Abstract
Synanthropic wild mammals can be important hosts for many vector-borne zoonotic pathogens. The aim of this study was determine the exposure of synanthropic mammals to two types of tick-borne pathogens in Panama, spotted fever group Rickettsia (SFGR) and Borrelia relapsing fever (RF) spirochetes. One hundred and thirty-one wild mammals were evaluated, including two gray foxes, two crab-eating foxes (from zoos), four coyotes, 62 opossum and 63 spiny rats captured close to rural towns. To evaluate exposure to SFGR, serum samples from the animals were tested by indirect immunofluorescence assay (IFA) using Rickettsia rickettsii and Candidatus Rickettsia amblyommii antigen. Immunoblotting was performed using Borrelia turicatae protein lysates and rGlpQ, to assess infection caused by RF spirochetes. One coyote (25%) and 27 (43%) opossums showed seroreactivity to SFGR. Of these opossums, 11 were seroreactive to C. R. amblyommii. Serological reactivity was not detected to B. turicatae in mammal samples. These findings may reflect a potential role of both mammals in the ecology of tick-borne pathogens in Panama.
Introduction
Synanthropic mammals are a diverse group of wild animals that prosper in areas where humans are present, both in rural and urban conditions [1]. Rodents, opossum, and mid-sized wild carnivores inhabit or migrate throughout ecotones that contain forest, pasture, and human dwellings, and may be important components of the transmission ecology of different pathogenic microorganisms [2, 3]. Furthermore, blood-feeding ectoparasites such as ticks add a dimension of complexity to the ecology of infectious diseases. In North America and Europe, there is evidence of tick-borne pathogens (TBP) are associated with wild mammals close to anthropogenic areas [4, 5, 6]. Unfortunately, throughout Central America there is a paucity of information regarding the roles of synanthropic mammals in the transmission of TBP.
There is evidence regarding the prevalence and significance of TBP in Panama [7, 8]. The first findings of TBP were cases of relapsing fever (RF) Borrelia in the early 1900s [9], with more than 100 cases confirmed in Panama City and neighboring localities [10]. Moreover, detection of RF spirochetes in the blood of small and large mammals including opossums, monkeys, armadillos, horses, and calves was also reported in the country [11, 12]. Currently, it remains unclear whether the pathogens continue to circulate in Panama.
From 1950–1953 five confirmed cases of Rickettsia rickettsii spotted fever were reported in Panama [13, 14, 15], while eight were diagnosed between 2004–2014 [8, 16, 17, 18]. Of these 13 cases, nine were fatal. In addition, there are also records of the Candidatus “Rickettsia amblyommii,” a member of the spotted fever group Rickettsai (SFGR) and other TBP such as ehrlichiosis and anaplasmosis in domestic mammals and ixodid ticks [18, 19, 20].
Given that TBP pose a risk to human public health in Panama, it is important to understand the role of mammals as putative hosts. In this current study, we evaluated serological responses of three carnivore species (coyotes, grey foxes, crab-eating foxes), common opossums, and spiny rats to SFGR and RF Borrelia antigens in Panama. Our findings indicate the importance of further defining the ecology of TBP in Panama.
Materials and Methods
Field sites
From 2013–2015, samples were taken opportunistically from wild carnivores relocated from anthropogenic localities to zoos: four coyotes (Canis latrans), two gray foxes (Urocyon cinereoargenteus) in El Níspero zoo (Coclé province), and two crab-eating foxes (Cerdocyon thous) in Summit Municipal Park (Panamá province). One coyote (No. 4) was removed from a highway two days prior to sampling. The animal was weak and lethargic, presumably hit by a car. However, traumatic injuries were not found; yet a skin rash was observed in trunk, abdomen, and inguinal area. Table 1 indicates origin of coyotes and foxes analyzed in the study. After chemical immobilization [intramuscular acepromazine (0.5 ml), plus ketamine 10% (0.5 ml) for coyotes], all animals were evaluated for the presence of ticks and additional ectoparasites. Blood was drawn from the femoral vein of each wild canid.
