Abstract
To clarify hematological abnormalities following infection with human immunodeficiency virus (HIV), we examined the hematopoietic capability of bone marrow by using cynomolgus monkeys infected with pathogenic simian/human immunodeficiency virus (SHIV) strain C2/1, an animal model of HIV infection. The relationship between the progress of the infection and the CD4/CD8 ratio of T lymphocytes or the amount of SHIV C2/1 viral load in the peripheral blood was also investigated. A colony assay was performed to assess the hematopoietic capability of bone marrow stem cells during the early and advanced phases of the infection. Colonies of granulocytes-macrophages (GM) were examined by PCR for the presence of the SIVmac239 gag region to reveal direct viral infection. There was a remarkable decrease in the CFU-GM growth on days 1 and 3 postinoculation, followed by recovery on day 56. During the more advanced stage, the CFU-GM growth decreased again. There was minimal evidence of direct viral infection of pooled cultured CFU-GM despite the continuously low CD4/CD8 ratios. These results indicate that the decrease in colony formation by bone marrow stem cells is reversible and fluctuates with the advance of the disease. This decrease was not due to direct viral infection of CFU-GM. Our data may support the concept that, in the early phase, production of inhibitory factors or deficiency of a stimulatory cytokine is responsible for some of the bone marrow defects described in the SHIV C2/1 model.
It is generally known as a feature of human immunodeficiency virus (HIV) infection that CD4-positive T lymphocytes and monocytes infected with pathogenic HIV or simian/human immunodeficiency virus (SHIV) decrease in number and disappear. Infected hosts will thus become immunodeficient. Moreover, it has been reported that, after HIV infection is contracted, hematological abnormalities in the bone marrow and the peripheral blood such as anemia, lymphopenia, and thrombocytopenia ensue and correlate with the advance of the illness (36). Several possibilities have been noted as the cause of such hematological abnormalities: the apoptosis of virus-infected cells, changes in the hematological environment, dysfunction of the thymus or the lymphoid system, change of cell division, or dysfunction of hematopoietic progenitor cells (1). Furthermore, a few reports have shown that the bone marrow of patients with AIDS displays morphological alterations similar to those of patients with myelodysplastic syndrome (2, 31). The term “HIV myelopathy” has been used for this bone marrow pathology by some investigators (10, 22).
Reduced numbers of CFU (burst-forming units-erythrocytes [BFU-E] or CFU-granulocytes-macrophages [CFU-GM]) have been reported in bone marrow samples from patients infected with HIV (9, 16, 27). Moreover, the reduction in CFU-GM resembles that of an animal model of AIDS experimentally induced by simian immunodeficiency virus (SIV) (13, 30, 32). While the precise mechanisms of such hematopoietic abnormalities remain unclear, several hypotheses have been proposed: (i) decreased levels of appropriate cytokines secondary to altered numbers of T-cell subsets or macrophages, which are commonly seen in HIV type 1 (HIV-1) infection (28); (ii) production of inhibitory factors (14, 29); (iii) cytotoxic elimination of the precursor cells by the antibody-dependent cell-mediated cytolytic mechanism (7); and (iv) infection of hematopoietic precursor cells with viruses, which leads to death of these cells or their metabolic alteration (7). On the other hand, it has been suggested that primitive bone marrow progenitor cells are most likely not a major reservoir for HIVs (6, 13, 28).
Despite mounting data supporting the above-mentioned hypotheses, a unifying explanation remains elusive. We studied bone marrow samples from cynomolgus monkeys (Macaca fascicularis) experimentally infected with an SHIV strain in order to evaluate possible cellular and molecular events that affect hematopoiesis in SHIV infection.
MATERIALS AND METHODS
Animals.
Twenty cynomolgus monkeys (nine males and 11 females) used in this study were maintained in our facility according to the guidelines of the Institutional Animal Care and Use Committee of the National Institute of Infectious Diseases (NIID), Tokyo, Japan. All treatments were performed according to the standard operating procedures for monkeys for evaluation of human vaccines in the Tsukuba Primate Center, NIID, Tsukuba, Ibaraki, Japan. Their ages were 3 to 6 years, and their weights were approximately 3 to 5 kg (Table 1). Four sham-inoculated monkeys were included as a control. They were inoculated with saline alone instead of virus-containing saline solution. Two additional monkeys without sham treatment also served as a negative control. Low-dose ketamine (intramuscular dose of 10 mg/kg of body weight) was used as an anesthetic for blood and bone marrow sampling.
