Abstract
19F magnetic resonance imaging (MRI) is a powerful noninvasive, sensitive, and accurate molecular imaging technique for early diagnosis of diseases. The major challenge of 19F MRI is signal attenuation caused by the reduced solubility of probes with increased number of fluorine atoms and the restriction of molecular mobility. Herein, we present a versatile one-pot strategy for the fabrication of a multifunctional nanoprobe with high 19F loading (~2.0 × 108 19F atoms per Cu1.75S nanoparticle). Due to the high 19F loading and good molecular mobility that results from the small particle size (20.8 ± 2.0 nm) and ultrathin polymer coating, this nanoprobe demonstrates ultrahigh 19F MRI signal. In vivo tests show that this multifunctional nanoprobe is suitable for 19F MRI and photothermal therapy. This versatile fabrication strategy has also been readily extended to other single-particle nanoprobes for ablation and sensitive multimodal imaging.
Keywords: 19F MRI, photothermal imaging and therapy, multifunctional nanoprobes, copper sulfide nanoparticle
Graphical Abstract
Among many in vivo imaging modalities, magnetic resonance imaging (MRI) is advantageous because it is noninvasive and can provide valuable information about deep tissues in living subjects with high spatial resolution without using radioactivity.1,2 19F MRI is anticipated to be a promising alternative to conventional 1H MRI because of its high sensitivity (0.83 relative to 1H),3–6 negligible background signals,7 and cost-effectiveness, which allows direct detection of 19F-labeled probes such as cells6,8 and drug capsules9 for unambiguous identification and quantification, unlike typical metal-based contrast agents.10,11 In order to obtain highly sensitive 19F MRI probes for their practical implications, it is important to increase the number of fluorine atoms in the MRI probes. Owing to the large number of fluorine atoms, current 19F MRI contrast agents focus on perfluoropolyether (PFPE) in either linear or macrocyclic structures. Unfortunately, the split multiple fluorine signals of linear PFPE may result in low signal intensity and imaging artifacts. In the case of macrocyclic PFPE, the fluorophilic feature makes it insoluble in most solvents. Besides, increasing number of fluorine atoms would result in larger molecular size as well as higher restriction of molecular mobility, which, in turn, causes attenuation of the 19F MRI signal.10,12,13 PFPE has been successfully encapsulated in nanoemulsion as a 19F MRI contrast agent for in vivo imaging.6,14–17 However, the performance of these nanoemulsions is dramatically impaired due to their poor stability, which originates from the molecular diffusion induced particle size expansion. To overcome this limitation, Kikuchi’s group reported a multifunctional 19F MRI nanoprobe with an average size of 76 ± 9 nm by coating the nanoemulsion with a silica shell.12 Another strategy to increase the fluorine content in the probe while maintaining high segmental molecular mobility is to fabricate supramolecular or polymer 19F nanoprobes.2,18,19
In addition, dual-modality probes with a high number of fluorine atoms have also been explored in the past decades.20–23 A facile and robust method has been proposed by loading the fluorous dendron tethered with a near-infrared (NIR) cyanine dye (F27-Cy5.5) into a polymeric nanoparticle with an average size of 130 nm for simultaneous 19F MR imaging and NIR fluorescence imaging.24 The 19F polymeric nanoparticles modified with fluorescent dyes were also used for MRI-optical multimodal imaging.25,26 By attaching the perfluorocarbon (PFC) onto quantum dots and then fabricating into a magneto-fluorescent nanocomposite emulsion with size of 290 nm, 19F MRI and fluorescence dual-modality imaging nanoprobe was achieved.27 Despite the effort, it is still a great challenge to synthesize single-particle multifunctional nanoprobes with high fluorine content, strong 19F MRI signal, small dynamic light scattering (DLS) particle size, good stability, and biocompatibility.
