Abstract
Aim
Most pediatric in-hositalcardiac arrests(IHCAs) occur in ICUs where invasive hemodynamic monitoring is frequently available. Titrating cardiopulmonary resuscitation (CPR) to the hemodynamic response of the individual improves survival in preclinical models of adult cardiac arrest. The objective of this study was to determine if titrating CPR to systolic blood pressure (SBP) and coronary perfusion pressure (CoPP) in a pediatric porcine model of asphyxia-associated ventricular fibrillation (VF) IHCA would improve survival as compared to traditional CPR.
Methods
After 7 minutes of asphyxia followed by VF, 4-week-old piglets received either Hemodynamic-Directed CPR (HD-CPR; compression depth titrated to SBP of 90mmHg and vasopressor administration to maintain CoPP ≥20mmHg); or Standard Care (compression depth 1/3 of the anterior-posterior chest diameter and epinephrine every 4 minutes). All animals received CPR for 10 minutes prior to the first defibrillation attempt. CPR was continued for a maximum of 20 minutes. Protocolized intensive care was provided to all surviving animals for 4 hours. The primary outcome was 4-hour survival.
Results
Survival rate was greater with HD-CPR (12/12) than Standard Care (6/10; p=0.03). CoPP during HD-CPR was higher compared to Standard Care (point estimate +8.1mmHg, CI95: 0.5–15.8mmHg; p=0.04). Chest compression depth was lower with HD-CPR than Standard Care (point estimate 14.0mm, CI95: 9.6–18.4mm; p<0.01). Prior to the first defibrillation attempt, more vasopressor doses were administered with HD-CPR versus Standard Care (median 5 versus 2; p<0.01).
Conclusions
Hemodynamic-directed CPR improves short-term survival compared to standard depth-targeted CPR in a porcine model of pediatric asphyxia-associated VF IHCA.
Keywords: Pediatric Cardiac Arrest, Cardiopulmonary Resuscitation
Introduction
Approximately 6000 children receive cardiopulmonary resuscitation (CPR) for in-hospital cardiac arrest (IHCA) annually in the United States.1 These events occur primarily in intensive care units (ICUs), where 1.4–6% of patients suffer an IHCA.2-5 Despite considerable improvements in outcomes in recent decades, only 39–52% of children survive to hospital discharge.5-10
Standard CPR includes absolute depth and rate targets with time-based epinephrine dosing.11-14 However, greater than 90% of pediatric IHCA events occur in an ICU setting, where CPR is generally provided by individuals with advanced training and where patient-specific physiologic monitoring (e.g., indwelling arterial catheters, end tidal carbon dioxide [ETCO2] monitoring) is often available.2 Therefore, it is reasonable for clinical teams to adjust resuscitation efforts based on an individual patients hemodynamics during CPR. Notably, “personalized” hemodynamic-directed CPR has improved survival outcomes in pre-clinical adult models of IHCA,15-17 but remains understudied in pediatric models.
The primary objective of this study was to determine if actively titrating CPR to hemodynamics (i.e., invasive arterial blood pressure [BP]) in a porcine model of pediatric asphyxia-associated ventricular fibrillation (VF) IHCA would improve survival compared with optimized standard CPR. We hypothesized that rates of the return of spontaneous circulation (ROSC) and 4-hour survival would be higher in animals treated with a method of hemodynamic-directed CPR (HD-CPR) compared with standard care. We further hypothesized that coronary perfusion pressure (CoPP) during HD-CPR would be higher compared to standard CPR, providing a potential physiologic mechanism for improved outcomes.
Methods
Animal Preparation
The Children's Hospital of Philadelphia Institutional Animal Care and Use Committee approved this experimental protocol. Twenty-two healthy 1-month old female domestic piglets were utilized for the study. All piglets underwent an overnight fast and were then anesthetized and tracheally intubated. They were mechanically ventilated with an anesthesia machine (Modulus SE; Datex Ohmeda, Madison, WI) on a mixture of room air and titrated isoflurane (approximately 1%) to maintain anesthesia. Ventilator settings included tidal volumes of 10mL/kg, positive end-expiratory pressure (PEEP) of 6cmH2O, and the titration of respiratory rates to maintain continuously monitored ETCO2 values between 38 and 42mmHg (NICO; Novametrix Medical Systems Inc., Wallingford, CT). Weights and anterior-posterior (AP) chest diameters were measured and recorded.
