Abstract
Obesity, the major cause of the current global epidemic of Type 2 diabetes (T2D), induces insulin resistance in peripheral insulin target tissues. Several mechanisms have been identified related to cross-talk between adipose tissue, skeletal muscle and liver. These mechanisms involve both increased free fatty acid release and altered secretion of adipokines from adipose tissue. A major determinant of metabolic health is the ability of subcutaneous adipose tissue (SAT) to store excess fat rather than allowing it to accumulate in ectopic depots including liver (i.e. in non-alcoholic fatty liver disease), muscle and heart, or in epicardial/pericardial and visceral fat depots which promote the metabolic complications of obesity. The ability to recruit and differentiate precursor cells into adipose cells (adipogenesis) in SAT is under genetic regulation and is reduced in high-risk individuals who have first-degree relatives with T2D. Early recruitment of new adipose cells is dependent on the cross-talk between canonical WNT and BMP4 signaling; WNT enhances their undifferentiated and proliferative state whereas BMP4 induces their commitment to the adipogenic lineage. Dysregulation of these signaling pathways is associated with impaired adipogenesis and impaired ability to respond to the need to store excess lipids in SAT. This leads to hypertrophic, dysfunctional and insulin-resistant adipose cells with a reduced content of GLUT4, the major insulin-regulated glucose transporter, which in turn reduces adipose tissue glucose uptake and de novo lipogenesis. We recently identified that reduced GLUT4 and lipogenesis in adipocytes impairs the synthesis of a novel family of lipids secreted by adipose tissue (and potentially other tissues), the fatty acid esters of hydroxy fatty acids (FAHFAs). FAHFAs have beneficial metabolic effects, including enhancing insulin-stimulated glucose transport and glucose-stimulated GLP1 and insulin secretion, as well as powerful anti-inflammatory effects. FAHFA levels are reduced in subcutaneous adipose tissue in insulin-resistant individuals, and this novel family of lipids may become of future therapeutic use.
Keywords: adipose tissue, glucose, insulin resistance, lipogenesis, Type 2 diabetes
Type 2 diabetes global epidemic: ethnic influence on the consequence of a high body mass index
The current global epidemic of Type 2 diabetes (T2D) is primarily a consequence of overweight/obesity and a sedentary lifestyle. It is predicted that by 2030 there will be over 500 million and by 2040 over 640 million individuals with diabetes, primarily T2D [1]. Diabetes leads to serious complications including cardiovascular disease (CVD), renal disease, cancer and cognitive dysfunction. Diabetes also has major effects on health costs. The costs for diabetes and its complications in the USA in 2014 were reported to be $612 billion [1].
Overweight in Caucasians is defined as a body mass index (BMI) of 25.0–29.9 and obesity as a BMI >29.9 kg/m2. However, in Asian populations, in which T2D is increasing at an epidemic rate, the risk of diabetes and CVD increases at a BMI of ≥ 24 kg/m2 [2]. This suggests that Asians are more sensitive to the negative consequences of increased fat accumulation and, in fact, the International Diabetes Federation definition of the metabolic syndrome in Asians requires a smaller waist circumference than in Caucasians [3]. Thus, BMI is a good epidemiologic marker in defined populations but not a well-defined marker of increased T2D risk from a global perspective across different ethnic groups.
The majority, although not all, overweight/obese individuals (as defined in different ethnic groups) have insulin resistance, i.e. reduced cellular sensitivity/response to insulin, which is a well-established precursor of T2D. Important advances have been made in recent years in understanding the mechanisms by which expanded adipose tissue leads to insulin resistance. Here we focus on the ability of subcutaneous adipose tissue (SAT) to store/release excess fat and the endocrine functions of this tissue. Although other fat depots also have endocrine functions and secrete cytokines and hormones, there are both qualitative and quantitative differences between the various sites. Furthermore, SAT is by far the largest adipose depot and the most important from the point of view of its quantitative contribution to lipid storage/release and endocrine function.
Adipose tissue has many functions and cells
The main function of white adipose tissue is to store and release lipids/fatty acids (FAs) as needed depending on fuel/food availability. Degradation or hydrolysis of the stored triglycerides is carefully regulated but the insulin resistance in obesity is associated with impaired inhibition of lipolysis by insulin resulting in increased release of FAs and glycerol. Glycerol is an important substrate for hepatic gluconeogenesis and FAs are important energy substrates for peripheral tissues. Excess lipids accumulate not only in the adipose tissue but also in several other tissues and they are also converted to very low-density lipoprotein (VLDL) triglycerides and secreted by the liver, resulting in the typical dyslipidemia associated with obesity and T2D. Accumulation of lipids in liver, skeletal muscle and other non-SAT tissues is an important mechanism for the development of insulin resistance in these tissues [4, 5].