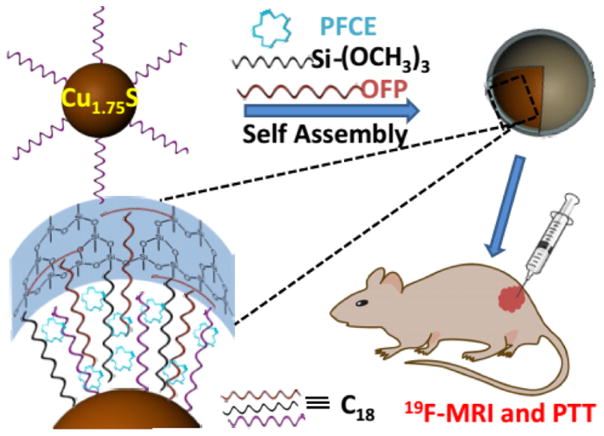
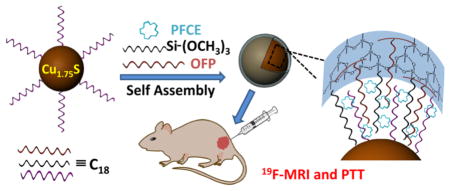
Development of multifunctional platforms for theranostics such as imaging-guided photothermal therapy (PTT) is very active and has attracted much attention in recent years.28–30 Herein, we have rationally designed a multifunctional Cu1.75S–19F@OFP–SiO2 nanoprobe with an average size of 20.8 ± 2.0 nm (DLS), which contains up to ~2.0 × 108 fluorine atoms per particle with ultrahigh 19F MRI signal for simultaneous 19F MRI, photothermal imaging (PTI), and PTT. Briefly, the perfluoro-15-crown-5-ether (PFCE) was anchored to the Cu1.75S nanoparticles (NPs)31–33 through a one-pot encapsulation method by utilizing oleylamine-functionalized polysuccinimide (OFP) and trimethoxy(octadecyl)silane (TMOS) as coencapsulation agents, where the PFCE was anchored through hydrophobic–hydrophobic interactions and then trapped within the silica shell (Scheme 1). These Cu1.75S–19F@OFP–SiO2 nanoprobes are highly stable, biocompatible, and capable of both in vivo imaging (19F MRI and PTI) and photothermal ablation.
Scheme 1.
Illustration Scheme for the Fabrication and Bioapplications of a Multifunctional Cu1.75S–19F@OFP–SiO2 Nanoprobe, Including 19F MRI, PTI, and PTT
RESULTS AND DISCUSSION
TEM and DLS Characterization of Nanoprobes
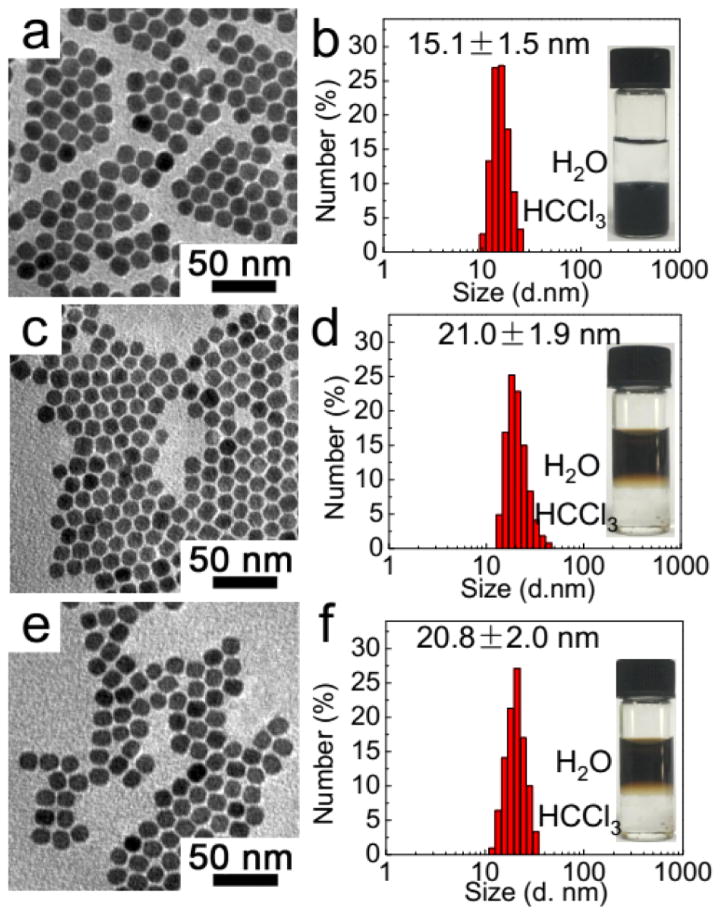
As shown in the transmission electron microscopy (TEM) image (Figure 1a), the as-prepared hydrophobic Cu1.75S NPs, whose crystal structure is confirmed by X-ray diffraction (XRD) (Figure S1), demonstrate uniform shape with an average DLS size of 15.1 ± 1.5 nm (Figure 1b). After being coencapsulated with PFCE using OFP, the Cu1.75S–19F@OFP nanoprobes are well-dispersed with an average DLS diameter of 21.0 ± 1.9 nm (Figure 1c,d). Compared with the hydrophobic Cu1.75S NPs without surface functionalization, these Cu1.75S–19F@OFP nanoprobes are stable in water (insets of Figure 1d,f) and the slight increase in the particle size can be attributed to the coating shell. To avoid leakage of PFCE from nanoprobes, an ultrathin SiO2 shell was introduced by hydrolysis of TMOS, which has no observable effects on the particle shape and size (Figure 1e,f) compared with that of Cu1.75S–19F@OFP (Figure 1c,d).
Figure 1.