An external jugular vein, femoral artery, and bilateral femoral veins were cannulated with vascular introducer sheaths (Cordis Corp., Fremont, CA) under ultrasound guidance using percutaneous Seldinger technique. High fidelity, solid-state, micromanometer-tipped catheters (4.5–6F; Millar Instruments, Houston, TX) were advanced through the femoral artery and vein access sites to measure continuous aortic and right atrial pressures, respectively. A balloon-tipped pulmonary artery thermodilution catheter (Edwards Lifesciences, Irvine, CA) was advanced to the pulmonary artery via a femoral vein and a bipolar pacing wire (Edwards Lifesciences) was advanced into the right ventricle via the external jugular vein. All catheter positions were confirmed by fluoroscopy. Unfractionated heparin (200U/kg) was administered to prevent catheter clotting. In an effort to replete intravascular volume to a euvolemic state after the overnight fast, 20mL/kg of 0.9% sodium chloride solution was administered to all animals.
Measurements
Prior to and during the experimental protocol, the electrocardiograph, aortic blood pressure, right atrial pressure, pulse oximetry, and ETCO2 waveforms were displayed and recorded (PowerLab; ADInstruments, Colorado Springs, CO). CoPP was calculated and displayed in real time by subtracting the mid-diastolic (release) right atrial pressure from the mid-diastolic aortic pressure18,19 (Figure 1). Arterial blood gas specimens were obtained from the thoracic aorta at baseline (prior to start of experimental protocol), 6.5 minutes into the asphyxial period, and 6 minutes into the resuscitation period.
Figure 1. Coronary Perfusion Pressure during Cardiopulmonary Resuscitation.
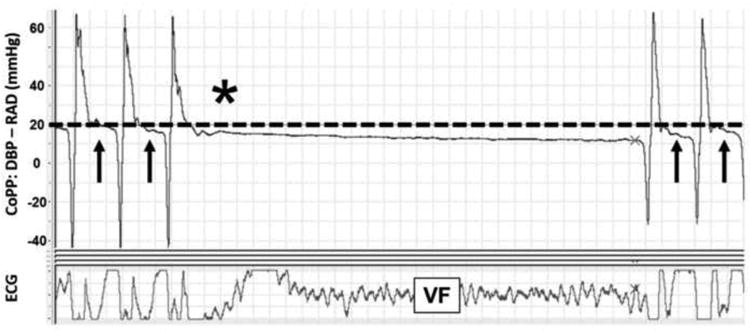
Representative sample during experimental protocol from PowerLab data acquisition system. Dotted line indicates coronary perfusion pressure of 20mmHg, goal coronary perfusion pressure in the HD-CPR group. Arrows represent period of coronary perfusion pressure assessment in mid-diastole. *Pause in CPR as per resuscitation protocol. Definitions of abbreviations: ECG = electrocardiogram; CoPP = coronary perfusion pressure; DBP = aortic diastolic pressure; RAD = right atrial diastolic pressure; VF = ventricular fibrillation.
A CPR recording defibrillator (Philips Heart Start MRx defibrillator with Q-CPR option [Philips Health Care, Andover, MA; and Laerdal Medical Corporation, Stavanger, Norway] or Zoll R Series Plus [Zoll Medical Corporation, Chelmsford, MA]) was utilized during CPR. These devices are validated to accurately measure chest compression depth within 1.6mm20 and 6mm21, respectively. The defibrillator displayed and recorded chest compression depth (mm) and rate (min-1).
Model Justification
A porcine model was selected because of the similarity between swine and humans in relation to AP chest diameter and chest compression characteristics.22 One-month old piglets are neurodevelopmentally similar to human toddlers,23 who account for 42% of pediatric IHCAs.6 Likewise, these animals are similar in body mass to children between 1 and 2 years of age.24 The injury model utilized in this study was designed to simulate pediatric IHCA preceded by an asphyxial event, as 42–52% of pediatric IHCAs have an underlying respiratory etiology.2,9,10,25,26 The duration of asphyxia (7 minutes) was intended to produce severe arterial hypoxemia and hypercarbia.15,17 While induction of VF is somewhat artificial, it was induced in order to ensure that the cardiac arrest would be maintained for a uniform 10-minute period to evaluate the effects CPR in both groups. Ten minutes is the median duration of CPR among pediatric survivors in the Get With The Guidelines-Resuscitation registry.26 Additionally, as nearly 1/3 of pediatric IHCAs will have VF documented at some point during CPR, a VF model has relevance to the in-hospital pediatric rescuer.27 The epinephrine dose (0.02mg/kg) utilized in this protocol, though twice the dose recommended by Pediatric Advanced Life Support (PALS) guidelines, is the long-standing standard for swine resuscitation studies.28-31 The Standard Care treatment group was designed to mimic optimal care with chest compression depth-directed CPR per PALS guidelines.12
Experimental Protocol
Asphyxia was induced by clamping the endotracheal tube and confirmed by absence of exhaled CO2. Throughout the 7-minute asphyxial period, fentanyl(1–10 mcg/kg/hr) was administered intravenously to maintain anesthesia. The tube remained clamped for 7 minutes to provoke severe pre-arrest hypoxemia and hypercarbia. At the end of 7 minutes, VF was induced by electrical pacing. After confirmation of VF, the endotracheal tube was unclamped and CPR commenced.
