes 1 and 2). At this drug concentration no effect was observed in cell viability, and only a very modest effect was seen on MG expression mediated by the LCMV polymerase (Fig. 2A, Transfection). To test whether Z was the target of the inhibitory effect of 2-OHM on VLP formation, we transiently expressed LCMV and LFV Z-HA in the presence or absence of 2-OHM (100 μM). As a control we used a plasmid expressing a truncated (amino acids 30 to 485) version of the nonmyristoylated protein α-dystroglycan, lacking also its transmembrane domain and tagged with an HA epitope (αDGH) (19). Forty-eight hours after transfection cell lysates were prepared, culture media SP was harvested and clarified by centrifugation at low speed, and VLP were collected by ultracentrifugation through a 20% sucrose cushion. Viral particles in the pellet produced by high-speed centrifugation were analyzed by Western blotting using an antibody to HA. The presence of 2-OHM did not affect steady-state expression levels of Z proteins (Fig. 2B, CL) but completely inhibited self-budding of LCMV and LFV Z proteins (Fig. 2B, VLP). Likewise, and as predicted, mutant Z G2A proteins were not detected in the SP. These results indicate that the self-budding property of Z requires its myristoylation; hence, the strict requirement of a G residue in position 2 for Z-mediated budding. The amount of αDGH protein detected in the SP of transfected cells was not significantly affected by the treatment with 2-OHM (Fig. 2B, VLP), a finding supporting the idea that inhibition by 2-OHM of Z-mediated budding was not due to a nonspecific impairment of protein trafficking and secretion.
FIG. 2.
Inhibitors of myristoylation abolish VLP formation. (A) Treatment with 2-OHM eliminates VLP production. 293T cells were transfected with the indicated combination of plasmids in the presence (+) or absence (−) of 100 μM 2-OHM. Forty-eight hours later, SP were harvested and cell lysates were prepared. The presence of VLP in the SP was assessed as described for Fig. 1C. Aliquots from lysates of the transfected 293T (Transfection) and VLP-infected (Passage) cells were assayed for CAT activity. (B) Treatment with 2-OHM impairs self-budding activity of LCMV and LFV Z. 293T cells were transfected with the indicated plasmids, and 48 h later SP were saved and cell lysates were prepared. VLP-containing SP were subjected to centrifugation through a 20% sucrose cushion, and pellets resuspended in SDS-PAGE loading buffer. Aliquots of CL and VLP pellets (VLP) were analyzed by Western blot using an anti-HA antibody. (C) The first 10 amino acids of Src are sufficient to restore self-budding properties of G2A mutant Z proteins. 293T cells were transfected with the indicated plasmids in the presence (+) or absence (−) of 100 μM 2-OHM. Presence of Z protein in CL or medium SP was analyzed as in Fig. 2B. (D) Fusion of the myristoylation domain of Src to the N terminus of G2A mutant Z protein restored its function in VLP production. 293T cells were transfected with the indicated plasmids, and 48 h later SP were saved and CL were prepared. VLP-containing SP were used to infect fresh cells as described for Fig. 1C. Aliquots from lysates of the transfected 293T (Transfection) and VLP-infected (Passage) cells were assayed for CAT activity. NAc, nonacetylated chloramphenicol; MAc, monoacetylated chloramphenicol.
We next sought to rule out that the phenotype of the G2A mutation was related to its effects on the structure of the Z protein or other Z functions associated with its most N-terminal sequence and affected by the G2A mutation. For this goal, we fused the first 10 amino acids of the N terminus of the cellular Src tyrosine protein kinase to the N terminus of the LCMV and LFV Z G2A proteins, maintaining also the HA epitope at their C termini. These proteins would be expected to be myristoylated, because it has been shown that the first seven amino acids of Src are sufficient for myristoylation and have been used to direct myristoylation and membrane targeting of heterologous proteins (17, 36).
293T cells were transfected with plasmids encoding different srcZ G2A fusion proteins in the presence or absence of 2-OHM (100 μM). After 48 h, SP were harvested and cell lysates were prepared. VLP were obtained as previously described and were analyzed by Western blotting using the anti-HA antibody. Both fusion proteins were expressed at the same level as the parental wt protein (Fig. 2C, CL, and data not shown). Addition of the Src myristoylation signal restored self-budding activity of LFV Z protein (Fig. 2C, VLP) as well as LCMV Z protein (data not shown). Self-budding of the srcZ protein was sensitive to treatment with 2-OHM (Fig. 2C, VLP). In addition, Z-mediated formation of MG-containing VLP was restored by fusing the myristoylation domain of Src to the N termini of the G2A mutant LCMV or LFV Z proteins (Fig. 2D, Passage, and data not shown).
We detected small amounts of srcZ-HA protein in the SP of cells transiently expressing the protein in the presence of 2-OHM (Fig. 2C, VLP). This was likely due to the significantly higher amount of protein expressed by the corresponding cell lysates (Fig. 2C, CL) and the fact that in some cases complete inhibition of myristoylation is required to abolish budding (26). It is plausible that 2-OHM inhibits myristoylation of Z to a greater degree than srcZ G2A; hence, the residual srcZ G2A-mediated budding detected in our assay.