TEM images (a,c,e) and DLS size distribution (b,d,f) of hydrophobic Cu1.75S (a,b), Cu1.75S–19F@OFP (c,d), and Cu1.75S–19F@OFP–SiO2 (e,f). Insets of (b,d,e) are the photographs of the nanoparticle colloids before and after surface modification.
FTIR, MRI, and Photothermal Efficiency Characterization of Nanoprobes
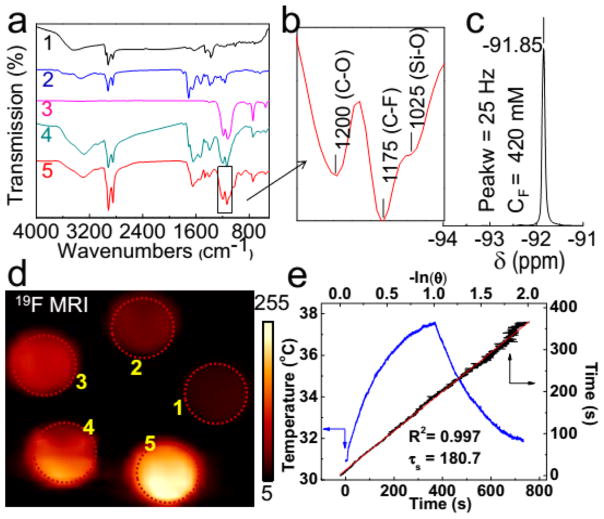
The encapsulation of PFCE and silica coating has been characterized via Fourier transform infrared (FTIR) spectroscopy (Figure 2a,b). The bands at 1175, 1200, and 1025 cm−1 correspond to the C–F, C–O, and Si–O bond vibrations, respectively, indicating the successful fabrication of Cu1.75S–19F@OFP–SiO2 nanoprobes. Additionally, the 19F moieties were confirmed by a 19F NMR spectrum with a single peak at −91.85 ppm (Figure 2c) and a half peak width of ~25 Hz that is narrow enough for MRI applications.6 The preliminary 19F MRI results indicated that stronger signals were observed along with the increase of nanoprobe concentration (Figure 2d). The photothermal profile (Figure 2e) illustrated superior thermal effects of the nanoprobe in aqueous medium under the irradiation of a NIR laser (808 nm). The photothermal conversion efficiency (η) was calculated to be 54.13% (Figure 2e) according to the reported method,32,34 which is comparable to or even higher than most of the reported photothermal agents.35–42
Figure 2.
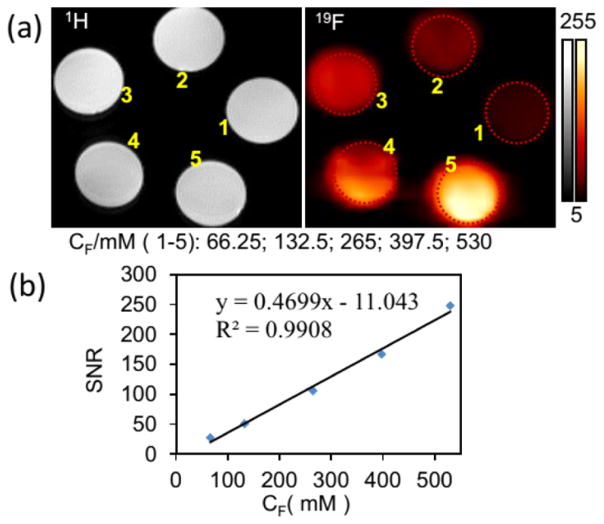
(a) FTIR spectra of hydrophobic Cu1.75S (a1), OFP (a2), PFCE (a3), Cu1.75S–19F@OFP (a4), and Cu1.75S–19F@OFP–SiO2 (a5). (b) Magnification of FTIR spectrum in the box of (a5). (c) 19F NMR spectrum of Cu1.75S–19F@OFP–SiO2 aqueous solution (11.0 mg/mL). (d) 19F MR images of Cu1.75S–19F@OFP–SiO2 colloidal solution with various 19F concentrations (CF). (1–5): 66.25, 132.5, 265, 397.5, 530 mM. (e) Temperature profile (blue) of Cu1.75S colloids (4 mg/mL) irradiated by 808 nm laser (0.45 W/cm2) for 360 s, followed by natural cooling for calculating the photothermal conversion efficiency (η = 54.13%). The linear fitting (red) of time from the cooling period versus negative natural logarithm of driving force temperature.