In all piglets, chest compressions were provided with a target rate of 100 per minute, with audio-guidance from a metronome and real-time visual feedback from the CPR quality-monitoring defibrillator. During CPR, mechanical ventilation was provided with a PEEP of 6cmH2O,100% inspired oxygen, and 6 breaths per minute with a constant tidal volume of 10mL/kg for both groups. Animals were randomized to one of two treatment strategies (Figure 2):
Hemodynamic-Directed CPR (“HD-CPR”) – Chest compression depth was actively titrated to maintain a systolic blood pressure (SBP) of 90mmHg, with the chest compressor continuously adjusting the manual force of compression to target this goal. As such, deeper compressions up to a maximal depth of 50% AP chest depth32,33 (as indicated by visual feedback from the CPR quality-monitoring defibrillator) were provided when SBP was less than 90mmHg, and shallower compressions were provided when SBP exceeded 90mmHg. This SBP goal is based on SBP values observed during successful CPR in children with IHCA34 and in previous piglet models of cardiac arrest.35-37 Vasopressors were titrated to maintain a CoPP of at least 20mmHg (Figure 1), based on human and animal data demonstrating improved rates of survival with CoPP greater than 20mmHg38,39 and prior success with this CoPP goal in related animal studies.15-17 These animals only received vasopressor boluses when CoPP was less than 20mmHg during mid-diastole for at least 3 consecutive chest compressions, beginning 2 minutes into the resuscitation period. The order of vasopressor administration was epinephrine (0.02mg/kg) first, additional epinephrine (0.02mg/kg) second, and vasopressin (0.4U/kg) third. Vasopressin was used in this group after two doses of epinephrine “failed” to maintain a CoPP above the goal of 20mmHg. The minimal dosing interval was one minute after epinephrine doses and two minutes after vasopressin doses, after which time the cycle was restarted in the same order.
Standard Care – Chest compression depth was targeted to the American Heart Association (AHA)-recommended depth of 1/3 of the AP chest diameter with the chest compressor actively adjusting the force of the compression in response to visual feedback from the CPR quality-monitoring defibrillator. Epinephrine (0.02mg/kg) was administered at PALS-recommended dosing intervals (every 4 minutes)12, beginning 2 minutes after the start of the resuscitation period to simulate the interval from recognized cardiac arrest to epinephrine administration in actual hospital settings.40
Figure 2. Protocol Design.
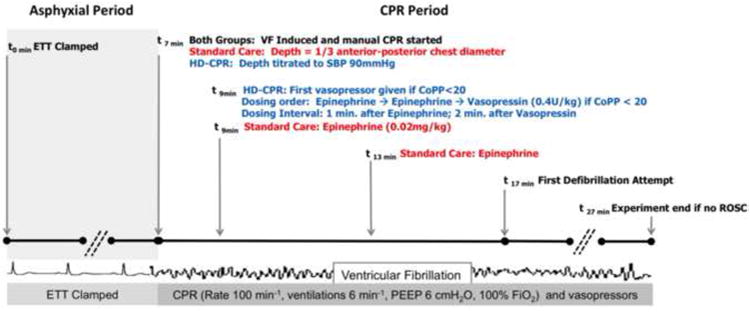
Depiction of experimental protocol for both treatment groups. Definitions of abbreviations: CPR = cardiopulmonary resuscitation; ETT = endotracheal tube; VF = ventricular fibrillation; CPR = cardiopulmonary resuscitation; HD = hemodynamic-directed; SBP = systolic blood pressure; AP = anterior-posterior; CoPP = coronary perfusion pressure; ROSC = return of spontaneous circulation.
In both groups, after 10 minutes of CPR, an initial 5 Joule/kg (50% of AHA recommended maximal pediatric defibrillation dose)12 biphasic waveform defibrillation attempt was provided because of previous data showing safety and efficacy of this dosage in piglets of similar size.36 Resuscitation according to treatment regimens continued with defibrillation attempts up to every two minutes until sustained ROSC was achieved or until an additional 10 minutes of resuscitation efforts after the initial defibrillation attempt failed to result in ROSC (Figure 2).
Animals in which ROSC was attained received algorithmic post-cardiac arrest care. This protocol included mechanical ventilation titrated to maintain ETCO2 of 38–42mmHg, inhaled oxygen titrated to maintain SpO2 94–99%, isoflurane titrated to inhibit a toe-pinch reflex, and intravenous dopamine titrated to maintain a mean aortic blood pressure (MAP) of at least 55mmHg. Four hours after ROSC, animals were euthanized under general anesthesia with potassium chloride. All animals received a post-mortem examination for assessment of visceral injuries.