Table 1. Sites where wild canids were originally captured.
| Species | Zoo location | Time in zooa | Capture site | Characteristic | Ticks |
|---|---|---|---|---|---|
| Canis latrans | El Níspero | >300 | Natá, Coclé | Rural town | No |
| Canis latrans | El Níspero | >300 | Natá, Coclé | Rural town | No |
| Canis latrans | El Níspero | >180 | Chepo, Panamá | Rural town | No |
| Canis latrans | El Níspero | 2 | Arraiján, Panamá Oeste | Urban town around secondary forest | Yes b |
| Cerdocyon thous | Summit MP | Unknown | Unknown | Unknown | No |
| Cerdocyon thous | Summit MP | Unknown | Unknown | Unknown | No |
| Urocyon cinereoargenteus | El Níspero | ~30 | Santiago, Veraguas | Urban city, around pasture areas | No |
| Urocyon cenereoargenteus | El Níspero | ~ 30 | Santiago, Veraguas | Urban city, around pasture areas | No |
a Days that each animal was in the zoo prior to the extraction of blood.
b Ticks collected were 101 Amblyomma cf. oblongoguttatum and one Rhipicephalus sanguineus s.l.
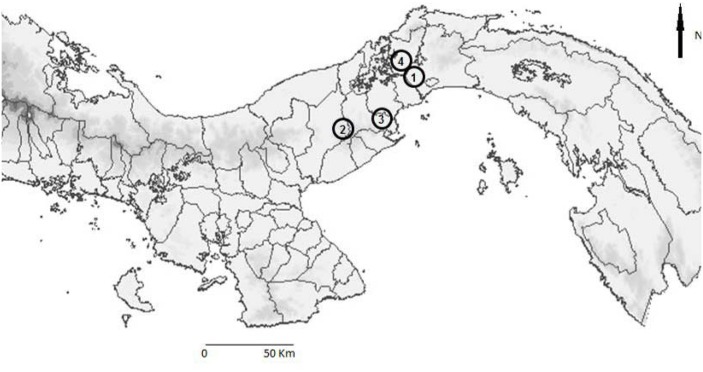
In addition, during 2013–2014, Tomahawk and Sherman traps were used to catch small mammals in the towns Gamboa (9° 07´25´´ N– 79° 43´ 02´´ W) (Colon province), Trinidad de las Minas (8° 46′ 32″ N—79° 59′ 45″ W), and Las Pavas (9° 6’ 15” N—79° 53’ 9” W) (Capira, Western Panama province). For the trapping and handling of the captured mammals, we followed methods cited by González et al. [21]. The ecology of Gamboa consists of rainforest [22], while Trinidad de las Minas and Las Pavas are rural towns surrounded by secondary forest and pasture [23, 24]. Fig 1 indicates the localities. The blood samples were centrifuged and serum was collected to detect the presence of circulating antibodies to SFGR and RF Borrelia.
Fig 1. Map of Panama showing the geographical sites: Summit Municipal Park (1), El Níspero (2), Capira (3), Gamboa (4).
Immunofluorescence assay (IFA) to verify previous exposure to SFGR
Serum samples were tested using crude antigens derived from Brazilian strains of R. rickettsii (Taiacu) and C. “R. amblyommii” (AC37) from the collection of the Center for Research in Tropical Diseases, Faculty of Microbiology at the University of Costa Rica, as previously described [25, 26]. Slides were incubated with fluorescein isothiocyanate labeled sheep anti-dog IgG, sheep anti-opossum IgG (derived from Didelphis aurita) (CCZ, SP, Brazil), and rabbit anti-guinea pig IgG, for wild canids, marsupials and rodents, respectively, following procedures described by Horta et al. [25] and Labruna et al. [26]. Initial screenings were at a 1 to 64 dilution, and the slides were read using an ultraviolet microscope (Nikkon Eclipse E-400) at 1,000 X magnification. Fluorescence was compared to positive and negative controls as previously described (25), and reactive samples were subsequently tested in serial two-fold dilution to determine the endpoint titer. Given the serological cross reactivity observed against species of SFGR, serum that showed a Rickettsia species titer of at least 4-fold higher compared to the other species was considered as the possible causative agent [26, 27]. For IFAs, negative and positive controls were from uninfected animals and laboratory infected dogs, respectively, and were provided by the University of San Palo.