Optimization of the Nanoprobe Fabrication Method
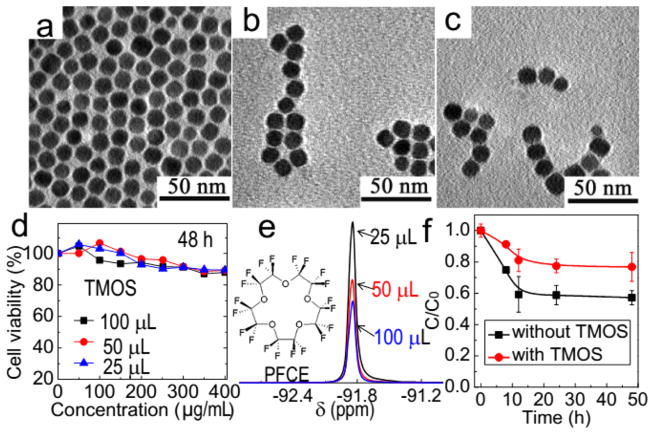
For biomedical applications, the biocompatibility of nanoprobes is of crucial importance. Various approaches have been attempted to improve biocompatibility as well as colloidal stability of our material. Thiolated polyethylene glycol (thiol-PEG), which is frequently used for modification of NPs, was first explored. It was found that monodispersed Cu1.75S–19F@OFP–thiol-PEG NPs could be successfully afforded; yet, the as-prepared NPs were rather cytotoxic (Figure S2). Then, polyvinylpyrrolidone (PVP) was used as a surface ligand to fabricate the Cu1.75S–19F@OFP–PVP NPs. Compared with Cu1.75S–19F@OFP–thiol-PEG NPs, the biocompatibility of Cu1.75S–19F@OFP–PVP NPs was somewhat improved (Figure S3) but still not satisfactory. We presumed that the exposure of nanoparticle surface and thus the leakage of PFCE may account for the cytotoxicity. Therefore, we decided to wrap the nanoparticles with a thin SiO2 shell, which, on the one hand, would maintain the hydrophilic properties of the probes and, on the other hand, anchors the PFCE on the NPs. The Cu1.75S–19F@OFP–SiO2 NPs with a SiO2 shell of different thicknesses were prepared, and the TEM images indicated that all the nanoprobes were well-dispersed in water (Figure 3a–c). As expected, the biocompatibility of the nanoprobes was significantly improved after the introduction of SiO2 coating. Over 90% of the cells remained alive even after incubation with the nanoprobe as concentrated as 400 μg/mL (Figure 3d). All the nanoprobes were purified via dialysis and centrifugation before cytotoxicity tests and bioapplications.
Figure 3.
TEM images of Cu1.75S–19F@OFP–SiO2 NPs prepared with 25 (a), 50 (b), and 100 μL (c) of TMOS. (d) Cytotoxicity tests of Cu1.75S–19F@OFP–SiO2 NPs after incubation with HeLa cell lines for 48 h. (e) 19F NMR spectra of Cu1.75S–19F@OFP–SiO2 prepared with different amounts of TMOS. Inset of (e) is the chemical structure of PFCE. (f) Plots for the PFCE leakage tests via dialysis. C and C0 represent the fluorine concentration in Cu1.75S–19F@OFP–SiO2 colloidal solution after and before dialysis in PBS for different time intervals, respectively.
In addition, little difference on the cytotoxicity of the nanoprobes with SiO2 shells of different thicknesses was observed. Nevertheless, the 19F NMR signals decreased with a thicker silica shell (Figure 3e). It is reasonable since the NMR signals are attenuated as the SiO2 shell gets thicker, which limits the mobility of the encapsulated 19F moieties.10,12 Prior to the bioapplications, we also carried out the 19F leakage test by dialysis in phosphate buffer solution (PBS, pH 7.4) at room temperature. As shown in Figure 3f, if the nanoprobes were encapsulated with a thin layer of silica via the hydrolysis of TMOS (25 μL), <20% of PFCE on the nanoprobes was released within 12 h. During the next 36 h, no obvious leakage was observed. This leakage can be attributed to the release of PFCE absorbed on the outer face of nanoprobes. Therefore, the dialysis is necessary before bioapplications of these nanoprobes. In the absence of TMOS, about 40% of the PFCE was released from the nanoprobes without silica coating within 12 h. Unlike the silica-coated nanoprobes, a continuous PFCE leakage from the nanoprobes without silica coating was observed even after 48 h. All of the results further indicated that the silica coating is crucial to prevent the PFCE leakage and thus reduce the cytotoxicity as well as maintain the strong MRI signal.
Meanwhile, the preparation procedures were optimized by investigating the impact of different factors. Interestingly, it was found that the introduction of PFCE would affect the phase transfer process. In the absence of PFCE, the dosage of NaOH (0.1 M) in the range of 0.4–1.0 mL has no obvious influence on the particle size and shape (Figure S4). However, in the presence of PFCE, the Cu1.75S–19F@OFP NPs will aggregate if the NaOH is less than 1.0 mL. In another aspect, the MR signals are linearly correlated with PFCE dosage, yet such enhancement would meet its limit when the dosage is over 15 μL (Figure S5). Hence, a total volume of 15 μL of PFCE was used for nanoprobe fabrication. It is noted that the fluorine concentration (CF) was calculated by an external standard method, where CF3COONa was used as a standard compound, and the calibration curve was plotted by using CF against the integration area of the MR signal.
