SUMMARY
DNA double strand breaks (DSBs) are cytotoxic lesions that must be accurately repaired to maintain genome stability. Replication protein A (RPA) plays an important role in homology-dependent repair of DSBs by protecting the single-stranded DNA (ssDNA) intermediates formed by end resection and facilitating Rad51 loading. We found that hypomorphic mutants of RFA1 that support intra-chromosomal homologous recombination are profoundly defective for repair processes involving long tracts of DNA synthesis, in particular, break-induced replication (BIR). The BIR defects of the rfa1 mutants could be partially suppressed by eliminating the Sgs1-Dna2 resection pathway, suggesting that Dna2 nuclease attacks the ssDNA formed during end resection when not fully protected by RPA. Over-expression of Rad51 was also found to suppress the rfa1 BIR defects. We suggest Rad51 binding to the ssDNA formed by excessive end resection and during D-loop migration can partially compensate for dysfunctional RPA.
Keywords: Homologous recombination, DSB repair, Break-induced replication, RPA, Rad51, Dna2
eTOC blurb
RPA plays an important role during homology-dependent repair (HDR) to protect fragile single-stranded DNA (ssDNA) intermediates. Here, Ruff et al show that partial destabilization of RPA binding to ssDNA has modest effects on efficient intra-chromosomal HDR reactions, but causes profound defects in HDR events that involves long-lived ssDNA intermediates, such as break-induced replication (BIR). The BIR defect caused by dysfunctional RPA can be partially overcome by preventing excessive end resection or by Rad51 over-expression.

INTRODUCTION
DNA double strand breaks (DSBs) are highly cytotoxic lesions in which both strands of the phosphodiester backbone are broken within close proximity. Failure to repair a DSB results in loss of genetic information and aberrant repair can cause chromosome rearrangements. Homologous recombination (HR) is the main mechanism for error-free repair of DSBs and defects in this process are associated with increased mutagenesis and cancer prone syndromes in humans (Kass et al., 2016; Prakash et al., 2015).
HR initiates by nucleolytic degradation of the 5′-terminated strands to generate 3′ single-stranded DNA (ssDNA) tails, a process termed end resection (Symington et al., 2014). The 3′ ssDNA tails are initially bound by the ubiquitous and abundant ssDNA binding protein, Replication Protein A (RPA). RPA is subsequently replaced by the conserved Rad51 recombinase, which is essential for the homology search and pairing of ssDNA with the complementary strand of the donor duplex (San Filippo et al., 2008). The 3′ end of the invading strand can then be extended by DNA synthesis, copying sequence from the donor duplex. There are several possible fates of the strand invasion (D-loop) intermediate. After limited DNA synthesis, the D-loop can be disrupted by a helicase and the newly synthesized DNA can anneal to the other resected end, resulting in a non-crossover gene conversion product. Alternatively, the displaced strand of the D-loop can pair with the other resected end to form a joint molecule that can be processed to form crossover or non-crossover products. However if one of the break ends is lost or homology is limited to only one DSB end, a so-called single-ended break, DNA synthesis within the context of the D-loop continues to the end of the donor chromosome, a form of DSB repair known as break-induced replication (BIR) (Llorente et al., 2008; Malkova and Ira, 2013). BIR can result in extensive loss of heterozygosity when it occurs between non-sister chromatids, and non-reciprocal translocation when dispersed repeats are involved (Bosco and Haber, 1998; Ho et al., 2010). Furthermore, microhomology-mediated BIR has been suggested to generate complex rearrangements associated with a variety of human diseases (Hastings et al., 2009).
In yeast, BIR is a rare repair outcome at two-ended DSBs accounting for ~1% of events (Ho et al., 2010; Malkova et al., 2005). However, in assay systems engineered to generate a DSB with only one end homologous to the donor sequence (Bosco and Haber, 1998; Davis and Symington, 2004; Donnianni and Symington, 2013; Lydeard et al., 2007; Malkova et al., 2005), BIR is the primary outcome. A replication fork encountering a nick and collapsing would produce a single-ended break and thus BIR was proposed as a mechanism for fork restart. However, recent evidence suggests that the contribution of BIR to collapsed fork repair is minimal due to D-loop resolution by structure-selective nucleases and converging forks (Mayle et al., 2015). Perhaps the most physiologically relevant role for BIR is in repair of breaks that occur near telomeres or to elongate telomeres in the absence of telomerase (Malkova and Ira, 2013; Pickett and Reddel, 2015; St Charles et al., 2012). Most studies of BIR have come from Saccharomyces cerevisiae, but a BIR-like mechanism has also been shown in cultured human cells undergoing high levels of replication stress (Costantino et al., 2014).
Previous work has shown that BIR DNA synthesis is conservative and that the leading and lagging strand are uncoupled resulting in a long-lived ssDNA intermediate (Donnianni and Symington, 2013; Saini et al., 2013; Wilson et al., 2013). The ssDNA generated during BIR is highly mutagenic and subjected to clustered mutations; it can also engage in secondary recombination events causing chromosome rearrangements (Sakofsky et al., 2014; Smith et al., 2007). In addition, the frequency of BIR is inversely correlated to the DNA synthesis length, such that the further the donor homology is from the telomere, the lower the frequency of BIR (Donnianni and Symington, 2013; Lydeard et al., 2007). Based on the DNA synthesis length dependency of BIR, as well as the formation of long ssDNA intermediates, we hypothesized that RPA would play an important role in BIR, and might even be a limiting factor to protect the ssDNA intermediate.
RPA is a heterotrimeric complex encoded by the RFA1, RFA2, and RFA3 genes in S. cerevisiae and is essential for many DNA metabolic processes involving ssDNA intermediates, including DNA replication during S-phase (Wold, 1997). Although RPA is essential, several hypomorphic alleles of RFA1 and RFA2 have been identified that are proficient for DNA replication but impaired for DNA repair functions (Firmenich et al., 1995; Santocanale et al., 1995; Smith and Rothstein, 1995; Umezu et al., 1998). In this study, we utilized two hypomorphic alleles of RFA1, which encodes the large subunit of the complex with the major DNA binding domains, to assess the role of RPA during BIR. The rfa1-D228Y allele was identified in a screen for suppression of the rad1 rad52 defect in spontaneous recombination between direct repeats, while rfa1-t33 was found in a screen for DNA damage sensitive alleles of RFA1 (Smith and Rothstein, 1995; Umezu et al., 1998). In this study, we refer to rfa1-t33 by the amino acid substitution (S373P) to use consistent nomenclature. Asp 228 and Ser 373 are located in DNA binding domains A and B of Rfa1, respectively, but do not directly contact DNA (Fan and Pavletich, 2012). In vitro, the RPAD228Y and RPAS373P mutant complexes show reduced affinity for ssDNA and RPAS373P is less effective in removing secondary structure from ssDNA than wild-type RPA (Deng et al., 2014; Smith and Rothstein, 1995). Here, we show that BIR and gene conversion requiring extensive DNA synthesis are severely compromised when RPA is dysfunctional while gene conversion involving limited DNA synthesis is less affected.