Immunoblotting to detect exposure to RF spirochetes
Protein lysates from the 91E135 strain of B. turicatae were used for SDS-PAGE and immunoblotting [28] because the strain originated from Texas and would be the closest known genetic match to species and isolates distributed in Panama. Assays were performed as previously described with 1 x 107 spirochetes and 1 μg of rGlpQ electrophoresed per lane [28, 29]. All serum samples were diluted 1:200 for assays. Protein G-HRP (Life Technologies, Carlsbad, CA) or anti-opossum HRP (Alpha Diagnostics International Inc., San Antonio, TX) at a 1:4,000 dilution were used to determine serum reactivity to Borrelia antigens. Positive control serum samples originated from rodents and a canine infected with B. turicatae by tick bite. Animals were considered positive if serological reactivity was detected to five or more proteins in B. turicatae lysates and rGlpQ, as previously described [30].
Ethical approval
This work was authorized by the National Bioethics Committee of Investigation (561/CNBI/ICGES/06) and Institutional Animal Care and Use Committe (IACUC, 2006/02) of the Gorgas Research Institute. Wild canids from the zoo were analyzed with authorization from the respective administrations.
Results
Serum samples from 131 wild mammals, corresponding to the eight wild canids, 63 spiny rats (Proechymys semispinosus) and 62 common opossums (Didelphis marsupialis) were collected. Of the four coyotes, only No. 4 was reactive against SFGR; however, because this canid had similar titers for R. rickettsii and C. “R. amblyommii”, we could not discriminate the infectious agent (Table 2). One male of Rhipicephalus sanguineus s.l. and 101 Amblyomma cf. oblongoguttatum were collected from coyote No. 4, while ticks were not identified on the remaining canids (Table 1).
Table 2. IFA reactivity of coyote and opossum serum samples to SFGR.
| No. Animal | Sites | Antigens slides | Putative pathogen c | |
|---|---|---|---|---|
| R. rickettsii a | R. amblyommii a | |||
| Coyote 4 | Arraiján | 2048 | 512 | SFGR |
| Opossum 2 | Capira | 512 | 256 | SFGR |
| Opossum 5 | Capira | - b | 256 | C. R. amblyommii |
| Opossum 7 | Capira | 512 | 512 | SFGR |
| Opossum 9 | Capira | - b | 64 | SFGR |
| Opossum 10 | Capira | - b | 512 | C. R. amblyommii |
| Opossum 11 | Capira | 128 | 512 | SFGR |
| Opossum 14 | Capira | - b | 256 | C. R. amblyommii |
| Opossum 18 | Capira | 128 | 1024 | C. R. amblyommii |
| Opossum 19 | Capira | 64 | 64 | SFGR |
| Opossum 22 | Capira | 128 | 1024 | C. R. amblyommii |
| Opossum 27 | Capira | 64 | 128 | SFGR |
| Opossum 28 | Capira | 64 | 64 | SFGR |
| Opossum 33 | Capira | 64 | 1024 | C. R. amblyommii |
| Opossum 35 | Capira | - b | 64 | SFGR |
| Opossum 37 | Capira | 128 | 1024 | C. R. amblyommii |
| Opossum 43 | Capira | 128 | 128 | SFGR |
| Opossum 46 | Capira | 64 | 128 | SFGR |
| Opossum 49 | Capira | 64 | 1024 | C. R. amblyommii |
| Opossum 50 | Gamboa | 128 | 64 | SFGR |
| Opossum 51 | Gamboa | 64 | - b | SFGR |
| Opossum 53 | Gamboa | 128 | 1024 | C. R. amblyommii |
| Opossum 55 | Gamboa | 64 | 64 | SFGR |
| Opossum 58 | Gamboa | 128 | 128 | SFGR |
| Opossum 59 | Gamboa | 256 | 1024 | C. R. amblyommii |
| Opossum 60 | Gamboa | - b | 512 | C. R. amblyommii |
| Opossum 61 | Gamboa | 64 | 1024 | C. R. amblyommii |
| Opossum 62 | Gamboa | 256 | 256 | SFGR |
a Titer was defnied as the inverse of the greatest serum sample dilution.