In addition to regulating lipid release and storage, adipose tissue functions as a large endocrine organ. In insulin-resistant obesity (around 70% of obese Caucasians), adipose tissue secretes molecules which antagonize insulin-action including cytokines and other proinflammatory molecules (e.g. Retinol Binding Protein 4 (RBP4), TNFα, IL-6 and IL-1b) which promote adipose tissue and systemic inflammation. Increased levels of C-reactive protein (CRP) are a marker of this inflammatory state which is also associated with other metabolic and cardiovascular abnormalities or risk factors including dyslipidemia, elevated uric acid and hypertension. These abnormalities constitute the metabolic syndrome which is defined on the basis of insulin resistance and its metabolic consequences, and increased BMI and waist circumference [3]. The metabolic syndrome is a predictor of risk of developing both T2D and CVD [2–4]. However, adipose tissue can also secrete molecules that are associated with enhanced insulin sensitivity such as adiponectin and the recently discovered branched fatty acid esters of hydroxy fatty acids (FAHFAs) (discussed below). The levels of these ‘metabolically favorable’ molecules are decreased in obesity that is associated with insulin-resistance.
The dysfunctional SAT in insulin resistance has reduced adipose cell GLUT4 protein – the insulin-regulated glucose transporter
Levels of the insulin-regulated glucose transporter protein GLUT4 are reduced in adipose cells in insulin-resistant obesity and in prediabetes [e.g. in high-risk individuals with first-degree relatives (FDRs) with T2D], as well as in T2D itself. Adipose tissue GLUT4 protein levels are also a marker of whole-body insulin sensitivity measured with the euglycemic clamp technique [6, 7]. This is surprising because the amount of glucose taken up by adipose tissue is small in comparison to skeletal muscle and accounts for only around 10% of a prandial glucose load. However, novel mechanisms have recently been identified whereby GLUT4 expression and glucose metabolism in adipose cells can regulate insulin sensitivity by altering endocrine function, metabolism of substrates, de novo lipogenesis or release of the novel FAHFA lipids as discussed below.
SAT is the ‘safest’ site for storing excess body fat
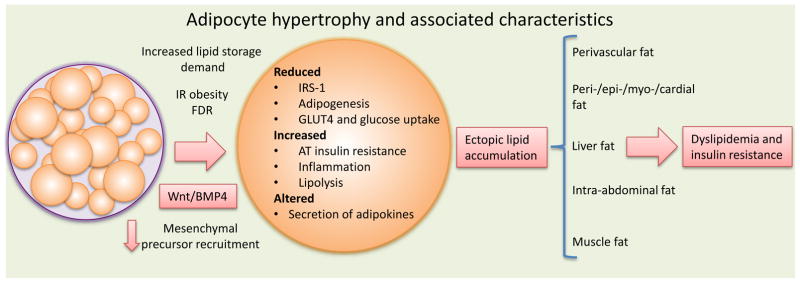
Not only is SAT the largest adipose tissue depot but it is also the least harmful site for lipid storage. However, it has a limited capacity to expand dependent on the ability of the adipose cells to enlarge and/or the ability to recruit new cells for storing excess fat. When storage capacity is exceeded, fat is stored outside SAT in the so-called ectopic fat depots which are important contributors to the obesity-associated insulin resistance and inflammation. Ectopic fat depots include the liver [in non-alcoholic fatty liver disease (NAFLD)], heart and skeletal muscle. Fat accumulation in non-SAT adipose tissues such as the epicardial/pericardial and intra-abdominal (visceral) depots is also correlated with ectopic fat accumulation and considered detrimental. Furthermore, abdominal fat distribution, usually measured as waist circumference, is a clinical marker of increased visceral fat and increases the risk of developing T2D by 2- to 3-fold for a given BMI [8, 9]. Asian populations have a reduced ability to expand the SAT and, therefore, are more prone to visceral/abdominal accumulation of their excess body fat. This is likely to be an important reason for their increased sensitivity to the negative consequences of even small increases in fat accumulation as discussed above [10, 11].
NAFLD is not only a risk factor for T2D but also for CVD and non-alcoholic steatohepatitis (NASH), a more severe form of liver disease with increased risk of cirrhosis and cancer of the liver. The increased ectopic fat accumulation at different sites elicits specific metabolic and vascular consequences which promote the development of various aspects of the metabolic syndrome (summarized in Fig. 1). The consequences of ectopic fat accumulation in different depots have been reviewed recently [12, 13].
Fig. 1.
Adipocyte hypertrophy and associated characteristics. IR, insulin resistant; FDR, first-degree relative., AT, adipose tissue Reproduced from Gustafson et al. (52), with permission.