TABLE 1.
Protocol for control and infection of cynomolgus monkeys with SHIV C2/1 and subsequent bone marrow harvestinga
| Monkey no. | Age (yr) | Sex | Day of bone marrow harvesting | Virus adminis- tration | Dose of inoculated virus (TCID50) |
|---|---|---|---|---|---|
| 13 | 5 | Male | |||
| 44 | 4 | Male | |||
| 181 | 6 | Male | |||
| 1037 | 5 | Male | |||
| 1091 | 5 | Male | |||
| 759 | 4 | Male | |||
| 4345 | 4 | Male | 1 | Intravenous | 20 |
| 1 | 5 | Female | 3 | Intravenous | 20 |
| 2 | 5 | Female | 3 | Intravenous | 20 |
| 90c | 5 | Female | 56 | Intravenous | 20 |
| 560 | 4 | Female | 56 | Intrarectal | 2,000 |
| 430 | 4 | Female | 56 | Intrarectal | 2,000 |
| 442 | 3 | Female | 56 | Intrarectal | 2,000 |
| 200 | 5 | Female | 56 | Intravenous | 20 |
| 944 | 5 | Male | 56 | Intravenous | 20 |
| 520 | 5 | Female | 56 | Intrarectal | 20 |
| 844 | 4 | Female | 56 | Intrarectal | 20 |
| 0634 | 4 | Female | 56 | Intravenous | 10 |
| 054 | 4 | Female | 113 | Intravenous | 2,000 |
| 039 | 4 | Male | 380 | Intravenous | 20 |
Monkeys 13 to 759 were controls that were not infected with SHIV. Monkeys 4345 to 039 were inoculated intravenously or intrarectally with the doses of SHIV C2/1 shown in the table. Bone marrow harvesting was performed on the indicated day after inoculation. TCID50, 50% tissue culture infective dose.
Viruses.
A highly pathogenic SHIV strain, designated C2/1, was obtained by serum passages in cynomolgus monkeys. The SHIV C2/1 strain contains the env gene of pathogenic HIV-1 strain 89.6. This chimeric virus was propagated in concanavalin A-activated peripheral blood mononuclear cells (PBMC) from healthy monkeys or in a cell line, M8166. Cell-free virus stocks were stored at −120°C (25).
Antibodies.
The mouse monoclonal antibodies (MAbs) used in this study were fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated MAbs to monkey CD3 (NF-18; BioSource) and to human CD4 (Nu-T H/I; Nichirei), CD8 (Nu-T S/C; Nichirei), CD16 (3G8; Pharmingen), and CD20 (Leu-16; Becton Dickinson).
Preparation of bone marrow cells.
Fourteen monkeys were infected with SHIV C2/1 at three 50% tissue culture infective doses by intravenous or intrarectal inoculation (Table 1). Bone marrow samples were aspirated from their femoral bone during autopsy. For a sham-inoculated control, monkeys received 0.5 ml of saline alone. One day or 3 days later, bone marrow samples were aspirated from their pelvic bones. Non-sham-control monkeys received only ketamine anesthesia for bone marrow aspiration.
Preparation of blood samples for cell surface antigen analysis by flow cytometry.
Peripheral blood was mixed with lysis buffer (Becton Dickinson) and centrifuged at 300 × g for 5 min. Viable cells were counted by the trypan blue dye-exclusion method. The cell surface antigens CD3, CD4, CD8, CD16, and CD20 were stained with their respective MAbs. After being washed with staining buffer, 5 × 104 cells in each labeled sample tube were analyzed by a FACSCalibur flow cytometer (Becton Dickinson) with use of Cell Quest software (Becton Dickinson). Absolute PBMC count was determined as follows. Fifty milliliters of each whole-blood sample, containing FITC-conjugated anti-CD3 MAb (BioSource), PE-conjugated anti-CD4 MAb (Becton Dickinson), and peridinin-chlorophyll protein-conjugated anti-CD8 MAb (Becton Dickinson), was added to a TRUCOUNT tube and incubated at room temperature. Contaminating red blood cells were lysed, and each sample was analyzed by flow cytometry as described above. All measurements were made under the same instrumental setting.