Inhibitors of myristoylation, like 2-OHM, have been reported to affect the multiplication of viruses known to produce myristoylated proteins, such as human immunodeficiency virus (5), Varicella-Zoster virus (15), and hepatitis B virus (29). In addition, two New World arenaviruses, Junin and Tacaribe, were inhibited by treatment with inhibitors of myristoylation (9), although the viral proteins targeted by the inhibitors were not identified. Our data indicated that Z is the viral protein targeted by the myristoylation inhibitor 2-OHM, and this results in impaired budding of VLP. This model would predict that 2-OHM affects production of infectious virus without altering virus RNA synthesis. To test this we examined the effect of 2-OHM on LCMV-infected BHK-21 cells. We infected BHK-21 cells with LCMV at low (0.1 PFU/cell) and high (1 PFU/cell) MOI in the presence of 0 or 100 μM 2-OHM. At 48 h after infection, cell SP were collected for determination of viral titers as described previously (12), and total cellular RNA was isolated using TriReagent and analyzed by Northern blot hybridization using a [32P]-labeled NP DNA probe.
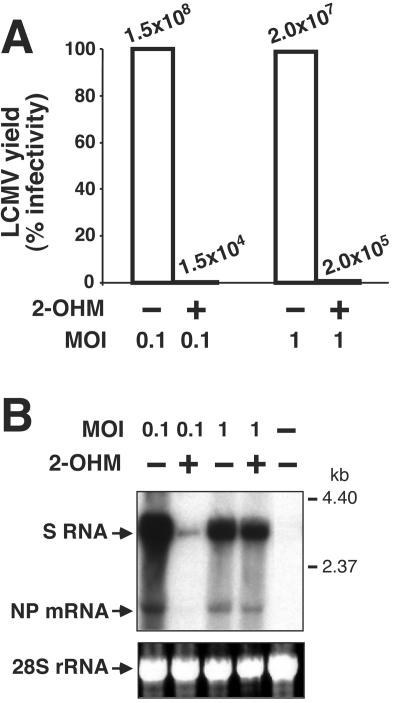
Cells treated with 100 μM 2-OHM produced only 0.01% (MOI = 0.1) or 1% (MOI = 1) of the virus released from the respective untreated infected cells (Fig. 3A). This represents a reduction of 4 log10 (MOI = 0.1; from 108 to 104) and two log10 (MOI = 1; from 107 to 105) in virus production. In contrast, 2-OHM had no significant effect on viral transcription and replication, as treated or untreated cells infected at a high MOI (MOI = 1) showed no differences in levels of S RNA and NP mRNA, which represent replication and transcriptional biosynthetic processes, respectively (Fig. 3B). The dramatic reduction in LCMV RNA replication and transcription observed in the cells infected at a low MOI and treated with 2-OHM reflects a defect in virus production by the small fraction of the cell population initially infected. This would negatively affect subsequent rounds of virus infection required to reach levels of viral RNA synthesis seen at high-MOI infection, where the majority (>99%) of the cells are infected during the first round.
FIG. 3.
Myristic acid analogs inhibit LCMV multiplication. (A) Treatment of LCMV-infected cells with 2-OHM eliminates virus production. BHK-21 cells were infected with LCMV at the indicated MOI in the presence (+) or absence (−) of 2-OHM (100 μM). Forty-eight hours later, SP were harvested and viral titers were determined by plaque assay. Virus production is represented as a percentage of the infectivity observed in LCMV-infected untreated controls (100%). (B) Treatment with 2-OHM does not affect LCMV transcription and replication. RNA isolated from treated and untreated LCMV-infected cells was analyzed by Northern blot using a 32P-labeled NP DNA probe.
Protein myristoylation has been shown to play an important role in different steps of the life cycle of several viruses, including entry, replication, assembly, and budding. Therefore, interfering with protein myristoylation in virally infected cells, either by inhibiting NMT or by using myristic acid antagonists, would be expected to cause severe impairment or elimination of virus multiplication. The high stability of cellular myristoylproteins (25) raises the prospect of using transient inhibition of protein myristoylation as a potential antiviral targeting strategy compatible with acceptable adverse side effects on cell physiology. Our results indicate that such an approach, if implemented, might be used to combat arenaviruses.
Acknowledgments
We thank S. Kunz for the αDGH construct and P. B. Lam for excellent technical assistance.
This work was entirely supported by National Institutes of Health grants AI55627 and AI53455.