To ensure that such an amount of PFCE was enough for 19F MR imaging, phantom studies were performed. As shown in Figure 4, very strong 19F MRI signals were observed from Cu1.75S–19F@OFP–SiO2 colloidal solution (Figure 4a), and the 19F MRI signal intensity was proportional to CF (Figure 4b). Alternatively, the photothermal imaging capability of the nanoprobes was also evaluated. Under the irradiation of 808 nm with a power density of 0.5 W/cm2, the temperature of the Cu1.75S–19F@OFP–SiO2 colloids increased rapidly (Figure S6). In addition, the more dosage of the colloids, the quicker and higher the temperature increased (Figure S6b). To be specific, with 80 μg/mL of nanoprobes, the temperature would increase from 29 to 68 °C within 3 min (Figure S6b). The corresponding photothermal images at different time intervals under different concentrations were also recorded (Figure S6a). All of the results clearly indicated that these multifunctional nanoprobes are applicable for 19F MRI, photothermal imaging, and photothermal ablation.
Figure 4.
(a) 1H and 19F magnetic resonance images of Cu1.75S–19F@OFP–SiO2 colloidal solution with various 19F concentrations (CF). (1–5): 66.25, 132.5, 265, 397.5, 530 mM. (b) Plot of the 19F MRI signal-to-noise ratio (SNR) versus 19F concentration (CF/mM).
In Vivo 19F MRI and Photothermal Therapy Tests
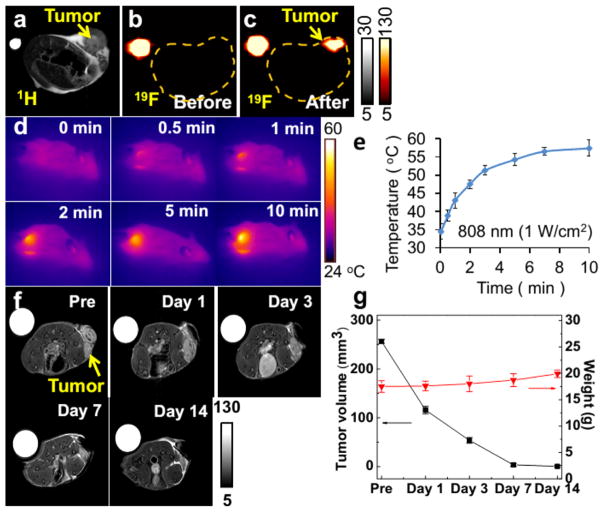
Encouraged by the aforementioned promising results, we explored the possibility of using these nanoprobes for in vivo studies. As shown in Figure 5a, 1H MRI was carried out to show the anatomic structure of the mouse and the profile of the tumor; however, it is hard to clearly delineate the boundary of the tumor from the surrounding normal tissues. As expected, a distinct difference can be observed in 19F MR images before and after administration of Cu1.75S–19F@OFP–SiO2 NPs (Figure 5b,c). Additionally, photothermal properties were also examined by irradiation of tumor xenografted mice with NIR light for different time intervals. The tumor site displays much brighter photothermal images compared with the surrounding medium (Figure 5d). The temperature at the tumor sites quickly increased from 34.5 to 57.4 °C within 10 min (Figure 5e), which is highly desirable for photothermal ablation. After photothermal treatment, the changes of tumors with an initial volume of ~256 mm3 were tracked by 1H MRI. As shown in Figure 5f,g, the tumors have completely disappeared after 2 weeks. In the meantime, the body weight of the mice slightly increased (Figure 5g). As a control, the tumor injected with PBS solution and Cu1.75S–19F@OFP–SiO2 colloids was further treated with and without 808 nm light, respectively. As shown in Figure S7a, after injection with Cu1.75S–19F@OFP–SiO2 NPs but without laser irradiation, the tumor would grow day by day. If the Cu1.75S–19F@OFP–SiO2 NPs were replaced with PBS and then irradiated with 808 nm light (1.0 W/cm2) for 10 min, the tumor size decreased in the next day and then increased step-by-step. Meanwhile, the body weight of mice decreased with the growth of the tumor (Figure S7b). In addition, from Figure S7a, it is clear that although the initial tumor size is smaller than that shown in Figure 5g, it still grew gradually if no NIR irradiation or photothermal agent was introduced. These observations demonstrate that the Cu1.75S–19F@OFP–SiO2 NPs hold great potentials as multifunctional platforms for diagnosis and therapy of tumors.
Figure 5.