Data Analysis and Outcomes
The primary outcome of this study was 4-hour survival post-ROSC. Secondary outcomes included (1) sustained ROSC ≥20 minutes; (2) hemodynamic measures (specifically CoPP); (3) chest compression depth (mm); and (4) arterial blood gas measurements. Continuous waveform data were captured in PowerLab and transformed into numerical values that were then collapsed into 15-second data epochs utilizing a custom code (Python, Enthought Canopy; Austin, TX). Statistical analyses were performed with the Stata-IC statistical package (Version 14; StataCorp, College Station, TX). Dichotomous variables (e.g., survival outcomes) were compared utilizing Fisher's exact test. Normality of continuous variables was assessed with the Skewness-Kurtosis test. Normally distributed continuous variables were described as mean (+/- standard error of the mean) and compared at predetermined times in the experimental protocol (baseline, end of asphyxial period, and end of resuscitation period) by Student's t-test. Non-normally distributed continuous variables were described as median with interquartile ranges and compared at these times utilizing Wilcoxon rank-sum tests. CoPP and chest compression depth throughout the duration of the CPR period were compared using a generalized estimating equation regression model, accounting for clustering of CPR epochs within individual piglets.
Results
Rates of sustained ROSC and 4-hour ICU survival were both significantly higher with HD-CPR than with Standard Care (Table 1). All animals in which sustained ROSC was achieved survived for the full 4 hours. During CPR, CoPP was significantly higher in the HD-CPR group than in the Standard Care group (point estimate +8.1mmHg, CI95: 0.5–15.8mmHg; p=0.04) (Figure 3). CoPP was also higher in survivors compared with non-survivors (point estimate +13.7mmHg, CI95: 4.6– 22.7; p<0.01), irrespective of treatment group.
Table 1. Rates of Survival between Treatment Groups.
| HD-CPR (n=12) | Standard Care (n=10) | p | |
|---|---|---|---|
| Sustained ROSC, n (%) | 12 (100) | 6 (60) | 0.03 |
| 4-hour ICU Survival, n (%) | 12 (100) | 6 (60) | 0.03 |
Comparisons between groups performed with Fisher's exact test. Definition of abbreviations: HD-CPR = hemodynamic-directed cardiopulmonary resuscitation; ROSC = return of spontaneous circulation; ICU = intensive care unit.
Figure 3. Coronary Perfusion Pressure during Cardiopulmonary Resuscitation.
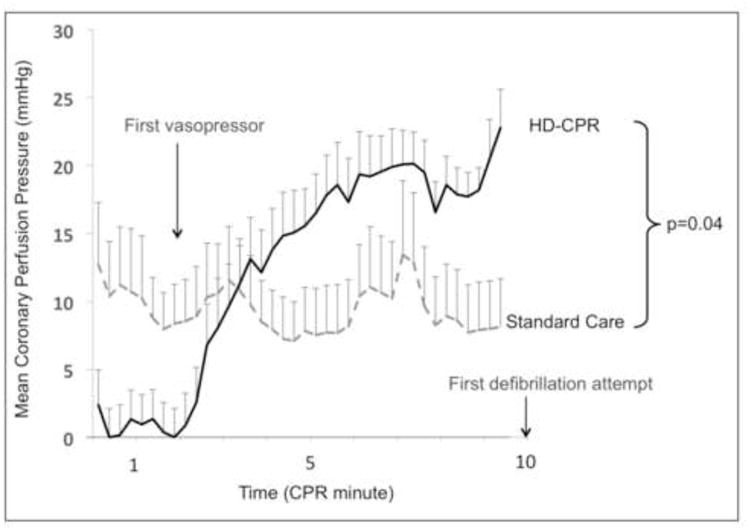
Mean coronary perfusion pressure during CPR across treatment groups. Error bars represent SEM. Comparison between groups performed with generalized estimating equation regression model. Definitions of abbreviations: CPR = cardiopulmonary resuscitation; HD = hemodynamic-directed.
At baseline, animals in the HD-CPR group had lower aortic SBP (95.3 ± 3.8mmHg vs. 108.6 ± 4.8mmHg, p=0.04) (Table 2). There were no other differences in hemodynamic measures between treatment groups at baseline or at the end of the asphyxial period. At the conclusion of the asphyxial period, animals in both groups were severely hypoxemic (median PaO2 <10mmHg) and hypercarbic (mean PaCO2 >75mmHg) (Table 3). Just prior to the first defibrillation attempt, piglets in the HD-CPR group had higher values of aortic diastolic pressure (DBP; 36.1 ± 3.2mmHg vs. 17.1 ± 3.6mmHg, p<0.01), right atrial diastolic pressure (RAD; 15.9 ± 1.5mmHg vs. 9.5 ± 2.1mmHg, p=0.02), and CoPP (22.8 ± 2.8mmHgvs. 8.1 ± 3.5mmHg, p<0.01) than those treated with Standard Care (Table 2). There were no significant differences between the treatment groups among arterial blood gas measurements obtained at baseline, at the end of the asphyxial period, or after 6 minutes of CPR (Table 3).