Quantification of cell-associated and plasma viral load.
Plasma viral RNA was extracted and purified using a QIAamp viral RNA minikit (Qiagen, Valencia, Calif.). For quantitative analysis of the RNA, reverse transcriptase-PCR (RT-PCR) was performed with primers and probes targeting the SIVmac239 gag region, designed by computer with the Primer Express software (PE Biosystems). The viral RNA was reverse transcribed and amplified using a Taqman EZ RT-PCR kit (PE Biosystems) with the designed primers (forward primer, 5′-AATGCAGAGCCCCAAGAAGAC-3′, and reverse primer, 5′-GGACCAAGGCCTAAAAAACCC-3′) and detected with a probe, FAM-5′-ACCATGTTATGGCCAAATGCCCAGAC-3′-TAMRA. Probed products were quantitatively monitored by their fluorescence intensity with ABI 7700 (PE Biosystems). For a positive-control RNA, SIVmac239 gag RNA was synthesized and purified using a MEGAscript kit (Ambion, Austin, Tex.) with template plasmid pKS460. This template contained the SIVmac239 gag sequence within the T7 promoter region. Plasma viral load, measured in duplicate, was estimated based on a standard curve of the control RNA and the RNA recovery rate (19).
Performance of colony assays on bone marrow specimens and detection of the SIVmac gag sequence by PCR in pooled cultured CFU-GM.
Bone marrow samples (n = 20) were obtained by aspiration from the femoral or pelvic bones of monkeys. An approximately 10-ml bone marrow sample diluted with phosphate-buffered saline was slowly layered on top of 10 ml of sterile Ficoll-Hypaque in a 15-ml conical tube. The tubes were then centrifuged at 400 × g for 30 min at room temperature. With use of a pipette, a top plasma layer was removed, and a mononuclear cell layer was transferred in a small volume to a tube. After two washes with 2% fetal calf serum-Iscove's medium (code no. HBM-3160; Stem Cell Technologies Inc.), cell density was adjusted to 106 mononuclear cells/ml. The cell suspensions were then mixed with methylcellulose medium (Methocult HF4434; Stem Cell Technologies) so that it gave a final concentration of 105 cells per 1.1 ml for final plating. The cell culture was performed in duplicate in 35-mm-diameter plastic dishes at 37°C, 5% CO2, and 100% humidity for 10 days, and colonies (BFU-E, CFU-GM, and CFU-granulocytes-erythroids-macrophages-megakaryocytes) were counted by inverted microscopy. CFU-GM were plucked from the methylcellulose culture and collected in pools and then subjected to PCR analysis by the method described above.
RESULTS
Figure 1 shows the relationship between the CD4/CD8 ratios of the peripheral blood T cells of infected monkeys and the postinoculation time. In general, the CD4/CD8 ratio decreased in 14 to 21 days after inoculation. It has been reported that monkeys inoculated with SHIV C2/1 had transient decreases of CD4+ T lymphocytes within several days after infection (25). In this study, one monkey showed a decrease in CD4/CD8 ratio even within several hours: namely, the CD4/CD8 ratio of monkey 4345 decreased to 1.28 in 6 h after inoculation and went up to 1.80 in 24 h (Fig. 1). Control monkeys showed only negligible declines (Fig. 1).
FIG. 1.