In vivo 1H (a) and 19F (b,c) MRI of the tumor-bearing mice before (a,b) and after (c) injection of Cu1.75S–19F@OFP–SiO2 colloidal solution (CF = 530 mM, 100 μL). Photothermal images (d) and temperature evolution profile (e) of the tumor under 808 nm laser irradiation (1.0 W/cm2) for 0–10 min. (f) 1HMRI of the tumor xenografted mice at different days after photothermal treatment. (g) Evolution plots of the tumor size and the mouse body weight after photothermal treatment.
Generality Tests of This Facile Fabrication Strategy
We also explored the possibility of using this facile nanoprobe fabrication strategy for the preparation of other multifunctional nanoprobes such as ZnS:Mn2+ quantum dots (Figure 6a), NaYF4:Yb3+/Er3+ upconversion luminescence nanoparticles (Figure 6b), and Ag2S near-infrared fluorescence quantum dots (Figure 6c). As shown in the TEM images, all three kinds of hydrophobic nanoparticles were successfully encapsulated in the hydrophilic micelle with one particle per micelle, and no aggregation was observed. Meanwhile, the 19F NMR spectra of these nanoprobes demonstrated a symmetric peak at −91.85 ppm, which is very similar to that of the as-prepared Cu1.75S–19F@OFP–SiO2 nanoprobes despite the slight influence of the paramagnetic element. All the results indicate that our nanoprobe fabrication method is a general and facile strategy that can be easily extended to other kinds of multifunctional nanoprobes.
Figure 6.
TEM images with low (a–c) and high magnification (a1–c1) and 19FNMRspectra (a2–c2) of ZnS:Mn2+ (a–a2), NaYF4:Yb3+/Er3+ (b–b2), and Ag2S (c–c2), respectively.
CONCLUSIONS
In conclusion, we have presented the Cu1.75S–19F@OFP–SiO2 nanoprobes with strong 19F MRI signals and small DLS size, which can be applied as 19F MRI and photothermal imaging contrast agents and for photothermal therapy. Preliminary experiments show that the multimodal nanoprobes are highly biocompatible under the conditions tested, which can be attributed to encapsulation of the Cu1.75S core within the biocompatible poly(amino acid) and silica shell. We believe that this new class of multifunctional agents creates the opportunity to significantly improve localized therapy using focused photothermal effects under sensitive 19F MR imaging guidance. The targeted imaging study by grafting specific biological moieties such as peptides is underway. This versatile fabrication strategy can be readily extended to other single-particle nanoprobes for ablation and multimodal imaging such as MRI fluorescence and MRI computer tomography.
EXPERIMENTAL SECTION
Chemicals and Reagents
General chemicals were of the highest grade available (at least analytical grade) and used as received without further purification. Absolute ethanol, methanol, chloroform, dimethyl-formamide (DMF), NaOH, NaH2PO4·2H2O, Na2HPO4·12H2O, and Cu(NO3)2·3H2O were supplied by Beijing Chemical Reagent Company. Polyvinylpyrrolidone (Mw ~ 10 000) was purchased from Aldrich. Oleylamine was obtained from Acros. mPEG-SH (Mw ~ 5000) was purchased from Tianjin Golden Biotechnology Co. Ltd. TMOS and PFCE were obtained from Fluorochem. Ltd. Polysuccinimide (Mw ~ 6000) was supplied by Shijiazhuang Desai Chemical Company (China). Dibutyl dithiocarbamate was supplied by Pacific Ocean United (Beijing) Petro-Chemical Co. Ltd. Diatomite was purchased from Tianjin Fuchen Chemical Reagents Factory. Ultrapure water was made by a Milli-Q water purification system from Millipore (Bedford, MA, USA).
Characterization
19F NMR spectra were measured on a Bruker Avance-III 400 instrument at 376 MHz. The chemical shift is relative to neat CFCl3 (external reference). FTIR spectra were acquired on a Nicolet 670 FTIR spectrophotometer. XRD patterns were measured on a Rigaku SmartLab X-ray diffractometer using Cu Kα radiation (λ = 1.5418 Å) over the range of 2θ from 5 to 90°. TEM images were acquired by using a JEOL JEM-1200EX (200 kV). DLS particle size analysis was carried out using a Zetasizer Nano-ZS90 zeta and size analyzer from Malvern. UV–vis–NIR absorption spectra were acquired on a UV-3600 spectrophotometer (Shimadzu) equipped with a plotter unit over the range of wavelength from 300 to 3300 nm.