Table 2. Hemodynamic Variables: HD-CPR vs. Standard Care.
| HD-CPR (n=12) | Standard Care (n=10) | p | |
|---|---|---|---|
| Baseline | |||
| Weight (kg) | 9.9 (0.3) | 9.6 (0.5) | 0.59 |
| SBP | 95.3 (3.8) | 108.6 (4.8) | 0.04 |
| DBP | 63.6 (3.4) | 71.9 (3.8) | 0.12 |
| RAD | 11.63 (1.8) | 8.7 (2.3) | 0.32 |
| CoPP | 56.9 (5.1) | 70.7 (4.9) | 0.07 |
| End of Asphyxial Perioda | |||
| SBP | 61.3 (9.9) | 58.4 (10.9) | 0.84 |
| DBP | 20.3 [17.9, 27.3] | 23.1 [17.4, 24.5] | 0.27 |
| RAD | 14.8 (1.7) | 11.7 (2.6) | 0.30 |
| CoPP | 12.4 (2.8) | 17.7 (4.4) | 0.31 |
| End of Resuscitation Periodb | |||
| SBP | 102.1 (4.3) | 83.4 (13.7) | 0.18 |
| DBP | 36.1 (3.2) | 17.1 (3.6) | <0.001 |
| RAD | 15.9 (1.5) | 9.5 (2.1) | 0.02 |
| CoPP | 22.8 (2.8) | 8.1 (3.5) | <0.01 |
| ETCO2 | 30.7 (2.8) | 33.0 (3.7) | 0.63 |
15-second epoch during asphyxial period from 6:30 to 6:45;
15-second epoch during protocol resuscitation period from 16:15 to 16:30, just prior to first defibrillation attempt. Pressures and ETCO2 are in mmHg. Data presented as mean (SEM) or median [IQR]. Comparisons between groups performed with Student's t-test or Wilcoxon rank-sum test.
Definition of abbreviations: HD-CPR = hemodynamic-directed cardiopulmonary resuscitation; SBP = aortic systolic pressure; DBP = aortic diastolic pressure; RAD = right atrial diastolic pressure; CoPP = coronary perfusion pressure; ETCO2 = end-tidal carbon dioxide.
Table 3. Arterial Blood Gases.
| HD-CPR (n = 12) | Standard Care (n = 10) | p | |
|---|---|---|---|
| Baseline | |||
| pH | 7.52 (0.03) | 7.49 (0.03) | 0.49 |
| PaCO2 (Torr) | 41.7 (2.4) | 39.0 (1.6) | 0.37 |
| PaO2 (Torr) | 132.3 (5.9) | 138.7 (13.2) | 0.64 |
| End of Asphyxial Perioda | |||
| pH | 7.20 (0.03) | 7.19 (0.02) | 0.84 |
| PaCO2 (Torr) | 86.8 (8.8) | 77.4 (4.5) | 0.49 |
| PaO2 (Torr) | 9.3 [6.4, 16.9] | 7.8 [5.6, 11.1] | 0.48 |
| After 6 Minutes of CPRb | |||
| pH | 7.22 (0.03) | 7.31 (0.03) | 0.09 |
| PaCO2 (Torr) | 56.6 (4.3) | 45.4 (5.9) | 0.15 |
| PaO2 (Torr) | 144.0 [101.4, 160.9] | 116.6 [69, 165.1] | 0.53 |
| 45 Minutes Post-ROSC | |||
| pH | 7.38 (0.03) | 7.39 (0.07) | 0.82 |
| PaCO2 (Torr) | 39.8 [34.3, 52.1] | 37.2 [34.6, 43.0] | 0.73 |
| PaO2 (Torr) | 119.7 [102.4, 138.0] | 114.6 [101.0, 137.3] | 0.99 |
Arterial blood gas samples were drawn at baseline,
6min, 30s into the asphyxial period, and
6 minutes into the resuscitation period. Data presented as mean (SEM) or median [IQR]. Comparisons between groups performed with Student's t-test or Wilcoxon rank-sum test.
Definitions of abbreviations: BP = blood pressure; PaCO2 = arterial carbon dioxide tension; PaO2 = arterial oxygen tension; CPR = cardiopulmonary resuscitation; ROSC = return of spontaneous circulation.
The depths provided in the Standard Care group achieved the protocol goal of 1/3 of the AP chest depth (actual achieved depth: 44.4 ± 1.1mm vs. 1/3 AP chest depth: 45.8 ± 1.1mm; p=0.49). Chest compression rate did not differ between the HD-CPR and Standard Care groups (99.4 ± 0.3min-1 vs. 99.4 ± 0.7min-1; p=0.99). Animals in the HD-CPR group received shallower chest compressions (30.4 ± 2.1mm vs. 44.4 ± 1.1mm, p<0.01). More vasopressor doses were administered prior to the first defibrillation attempt with HD-CPR than with Standard Care (median 5 [range 3–6] vs. 2 [range 2–2], p<0.01). One animal in each group required a dopamine infusion to maintain MAP >55mmHg after ROSC. There were no deviations from the protocol as described in the Methods section.