RESULTS
The rfa1-D228Y and rfa1-S373P mutants are proficient for intra-chromosomal recombination
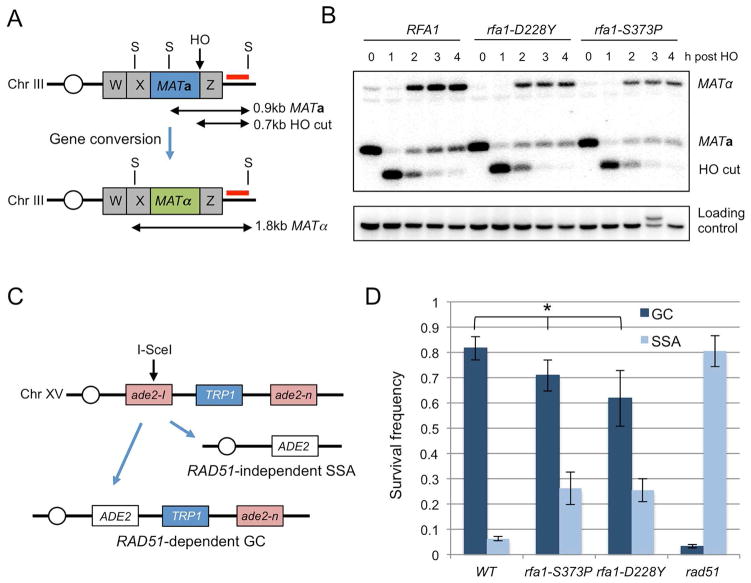
All forms of HR involve ssDNA intermediates; thus, we expected even partial disruption of RPA binding to ssDNA to reduce the frequency of repair. The rfa1-D228Y and rfa1-S373P alleles have previously been shown to encode proteins with reduced ssDNA binding affinity, but exhibit no defect in 5′–3′ end resection, an essential early step of HR (Deng et al., 2014; Smith and Rothstein, 1995). To test whether the rfa1 mutants are capable of gene conversion, we used a mating-type switching assay. Mating-type switching in S. cerevisiae is an intra-chromosomal recombination reaction in which a DSB at the MAT locus is repaired by one of two transcriptionally silent homologous donors resulting in a change in mating type, which can be scored by restriction digestion and Southern blotting (Fig 1A) (Haber, 2012). The switching efficiency measured 4 hours (h) after HO induction was 94% for wild type, 68% for rfa1-S373P and 65% for the rfa1-D228Y mutant, indicating that the rfa1 mutants are largely proficient for Rad51-dependent HR (Fig 1B, Fig S1). The kinetics of MAT switching were comparable in wild type and the rfa1 mutants with switched products visible 2 h after HO induction.
Figure 1. The rfa1 mutants are proficient for intra-chromosomal HR.
A. Schematic of the MAT locus showing the location of the HO cut site and the StyI restriction sites used to distinguish between MATa and MATα alleles. The red line indicates the location of the probe used. B. Genomic blot of DNA extracted from the indicated strains at different times after HO induction. C. Cartoon of the direct repeat recombination reporter. After I-SceI cleavage repair can occur by gene conversion (GC), retaining the TRP1 marker between the repeats, or by SSA resulting in deletion of TRP1 and one of the repeats. Repair products are mostly Ade+. D. The frequency of GC and SSA repair of the indicated strains is shown. WT refers to wild type. Error bars indicate SD (n=4–6). * indicates p values of <0.05. See also Fig S1
To further test functionality of the rfa1-D228Y and rfa1-S373P mutants, we measured HR between ade2 repeats that are separated by 5 kb, including the selectable TRP1 marker (Mimitou and Symington, 2008; Mozlin et al., 2008). One of the ade2 alleles contains an I-SceI cut site and the strains express I-SCEI from the galactose-inducible GAL1 promoter (Fig 1C). The DSB generated after growth on galactose-containing medium can be repaired by RAD51-dependent gene conversion (GC, Trp+) or by RAD51-independent single-strand annealing (SSA, Trp−). GC could occur between repeats on the same chromatid or between misaligned repeats of sister chromatids. Repair was measured by the plating efficiency on galactose-containing medium (I-SCEI constitutively induced) relative to the glucose-containing medium (I-SCEI repressed), and the proportion of survivors that repaired by GC or SSA was determined by the Trp phenotype. All strains exhibited comparable survival on galactose containing medium, but the distribution of events significantly differed (Fig 1D, Fig S1B). GC was the primary outcome from wild-type cells, whereas most DSBR occurred by SSA in the rad51Δ mutant. The rfa1-D228Y and rfa1-S373P mutants both showed a small but significant reduction in GC and a corresponding increase in the frequency of SSA, as compared to wild type, and the frequency of GC was ~20-fold higher than the rad51Δ mutant (p=0.0001). Collectively, these data show that the rfa1-D228Y and rfa1-S373P mutants are largely proficient for intra-chromosomal GC and SSA, in contrast to the previously characterized rfa-t11 mutant, which is highly defective for both repair processes (Umezu et al., 1998; Wang and Haber, 2004). The rfa1-t11 mutation results from a substitution in the N-terminal domain of Rfa1, which does not impact ssDNA binding in vitro (Kantake et al., 2003; Umezu et al., 1998).