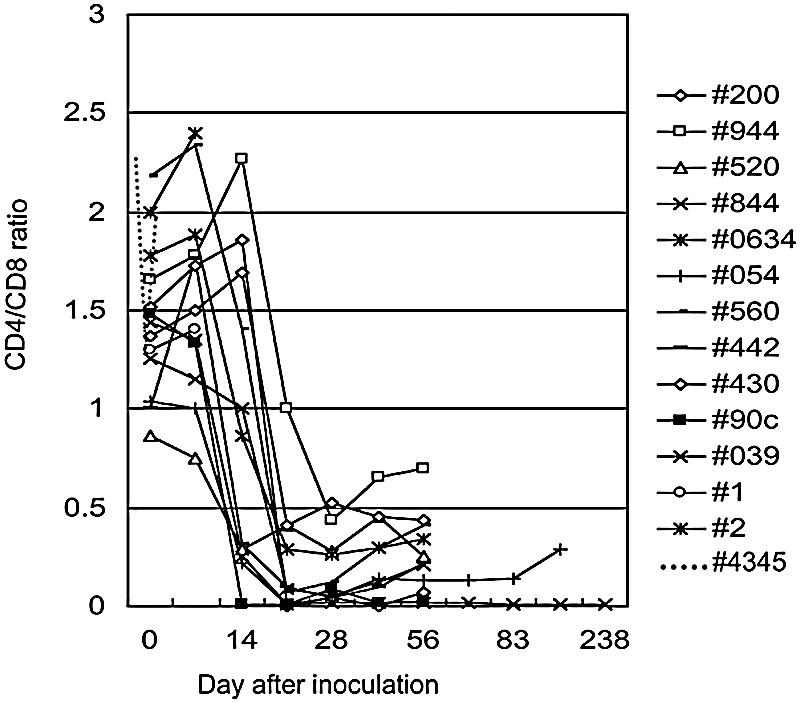
Changes of CD4/CD8 ratio in monkeys inoculated with SHIV C2/1. All monkeys showed decreased CD4/CD8 ratios between day 14 and day 21 after inoculation. Monkey 4345 had a decrease in CD4/CD8 ratio in the first 24 h. Control monkeys showed only negligible declines in the first 24 h (preinoculation, 1.26 ± 0.400 [mean ± standard deviation]; 6 h, 1.24 ± 0.259; 24 h, 1.11 ± 0.323; n = 4).
The number of viral copies was estimated for four animals (200, 944, 520, and 844) by real-time PCR (Fig. 2). It has been shown that the reduction of the CD4/CD8 ratio correlates with the increase of SHIV viral copies (15, 25). These four monkeys showed that the peak of viral copies occurred on the 14th day after inoculation and declined to 500 copies or less on the 56th day (Fig. 2).
FIG. 2.
Plasma viral load in the infected monkeys. Plasma viral RNA of four monkeys (200, 944, 520, and 844) was analyzed by PCR for the presence of the SIVmac239 gag region.
We arbitrarily defined a postinoculation period of day 1 through 3 as “early stage” (covering monkeys 4345, 1, and 2 in Fig. 3) and that of day 56 or later as “advanced stage” (covering monkeys from 90c to 039 in Fig. 3). Monkey 4345 showed a remarkable reduction in the number of colonies in 24 h (Fig. 3). Monkeys 1 and 2 also had such a dramatic decline on day 3 (Fig. 3). However, nine monkeys (90c through 0634 in Table 1 and Fig. 3) maintained colony formation during the advanced stage at a level comparable to that of the control monkeys (Fig. 3). Compared with sham-inoculated controls, monkey 054 had a somewhat lower number of colonies on the 113th day. Monkey 039, which died of AIDS on day 238, showed more reduced colony formation, especially CFU-GM formation, than did monkey 054 or the sham-inoculated control monkeys (Fig. 3). At the advanced stage, no difference in the morphology or the number of colonies was noted between the noninfected and the infected monkeys (Fig. 4).
FIG. 3.
Colony assay on monkeys inoculated with SHIV C2/1. A period of days 1 through 3 after inoculation was defined as early stage, whereas days 56 through 238 were defined as advanced stage. P was <0.005 for CFU-GM, and P was <0.02 for CFU-E in comparison of early stage and advanced stage and of virus-inoculated monkeys and sham-inoculated controls at days 1 and 3. P values were calculated according to Kruskal-Wallis analysis. There was no statistically significant difference between sham-inoculated controls and non-sham-treated controls by Mann-Whitney analysis.
FIG. 4.