Hypertrophic and hyperplastic obesity
Much evidence, including from prospective studies, has shown that the accumulation of fat in non-SAT and ectopic depots, rather than in the subcutaneous depot, is associated with the subsequent development of T2D and CVD [13, 14]. In principle, SAT can expand by increasing the size of the cells (hypertrophic obesity) and/or by recruiting new cells (hyperplastic obesity). A hyperplastic response protects against cell expansion and the development of dysregulated and dysfunctional SAT. Hypertrophic, rather than hyperplastic, obesity has long been known to be a marker of metabolic risk in obesity [8] and has also been shown to be an independent risk factor for the development of T2D [15]. Thus, preventing adipose cell expansion by recruiting new cells is protective against the metabolic complications of obesity. However, we showed for the first time several years ago that there were large inter-individual differences in the ability to recruit and differentiate new adipose cells (adipogenesis) in SAT and that this ability was a marker of how the adipose tissue and cells expanded, i.e. resulting in the development of hyperplastic or hypertrophic obesity [16].
In an elegant study, Spalding et al. demonstrated that the number of adipose cells in humans is established around puberty and that there is an annual turnover of the cells in SAT of ~10% [17]. Hypertrophic obesity was associated with a reduced turnover of the adipose cells [18] which is consistent with our findings of reduced adipogenesis in hypertrophic obesity [16, 19]. Importantly, we have also found that the number of precursor cells is not reduced in hypertrophic obesity but that there is an impairment of the ability of the precursors to enter adipogenesis and adipose cell development [19].
An important step in initiating adipogenesis is to commit available precursor (mesenchymal stem) cells to the adipogenic lineage where bone morphogenetic protein (BMP)4 plays a key role [20]. BMP4 is secreted by several different cells, including mature adipose cells, and increased in hypertrophic obesity [21]. This is likely to be part of a feedback cycle aimed at recruiting new cells to adipogenesis to prevent the inappropriate expansion and dysregulation of mature adipocytes in SAT as the need to store excess fat increases. However, this obviously does not function adequately in hypertrophic obesity, probably due to increased secretion of endogenous BMP4 antagonists as discussed below [21].
Adipose tissue precursor cells are present in the stromal vascular tissue but it has been unclear whether these are permanent resident cells and/or bone marrow-derived cells which are recruited into the adipose tissue to enter adipogenesis. In a recent study, performed in individuals who had undergone bone marrow transplantation, it was found that bone marrow-derived cells accounted for around 10% of the SAT cell population and this was increased to 25% in obese individuals [22]. Thus, it is likely that the precursor cells that can undergo adipogenesis are of different origins. Whether these cells require different signals to enter the adipogenic program and whether this has consequences for the development of hypertrophic versus hyperplastic obesity are interesting questions.
Hypertrophic obesity is associated with T2D and family history
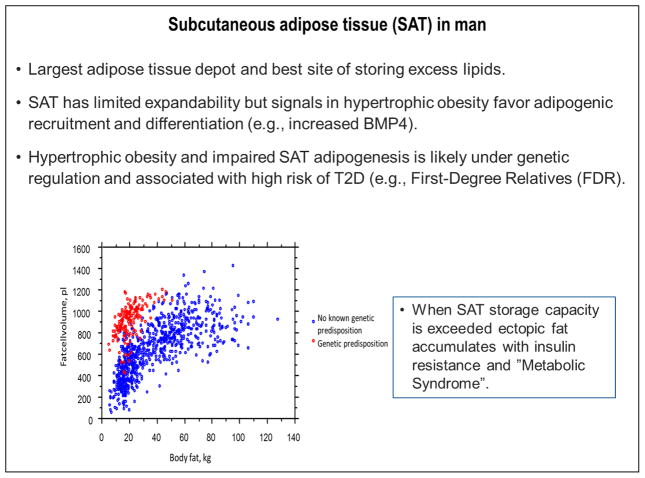
As mentioned above, having an FDR with T2D is a major risk factor for developing T2D in the future. In fact, FDRs have a higher risk of developing T2D than the pooled risk induced by the identified diabetes risk genes [23]. We examined many of the known genetic risk markers in a large cohort of FDRs but found only a small association with their high risk of T2D [24]. Thus, the currently identified genetic risk markers cannot account for the very high risk of developing T2D in individuals with an FDR with the disease. We also examined SAT adipose cell size (a marker of hypertrophic obesity) in cohorts of carefully matched FDRs and individuals lacking a known family history of T2D [25]. Intriguingly, we found that FDRs were characterized by hypertrophic obesity and several markers of insulin resistance even before becoming obese. This is further supported by data showing the association in our cohort of FDRs between amount of body fat and SAT adipose cell size (Fig. 2). Using similar methodology to measure the adipose cell size, Arner et al. demonstrated a marked and inappropriate increase in adipose cell size in FDRs compared to a large cohort of individuals without a known family history of T2D [18]. Furthermore, it was recently shown that non-obese individuals with T2D were also characterized by this risk marker, i.e. hypertrophic obesity [26]. Taken together, these findings strongly support the importance of genetic regulation of SAT adipogenesis but this is outside the currently identified T2D genetic risk markers. Understanding the genetic basis for this will likely open up new diagnostic and therapeutic possibilities.