The rfa1 mutants are highly defective for BIR
Because BIR involves long ssDNA intermediates, we anticipated that the rfa1 mutants would be more defective for this mode of repair than for GC. The BIR assay used consists of a recipient 3′ truncated lys2 gene on chromosome (Chr) V with a 36-bp HO recognition site that shares 2.2 kb of homology on only one side of the DSB to a donor 5′ truncated lys2 gene on a different chromosome (Fig 2A) (Donnianni and Symington, 2013). Because the genes telomere-proximal to the DSB on the recipient chromosome are not essential, the non-homologous end can be lost from haploid cells. In these strains, HO endonuclease is expressed from a galactose-inducible promoter; additionally, the strains have the MATα-inc allele to prevent HO cleavage at the endogenous MAT locus. After DSB formation, the 3′ truncated lys2 gene invades homologous sequence on the donor chromosome forming a D-loop, and DNA synthesis continues until the end of the donor chromosome. The BIR frequency is determined by the plating efficiency on galactose-containing medium relative to glucose-containing medium, and >99% of the colonies formed on galactose-containing medium are Lys+, indicating reconstitution of the LYS2 gene by BIR. Three different strain backgrounds were used in which the donor cassette was inserted 15, 70, or 128 kb from the telomere.
Figure 2. BIR is defective in rfa1 hypomorphic mutants.
A. Schematic of the ectopic BIR assay. The locations of the primers used to monitor DSB formation (D1, D2), BIR product (P1, P2) and control primers (C1, C2) are indicated by horizontal arrows. B. BIR frequencies of the indicated strains with the donor cassette located 15, 70 or 128 kb from the telomere. Error bars show SD (n=3–5). C. Detection of BIR intermediates by PCR. Error bars show SD (n=3). D. Schematic showing the predicted sizes of genomic EcoRV fragments before and after BIR. E. Genomic blot of the indicated strains showing formation of completed BIR product in real time. See also Figs S2 and S3
Consistent with previous results (Donnianni and Symington, 2013), the BIR frequency of the wild-type strains decreased as the distance from the donor to the chromosome end increased (Fig 2B). Even for the 15 kb donor, the rfa1 mutants exhibited a profound defect (20 to 50-fold lower than wild-type), similar to the low frequency BIR observed for the pol32Δ mutant (Donnianni and Symington, 2013; Lydeard et al., 2007). Even greater decreases were observed for the 70 kb and 128 kb donor variants (Fig 2B). Results from the genetic assay were verified by physical assays in which BIR product formation was measured in real time. PCR primers were designed such that one primer was upstream of the break on the recipient chromosome while the reverse primer was downstream of the homologous sequence on the donor chromosome (Fig 2A). BIR product formation as measured by PCR requires strand invasion and at least 278 nucleotides of DNA synthesis. BIR product formation was greatly reduced for the rfa1 15 kb donor strains (Fig 2C) and almost undetectable for the 70 kb donor strains (data not shown), mirroring results of the genetic assay. The BIR defect of the rfa1-S373P and rfa1-D228Y mutants was verified by restriction digestion and Southern blot (Fig 2D, E and Fig S2A).
RPA is not limiting for BIR
One potential explanation for the length-dependent decrease in the BIR frequency is that the long tracts of ssDNA generated are not fully protected by RPA and are susceptible to cleavage (Chen et al., 2013; Toledo et al., 2013). To test whether RPA is limiting for BIR, we constructed a high-copy number plasmid containing the RFA1, RFA2, and RFA3 genes all under their endogenous promoters and measured the BIR frequency. Because the antibodies used to detect RPA only recognize the large subunit, we cannot state that all three subunits are over-expressed, only that Rfa1 is expressed at 3.6-fold higher level than in the empty vector strain (Fig S2B). Despite over-expression of Rfa1, there was no difference in BIR frequency at any of the donor distances tested compared to the empty vector control (Fig S2C), suggesting that RPA is not limiting for BIR.
Long tract gap repair is defective in the rfa1 hypomorphic mutants
To further understand why BIR is more sensitive to RPA dysfunction than GC, we determined whether the rfa1-S373P mutant is defective for gap repair, which is expected to involve a long-lived ssDNA intermediate, similar to BIR. Jain et al. (Jain et al., 2009) had previously described an ectopic HR assay involving a recipient cassette and two donor homology regions with varying lengths of DNA between them, and had shown that as the amount of DNA between the donor fragments increases, the frequency of repair decreases and cells switch from gene conversion to BIR. However, even when the donor homologies are 18 kb apart, 75% of cells complete repair by gene conversion. Briefly, the assay system consists of a leu2 recipient cassette harboring an HO cut site inserted at the CAN1 locus on Chr V; there are no essential genes telomere proximal to the DSB. The donor cassette on Chr III has an intact LEU2 gene, or LEU2 is split with the fragments separated by different distances. The BIR strain has only the 5′ truncated half of the leu2 donor (Fig 3A). Repair was measured by plating efficiency on galactose-containing medium relative to the glucose-containing medium, and the proportion of survivors that repaired by BIR was determined by loss of Chr V sequence centromere distal to the DSB.
Figure 3. The rfa1-S373P mutant is defective for long-tract gene conversion.
A. Schematic showing the recipient LEU2 gene targeted by HO on Chr V (black line) and the donor cassettes on Chr III (grey lines) with varying distance between LE and U2. B. Percent survival of the indicated strains. Error bars show the SD (n=3).
Repair from the intact LEU2 donor (no gap) was reduced by 3-fold in the rfa1-S373P mutant relative to wild type (Fig 3B), a greater defect than observed for the intra-chromosomal HR assays. As the amount of DNA to be synthesized during repair was increased, there was a marked decrease in survival of the rfa1-S373P derivatives. Although a similar trend was seen in the wild-type strain, the fold decrease in survival was greater in the rfa1-S373P derivatives. Additionally, almost all repaired colonies in the rfa1-S373P background retained the other break end, indicating that the decrease in viability was associated with loss of both gap repair and BIR. As expected, the viability of the wild-type BIR only strain was ~24%, and was reduced by >20-fold in the rfa1-S373P derivative. Interestingly, when viable colonies arising from the BIR-only strain were analyzed for loss of the DSB-distal sequence, all of the colonies tested from the wild-type strain had lost the terminal fragment (20/20 tested) while several colonies from the rfa1-S373P strain did not (3/20 tested). Since survival of the rfa1-S373P BIR strain is very low (<1%) it is possible that some cells repaired the DSB by interstitial deletion between microhomologies, which is known to increase in the rfa1-S373P mutant and to occur at a similar frequency (Deng et al., 2014; Deng et al., 2015).