Morphology of colonies produced by BFU-E and CFU-GM. Photographs of colonies cultured in nitrocellulose medium are shown at an ×75 magnification by a microscope. The left column shows BFU-E, and the right column shows CFU-GM. The upper section shows colonies from uninfected monkeys, while the lower shows colonies from monkeys infected with SHIV C2/1.
Taken together, a reduction of CD4/CD8 ratio and CFU-GM growth occurred in the early phase of the postinoculation period. However, the CFU-GM growth tended to increase following viremia while CD4+ T lymphocytes continuously declined. The colony growth of the infected monkeys during the advanced stage recovered up to a level comparable to that of the control monkeys.
Infection of CFU-GM with SHIV C2/1 virus was tested by a PCR technique as described in Materials and Methods. Of the 14 cynomolgus monkeys infected with SHIV C2/1 virus, only three were positive, suggesting that the direct infection of bone marrow progenitor cells was minimal (Fig. 5). There was no positive case in the control monkey group.
FIG. 5.
Detection of SIVmac239 gag sequence in bone marrow colonies by PCR. Lanes: 1, a DNA ladder marker, HincII; 2, a negative control; 3, a positive control, a DNA sample from cell line M8166; 4, CFU-GM from monkey 4345 at the early stage; 5, CFU-GM from monkey 430 at the advanced stage; 6, CFU-GM from monkey 039 at the advanced stage (this monkey died of AIDS on day 238).
DISCUSSION
Hematological abnormalities such as anemia, lymphopenia, and thrombocytopenia have been documented in a variety of retrovirus infections in both humans and experimental animals. While the precise mechanisms for such hematological abnormalities remain to be elucidated, several hypotheses have been postulated: (i) destruction of infected cells by a virus itself or by the antibody-dependent cell-mediated cytolytic mechanism, (ii) damage of the thymus or the lymphoid tissue, (iii) abnormal turnover of infected cells in the peripheral blood (i.e., apoptosis), and (iv) suppression of hematopoietic progenitor cells (23).
In this report, we showed that the remarkable decrease in the colony formation occurred during the early stage of infection with SHIV C2/1 (days 1 through 3 postinoculation). These results suggest that the hematopoietic progenitor cells are damaged or defective during such an early phase of infection. Furthermore, the CD4/CD8 ratio in monkey 4345 decreased within several hours, compared with controls (Fig. 1). We used ketamine for viral or saline inoculation, blood sampling, and autopsy. Ketamine has safely been used for bone marrow aspiration in humans and monkeys (21, 22, 26, 34, 35). It would be unlikely, therefore, that such bone marrow suppression occurred as a result of the anesthetic agent. However, our anesthetic procedure probably induces the release of corticosteroids in animals by the stress of capture and injection, which may have a negative impact on the colony formation. Our observation of the ability of bone marrow cultures from sham-inoculated controls to produce BFU-E and CFU-GM at days 1 and 3 proved otherwise.
After day 56, the ability of the bone marrow to form colonies recovered despite the preceding viremia and the continuing reduction of CD4/CD8 ratio. Furthermore, the colony formation was maintained at a level comparable to that of the control monkeys toward the terminal stage. Many reports have noted that CFU-GM growth continuously declines in SHIV infection, and such a decline appears to correlate with disease activity (20). However, CD4/CD8 ratio may not reflect the ongoing status of the bone marrow. A reason for the continuous reduction of CD4/CD8 ratio could be that infected T lymphocytes were destroyed in the peripheral blood more than they were produced in the bone marrow. This could be due to enhanced apoptosis or ongoing destruction of T cells by the antibody-dependent cell-mediated cytolytic mechanism in SHIV infection (24).
In contrast to previous reports, our results clearly showed that the decreasing CFU-GM growth recovered in the advanced stage, suggesting that the damage to colony formation during the early stage is reversible. We showed by PCR in this report that the direct infection of bone marrow progenitor cells with SHIV C2/1 was minimal (3, 5, 13, 18). It is possible, however, that the number of colonies was too low for detection of SHIV C2/1 virus or that SHIV C2/1 virus-infected cells were already removed by the host immune system before the assays (6, 8, 13).