Fig. 2.
Subcutaneous adipose tissue in humans.
Regulation of adipose precursor cell proliferation, commitment and differentiation
Multipotent adipose precursor cells are present in the perivascular stroma in adipose tissue and may be differentially recruited to adipogenesis at various stages of development. As discussed, it has also been shown that the bone marrow precursor cells contribute 10–25% of the adipose cell population in SAT in humans [22]. However, very limited information is available about factors that regulate the proliferation of these precursor cells which then determine a hyperplastic versus hypertrophic response in obesity. The canonical WNT pathway is a fundamental regulator of undifferentiated precursor cell growth and differentiation [27]. We recently identified a WNT-associated molecule, termed WISP2, which is secreted by mesenchymal precursor cells and, in an autocrine/paracrine fashion, enhances their proliferation [28]. WISP2 is most highly expressed in the SAT in humans. WISP2 needs to be inhibited to allow the differentiation of adipogenic precursor cells and we found that it also regulates the induction by BMP4 of PPARγ, the master regulator of adipogenesis. Thus WISP2 is a regulator of adipogenic commitment of precursor cells [28].
BMP4 and BMP7 regulate white and beige/brown adipogenesis
Several studies have shown that BMP4 plays a critical role in inducing commitment of mesenchymal stem cells into the white adipogenic lineage [20, 21]. Importantly, BMP4 activates nuclear entry of the PPARγ transcriptional activator ZNF423 [28], through the BMP4-induced dissociation of an intracellular protein complex between WISP2 and ZNF423 which retains ZNF423 in the cytosol. BMP4-induced dissociation of this protein complex allows ZNF423 to enter the nucleus and drive the transcriptional activation of PPARγ [28]. This is an early step in committing the precursor cells to the adipogenic lineage. However, PPARγ then also needs to be activated by endogenous ligands, the nature of which is incompletely understood. The currently used antidiabetic agents, the thiazolidinediones (TZDs), are PPARγ ligands and their positive effect on insulin sensitivity and glucose control in diabetes is largely dependent on their effect in adipose tissue [29]. Treatment with TZDs reduces fat accumulation in the visceral depot and liver, and increases the storage capacity of SAT. However, TZDs can only increase the differentiation of already committed and differentiated precursor cells and not the recruitment of new adipose cells. Furthermore, the side effects of TZDs, including increased risk of bone fractures, have limited their use and novel insulin-sensitizing agents are greatly needed.
BMP4 is highly expressed in, and secreted by, mature adipose cells and in particular in hypertrophic obesity [21]. This is likely a feedback signal aimed at recruiting new cells to avoid further expansion of the adipose cells. However, it is clear that this does not function in hypertrophic obesity, at least in part due to an inability to induce the downstream signals for BMP4. In an extensive study, we identified an endogenous BMP inhibitor, Gremlin1, that was upregulated in hypertrophic obesity [21]. Gremlin1 is a powerful, secreted BMP4 antagonist that prevents the positive effect of BMP4 on adipogenesis. Reducing Gremlin1, either genetically or by specific antibodies, improved BMP4-induced recruitment and differentiation of new adipose cells. Thus, endogenous BMP4 antagonists are powerful regulators of the cellular response and we concluded that hypertrophic obesity in humans is a state of BMP4 resistance, at least in part mediated by Gremlin1 [21].
BMP4 signaling is important not only for the recruitment of new adipose cells and their differentiation but also for the development of a beige/brown and oxidative phenotype of the adipose cells [21] which protects against obesity. Transgenic mice overexpressing BMP4 in adipose tissue assume an oxidative phenotype in their white adipose tissue, a so-called beiging/browning of the white adipose tissue [30]. Thus BMP4, together with its downstream signaling pathway, is an important regulator not only of white adipogenic cell commitment and differentiation but also of the induction of a brown and oxidative phenotype of the lipid-storing white adipose cells. We are presently focusing on understanding the detailed molecular mechanisms involved, as this may open up new possibilities to prevent and/or treat obesity and T2D. A beige/brown oxidative phenotype in SAT, with increased energy expenditure, has also been seen in humans following severe burns accompanied by increased adrenergic activation, demonstrating that these adaptations of SAT are indeed possible in humans [31]. Taken together, several studies have shown that SAT may change its phenotype from that of a primarily lipid-storing organ to that of both an oxidative and a lipid-storing organ.
In contrast to BMP4 which does not target the classical brown fat, BMP7 targets brown adipose tissue growth and protects mice from obesity [32]. Certain effects of BMP7 in vitro to increase the differentiation of human mesenchymal stem cells into a beige/brown adipose phenotype have been reported [33]. However, we have found virtually no expression at all of BMP7 in human SAT, arguing against an important endogenous regulatory role of BMP7 in humans [21].