Rad51 over-expression suppresses the BIR and gap repair defects of the rfa1 mutants
We considered several possibilities to explain the BIR and gap repair defects of the rfa1 hypomorphic mutants. First, BIR and long-gap repair are slow processes, and if cells failed to arrest in response to the DSB and progressed to the next cell cycle it could impact repair and cell viability. While RPA is required for the Mec1-dependent DNA damage checkpoint leading to cell cycle arrest at G2/M in response to DSBs (Zou and Elledge, 2003), we found that the checkpoint was activated and maintained normally in the rfa1-D228Y and rfa1-S373P mutants, suggesting that the BIR defect is not caused by abnormal cell cycle progression (Fig S3).
Second, even though the rfa1 mutants can perform intra-chromosomal HDR, Rad51 recruitment to the DSB and the subsequent homology search during inter-chromosomal HDR could be defective in the BIR strains. If so, we predicted that Rad51 over-expression (OE) might be able to suppress the BIR defect of the rfa1 mutants. Indeed, expressing RAD51 from the native promoter on a high copy number plasmid was found to significantly increase BIR in rfa1-D228Y and rfa1-S373P mutants (Fig 4A) (p<0.005 for both strains). Although Rad51 OE did not significantly increase BIR in the wild type 15 kb donor strain, it was found to improve BIR in the 70 kb and 128 kb donor strains, consistent with a previous study (Fig S4A, B) (Lydeard et al., 2010). We also found that Rad51 OE increased the efficiency of gap repair in wild type cells by 2-fold and in rfa1-S373P cells by 5-fold (Fig. 4C). Over-expression of Rad52, a DNA binding protein that interacts with RPA and mediates assembly of Rad51 nucleoprotein filaments (San Filippo et al., 2008), was also found to suppress the rfa1 BIR defect (Fig S5).
Figure 4. Rad51 over-expression, but not mph1Δ, suppresses the rfa1 BIR defect.
A. Percent BIR for the indicated strains expressing empty vector (EV) or RAD51 from a high copy number vector. Error bars show the SD (n=3). B. Rad51 enrichment at sequence 2 kb upstream of the DSB in the indicated strains, 0, 2 or 4 h after HO induction. Error bars show SD (n=3). C. Percent repair for the indicated 5 kb gap strains expressing EV or RAD51 from a high copy number vector. Error bars show SD (n=3). D. Percent BIR for the indicated MPH1 and mph1Δ strains. Error bars show SD (n=3). See also Figs S4 and S5
The magnitude of the rfa1-D228Y and rfa1-S373P BIR defects is similar to that reported for the pol32Δ mutant (Deem et al., 2008; Donnianni and Symington, 2013; Lydeard et al., 2007). We found that RAD51 overexpression had no significant impact on the BIR frequency of the pol32Δ mutant (p=0.15) (Fig 4A), indicating that the rfa1 defect in BIR is different to that resulting from loss of Pol32.
To directly test whether Rad51 recruitment is defective in the BIR strains, we measured Rad51 enrichment at a sequence adjacent to the HO cut site on the recipient chromosome by chromatin immunoprecipitation (ChIP) (Fig 4B). Two hours after HO induction, Rad51 was enriched 220 to 340-fold in wild type and rfa1-D228Y strains, respectively, and by 190-fold in the rfa1-S373P mutant; at 4 h, Rad51 enrichment continued to increase in all of the strains, but not to the same extent in the rfa1 cells as observed in wild type. As described below, the decrease in Rad51 binding at later times in the rfa1 mutants could be due to loss of the resected end. These data suggest that the rfa1 BIR defect is not due to failure to assemble Rad51 nucleoprotein filaments, which is consistent with their ability to perform intra-chromosomal HR.
Since Rad51 loading to sequence adjacent to the DSB appeared to be normal in the rfa1-D228Y and rfa1-S373P mutants, it was not obvious why over-expressing RAD51 would suppress their BIR defects. During BIR there are multiple rounds of strand invasion and D-loop dissociation that would require repeated rounds of Rad51 loading (Smith et al., 2007). We reasoned that the subtle defect in Rad51-dependent strand invasion in the rfa1 mutants would be compounded during BIR, and might be alleviated by eliminating Mph1, a helicase responsible for dismantling Rad51-catalyzed D-loop intermediates (Prakash et al., 2009). Previous studies have shown that the mph1Δ mutant has a higher frequency of BIR and reduced template switching (Jain et al., 2016; Luke-Glaser and Luke, 2012; Stafa et al., 2014). However, in mph1Δ rfa1-S373P and mph1Δ rfa1-D228Y double mutant strains there was no significant difference in BIR frequency compared to the rfa1 single mutants (Fig 4D), implying that repeated cycles of strand invasion do not contribute to the rfa1 defect. The suppressive effect of mph1Δ in the wild-type 15 kb donor strain is quite small, but is magnified for the strains with donors more distant from the telomere (Fig S4C, D).
Instability of the invading end contributes to the BIR defect of the rfa1-S373P mutant
Stabilization of ssDNA is expected to be required at two stages of BIR: first, to shield the 3′ ssDNA formed by end resection (Chen et al., 2013), and second, to protect the ssDNA formed by D-loop migration until completion of second strand synthesis (Saini et al., 2013). Because BIR and gene conversion repair of long gaps are slow processes, resection is expected to continue for several hours producing long ssDNA tracts, and if these were unstable, the short sequence homology present at the invading end in the ectopic assays could be degraded. We used real-time PCR with primers located immediately upstream of the lys2 homology on the recipient chromosome to test 3′ end loss of the rfa1-S373P mutant. Resection is expected to reduce the PCR product by 50% relative to the 0 h time point, and when repair is complete, the signal should be restored to a value corresponding to the repair efficiency of the cells. For wild type cells, the 3′ end signal was 45% at 2 h and was restored to 75% by 10 h, when repair is completed. By contrast, only 24% the 3′ end signal was retained at 2 h in the rfa1-S373P cells and was reduced to 18% at 6 h and 10% at 10 h, indicating eventual loss of both 5′ and 3′ strands (Fig 5A). Thus, the reduced BIR frequency is due in part to loss of the short homology on the recipient chromosome.
Figure 5. 3′ strand loss contributes to the BIR defects of the rfa1 mutants.
A. Fraction of recipient locus retained in the indicated strains at 2, 6 and 10 h after HO induction. B. Percent BIR for the indicated strains, error bars show SD (n=3). * Indicates a p value of <0.05; ** indicates a p value of <0.01.