It has been reported that the cellularity of the bone marrow from patients with HIV does not always correlate with the peripheral blood abnormalities (4). The commonly seen pancytopenia is often associated with hypercellular bone marrow where the increased number of lymphocytes, plasma cells, or histiocytes is seen. The latter finding suggests either dysmyelopoiesis or increased peripheral destruction of blood cells. Yoshino et al. have recently reported that atypical lymphocytes and monocytes were observed in the peripheral blood following intense viremia on the 10th to 14th days of SHIV infection (33). They further found erythroid multinucleation and atypical mononuclear cells in the hypercellular bone marrow, suggesting direct viral infection of hematopoietic progenitor cells (33).
As mentioned above, the colony formation in the bone marrow of the infected monkeys recovered spontaneously following viremia, suggesting that the reduced colony formation capability was reversible. It has been reported that inhibition of SIV replication in bone marrow macrophages resulted in increased colony growth of progenitor cells (32), and administration of recombinant human GM colony-stimulating factor could reverse leukocytopenia (11). Our data thus support the concept that, in the early phase, production of inhibitory factors or a lack or an inhibition of stimulatory cytokine production from lymphoid cells may be responsible for some of the bone marrow kinetic defects previously described in HIV (14, 28, 29). It is necessary to determine and verify what factor is participating in the regulation and recovery of the bone marrow CFU-GM growth.
The highly pathogenic SHIV C2/1 virus is an interesting tool to study the effect of HIV and SHIV infection on hematopoietic progenitor cells (15, 17, 25). Such studies will help us understand the pathophysiology of AIDS and contribute to the development of vaccines in humans (12).
Acknowledgments
This work was supported by the AIDS Research Center, NIID, Japan.
We express our appreciation to T. Nakasone and Y. Izumi, NIID, and M. Teramura, Tokyo Women's Medical University, for their quantitative analysis of plasma viral load and instruction in the stem cell culture system. We also thank K. Suzuki, Nihon University School of Medicine, Department of Public Health, for his statistical analysis and R. Suenaga for critical reading of the manuscript.
REFERENCES
- 1.Bagnara, G. P., G. Zauli, M. Giovannini, M.C. Re, and G. Furlini. 1990. Early loss of circulating haematopoietic progenitors in HIV-1-infected subjects. Exp. Hematol. 18:426-430. [PubMed] [Google Scholar]
- 2.Candido, A., P. Rossi, G. Menichella, L. Pierelli, E. Pizzigallo, G. Camilli, C. Rumi, and G. Mango. 1990. Indicative morphological myelodysplastic alterations of bone marrow in overt AIDS. Haematologica 75:327-333. [PubMed] [Google Scholar]
- 3.Chelucci, C., H. J. Hassan, C. Locardi, D. Bulgarini, E. Pelosi, G. Mariani, U. Testa, M. Federico, M. Valtieri, and C. Peschle. 1995. In vitro human immunodeficiency virus-1 infection of purified haematopoietic progenitors in single-cell culture. Blood 85:1181-1187. [PubMed] [Google Scholar]
- 4.Costello, C. 1998. Haematological abnormalities in human immunodeficiency virus (HIV) disease. J. Clin. Pathol. 41:711-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, R. D., D. H. Schwartz, J. C. Marx, C. E. Johnson, J. M. Berry, J. Lyding, T. C. Mergan, and A. Zander. 1991. Absent or rare human immunodeficiency virus infection of bone marrow stem/progenitor cells in vivo. J. Virol. 65:1985-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLuca, A., L. Teofili, A. Antinori, M. S. Iovino, P. Mencarini, E. Visconti, E. Tamburrini, G. Leone, and L. Ortona. 1993. Haematopoietic CD34+ progenitor cells are not infected by HIV-1 in vivo but show impaired clonogenesis. Br. J. Haematol. 85:20-24. [DOI] [PubMed] [Google Scholar]