REFERENCES
- 1.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borden, K. L., E. S. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391-2405. [DOI] [PubMed] [Google Scholar]
- 4.Broussard, L. A. 2001. Biological agents: weapons of warfare and bioterrorism. Mol. Diagn. 6:323-333. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, M. L., R. O. Heuckeroth, J. T. Kimata, L. Ratner, and J. I. Gordon. 1989. Replication of human immunodeficiency virus 1 and Moloney murine leukemia virus is inhibited by different heteroatom-containing analogs of myristic acid. Proc. Natl. Acad. Sci. USA 86:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchmaier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the virus and their replication, p. 1635-1668. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 7.Campbell Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. Borden. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 9.Cordo, S. M., N. A. Candurra, and E. B. Damonte. 1999. Myristic acid analogs are inhibitors of Junin virus replication. Microbes Infect. 1:609-614. [DOI] [PubMed] [Google Scholar]
- 10.Cornu, T. I., and J. C. de la Torre. 2002. Characterization of the arenavirus RING finger Z protein regions required for Z-mediated inhibition of viral RNA synthesis. J. Virol. 76:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornu, T. I., and J. C. de la Torre. 2001. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J. Virol. 75:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689-1698. [DOI] [PubMed] [Google Scholar]
- 13.Farazi, T. A., G. Waksman, and J. I. Gordon. 2001. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 276:39501-39504. [DOI] [PubMed] [Google Scholar]
- 14.Garcin, D., S. Rochat, and D. Kolakofsky. 1993. The Tacaribe arenavirus small zinc finger protein is required for both mRNA synthesis and genome replication. J. Virol. 67:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper, D. R., R. L. Gilbert, C. Blunt, and R. A. McIlhinney. 1993. Inhibition of varicella-zoster virus replication by an inhibitor of protein myristoylation. J. Gen. Virol. 74:1181-1184. [DOI] [PubMed] [Google Scholar]
- 16.Isaacson, M. 2001. Viral hemorrhagic fever hazards for travelers in Africa. Clin. Infect. Dis. 33:1707-1712. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan, J. M., G. Mardon, J. M. Bishop, and H. E. Varmus. 1988. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol. Cell. Biol. 8:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kentsis, A., E. C. Dwyer, J. M. Perez, M. Sharma, A. Chen, Z. Q. Pan, and K. L. Borden. 2001. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J. Mol. Biol. 312:609-623. [DOI] [PubMed] [Google Scholar]
- 19.Kunz, S., N. Sevilla, D. B. McGavern, K. P. Campbell, and M. B. Oldstone. 2001. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 155:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de La Torre. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, K. J., M. Perez, D. D. Pinschewer, and J. C. de la Torre. 2002. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 76:6393-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez, N., R. Jacamo, and M. T. Franze-Fernandez. 2001. Transcription and RNA replication of Tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol. 75:12241-12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurer-Stroh, S., B. Eisenhaber, and F. Eisenhaber. 2002. N-terminal N-myristoylation of proteins: refinement of the sequence motif and its taxon-specific differences. J. Mol. Biol. 317:523-540. [DOI] [PubMed] [Google Scholar]
- 24.Maurer-Stroh, S., and F. Eisenhaber. 2004. Myristoylation of viral and bacterial proteins. Trends Microbiol. 12:178-185. [DOI] [PubMed] [Google Scholar]
- 25.McIlhinney, R. A. 1990. The fats of life: the importance and function of protein acylation. Trends Biochem. Sci. 15:387-391. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa, Y., S. Hinata, H. Tomoda, T. Goto, M. Nakai, C. Aizawa, H. Tanaka, and S. Omura. 1996. Complete inhibition of human immunodeficiency virus Gag myristoylation is necessary for inhibition of particle budding. J. Biol. Chem. 271:2868-2873. [DOI] [PubMed] [Google Scholar]
- 27.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 28.Paige, L. A., G. Q. Zheng, S. A. DeFrees, J. M. Cassady, and R. L. Geahlen. 1990. Metabolic activation of 2-substituted derivatives of myristic acid to form potent inhibitors of myristoyl CoA:protein N-myristoyltransferase. Biochemistry 29:10566-10573. [DOI] [PubMed] [Google Scholar]
- 29.Parang, K., L. I. Wiebe, E. E. Knaus, J. S. Huang, D. L. Tyrrell, and F. Csizmadia. 1997. In vitro antiviral activities of myristic acid analogs against human immunodeficiency and hepatitis B viruses. Antivir. Res. 34:75-90. [DOI] [PubMed] [Google Scholar]
- 30.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez, M., and J. C. de la Torre. 2003. Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 77:1184-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinschewer, D. D., M. Perez, A. B. Sanchez, and J. C. de la Torre. 2003. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. USA 100:7895-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajala, R. V., R. S. Datla, T. N. Moyana, R. Kakkar, S. A. Carlsen, and R. K. Sharma. 2000. N-myristoyltransferase. Mol. Cell Biochem. 204:135-155. [DOI] [PubMed] [Google Scholar]
- 34.Salvato, M. S., K. J. Schweighofer, J. Burns, and E. M. Shimomaye. 1992. Biochemical and immunological evidence that the 11-kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 22:185-198. [DOI] [PubMed] [Google Scholar]
- 35.Strecker, T., R. Eichler, J. Meulen, W. Weissenhorn, H. Dieter Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wills, J. W., R. C. Craven, R. A. Weldon, Jr., T. D. Nelle, and C. R. Erdie. 1991. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J. Virol. 65:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]