Endocrine effects of Adipose Tissue
Adipose tissue is an endocrine organ secreting factors that can both improve and impair insulin sensitivity. In general, well-functioning adipose tissue secretes adipokines and other molecules with important regulatory effects such as leptin [34], adiponectin [35] and the recently described novel family of lipids, the FAHFAs [36]. However, hypertrophic and dysregulated adipose tissue secretes more pro-inflammatory and insulin-antagonistic molecules, including amongst others RBP4, IL-6 and IL-8, while the production of adiponectin and FAHFAs is attenuated.
There is much evidence for a strong association between adiponectin and insulin sensitivity in mice and studies have also shown that adiponectin increases lipid oxidation and is anti-inflammatory [37]. However, attempts to evaluate causality between insulin resistance and adiponectin in humans using Mendelian randomization analysis have yielded inconsistent results [38, 39].
RBP4, the sole retinol transporter in blood, was identified as an adipocyte- and liver-secreted molecule, the levels of which are elevated in insulin-resistant obesity and T2D [40, 41]. We showed that elevated RBP4 causes insulin resistance via induction of inflammation in monocytes, macrophages and dendritic cells resulting in antigen presentation and activation of both the innate and adaptive immune systems [42, 43]. Large epidemiologic studies have demonstrated that RBP4 is a circulating biomarker of insulin resistance and risk of CVD and metabolic syndrome [44, 45]. A genetic alteration in the RBP4 promoter which increases RBP4 expression in adipose tissue is associated with nearly a 2-fold increased risk of T2D [46].
RBP4 was identified by examining the gene expression pattern in mice with high or low GLUT4 in the adipose tissue [41]. Since the adipose tissue accounts for only around 10% of whole-body glucose uptake following a meal, it was a highly unexpected finding that mice lacking GLUT4 in the adipose tissue were as insulin-resistant as mice lacking GLUT4 in the skeletal muscle [41], the major site of insulin-stimulated glucose disposal. In addition, overexpressing GLUT4 specifically in the adipose cells was associated with lower ambient glycemia and insulinemia and enhanced glucose tolerance despite increased adiposity in the mice [47]. The adipose tissue of GLUT4-overexpressing mice was characterized by hyperplasia, providing another example of how hyperplastic obesity can protect against insulin resistance. Furthermore, overexpressing GLUT4 in the adipose tissue rescued insulin sensitivity in mice lacking GLUT4 in the skeletal muscles [48]. Taken together, these and other data show that adipose tissue is an important regulator of whole-body insulin sensitivity and glucose uptake but its regulatory role cannot be accounted for by the number of moles of glucose taken up by the adipose tissue itself. Extensive studies in these mice have subsequently shown that GLUT4 expression in the adipose cells is a regulator of the endocrine secretion pattern and the metabolic function of the cells.
Identification of a novel class of lipids (FAHFAs) that improve insulin sensitivity and insulin secretion
To further clarify the mechanisms underlying the insulin-sensitizing effect of high GLUT4 levels in the adipose cells on whole-body insulin sensitivity we, together with Dr. Alan Saghatelian’s lab, performed an untargeted lipidomic analysis of adipose tissue of mice with high GLUT4 expression. Intriguingly, we identified a family of novel lipids, the FAHFAs (Fig. 3) [36]. FAHFAs are present in many tissues but levels are highest in white and brown adipose tissue. Some of these novel lipids enhance the effect of insulin on glucose uptake in adipocytes and augment glucose-stimulated GLP1 secretion from entero-endocrine cells and insulin secretion by pancreatic beta cells (Fig. 4) [36]. These lipids also exert anti-inflammatory effects. Adipose tissue from mice with high GLUT4 levels had high levels of the FAHFAs whereas the opposite was found in adipose tissue from mice with low GLUT4 levels, such as in obese mice fed a high-fat diet. The relevance of these findings to humans was verified as human adipose tissue and serum also contain FAHFAs. We investigated isomers of one family of FAHFAs, palmitic acid esters of hydroxy fatty acids (PAHSAs), and found that they are low in serum and adipose tissue in insulin-resistant compared to insulin-sensitive individuals. Serum PAHSA levels were closely positively correlated with the degree of insulin sensitivity measured with the euglycemic clamp technique [36]. Taken together, we identified a novel class of lipids synthesized by adipose tissue, as well as other tissues, which may provide a mechanism contributing to the tight correlation between adipose tissue GLUT4 levels and whole-body insulin sensitivity in spite of the relatively small amount of glucose taken up by adipose tissue.