b (-) indicates that the serum sample was unreactive to the antigens used in the assay.
c The SGRF designation indicates that the putative species causing infection was indistinguishable in 16 animals.
Of the 62 D. marsupialis, 27 (43%, 95% CI [31, 57%]) opossums demonstrated serological reactivity against SFGR, 18 originated from Capira (36%, 95% CI [24, 52%], N = 49) and nine from Gamboa (69%, 95% CI [39, 90%], N = 13). Of these, 12 were reactive to C. R. amblyommii, and 15 samples were impossible to discriminate the infectious agent (Table 2). Immature Amblyomma spp. and adult Ixodes luciae were collected from 12 opossums. None of the spiny rat (N = 63) samples were considered serologically positive SFGR, and serum samples from all the animals in this study were considered seronegative against RF Borrelia antigens.
Discussion
In this study, we evaluated serological responses to SFGR and RF spirochetes in mammals that have been overlooked as putative hosts in Panama. The results indicate that opossums and coyotes may be involved with the maintenance of SFGR in the mammals tested in this study. This work compliments previous findings that SFGR circulate in Capira, a western Panama province, and the implication of opossums in fatal cases of SF in this region [15, 16]. Moreover, seroreactivity of opossums in Gamboa presents a new location within the Colon province where SFGR may circulate.
Our results further suggest the relevance of Didelphis species as reservoir of SFGR. In Sao Paulo, Brazil 63% of the Didelphis aurita (N = 65) and 72% of Didelphis albiventris (N = 29) were naturally infected with SFGR [25], while 100% (N = 5) of D. albiventris were seropositive to SFGR from Paulicéia, Brazil [31]. Moreover, Didelphis species display extended periods of rickettsemia compared to rodents, and are asymptomatic during infection with R. rickettsia [32, 33]. Didelphis virginiana maintained R. rickettsii for three to four weeks [32], while D. aurita remained infected with this pathogen for 26 days [33]. Didelphis aurita is also considered an amplifying host for immature A. cajennense ticks [33]. Given the ability of these marsupials to thrive in wild, rural, and urban environments [34], they may play a significant role in the public health significance and ecology of SFGR in Panama.
The public health significance of C. “R. amblyommii” remains unclear; although it may also cause mild fever in humans [35, 36, 37]. Antibody titers of 12 opossums (Table 2) suggest the animals may maintain this species of SFGR. Candidatus “R. amblyommii.” Additionally, there is evidence that horses and dogs maintain this species of Rickettsia [38, 39], and the putative pathogen has also been detected in several species of ticks in the Americas [40]. Within Panama, the organism is widely distributed and found in the Dermacentor nitens, Amblyomma mixtum, R. sanguineus s.l., Amblyomma ovale, and Haemaphysalis juxtakochi [18, 19, 41]. Future work will evaluate the ticks collected in this study for microorganism detection.