Resection of the 5′ strand precedes loss of the 3′ strand when RPA is depleted from cells (Chen et al., 2013). Therefore, we hypothesized that if loss of the 3′ strand contributes to the BIR defect of the rfa1 mutants, then, by limiting 5′ end resection, we should observe a partial recovery of BIR. The MRX-Sae2 complex initiates end resection, followed by extensive processing of the 5′ strands by either Exo1 or Dna2-Sgs1 (Symington and Gautier, 2011). Because the rfa1-S373P and rfa1-D228Y alleles are inviable in combination with exo1Δ and sgs1Δ mutations (data not shown), we used a hypomorphic sgs1 allele, sgs1-D664Δ (Bernstein et al., 2013), which is specifically defective for end resection and that we found to be viable with exo1Δ and rfa1-S373P or rfa1-D228Y. Loss of Exo1 did not change the BIR frequency (Fig 5B); however, the sgs1-D664Δ allele resulted in a significant suppression of the BIR defect of the rfa1 mutants (Fig 5B). Together, exo1Δ and sgs1-D664Δ did not suppress the BIR defect of the rfa1-D228Y mutant further than sgs1-D664Δ alone, while exo1Δ significantly increased the BIR frequency of the rfa1-S373P sgs1-D664Δ mutant. These data show that the Sgs1-Dna2 pathway of end resection impairs BIR when RPA is dysfunctional.
DISCUSSION
RPA plays critical roles in several steps of homology-dependent DSB repair, including end resection, removal of secondary structure from ssDNA, recruitment of Rad52 and Rad51, and stimulation of Sgs1/BLM-Top3-catalyzed dissolution of double Holliday-junction containing intermediates (Cejka et al., 2010; Chen et al., 2013; Lisby et al., 2004; Niu et al., 2010; Plank et al., 2006). In this study, we employed hypomorphic alleles of RFA1 to determine the importance of RPA in HR processes predicted to involve varying amounts of ssDNA.
While the rfa1 mutants were proficient at intra-chromosomal break repair, they were defective for long-gap repair and BIR. Break repair is thought to involve limited tracts of DNA synthesis, helicase-mediated unwinding of the extended 3′ end, followed by annealing of the nascent ssDNA to ssDNA formed by resection of the other break end (Fig 6). Gap repair requires more extensive DNA synthesis in the context of the D-loop and most likely involves a migrating D-loop with the newly synthesized DNA extruding from the trailing end. Second end capture could occur by annealing of the helicase-dissociated invading end or the ssDNA emanating from the trailing end of the D-loop with the resected end. BIR occurs by a migrating D-loop to the end of the chromosome resulting in a long-lived ssDNA intermediate (Saini et al., 2013). In contrast to gene conversion where the captured 3′ end primes DNA synthesis, BIR requires de novo priming on the newly synthesized ssDNA to complete repair. We envision that the long ssDNA intermediates formed during gap repair and BIR are fragile and more dependent on protection by RPA, hence the reduced frequency of repair in the rfa1 hypomorphic mutants (Fig 6).
Figure 6. Model for Break Repair and BIR.
Break repair involves short ssDNA intermediates that are less susceptible to degradation when RPA is dysfunctional. By contrast, BIR involves long-lived ssDNA intermediates formed by end resection and migrating D-loop synthesis that are more dependent on fully functional RPA for protection from degradation. Naked ssDNA is prone to base damage or hydrolysis and forms secondary structure that could be targeted by structure-selective nucleases thereby destroying recombination intermediates. Rad51 OE suppresses the BIR defect of the rfa1 mutants, pre or post-synaptically, by protecting ssDNA and promoting strand invasion.
RPA is required to protect the 3′ end formed by end resection and loss of the 3′ end could also contribute to the rfa1 defect (Chen et al., 2013). We found reduced stability of the 3′ strand formed by end resection in the rfa1-S373P mutant relative to wild type. Physical analysis has shown a significant delay in formation of gap repair products when >5kb of DNA synthesis is required as compared to break repair (Jain et al., 2009). Similarly, BIR is generally slower than break repair (Donnianni and Symington, 2013; Lydeard et al., 2007; Malkova et al., 2005). The delay in long gap repair and BIR could occur during the DNA synthesis step or could be due to repeated cycles of invasion and dissociation of the extended invading end (Smith et al., 2007). Resection is expected to continue at the invading end during delayed repair and the ssDNA intermediates would be susceptible to hydrolysis or endonucleolytic cleavage if not fully protected by RPA (Fig 6). Because programmed break repair reactions, such as mating-type switching, are fast, the amount of ssDNA generated by end resection is likely to be minimal. We found a significant suppression of the rfa1 BIR defect by inactivating the Sgs1-Dna2 resection pathway, consistent with excessive resection contributing to the defect. Interestingly, loss of Exo1 did not suppress the low frequency BIR of the rfa1 mutants. Dna2 can attack the 3′ strand produced by end resection in vitro if it is not fully protected by RPA (Cejka et al., 2010; Niu et al., 2010; Zhou et al., 2015). Therefore, we propose Dna2 cleaves the 3′ overhang during end resection when ssDNA binding by RPA is compromised, resulting in reduced efficiency BIR. Secondary structures form in ssDNA that is not fully protected by RPA, but these are unlikely to be substrates for Dna2 because it requires a free end for degradation (Chen et al., 2013; Zhou et al., 2015). Instability of resected ends has also been suggested to contribute to the reduced repair efficiency between ectopic repeats located at distant sites in the nucleus (Lee et al., 2016). In the Lee et al study (Lee et al., 2016), exo1Δ was found to increase the repair efficiency of poorly used donors, but Rad51 OE did not, in contrast to our findings.
The rfa1 BIR defect is unlikely to be explained solely by 3′ end loss. Even 10 hours after HO induction we could still detect DNA at the recipient locus, and sgs1-D664Δ and exo1Δ did not fully suppress the rfa1 phenotype. Furthermore, a previous study reported a large increase in micro-homology mediated end joining of repeats adjacent to a DSB in rfa1-D228Y and rfa1-S373P mutants (Deng et al., 2014), and the rfa1 mutants are proficient for SSA, both processes requires retention of the 3′ strands for annealing.