- 7.Donahue, R. E., M. M. Johnson, L. I. Zon, S. C. Clark, and J. E. Groopman. 1987. Suppression of in vitro haematopoiesis following human immunodeficiency virus infection. Nature 326:200-203. [DOI] [PubMed] [Google Scholar]
- 8.Folks, T. M., S. W. Kessler, J. M. Orenstein, J. S. Justement, E. S. Jaffe, and A. S. Fauci. 1988. Infection and replication of HIV-1 purified progenitor cells of normal human bone marrow. Science 242:919-922. [DOI] [PubMed] [Google Scholar]
- 9.Geissler, R. G., O. G. Ottmann, K. Kleiner, U. Mentzel, A. Bickelhaupt, D. Hoelzer, and A. Ganser. 1993. Decreased haematopoietic colony growth in long-term bone marrow cultures of HIV-positive patients. Res. Virol. 144:69-73. [DOI] [PubMed] [Google Scholar]
- 10.Godwin, J. H. 1999. HIV/AIDS case histories: diagnostic problems. AIDS Patient Care STDs 13:303-305. [DOI] [PubMed] [Google Scholar]
- 11.Groopman, J. E., R. T. Mitsuyasu, M. J. DeLeo, D. H. Oette, and D. W. Golde. 1987. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 317:593-598. [DOI] [PubMed] [Google Scholar]
- 12.Hayami, M., T. Igarashi, T. Kuwata, M. Ui, T. Haga, Y. Ami, K. Shinohara, and M. Honda. 1999. Gene-mutated HIV-1/SIV chimeric viruses as AIDS live attenuated vaccines for potential human use. Leukemia 13:42-47. [DOI] [PubMed] [Google Scholar]
- 13.Hillyer, C. D., D. A. Lackey III, F. Villinger, E. F. Winton, H. M. McClure, and A. A. Ansari. 1993. CD34+ and CFU-GM progenitors are significantly decreased in SIVsmm9 infected rhesus macaques with minimal evidence of direct viral infection by polymerase chain reaction. Am. J. Hematol. 43:274-278. [DOI] [PubMed] [Google Scholar]
- 14.Leiderman, I. Z., M. L. Greenberg, B. R. Adelsberg, and F. P. Siegal. 1987. A glycoprotein inhibitor of in vitro granulopoiesis associated with AIDS. Blood 70:1267-1272. [PubMed] [Google Scholar]
- 15.Lu, Y., P. Brosio, M. Lafaile, J. Li, R. G. Collman, J. Sodroski, and C. J. Miller. 1996. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J. Virol. 70:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marandin, A., A. Katz, E. Oksenhendler, M. Tulliez, F. Picard, W. Vainchenker, and F. Louache. 1996. Loss of primitive hematopoietic progenitors in patients with human immunodeficiency virus infection. Blood 88:4568-4578. [PubMed] [Google Scholar]
- 17.Miller, C. J., M. Marthas, J. Greenier, D. Lu, P. J. Dailey, and Y. Lu. 1998. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J. Virol. 72:3248-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina, J. M., D. T. Scadden, M. Sakaguchi, B. Fuller, A. Woon, and J. E. Groopman. 1990. Lack of evidence for infection of or effect on growth of hematopoietic progenitor cells after in vivo or in vitro exposure to human immunodeficiency virus. Blood 76:2476-2482. [PubMed] [Google Scholar]
- 19.Mori, K., Y. Yasutomi, S. Ohgimoto, T. Nakasone, S. Takamura, T. Shioda, and Y. Nagai. 2001. Quintuple deglycosylation mutant of simian immunodeficiency virus SIVmac239 in rhesus macaques: robust primary replication, tightly contained chronic infection, and elicitation of potent immunity against the parental wild-type strain. J. Virol. 75:4023-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moses, A., J. Nelson, and G. C. Bagby, Jr. 1998. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood 91:1479-1495. [PubMed] [Google Scholar]
- 21.Parker, R. I., R. A. Mahan, D. Giugliano, and M. M. Parker. 1997. Efficacy and safety of intravenous midazolam and ketamine as sedation for therapeutic and diagnostic procedures in children. Pediatrics 100:732-733. [DOI] [PubMed] [Google Scholar]
- 22.Perkocha, L. A., and G. M. Rodgers. 1988. Hematologic aspects of human immunodeficiency virus infection: laboratory and clinical considerations. Am. J. Hematol. 29:94-105. [DOI] [PubMed] [Google Scholar]