Our findings comprise the first indication in Panama that coyotes may be involved with the ecology of SFGR in the country. Although determining the Rickettsia species that infected coyote No. 4 was not possible, high antibody titers (2,048 R. rickettsii and 516 C. “R. amblyommii”), the presence of a skin rash, and the animal’s physical condition indicated an active SFGR infection. Given the close relationship between coyotes and dogs, it is possible that both canids can manifest similar symptoms to pathogenic SFGR, especially R. rickettsia. These symptoms can include skin rash, petechial hemorrhage in eyes and mucosa, lethargy and paralysis [27, 42]. Alternatively, it is known that C. “R. amblyommii” can stimulate an immune response in both canids [39, 43, 44], but there is no evidence to support the pathogenicity of C. “R. amblyommii” in dogs. Although we cannot confirm that R. rickettsii infected coyote No. 4, the symptoms presented and the seroreactivity against SFGR can potentially rule out other TBP such as ehrlichiosis and anaplasmosis that affect wild and domestic canids [45, 46].
While we failed to detect exposure to RF Borrelia spirochetes in the coyotes and opossums evaluated, the sample size was small and additional work is needed. These vertebrates should be considered in the ecology of RF spirochetes, as Dunn and Clark reported the detection of spirochetes in the blood of 9.8% of opossums from Panama [12]. Additionally, Borrelia species distributed within the United States are infectious to domestic dogs [28, 47,48], suggesting that coyotes may be a likely host. Given that the Ornithodoros tick vector of RF Borrelia possesses a 10 to 20-year life history and the observed transovarial transmission of some species [49], it is likely that the pathogens continue to circulate within Panama.
Ticks and TBP in Panama are largely neglected by health care providers and the scientific community. As infectious diseases emerge and continue to threaten human health, it is important to understand how the pathogens are maintained. Our results show insight into the ecology of SFGR in Panama, and suggest the necessity to include synanthropic mammals, such as opossums and coyotes, in future field studies. Furthermore, as additional work is focused to collect Ornithodoros ticks, we will define and determine the distribution and disease burden of RF spirochetes in the country.
Acknowledgments
We thank the Environment Ministry for permission to collections, and Edgar Araúz for the authorization in SMP; Laya Hun, Francisco Vega (University of Costa Rica, CR) to facilitate the plates seeded with antigens of R. rickettsii and C. R. amblyommii; Marcelo Labruna and Mauricio Horta (Sao Paulo University, Br) for the positives controls; Julie Lopez for critical review of the manuscript. This work received financial support from Panamanian SENACYT grant, COL11-043 (NG, AS), and the United States National Institutes of Health grant, AI123652 (JEL). AS and JEC received funds of Sistema Nacional de Investigación (SNI-SENACYT).
Data Availability
All relevant data are within the paper.
Funding Statement
This work received financial support from Panamanian SENACYT grant, COL11-043 (NG, AS), and the United States National Institutes of Health grant, AI123652 (JEL). AS and JEC received funds of Sistema Nacional de Investigación (SNI-SENACYT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Khlyap LA, Warshavsky AA. Synanthropic and agrophilic rodents as invasive alien mammals. Russian J Biol Inv 2011; 1(4): 301–312. [Google Scholar]