Preparation of Cu1.75S Nanoparticles
The hydrophobic Cu1.75S nanoparticles with strong near-infrared localized surface plasmon resonance absorption (Figure S8) were prepared according to our previously developed strategy with some modification.32 Briefly, copper nitrate (Cu(NO3)2·3H2O, 242 mg, 1.0 mmol) and dibutyl dithiocarbamate (200 mg) were dissolved in 2 mL of ethanol under ultrasonication until the mixture became a transparent solution. Oleylamine (20 mL) and 1-octadecene (30 mL) were added into a three-necked flask with nitrogen (N2) purging for 15 min. Thereafter, the mixture was heated to 205 °C with continuous stirring, and the transparent solution prepared above was then injected into the flask quickly and the temperature kept at 190 °C for 15 min. After being cooled to room temperature, the Cu1.75S NPs were collected by precipitation with ethanol, followed by centrifugation for 10 min at the speed of 8000 rpm for two times (the solid was redispersed in cyclohexane before the second time). The final product was dispersed in chloroform (5.0 mL) and stored at 4 °C for further use.
Preparation of OFP
The OFP was synthesized according to our previous method with minor alteration.43 Polysuccinimide (1.6 g) was dissolved in 32 mL of DMF, and the mixture was stirred for 5 h at 90 °C. Then, oleylamine (1.63 mL) was added, and stirring continued for another 5 h at 100 °C. After being cooled to room temperature, the solid product was harvested by precipitation with methanol, followed by centrifugation at a speed of 7000 rpm for 5 min. The final product (OFP) was dispersed in chloroform (8.0 mL).
Preparation of Water-Dispersible Cu1.75S–19F@OFP–SiO2
Cu1.75S NPs (4.0 mg), PVP (2.0 mg), mPEG-SH (3.0 mg), TMOS (25 μL, 22.13 mg), PFCE (28 mg), and OFP (40 mg) were dissolved in 1.0 mL of CHCl3. Then, the mixture was added into NaOH aqueous solution (0.01 M, 10 mL) with ultrasonication treatment (300 W for 6 min, pulsed working as 3 s “on” and 3 s “off”). After the resultant mixture solution changed into an emulsion, the CHCl3 was removed at ambient temperature to get a clear solution which was then centrifuged with water three times at a speed of 18 000 rpm for 15 min. Along with the formation of Cu1.75S–19F@OFP–SiO2, the surface of Cu1.75S NPs was coated with a silica and OFP–COO− layer (the lactam rings were hydrolyzed to carboxylic groups under alkaline condition), which makes the entire nanocomposite water-dispersible. The final product was dispersed in PBS buffer (0.02 M, 1.0 mL) for further imaging experiments. Similarly, for comparison, Cu1.75S–19F@OFP NPs, Cu1.75S–19F@OFP–thiol-PEG NPs, and Cu1.75S–19F@OFP–PVP NPs without SiO2 were also prepared with the above-mentioned method for Cu1.75S–19F@OFP–SiO2 NPs.
19F NMR Measurements
Water-dispersible nanocomposites (Cu1.75S–19F@OFP, Cu1.75S–19F@OFP–SiO2, and Cu1.75S–19F@OFP–thiol-PEG) were prepared in water by using a coaxially D2O-filled capillary tube for locking the field. The external standard CF3COONa was also dissolved in water with D2O in a coaxial capillary tube for the field locking. All of the 19F magnetic resonance spectra were recorded by utilizing a “single-pulse” sequence (a Bruker “zg” sequence) without decoupling of 1H. The major parameters were set as follows: the spectral width was 89 286 Hz; the size of FID was 64 k; the acquisition time was 0.367 s; the number of accumulation was 128; the relaxation delay was 2 s; and the pulse angle was 45°.
MRI Measurements
All the MRI images were taken on a 9.4 T Bruker BioSpec MRI system. The RARE-T2 (rapid acquisition with refocused echoes) method was employed for 1H MRI. The parameters for 1H MRI were set as follows: the number of accumulations was 2, and the repetition time (TR) and the effective time (TE) were 2500 and 3.3 ms, respectively. The field of view was set as 40 mm × 40 mm, and the slice thickness was 1 mm. The matrix size was 256 × 256. Correspondingly, the FLASH method was applied for 19F MRI, and the parameters were as follows: the number of accumulations was 64, and TR and TE were 800 and 1.3 ms, respectively. The field of view was 40 mm × 40 mm with the slice thickness of 10 mm. The matrix size was 32 × 32, and the total accumulation time was approximately 27 min.