Discussion
This study demonstrates that a hemodynamic-directed method of CPR improves 4-hour survival in a porcine model of pediatric IHCA. The findings of higher CoPP during CPR among piglets in the HD-CPR group provide biologic plausibility to our findings. Interestingly, in this homogenous laboratory animal cohort, there was variability in the HD-CPR group in terms of the timing and number of vasopressor doses needed to maintain CoPP (range of 3–6 during first 10 minutes of CPR). This variability, in a group with 100% survival, underscores a potential benefit of a personalized medicine approach to CPR that provides resuscitative medications when they are needed and avoids medications when they are not physiologically necessary and potentially harmful.
A 2013 AHA consensus statement recommended physiologic monitoring during CPR in the form of targeting CoPP, DBP, or ETCO2.41 However, the authors acknowledged that “clinical studies supporting the optimal titration of these parameters during human CPR are lacking.” The 2015 PALS guidelines offer a Class IIb recommendation to use blood pressure to guide CPR quality.12 However, similar to the authors of the CPR Quality Consensus statement,41 they recognize a paucity of pediatric data contributing to this recommendation.12 Since >90% of pediatric IHCAs occur in ICUs and many of these patients have an arterial catheter in place at the time of arrest,2 the role for a hemodynamic-based method of CPR is especially relevant to pediatric intensivists.
The findings of this study are congruent with previous data regarding the importance of CoPP in cardiac arrest. CoPP, the difference between the aortic pressure and right atrial pressure during the relaxation phase of chest compressions (“diastole”) is the driving force for myocardial blood flow.38,42 Higher levels of CoPP during CPR are associated with higher rates of ROSC and meaningful survival outcomes in both large animal investigations15-17,19,38,43 and clinical studies.44 CoPP values in this study were higher in animals treated with HD-CPR when analyzed continuously throughout CPR (Figure 3) and at a point in time just prior to the first defibrillation attempt (Table 1). Substantially higher DBP values were responsible for the increased CoPP in the HD-CPR group, reflecting the importance of adequate systemic vascular resistance for myocardial perfusion. Interestingly, RAD, the downstream pressure for the coronary vasculature, was also increased in the HD- CPR group. We postulate that the increased RAD in these animals may have been due to: 1) increases in pulmonary vascular resistance due to more vasopressors provided to the HD-CPR group45 or 2) more effective CPR in these animals augmenting systemic venous return with resultant increases in right atrial filling.
In the HD-CPR group, chest compression depth was actively titrated to maintain a goal SBP of 90mmHg, whereas in the Standard Care group, depth was maintained at the PALS-recommended depth of 1/3 of the AP diameter of the chest. It is possible that targeting the lower limit of the PALS-recommended depth range (“at least one third of the chest depth”)12 did not provide adequate compression depths in this group. However, in the HD-CPR group, the depth necessary to achieve the SBP goal was substantially and consistently lower than the depth provided to the Standard Care group. Despite deeper compressions, DBP (p<0.001) and CoPP (p<0.01) were lower in the Standard Care group. This allows for the possibility that vasopressor dosing in the HD-CPR group generated higher systemic vascular resistance to improve DBP and SBP such that lesser force and depth were necessary to achieve the goal SBP. It is also plausible that the deeper compressions delivered to Standard Care animals were deleterious. Recent work has demonstrated increased rates of CPR-related injuries with deeper compressions46 and a trend toward lower survival rates with chest compression depths greater than 45.6mm in adults.47 Based on these findings, the 2015 AHA guidelines introduced a maximum depth of 6cm for adults.14 In our model that approximates the size and chest dimensions of a young toddler, the Standard Care group's mean compression depth of 4.4±0.1cm was similar to pediatric guideline recommendations (4cm for infants; 5cm for children).11 That animals in the HD-CPR group received substantially shallower chest compressions (3.0±0.2cm) and yet were more likely to survive warrants further investigation.