- 2.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol 2007; 22(2): 95–102. 10.1016/j.tree.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarlane R, Sleigh A, McMichael T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian–Australasian Region. EcoHealth 2012; 9: 24–35. 10.1007/s10393-012-0763-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girard YA, Swift P, Chomel BB, Kasten RW, Fleer K, Foley F, et al. Zoonotic vector-borne bacterial pathogens in California mountain lions (Puma concolor), 1987–2010. Vector Borne Zoonotic Dis. 2012; 12, 913–921. 10.1089/vbz.2011.0858 [DOI] [PubMed] [Google Scholar]
- 5.Cardoso L, Gilad M, Cortes H, Nachum-Biala H, Lopes J, Vila-Viçosa J, et al. 2015. First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasit Vectors. 2015: 8, 144 10.1186/s13071-015-0756-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millán J, Proboste T, Mera I, Chirife AD, de la Fuente J, Altet L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human-wildlife interface. Ticks Tick-Borne Dis. 2015; [DOI] [PubMed] [Google Scholar]
- 7.Méndez E, Chaniotis B. Reseña de las principales enfermedades transmitidas por garrapatas en Panamá. Rev Med Pan 1987; 12: 217–223. [PubMed] [Google Scholar]
- 8.De Luca J, García G, García E, Castro A, Lyons C, Bermúdez S. Nuevo caso de rickettsiosis humana en Panamá: primer reporte proveniente de un área silvestre. Rev Med Pan 2013; 34: 40–43. [Google Scholar]
- 9.Darling S. The relapsing fever of Panama. Proc. Canal Zone Med. Assoc. 1909; 4 [Google Scholar]
- 10.Calero C. Relapsing fever on the isthmus of Panamá; report of the 106 cases. Am J Trop Med Hyg 1946; 26: 761–769. [DOI] [PubMed] [Google Scholar]
- 11.Bates L, Dunn L, St. John J. Relapsing fever in Panama: the tick, Ornithodoros talaje, demonstrated to be the transmitting agent of relapsing fever in Panama by human experimentation. Am J Trop Med Hyg 1921; 1 (4): 183–210. [Google Scholar]
- 12.Dunn L, Clark H. Notes on relapsing fever in Panama, with special reference to animal host. Am J Trop Med Hyg 1933; 13 (2): 201–209. [Google Scholar]
- 13.Rodaniche E, Rodaniche A. Spotted fever in Panama; isolation of the etiologic agent from a fatal case. Am J Trop Med Hyg 1950; 30: 511–517. [DOI] [PubMed] [Google Scholar]
- 14.Hurtado LA, Calzada JE, Pineda V, González K, Santamaría AM, Cáceres L, Wald C, Saldaña A. Conocimientos y factores de riesgo relacionados con la enfermedad de Chagas en dos comunidades panameñas donde Rhodnius pallescens es el vector principal. Biomedica 2014; 34: 260–70. [DOI] [PubMed] [Google Scholar]
- 15.Rodaniche E Natural infection of the tick, Amblyomma cajennense, with Rickettsia rickettsii in Panama. Am J Trop Med Hyg 1953; 2(4): 696–699. [DOI] [PubMed] [Google Scholar]
- 16.Estripeaut D, Aramburú M, Saéz-Llórens X, Thompson H, Dasch G, Paddock C, et al. Rocky Mountain Spotted Fever, Panama. Emerg Infect Dis. 2007; 13: 1763–1765. 10.3201/eid1311.070931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tribaldos M, Zaldivar Y, Bermúdez S, Samudio F, Mendoza Y, Martinez A, et al. Rocky Mountain spotted fever in Panama: a cluster description. J Infect Dev Ctries. 2011; 5: 737–741. [DOI] [PubMed] [Google Scholar]
- 18.Bermúdez S, Castro A, Trejos T, García G, Gabster A, Miranda R, et al. Distribution of Spotted Fever Group Rickettsiae in hard ticks (Ixodida: Ixodidae) from Panamanian urban and rural environments. EcoHealth 2016; 1–11. [DOI] [PubMed] [Google Scholar]