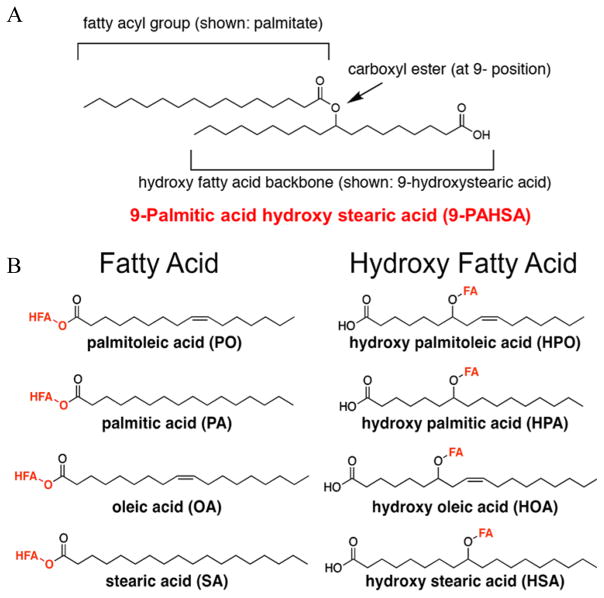
Fig. 3.
Structure of branched fatty acid esters of hydroxy fatty acids (FAHFAs). (A) FAHFAs are esters that combine a fatty acid such as palmitate with a hydroxy fatty acid backbone such as hydroxystearic acid. The FAHFA shown is 9-palmitic acid hydroxystearic acid (PAHSA) which is the most upregulated FAHFA family member in adipose tissue of mice that are overexpressing GLUT4 selectively in adipocytes. (B) A total of 16 FAHFA family members were identified in mouse serum. These lipids consist of four different fatty acid moieties and four hydroxy fatty acid moieties in different combinations. Adapted from ref. 36 and reproduced with permission from Cell Press.
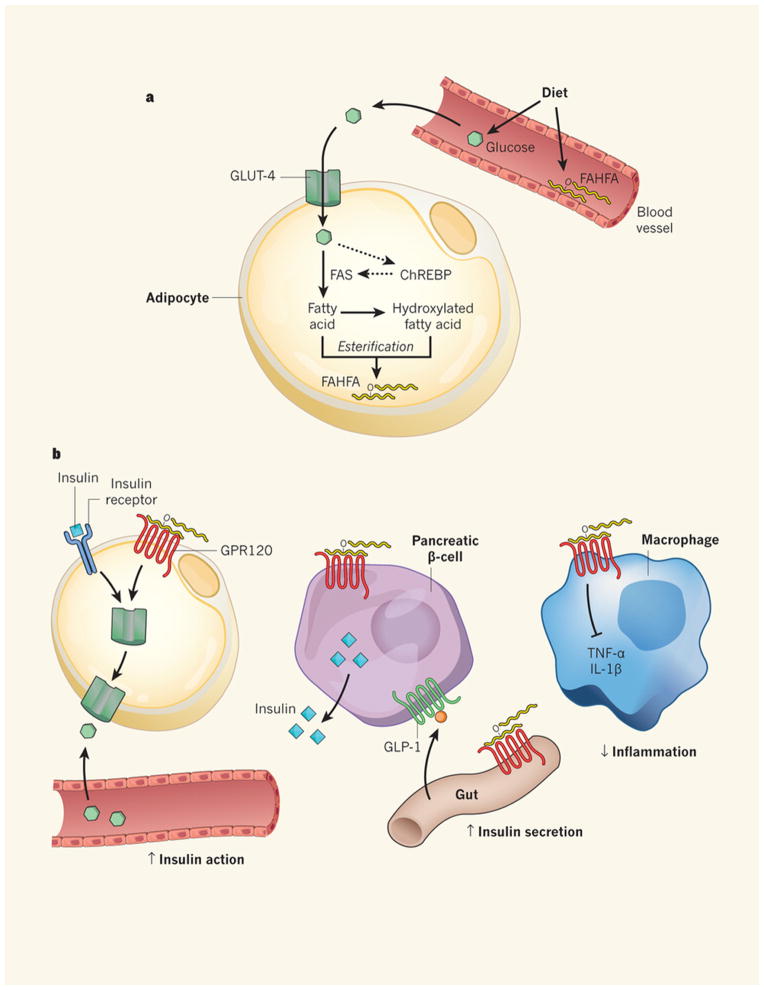
Fig 4.
Anti-diabetic and anti-inflammatory effects of branched fatty acid esters of hydroxy fatty acids (FAHFAs). (a) Glucose is transported into adipocytes by the GLUT4 glucose transporter. The increased glucose entry activates the transcription factor ChREBP, thereby enhancing de novo lipogenesis and synthesis of branched Fatty Acid esters of Hydroxy Fatty Acids (FAHFAs). (b) FAHFAs augment insulin-stimulated glucose transport in adipocytes and glucose-stimulated GLP1 secretion from the gut enteroendocrine cells and insulin secretion from pancreatic beta cells. FAHFAs also reduce inflammation by decreasing the production of pro-inflammatory cytokines from macrophages and dendritic cells. ChREBP, carbohydrate response element binding protein; FAS, fatty acid synthase; GPR120, G protein-coupled receptor 120. Diabetes: The good in fat. Muoio D.M & Newgard C.B. Nature 516:49–50, 2014. Reproduced with permission from Nature Publishing Group, license number 3851950681929.