- 23.Reinberger, S., M. Spring, T. Nißlein, C. Stahl-Hennig, G. Hunsmann, and U. Dittmer. 1999. Kinetics of lymphocyte apoptosis in macaques infected with different simian immunodeficiency viruses or simian/human immunodeficiency hybrid viruses. Clin. Immunol. 90:141-146. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki, Y., Y. Ami, T. Nakasone, K. Shinohara, E. Takahashi, S. Ando, K. Someya, Y. Suzaki, and M. Honda. 2000. Induction of CD95 ligand expression on T lymphocytes and B lymphocytes and its contribution to apoptosis of CD95-up-regulated CD4 T lymphocytes in macaques by infection with a pathogenic simian/human immunodeficiency virus. Clin. Exp. Immunol. 122:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinohara, K., K. Sakai, S. Ando, Y. Ami, N. Yoshino, E. Takahashi, K. Someya, Y. Sasaki, T. Nakasone, Y. Sasaki, M. Kaizu, Y. Lu, and M. Honda. 1999. A highly pathogenic simian/human immunodeficiency virus with genetic changes in cynomolgus monkey. J. Gen. Virol. 80:1231-1240. [DOI] [PubMed] [Google Scholar]
- 26.Slonim, A. D., and F. P. Ognibene. 1998. Sedation for pediatric procedures, using ketamine and midazolam, in a primarily adult intensive care unit: a retrospective evaluation. Crit. Care Med. 26:1900-1904. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg, H. N., C. S. Crumpacker, and P. A. Chatis. 1991. In vitro suppression of normal human bone marrow progenitor cells by human immunodeficiency virus. J. Virol. 65:1765-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stella, C. C., A. Ganser, and D. Hoelzer. 1987. Defective in vitro growth of the haematopoietic progenitor cells in the acquired immunodeficiency syndrome. J. Clin. Investig. 80:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stutte, H. J., H. Müller, S. Falk, and H. L. Schmidts. 1990. Pathophysiological mechanisms of HIV-induced defects in haematopoiesis: pathology of the bone marrow. Res. Virol. 141:195-200. [DOI] [PubMed] [Google Scholar]
- 30.Thiebot, H., F. Louache, B. Vaslin, T. D. Revel, O. Neildez, J. Larghero, W. Vainchenker, D. Dormont, and R. L. Grand. 2001. Early persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J. Virol. 75:11594-11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiele, J., T. K. Zirbes, P. Wiemers, J. Lorenzen, H. M. Kvasnicka, and R. Fischer. 1997. Incidence of apoptosis in HIV-myelopathy, myelodysplastic syndromes and non-specific inflammatory lesions of the bone marrow. Histopathology 30:307-311. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe, M., D. J. Ringler, M. Nakamura, P. A. Delong, and N. L. Letvin. 1990. Simian immunodeficiency virus inhibits bone marrow hematopoietic progenitor cell growth. J. Virol. 64:656-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshino, N., T. Ryu, Y. Ami, K. Shinohara, M. Kaizu, Y. Izumi, T. Nakasone, and M. Honda. 2000. Pathogenic SHIV induce dysplastic hematopoiesis which may be resulted from the effect on hematopoietic progenitor cells and may affect progression to disease-state in monkeys, p. 302. In Abstract book, vol. 1. The 13th International AIDS Conference, Durban, South Africa.
- 34.Young, S. S., A. M. Schilling, S. Skeans, and G. Ritacco. 1999. Short duration anaesthesia with medetomidine and ketamine in cynomolgus monkeys. Lab. Anim. 33:162-168. [DOI] [PubMed] [Google Scholar]
- 35.Zafer, T., S. Javaid, M. Saleem, Z. A. Malik, and M. F. Khattak. 1997. Ketamine for bone marrow aspiration and trephine biopsy in children. J. Pak. Med. Assoc. 47:304-305. [PubMed] [Google Scholar]
- 36.Zon, L. I., and J. E. Groopman. 1988. Hematologic manifestations of the human immune deficiency virus (HIV). Semin. Hematol. 25:208-218. [PubMed] [Google Scholar]