This study has limitations. First, these experiments were performed in a controlled laboratory setting in a relatively uniform group of healthy animals. Greater heterogeneity exists among children with IHCA in regard to underlying disease processes and the actual CPR delivered. Second, the 6 ventilations per- minute provided during CPR is less than that recommended by PALS guidelines12 or carried out in current practice.48 However, the mild degree of respiratory acidosis measured during and after CPR (Table 3) suggests that this was unlikely to significantly impact our findings. Third, baseline aortic SBP values were lower in the HD-CPR group than in the Standard Care group (95.3 ± 3.8mmHg vs. 108.6 ± 4.8mmHg, p=0.04; Table 2). CoPP values were also lower in the HD-CPR group at the onset of VF, until the time that the first vasopressor dose was administered (Figure 3). While these differences may represent unintended baseline differences between groups, such differences would be expected to result in worse outcomes among the HD-CPR group, rather than the better outcomes reported. Fourth, we examined 4- hour short-term survival in this proof-of-concept trial, but did not address long- term survival. With improvements in survival rates from pediatric IHCA,6 focus is appropriately shifting to long-term survival and meaningful neurologic outcomes. Notably, in our previous work with a similar adult model of asphyxia-associated cardiac arrest, nearly all of the animals that survived to 45 minutes survived to 24 hours with favorable neurologic outcomes.15 Fifth, piglets in the HD-CPR group received more doses of vasopressors on average than did those in the Standard Care group. Given the effects of vasopressors on both macrovascular49 and microvascular cerebral blood flow,50,51 the subsequent effect on long-term neurological outcomes remains an unanswered question. Nevertheless, in our previous swine studies with adult models, we demonstrated that cerebral perfusion pressures and cerebral blood flows were higher52 and that 24-hour survival with favorable neurologic outcomes was more likely15 with HD-CPR compared with Standard Care.
Conclusions
In this animal model of pediatric asphyxia-associated IHCA, short-term ICU survival was significantly more likely with hemodynamic-directed personalized CPR compared with Standard Care. Because most pediatric IHCAs occur in the ICU and many patients have arterial blood pressure monitoring at the time of arrest, such a method of HD-CPR is clinically feasible and warrants continued investigation.
Supplementary Material
Acknowledgments
Conflicts of Interest: Financial support was provided through Russell Raphaely Endowed Chair funds at The Children's Hospital of Philadelphia. Ryan W. Morgan receives University of Pennsylvania Institute of Translational Medicine and Therapeutics Scholarship funding that is financed by a National Institutes of Health grant (National Center for Advancing Translational Sciences – UL1TR000003). Robert M. Sutton is supported through a National Institutes of Health career development award (Eunice Kennedy Shriver National Institute of Child Health & Human Development – K23HD072629).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knudson JD, Neish SR, Cabrera AG, et al. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids' Inpatient Database*. Crit Care Med. 2012;40(11):2940–2944. doi: 10.1097/CCM.0b013e31825feb3f. [DOI] [PubMed] [Google Scholar]
- 2.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41(10):2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suominen P, Olkkola KT, Voipio V, Korpela R, Palo R, Rasanen J. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17–25. doi: 10.1016/s0300-9572(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 4.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med. 2016;44(4):798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Castillo J, Lopez-Herce J, Canadas S, et al. Cardiac arrest and resuscitation in the pediatric intensive care unit: a prospective multicenter multinational study. Resuscitation. 2014;85(10):1380–1386. doi: 10.1016/j.resuscitation.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Herce J, Del Castillo J, Matamoros M, et al. Factors associated with mortality in pediatric in-hospital cardiac arrest: a prospective multicenter multinational observational study. Intensive Care Med. 2013;39(2):309–318. doi: 10.1007/s00134-012-2709-7. [DOI] [PubMed] [Google Scholar]
- 9.Meert KL, Donaldson A, Nadkarni V, et al. Multicenter cohort study of in- hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10(5):544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe H, Zebuhr C, Topjian AA, et al. Interdisciplinary ICU cardiac arrest debriefing improves survival outcomes*. Crit Care Med. 2014;42(7):1688–1695. doi: 10.1097/CCM.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins DL, Berger S, Duff JP, et al. Part 11: Pediatric Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascula r Care. Circulation. 2015;132(18 Suppl 2):S519–525. doi: 10.1161/CIR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 12.de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint) Pediatrics. 2015;136(2):S176–195. doi: 10.1542/peds.2015-3373F. [DOI] [PubMed] [Google Scholar]
- 13.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S414–435. doi: 10.1161/CIR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 15.Sutton RM, Friess SH, Naim MY, et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190(11):1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41(12):2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84(5):696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Ann Emerg Med. 1984;13(2):79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 19.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12(10):871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Aase SO, Myklebust H. Compression depth estimation for CPR quality assessment using DSP on accelerometer signals. IEEE Trans Biomed Eng. 2002;49(3):263–268. doi: 10.1109/10.983461. [DOI] [PubMed] [Google Scholar]
- 21.Zoll Medical Corporation. R Series Technical Specifications. 2012 http://www.zoll.com/uploadedFiles/Public_Site/Products/R_Series_Defibrillators/r-series-spec-sheet.pdf.