How can the glucose transporter GLUT4 in adipose tissue regulate the production of this family of lipids? In a comprehensive mechanistic study, Herman et al. showed that GLUT4 and adipose tissue glucose uptake induce and activate the nuclear transcription factor carbohydrate response element binding protein (ChREBP), which enhances lipogenesis and the synthesis of these FAHFAs [36]. Thus, although fairly modest relative to whole-body glucose uptake, local adipose tissue glucose uptake and metabolism play an important role in the endocrine secretion of different adipokines as well as in other functions of adipose tissue. Glucose metabolism is also an important regulator of lipolysis. Increased lipolysis and elevated release of FAs can contribute to insulin resistance in both skeletal muscle and liver [4, 5]. Acetyl CoA and glycerol from adipocytes serve as gluconeogenic substrates. Furthermore, elevated FFA levels promote dyslipidemia due to increased hepatic VLDL triglyceride secretion [49].
As discussed previously, GLUT4 protein levels in human SAT are reduced in T2D, in insulin-resistant obesity and also in FDRs long before diabetes develops [7]. Thus, the very high risk of developing T2D in FDRs is associated with markers of impaired SAT adipogenesis which promotes hypertrophic obesity and ectopic fat accumulation even in non-obese individuals. Having an FDR with T2D is also associated with reduced GLUT4 in adipose cells, which influences the endocrine secretion pattern of these cells.
Anti-inflammatory effects of FAHFAs
In addition to the effects of the PAHSAs to enhance insulin-stimulated glucose uptake in adipocytes and augment glucose-stimulated GLP1 and insulin secretion, they also exert anti-inflammatory effects (Fig. 4). PAHSAs antagonize the pro-inflammatory response induced by lipopolysaccharides in bone marrow-derived dendritic cells and also reduce adipose tissue inflammation in obese mice [36]. These anti-inflammatory effects of PAHSAs may be mediated through GPR120 similar to the anti-inflammatory effects of ω-3 FAs [50, 51].
Because inflammation is involved in many chronic diseases, we investigated the effect of PAHSAs in a mouse model of chemically induced colitis; the results were quite dramatic (Lee J, Moraes-Vieira and Kahn BB, unpublished data). Treating the mice once daily with oral PAHSAs delayed the onset and reduced the severity of colitis. This was associated with reduced –immune cell activation and expression of pro-inflammatory cytokines. Thus, oral PAHSA treatment in this model regulates both the innate and adaptive immune systems to prevent intestinal mucosal damage and protect against colitis.
Conclusions
White adipose tissue is the key regulator of lipid storage and release as well as a large endocrine organ. We have found that individuals with FDRs with T2D, who are at very high risk of developing diabetes, are characterized by inappropriately enlarged adipose cells relative to their BMI (hypertrophic obesity) due to reduced adipogenesis, insulin resistance and adipose tissue inflammation. In addition, adipose GLUT4 levels are reduced in FDRs to the same extent as in adipocytes from individuals with T2D. Glucose uptake by adipose tissue is important for adipocytes to store and (re-)esterify FAs and, thus, reduce circulating FA levels. Elevated FA levels are associated with reduced glucose uptake by peripheral tissues and increased insulin resistance in both skeletal muscles and liver. Furthermore, elevated FA levels promote hepatic lipid accumulation and VLDL triglyceride synthesis and release, contributing to the dyslipidemic phenotype of the metabolic syndrome. Levels of the glucose transporter GLUT4 in adipose cells are negatively correlated with whole-body insulin sensitivity but this is not a direct consequence of the mass of glucose taken up by adipose tissue which only accounts for around 10% of whole-body insulin-stimulated glucose uptake. Instead, GLUT4 expression and glucose uptake are important to induce ChREBP expression and de novo lipogenesis (synthesis of FAs from glucose) and to regulate the release of FAs and other lipids as well as endocrine hormones and cytokines. Low GLUT4 in adipocytes is associated with elevated adipose and serum levels of RBP4 as well as reduced de novo lipogenesis and production of the newly identified lipid family, FAHFAs. These lipids exert several beneficial effects including augmentation of insulin-stimulated glucose uptake in adipocytes and increased glucose-dependent secretion of GLP1 by intestinal L cells and insulin from human islets as well as anti-inflammatory effects (Fig. 4). Levels of FAHFAs are decreased in the subcutaneous adipose tissue and serum in insulin-resistant individuals. The anti-inflammatory effects extend to other diseases in which we showed that oral administration of the lipids attenuates both the innate and adaptive immune systems. As little as 3 days of oral treatment with FAHFAs also reduces adipose tissue inflammation in obese mice. Thus, FAHFAs are potential therapeutic agents and their biosynthetic and degradative pathways are potential targets for treatment of insulin resistance and Type 2 diabetes.