We also found that Rad51 over-expression suppresses the rfa1 BIR defect. Because intra-chromosomal gene conversion is only mildly defective in the rfa1 mutants and Rad51 does localize to the DSB during BIR, it seems unlikely that Rad51 OE counters a defect in strand invasion. BIR involves multiple rounds of invasion and dissociation of the invading strand; thus, it is possible that there is a greater demand for Rad51 during BIR. Consistently, Rad51 OE increases the BIR frequency (Lydeard et al., 2010). Previous studies have shown that mph1Δ increases BIR, presumably by stabilizing the invading end (Jain et al., 2016; Luke-Glaser and Luke, 2012; Stafa et al., 2014). However, mph1Δ did not suppress the rfa1 BIR defects, arguing against the need for multiple strand invasions contributing to their defects. Instead, we favor the idea that Rad51 OE stabilizes fragile ssDNA intermediates, either pre or post-strand invasion, when RPA-ssDNA binding is compromised (Fig 6). RPA could also be important to protect the displaced strand of the D-loop intermediate during BIR and for recruitment of the DNA Polα-Primase complex to complete synthesis of the second strand (Wold, 1997).
In summary, our findings highlight the important role RPA plays in stabilization of ssDNA during HR. This stabilization is most important for repair reactions that involve long-lived ssDNA intermediates, for example, BIR, or that are naturally slow resulting in greater end resection. Rad51 OE improves the efficiency of BIR and gap repair both in wild type and rfa1 mutant settings. It is interesting to note that Rad51 is over-expressed in multiple types of cancer (Klein, 2008; Mason et al., 2014) and this could facilitate mutagenic HR processes, such as BIR and gap repair.
EXPERIMENTAL PROCEDURES
Yeast strains and plasmids
Saccharomyces cerevisiae strains used in this study are listed in Table S1. W303-derived strains were generated by crossing the appropriate haploid strains, and JKM-derived strains were generated by PCR fragment-mediated gene targeting. Strains were grown at 30° C except where noted. The rfa1-S373P allele confers a temperature sensitive growth defect (Umezu et al., 1998) and we found the maximal permissive temperature to be lower in the JKM strain background than in W303; consequently, the gap repair assay was performed at 24°. In order to overexpress the RPA complex in yeast, plasmid pRPA was constructed in which each RPA subunit (RFA1, RFA2, and RFA3) was expressed under its endogenous promoter. pRPA was constructed using plasmid pWJ583, which is a high-copy number vector (pRS425) containing RFA1 (Smith and Rothstein, 1995). RFA2 and RFA3 were each amplified using Phusion DNA polymerase (Thermo Fisher Scientific) from genomic DNA. The PCR products were then treated with Taq polymerase (Thermo Fisher Scientific) for subsequent T-A cloning into the pGEM-T easy vector (Promega). Clones were verified by restriction digestion and by DNA sequencing. The 1.9 kb ApaI and NaeI fragment from the pGEM-RFA2 clone was ligated to ApaI-NaeI digested pWJ583 to generate pWJ583-RFA2. The 1.3 kb RFA3 fragment was ligated into SalI-ApaI digested pWJ583-RFA2 in order to produce pRPA. The high copy number RAD51 and RAD52 plasmids were described previously (Davis and Symington, 2001; Johnson and Symington, 1995).
Gene conversion assays
For the mating-type switching assay, cells were grown in 1% yeast extract 2% peptone (YP) medium containing 2% lactate (YPL) to log phase. Galactose was added to a final concentration of 2% for HO induction. After 1 h, the cultures were washed and resuspended in YPD to stop HO induction. Cells were collected at 1 h intervals for genomic DNA extraction, and southern blot was performed as previously described (Mimitou and Symington, 2008). Band intensities were quantified by ImageJ and were normalized to a loading control band (probes corresponds to coordinates 173285–173654 on chromosome VII). Percentage of mating-type switching from MATa to MATα was calculated by [(MATα ti – MATα t1) / total MATa cut], where the total MATa cut was calculated by [(HO cut t1/ Total DNA t0) x MATa t0]. Significance was determined by a t test using the mean values of three independent trials and representative gels are shown. For the direct repeat recombination assay, cells were grown to exponential phase in synthetic complete medium lacking tryptophan (SC-trp) supplemented with raffinose, and then plated on YP medium containing 2% glucose (YPD) or 2% galactose (YPGal). Plates were incubated at 30°C and counted after 2–4 days. Colonies from YPGal were replica plated to synthetic complete SC-trp to determine the fraction due to gene conversion. Cell viability after I-SceI induction was determined by dividing the number of Trp+ and Trp− colony forming units (CFUs) on YPGal by that on YPD. Significance was determined by a t test using the mean values of at least three independent trials. For the gap repair assay, cells were grown to exponential phase in YPR, and then plated on YPD or YPGal. Plates were incubated at 24°C and counted after 3–5 days. Significance was determined by a t test using the mean values of three independent trials. Cell viability after HO induction was determined by dividing the number of CFUs on YPGal by that on YPD. Telomere proximal arm loss (a measure of BIR) in these strains was done by PCR using primers in the DLD3 gene locus ~16 kb from the left arm of chromosome V.
BIR assay
Cells were grown to exponential phase in YPR, and then plated on YPD or YPGal. Colonies were counted after 3 days and colonies growing on YPGal were replica-plated onto SC-lys or YPD containing geneticin. Cell viability after HO induction was determined by dividing the number of colony forming units (CFUs) on YPGal by that on YPD. The percentage of cells repairing by BIR was determined by dividing the number of Lys+ by the number of YPGal CFU, and then normalized to the number of CFU on YPD. BIR frequencies were determined at least three times for each strain and the mean values used to determine significance using the t test.
Detection of BIR Products by PCR and Southern Blot Hybridization
For PCR analysis, 25 ng of genomic DNA was amplified for 25 cycles and quantified using Bio-Rad Quantity One software. The percentage of BIR product formed (primers P1 and P2) was determined by dividing the BIR product signal to that amplified from an independent locus 66-kb centromere proximal to the DSB on chromosome V (primers C1 and C2) obtained in the same reaction. The BIR product was then normalized to the ratio from a BIR repaired Lys+ colony and plotted against time. At least three PCR reactions were performed for each strain. DSB formation was monitored using primers D1 and D2. The percentage of DSB formation was obtained by dividing the DSB signal to that amplified from the control primers obtained in the same reaction and then normalized to the ratio obtained before galactose induction. For Southern blot analysis, 5 μg genomic DNA was digested with EcoRV, DNA was transferred to nylon membrane and hybridized with a LYS2 probe.