- 22.Neurauter A, Nysaether J, Kramer-Johansen J, et al. Comparison of mechanical characteristics of the human and porcine chest during cardiopulmonary resuscitation. Resuscitation. 2009;80(4):463–469. doi: 10.1016/j.resuscitation.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Duhaime AC, Margulies SS, Durham SR, et al. Maturation-dependent response of the piglet brain to scaled cortical impact. J Neurosurg. 2000;93(3):455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37(7):2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matos RI, Watson RS, Nadkarni VM, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127(4):442–451. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
- 27.Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354(22):2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 28.Berg RA, Otto CW, Kern KB, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994;22(2):282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Brown CG, Werman HA, Davis EA, Hobson J, Hamlin RL. The effects of graded doses of epinephrine on regional myocardial blood flow during cardiopulmonary resuscitation in swine. Circulation. 1987;75(2):491–497. doi: 10.1161/01.cir.75.2.491. [DOI] [PubMed] [Google Scholar]
- 30.Pytte M, Kramer-Johansen J, Eilevstjonn J, et al. Haemodynamic effects of adrenaline (epinephrine) depend on chest compression quality during cardiopulmonary resuscitation in pigs. Resuscitation. 2006;71(3):369–378. doi: 10.1016/j.resuscitation.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez R, Urbano J, Botran M, et al. Adrenaline, terlipressin, and corticoids versus adrenaline in the treatment of experimental pediatric asphyxial cardiac arrest. Pediatr Crit Care Med. 2014;15(6):e280–287. doi: 10.1097/PCC.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 32.Sutton RM, Niles D, Nysaether J, et al. Pediatric CPR quality monitoring: analysis of thoracic anthropometric data. Resuscitation. 2009;80(10):1137–1141. doi: 10.1016/j.resuscitation.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Braga MS, Dominguez TE, Pollock AN, et al. Estimation of optimal CPR chest compression depth in children by using computer tomography. Pediatrics. 2009;124(1):e69–74. doi: 10.1542/peds.2009-0153. [DOI] [PubMed] [Google Scholar]
- 34.Sutton RM, French B, Nishisaki A, et al. American Heart Association cardiopulmonary resuscitation quality targets are associated with improved arterial blood pressure during pediatric cardiac arrest. Resuscitation. 2013;84(2):168–172. doi: 10.1016/j.resuscitation.2012.08.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg RA, Hilwig RW, Kern KB, Babar I, Ewy GA. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27(9):1893–1899. doi: 10.1097/00003246-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 36.Berg RA, Hilwig RW, Kern KB, Ewy GA. “Bystander” chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless “cardiac arrest”. Circulation. 2000;101(14):1743–1748. doi: 10.1161/01.cir.101.14.1743. [DOI] [PubMed] [Google Scholar]
- 37.Zuercher M, Hilwig RW, Ranger-Moore J, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Crit Care Med. 2010;38(4):1141–1146. doi: 10.1097/CCM.0b013e3181ce1fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16(4):241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 39.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14(6):521–528. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 40.Andersen LW, Berg KM, Saindon BZ, et al. Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. JAMA. 2015;314(8):802–810. doi: 10.1001/jama.2015.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 42.Raessler KL, Kern KB, Sanders AB, Tacker WA, Jr, Ewy GA. Aortic and right atrial systolic pressures during cardiopulmonary resuscitation: a potential indicator of the mechanism of blood flow. Am Heart J. 1988;115(5):1021–1029. doi: 10.1016/0002-8703(88)90071-3. [DOI] [PubMed] [Google Scholar]
- 43.Morgan RW, French B, Kilbaugh TJ, et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation. 2016;104:6–11. doi: 10.1016/j.resuscitation.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 45.Lindberg L, Liao Q, Steen S. The effects of epinephrine/norepinephrine on end-tidal carbon dioxide concentration, coronary perfusion pressure and pulmonary arterial blood flow during cardiopulmonary resuscitation. Resuscitation. 2000;43(2):129–140. doi: 10.1016/s0300-9572(99)00129-x. [DOI] [PubMed] [Google Scholar]
- 46.Hellevuo H, Sainio M, Nevalainen R, et al. Deeper chest compression - more complications for cardiac arrest patients? Resuscitation. 2013;84(6):760–765. doi: 10.1016/j.resuscitation.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Stiell IG, Brown SP, Nichol G, et al. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation. 2014;130(22):1962–1970. doi: 10.1161/CIRCULATIONAHA.114.008671. [DOI] [PubMed] [Google Scholar]
- 48.McInnes AD, Sutton RM, Orioles A, et al. The first quantitative report of ventilation rate during in-hospital resuscitation of older children and adolescents. Resuscitation. 2011;82(8):1025–1029. doi: 10.1016/j.resuscitation.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michael JR, Guerci AD, Koehler RC, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69(4):822–835. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 50.Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35(9):2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 51.Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37(4):1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 52.Friess SH, Sutton RM, French B, et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation. 2014;85(9):1298–1303. doi: 10.1016/j.resuscitation.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