- 19.Eremeeva M, Karpathy S, Levin M, Caballero M, Bermúdez S, Dasch G, et al. Spotted Fever Rickettsiae, Ehrlichia and Anaplasma in Peridomestic Environments in Panama. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2009; 15 Suppl 2: 12–14. [DOI] [PubMed] [Google Scholar]
- 20.Pérez A, Torres A, Lasso J. Prevalência de ehrlichiose canina entre animais atendidos no Complexo Hospitalário Veterinário do Corozal, Faculdade de Medicina Veterinária, Universidade de Panamá. Arq Inst Biologia 2013; 80(2): 207–211. [Google Scholar]
- 21.González K, Calzada JE, Saldaña A, Riggs C, Alvarado G, et al. Survey of Wild Mammal Hosts of Cutaneous Leishmaniasis Parasites in Panamá and Costa Rica. Trop Med Health 2015; 43(1) 75–78. 10.2149/tmh.2014-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermúdez-Mongue Y, Barrios H. Insectos asociados a Vriesea sanguinolenta (Bromeliaceae). Scientia 2011; 21 (2): 7–32 [Google Scholar]
- 23.Calzada JE, Saldaña A, Riggs C, Valderrama A, Romero L, Chaves LF Changes in Phlebotomine sand fly species composition following insecticide thermal fogging in a rural setting of Western Panama. PLoS ONE 2011; 8(1): e53289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calero C, Nuñez J, Silva R. Rocky Mountain spotted fever in Panama. Report of two cases. Am J Trop Med Hyg 1952; 1(4): 631–636. [PubMed] [Google Scholar]
- 25.Horta M, Labruna M, Pinter A, Linardi P, Schumaker T. Rickettsia infection in five areas of the state of São Paulo, Brazil. Mems Inst Oswaldo Cruz 2007; 102(7): 793–801. [DOI] [PubMed] [Google Scholar]
- 26.Labruna M, Pacheco R, Richtzenhain L, Szabó M Isolation of Rickettsia rhipicephali and Rickettsia bellii from Haemaphysalis juxtakochi ticks in the States of Sao Paulo, Brazil. Appl Env Microbiol. 2007; 73 (3): 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piranda E, Faccini J, Pinter A, Saito T, Pacheco R, Hagiwara M, et al. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mems Inst Oswaldo Cruz 2008; 103(7): 696–701. [DOI] [PubMed] [Google Scholar]
- 28.Lopez JE, Wilder HK, Boyle W, Drumheller LB, Thornton JA, et al. Sequence analysis and serological responses against Borrelia turicatae BipA, a putative species-specific antigen. PLoS Neglected Tropical Diseases 2013; 7(9): e2454 10.1371/journal.pntd.0002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder HK, Wozniak E, Huddleston E, Tata SR, Fitzkee NC, Lopez JE. Case Report: A retrospective serological analysis indicating human exposure to Tick-Borne Relapsing Fever Spirochetes in Texas. PLoS Neglected Tropical Diseases 2015; 9(4): e0003617 10.1371/journal.pntd.0003617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, McCoy BN, et al. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Neglected Tropical Diseases 2012; 6: e1924 10.1371/journal.pntd.0001924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silveira I, Martins T, Olegário M, Peterka C, Guedes E, Ferrerira F, et al. Rickettsial Infection in Animals, Humans and Ticks in Paulicéia, Brazil. Zoon Pub Health 2015; 1–9. [DOI] [PubMed] [Google Scholar]
- 32.Bozeman FM, Shirai A, Humphries JW, Fuller HS Ecology of Rocky Mountain spotted fever II. Natural infection of wild mammals and birds in Virginia and Maryland. Am J Trop Med Hyg. 1967; 16: 48–59. [PubMed] [Google Scholar]
- 33.Horta M, Morae-Filho J, Casagrande R, Saito T, Rosa S, Ogrzewalska M, et al. Experimental infection of opossums Didelphis aurita by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Vector-Borne Zoonotic Dis. 2009; 9(1): 109–117. 10.1089/vbz.2008.0114 [DOI] [PubMed] [Google Scholar]
- 34.Adler GH, Carvajal A, Davis-Foust SL, Dittel JW. Habitat associations of opossums and rodents in a lowland forest in French Guiana. Mamm Biol. 2012; 77: 84–89. [Google Scholar]