Western blot analysis
Trichloroacetic acid precipitations were performed on yeast whole cell extracts and were analyzed by SDS-PAGE and Western blotting. Antibodies to S. cerevisiae Rfa1 (Agrisera, AS07-214, 1:5,000) were used and Ponceau Red staining of the membrane was used as a loading control.
Rad51 Chromatin Immunoprecipitation (ChIP)
ChIP analysis was performed as described previously using polyclonal rabbit anti-Rad51 sera (Davis and Symington, 2003; Donnianni et al., 2010). Quantification was performed by qPCR using a primer pair 1 kb upstream of the HO cut site ChIP_F (ACGCCCACAACAAGAACCTTATACG) and ChIP_R (CAATGCAGCCCATACACTCAAAGC) on the recipient chromosome and a negative control primer pair NEG_ChIP_F (ACTGCATCGGAAAGGGAAACTACC) and NEG_ChIP_R (CAGAACAGCTCTAGGGAGCGTAC) on chromosome IV at coordinates 703739 to 703762 and 703850 to 703872, respectively. The same primers were used to measure strand loss at the recipient locus by qPCR.
Supplementary Material
Partial destabilization of RPA binding to ssDNA modestly decreases break repair
Stable RPA-ssDNA interaction is critical for break-induced replication and gap repair
The rfa1 BIR defects are suppressed by elimination of Sgs1-Dna2 catalyzed end resection
Rad51 over-expression suppresses the rfa1 BIR defect
Acknowledgments
We thank members of the Symington lab for discussions and critical reading of the manuscript. This study was supported by grants from the National Institute of Health (T32 CA09503, R01 GM094386 and R01 GM041784).
Footnotes
AUTHOR CONTRIBUTIONS
P.R. performed experiments in Figs 2 and 3 and contributed to Fig 4; R.A.D. contributed to experiments shown in Figs 1, 4, and 5; E.G. contributed to Figs. 4 and 5 and J.O. contributed to Fig 1. All authors contributed to the Supplemental Data, experimental design and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein KA, Mimitou EP, Mihalevic MJ, Chen H, Sunjaveric I, Symington LS, Rothstein R. Resection activity of the Sgs1 helicase alters the affinity of DNA ends for homologous recombination proteins in Saccharomyces cerevisiae. Genetics. 2013;195:1241–1251. doi: 10.1534/genetics.113.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Haber JE. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010;467:112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lisby M, Symington LS. RPA coordinates DNA end resection and prevents formation of DNA hairpins. Molecular cell. 2013;50:589–600. doi: 10.1016/j.molcel.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino L, Sotiriou SK, Rantala JK, Magin S, Mladenov E, Helleday T, Haber JE, Iliakis G, Kallioniemi OP, Halazonetis TD. Break-induced replication repair of damaged forks induces genomic duplications in human cells. Science. 2014;343:88–91. doi: 10.1126/science.1243211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Symington LS. The yeast recombinational repair protein Rad59 interacts with Rad52 and stimulates single-strand annealing. Genetics. 2001;159:515–525. doi: 10.1093/genetics/159.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Symington LS. The Rad52-Rad59 complex interacts with Rad51 and replication protein A. DNA repair. 2003;2:1127–1134. doi: 10.1016/s1568-7864(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Davis AP, Symington LS. RAD51-dependent break-induced replication in yeast. Molecular and cellular biology. 2004;24:2344–2351. doi: 10.1128/MCB.24.6.2344-2351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem A, Barker K, Vanhulle K, Downing B, Vayl A, Malkova A. Defective break-induced replication leads to half-crossovers in Saccharomyces cerevisiae. Genetics. 2008;179:1845–1860. doi: 10.1534/genetics.108.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng SK, Gibb B, de Almeida MJ, Greene EC, Symington LS. RPA antagonizes microhomology-mediated repair of DNA double-strand breaks. Nature structural & molecular biology. 2014;21:405–412. doi: 10.1038/nsmb.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng SK, Yin Y, Petes TD, Symington LS. Mre11-Sae2 and RPA Collaborate to Prevent Palindromic Gene Amplification. Molecular cell. 2015;60:500–508. doi: 10.1016/j.molcel.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnianni RA, Ferrari M, Lazzaro F, Clerici M, Tamilselvan Nachimuthu B, Plevani P, Muzi-Falconi M, Pellicioli A. Elevated levels of the polo kinase Cdc5 override the Mec1/ATR checkpoint in budding yeast by acting at different steps of the signaling pathway. PLoS genetics. 2010;6:e1000763. doi: 10.1371/journal.pgen.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnianni RA, Symington LS. Break-induced replication occurs by conservative DNA synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13475–13480. doi: 10.1073/pnas.1309800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Pavletich NP. Structure and conformational change of a replication protein A heterotrimer bound to ssDNA. Genes & development. 2012;26:2337–2347. doi: 10.1101/gad.194787.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmenich AA, Elias-Arnanz M, Berg P. A novel allele of Saccharomyces cerevisiae RFA1 that is deficient in recombination and repair and suppressible by RAD52. Molecular and cellular biology. 1995;15:1620–1631. doi: 10.1128/mcb.15.3.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191:33–64. doi: 10.1534/genetics.111.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS genetics. 2009;5:e1000327. doi: 10.1371/journal.pgen.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CK, Mazon G, Lam AF, Symington LS. Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Molecular cell. 2010;40:988–1000. doi: 10.1016/j.molcel.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Sugawara N, Lydeard J, Vaze M, Tanguy Le Gac N, Haber JE. A recombination execution checkpoint regulates the choice of homologous recombination pathway during DNA double-strand break repair. Genes & development. 2009;23:291–303. doi: 10.1101/gad.1751209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Sugawara N, Mehta A, Ryu T, Haber JE. Sgs1 and Mph1 Helicases Enforce the Recombination Execution Checkpoint During DNA Double-Strand Break Repair in Saccharomyces cerevisiae. Genetics. 2016;203:667–675. doi: 10.1534/genetics.115.184317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RD, Symington LS. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Molecular and cellular biology. 1995;15:4843–4850. doi: 10.1128/mcb.15.9.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantake N, Sugiyama T, Kolodner RD, Kowalczykowski SC. The recombination-deficient mutant RPA (rfa1-t11) is displaced slowly from single-stranded DNA by Rad51 protein. The Journal of biological chemistry. 2003;278:23410–23417. doi: 10.1074/jbc.M302995200. [DOI] [PubMed] [Google Scholar]