Taken together, adipose tissue and in particular the large SAT depot is not only important for the storage and release of lipids but also as a regulator of whole-body insulin sensitivity.
Acknowledgments
The studies summarized in this paper which were conducted by the authors’ laboratories were supported by grants from the Swedish Research Council, Novo Nordisk Foundation, Torsten Söderberg’s Foundation, the Swedish ALF, the Swedish Diabetes Foundation, the Edgar Sjölund Foundation, NIH/NIDDK (grants R37DK43051, R01DK106210 and R01DK098002) and the JPB Foundation.
Footnotes
Conflict of interest statement
BBK is an inventor on patents regarding RBP4 and FAHFA lipids.
References
- 1.IDF Diabetes Atlas. International Diabetes Federation. 7 Brussels, Belgium: 2015. [Google Scholar]
- 2.Li R, Lu W, Jia J, et al. Relationships between indices of obesity and its cardiovascular comorbidities in a Chinese population. Circ J. 2008;72:973–8. doi: 10.1253/circj.72.973. [DOI] [PubMed] [Google Scholar]
- 3.The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation; Brussels, Belgium: 2005. Apr 14, http://www.idf.org/webdata/docs/Metac_syndrome_def.pdf. [Google Scholar]
- 4.Perry RJ, Camporez JP, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–58. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25:255–62. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001;15:1101–3. [PubMed] [Google Scholar]
- 8.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–62. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Tokunaga K, Shimomura I, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107:239–46. doi: 10.1016/0021-9150(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 11.McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes Care. 2012;35:296–8. doi: 10.2337/dc11-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafson B, Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis. 2015;241:27–35. doi: 10.1016/j.atherosclerosis.2015.04.812. [DOI] [PubMed] [Google Scholar]
- 13.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–47. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24:326–31. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- 16.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–7. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 18.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafson B, Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61:1217–24. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A. 2006;103:13022–7. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson B, Hammarstedt A, Hedjazifar S, et al. BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes. 2015;64:1670–81. doi: 10.2337/db14-1127. [DOI] [PubMed] [Google Scholar]
- 22.Ryden M, Uzunel M, Hard JL, et al. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab. 2015;22:408–17. doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 23.InterAct C, Langenberg C, Sharp SJ, et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med. 2012;9:e1001230. doi: 10.1371/journal.pmed.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cederberg H, Stancakova A, Kuusisto J, Laakso M, Smith U. Family history of type 2 diabetes increases the risk of both obesity and its complications: is type 2 diabetes a disease of inappropriate lipid storage? J Intern Med. 2015;277:540–51. doi: 10.1111/joim.12289. [DOI] [PubMed] [Google Scholar]
- 25.Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS One. 2011;6:e18284. doi: 10.1371/journal.pone.0018284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta JR, Douagi I, Andersson DP, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59:560–70. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]
- 27.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 28.Hammarstedt A, Hedjazifar S, Jenndahl L, et al. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A. 2013;110:2563–8. doi: 10.1073/pnas.1211255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–91. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian SW, Tang Y, Li X, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A. 2013;110:E798–807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidossis LS, Porter C, Saraf MK, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–27. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsen M, Raschke S, Tennagels N, et al. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am J Physiol Cell Physiol. 2014;306:C431–40. doi: 10.1152/ajpcell.00290.2013. [DOI] [PubMed] [Google Scholar]
- 34.Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–45. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 36.Yore MM, Syed I, Moraes-Vieira PM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–32. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awazawa M, Ueki K, Inabe K, et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–12. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Gao H, Fall T, van Dam RM, et al. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes. 2013;62:1338–44. doi: 10.2337/db12-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaghootkar H, Lamina C, Scott RA, et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62:3589–98. doi: 10.2337/db13-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 41.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 42.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19:512–26. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norseen J, Hosooka T, Hammarstedt A, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010–9. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi Q, Yu Z, Ye X, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92:4827–34. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 45.Meisinger C, Ruckert IM, Rathmann W, et al. Retinol-binding protein 4 is associated with prediabetes in adults from the general population: the Cooperative Health Research in the Region of Augsburg (KORA) F4 Study. Diabetes Care. 2011;34:1648–50. doi: 10.2337/dc11-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia. 2008;51:1423–8. doi: 10.1007/s00125-008-1042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–6. [PubMed] [Google Scholar]
- 48.Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–61. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- 49.Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013;274:25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 50.Hara T, Kimura I, Inoue D, Ichimura A, Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev Physiol Biochem Pharmacol. 2013;164:77–116. doi: 10.1007/112_2013_13. [DOI] [PubMed] [Google Scholar]
- 51.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]