- 35.Dasch G, Kelly O, Richards A, Sanchez J, Rives C. Western Blotting analysis of sera from military personnel exhibiting serological reactivity to Spotted Fever Group Rickettsiae. Am J Trop Med Hyg 1993; 49. [Google Scholar]
- 36.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, et al. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008; 8:597–606. 10.1089/vbz.2007.0271 [DOI] [PubMed] [Google Scholar]
- 37.Jiang J, Yarina T, Miller M, Stromdahl E, Richards A. Molecular Detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector-Borne Zoonotic Dis. 2010; 10(4): 329–340. 10.1089/vbz.2009.0061 [DOI] [PubMed] [Google Scholar]
- 38.Bermúdez SE, Zaldívar Y, Spolidorio M, Moraes-Filho J, Miranda R, Caballero C, et al. Rickettsial infection in domestic mammals and their ectoparasites in El Valle de Antón, Coclé, Panamá. Vet Parasitol. 2011; 177: 134–138. 10.1016/j.vetpar.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 39.Barrett A, Little SE, Shaw E. ‘‘Rickettsia amblyommii” and R. montanensis Infection in Dogs Following Natural Exposure to Ticks. Vector-Borne Zoonotic Dis. 2014; 14(1): 20–25. 10.1089/vbz.2013.1325 [DOI] [PubMed] [Google Scholar]
- 40.Labruna M, Mattar S, Nava S, Bermúdez S, Venzal J, Dolz G, et al. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Rev MVZ Cordoba 2011; 16 (2): 2435–2457. [Google Scholar]
- 41.Castro A, García G, Dzul-Rosado K, Aguilar A, Castillo J, Gabster A, et al. Questing Amblyomma mixtum and Haemaphysalis juxtakochi (Acari: Ixodidae) Infected with Candidatus “Rickettsia amblyommii” from the Natural Environment in Panama Canal Basin, Panama. Trop Med Health 2015; 43 (4): 217–222. 10.2149/tmh.2015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin ML, Killmaster LF, Zemtsova GE, Ritter J. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One 2014; 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bischof R, Rogers DG Serologic Survey of Select Infectious Diseases in Coyotes and Raccoons in Nebraska. J Wildlife Dis. 2005; 41(4): 787–791. [DOI] [PubMed] [Google Scholar]
- 44.Starkey L, West M, Barrett A, Saucier J, O´Connor T, Paras K, et al. Prevalence of Antibodies to Spotted Fever Group Rickettsia spp. and Ehrlichia spp. in Coyotes (Canis latrans) in Oklahoma and Texas, USA. J Wildlife Dis. 2013; 49(3):670–673. [DOI] [PubMed] [Google Scholar]
- 45.Leschnik M, Kirtz G, Wille-Piazzai W, Duscher G. Acute granulocytic anaplasmosis in a captive timber wolf (Canis lupus occidentalis). J Zoo Wildlife Med. 2012; 43(3):645–8. [DOI] [PubMed] [Google Scholar]
- 46.Morales-Soto F, García-De la Peña C, Rodríguez-Vivas R, Rodríguez-Martínez R. Serosurvey of vector-borne diseases in the Mexican wolf (Canis lupus baileyi) in captivity. Ar Med Vet. 2016; 48: 129–131. [Google Scholar]
- 47.Breitschwerdt E, Nicholson W, Kiehl A, Steers C, Meuten D, Levine J. Natural infections with Borrelia spirochetes in two dogs from Florida. J Clin Microbiol 1994; 32(2): 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly A, Raffel S, Fischer R, Bellinghausen M, Stevenson C, Schwan TG. First isolation of the relapsing fever spirochete, Borrelia hermsii, from a domestic dog. Ticks Tick-Borne Dis. 2014; 5(2): 95–99. 10.1016/j.ttbdis.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis GE. Ornithodoros turicata: the male, feeding and copulation habits, fertility, span of life, and the transmission of relapsing fever spirochetes. Pub Health Rep. 1941; 56: 1799–1802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.