- Kass EM, Moynahan ME, Jasin M. When Genome Maintenance Goes Badly Awry. Molecular cell. 2016;62:777–787. doi: 10.1016/j.molcel.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA repair. 2008;7:686–693. doi: 10.1016/j.dnarep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Wang RW, Chang HH, Capurso D, Segal MR, Haber JE. Chromosome position determines the success of double-strand break repair. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E146–154. doi: 10.1073/pnas.1523660113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Llorente B, Smith CE, Symington LS. Break-induced replication: what is it and what is it for? Cell cycle. 2008;7:859–864. doi: 10.4161/cc.7.7.5613. [DOI] [PubMed] [Google Scholar]
- Luke-Glaser S, Luke B. The Mph1 helicase can promote telomere uncapping and premature senescence in budding yeast. PloS one. 2012;7:e42028. doi: 10.1371/journal.pone.0042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydeard JR, Jain S, Yamaguchi M, Haber JE. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature. 2007;448:820–823. doi: 10.1038/nature06047. [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Lipkin-Moore Z, Jain S, Eapen VV, Haber JE. Sgs1 and exo1 redundantly inhibit break-induced replication and de novo telomere addition at broken chromosome ends. PLoS genetics. 2010;6:e1000973. doi: 10.1371/journal.pgen.1000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Ira G. Break-induced replication: functions and molecular mechanism. Current opinion in genetics & development. 2013;23:271–279. doi: 10.1016/j.gde.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Naylor ML, Yamaguchi M, Ira G, Haber JE. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Molecular and cellular biology. 2005;25:933–944. doi: 10.1128/MCB.25.3.933-944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JM, Logan HL, Budke B, Wu M, Pawlowski M, Weichselbaum RR, Kozikowski AP, Bishop DK, Connell PP. The RAD51-stimulatory compound RS-1 can exploit the RAD51 overexpression that exists in cancer cells and tumors. Cancer research. 2014;74:3546–3555. doi: 10.1158/0008-5472.CAN-13-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle R, Campbell IM, Beck CR, Yu Y, Wilson M, Shaw CA, Bjergbaek L, Lupski JR, Ira G. DNA REPAIR. Mus81 and converging forks limit the mutagenicity of replication fork breakage. Science. 2015;349:742–747. doi: 10.1126/science.aaa8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozlin AM, Fung CW, Symington LS. Role of the Saccharomyces cerevisiae Rad51 paralogs in sister chromatid recombination. Genetics. 2008;178:113–126. doi: 10.1534/genetics.107.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett HA, Reddel RR. Molecular mechanisms of activity and derepression of alternative lengthening of telomeres. Nature structural & molecular biology. 2015;22:875–880. doi: 10.1038/nsmb.3106. [DOI] [PubMed] [Google Scholar]
- Plank JL, Wu J, Hsieh TS. Topoisomerase IIIalpha and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, et al. Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes & development. 2009;23:67–79. doi: 10.1101/gad.1737809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harbor perspectives in biology. 2015;7:a016600. doi: 10.1101/cshperspect.a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N, Ramakrishnan S, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature. 2013;502:389–392. doi: 10.1038/nature12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakofsky CJ, Roberts SA, Malc E, Mieczkowski PA, Resnick MA, Gordenin DA, Malkova A. Break-induced replication is a source of mutation clusters underlying kataegis. Cell reports. 2014;7:1640–1648. doi: 10.1016/j.celrep.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annual review of biochemistry. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- Santocanale C, Neecke H, Longhese MP, Lucchini G, Plevani P. Mutations in the gene encoding the 34 kDa subunit of yeast replication protein A cause defective S phase progression. Journal of molecular biology. 1995;254:595–607. doi: 10.1006/jmbi.1995.0641. [DOI] [PubMed] [Google Scholar]
- Smith CE, Llorente B, Symington LS. Template switching during break-induced replication. Nature. 2007;447:102–105. doi: 10.1038/nature05723. [DOI] [PubMed] [Google Scholar]
- Smith J, Rothstein R. A mutation in the gene encoding the Saccharomyces cerevisiae single-stranded DNA-binding protein Rfa1 stimulates a RAD52-independent pathway for direct-repeat recombination. Molecular and cellular biology. 1995;15:1632–1641. doi: 10.1128/mcb.15.3.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles J, Hazkani-Covo E, Yin Y, Andersen SL, Dietrich FS, Greenwell PW, Malc E, Mieczkowski P, Petes TD. High-resolution genome-wide analysis of irradiated (UV and gamma-rays) diploid yeast cells reveals a high frequency of genomic loss of heterozygosity (LOH) events. Genetics. 2012;190:1267–1284. doi: 10.1534/genetics.111.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafa A, Donnianni RA, Timashev LA, Lam AF, Symington LS. Template switching during break-induced replication is promoted by the Mph1 helicase in Saccharomyces cerevisiae. Genetics. 2014;196:1017–1028. doi: 10.1534/genetics.114.162297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annual review of genetics. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- Symington LS, Rothstein R, Lisby M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics. 2014;198:795–835. doi: 10.1534/genetics.114.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Altmeyer M, Rask MB, Lukas C, Larsen DH, Povlsen LK, Bekker-Jensen S, Mailand N, Bartek J, Lukas J. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell. 2013;155:1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Umezu K, Sugawara N, Chen C, Haber JE, Kolodner RD. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics. 1998;148:989–1005. doi: 10.1093/genetics/148.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Haber JE. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS biology. 2004;2:E21. doi: 10.1371/journal.pbio.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Kwon Y, Xu Y, Chung WH, Chi P, Niu H, Mayle R, Chen X, Malkova A, Sung P, et al. Pif1 helicase and Poldelta promote recombination-coupled DNA synthesis via bubble migration. Nature. 2013;502:393–396. doi: 10.1038/nature12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annual review of biochemistry. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- Zhou C, Pourmal S, Pavletich NP. Dna2 nuclease-helicase structure, mechanism and regulation by Rpa. eLife. 2015:4. doi: 10.7554/eLife.09832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.