Cell Viability
The cytotoxicity of the as-prepared imaging contrast agents, such as Cu1.75S–19F@OFP, Cu1.75S–19F@OFP–SiO2, Cu1.75S–19F@OFP–thiol-PEG, and Cu1.75S–19F@PVP, was evaluated via the methyl thiazolyltetrazolium (MTT) assay against the cultured HeLa cell lines according to previous reports.33,43,44 Briefly, HeLa cells (~5 × 104 cells/well) were seeded in a 96-well microtiter plate, and then different amounts of the aforementioned hydrophilic nanocomposites (calculated with CNPs from 0 to 400 μg/mL) were added and cultured at 37 °C for 24 or 48 h under 5% CO2 and a 95% relative humidity atmosphere, respectively. Thereafter, 10 μL of sterile-filtered MTT stock solution in PBS (4.0 mg/mL) was added to each well. The 96- well microtiter plate was incubated at 37 °C for another 3 h. The absorbance of the soluble colored formazan produced by cellular reduction of MTT (the reduction takes place only when the mitochondrial reductases are active, i.e., the cells are viable) in each well was measured at 490 nm using an ELISA plate reader (F50, TECAN). The larger viable cell number results in the increase in the amount of MTT formazan and the increase in absorbance.
Tumor Model and In Vivo Experimental Procedure for MRI and Photothermal Therapy
Animal experiments were performed using female mice (Balb/c) between 4 and 5 weeks old (18–21 g body weight). The tumor-bearing mice were prepared. First, the 4T1 cells were centrifuged at 1000 rpm for 5 min at 4 °C and resuspended in PBS (pH 7.4) to a final concentration of 1 × 107 cells/mL. Then, approximately 1 × 106 4T1 cells were subcutaneously inoculated on the right leg of mice and allowed to grow for 2 weeks, and typically, a solid tumor formed during this period. Prior to imaging experiments, the mice bearing tumors (size of the tumor was around 250 mm3) were administered 100 μL of Cu1.75S–19F@OFP–SiO2 solution (11 mg/mL in PBS) through intratumor injection. MRI measurements of live mice were carried out using 100 μL of 7% chloral hydrate through intraperitoneal injection for anesthetizing.
Photothermal imaging and therapy experiments were performed on an 808 nm laser instrument (STL808CF-10W). For the in vitro test for the evaluation of the photothermal properties of the as-prepared Cu1.75S–19F@OFP–SiO2 NPs, different concentrations were investigated under 0.5 W/cm2 power density of the irradiation for 10 min. For the in vivo measurements, after administration of 100 μL of Cu1.75S–19F@OFP–SiO2 solution, the tumor-bearing mice were exposed to an 808 nm laser irradiation with power density of 1.0 W/cm2 for 10 min under isoflurane. Then, the changes of the tumor volume and the body weight of the mice were monitored at different days (1, 3, 7, and 14) for PTT.
Supplementary Material
Acknowledgments
This research was supported in part by the National Natural Science Foundation of China (Grant Nos. 21475007, 21275015, and 21271030), the State Key Project of Fundamental Research of China (Grant No. 2011CB932403), and the intramural research program of the National Institute of Biomedical Imaging and Bioengineering. We also thank the support from the “Innovation and Promotion Project of Beijing University of Chemical Technology”, the “Public Hatching Platform for Recruited Talents of Beijing University of Chemical Technology”, the “High-Level Faculty Program of Beijing University of Chemical Technology (buctrc201507, buctrc201325)”, and BUCT Fund for Disciplines Construction and Development (Project No. XK1526).
Footnotes
The authors declare no competing financial interest.
- Rough calculation of 19F atoms on the nanoprobe surface, XRD pattern (Figure S1), TEM images and cytotoxicity tests of Cu1.75S–19F@OFP–thiol-PEG NPs (Figure S2), TEM images and cytotoxicity tests of Cu1.75S–19F@OFP–PVP NPs (Figure S3), TEM images of Cu1.75S@OFP NPs and Cu1.75S–19F@OFP NPs prepared with different amount of NaOH solution (Figure S4), TEM images of Cu1.75S–19F@OFP NPs prepared with various amounts of PFCE and calibration curve for 19F quantitation (Figure S5), photothermal images and temperature evolution profiles (Figure S6), evolution plots of the tumor size (a) and the body weight (b) of mice in control experiments (Figure S7), and UV–vis–NIR absorption spectrum (Figure S8) (PDF)
References
- 1.Chen H, Viel S, Ziarelli F, Peng L. F-19 NMR: A Valuable Tool for Studying Biological Events. Chem Soc Rev. 2013;42:7971–7982. doi: 10.1039/c3cs60129c. [DOI] [PubMed] [Google Scholar]
- 2.Takaoka Y, Sakamoto T, Tsukiji S, Narazaki M, Matsuda T, Tochio H, Shirakawa M, Hamachi I. Self-Assembling Nanoprobes that Display off/on F-19 Nuclear Magnetic Resonance Signals for Protein Detection and Imaging. Nat Chem. 2009;1:557–561. doi: 10.1038/nchem.365. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Ueki R, Nonaka H, Sugihara F, Matsuda T, Sando S. Design of Chemical Shift-Switching F-19 Magnetic Resonance Imaging Probe for Specific Detection of Human Monoamine Oxidase A. J Am Chem Soc. 2011;133:14208–14211. doi: 10.1021/ja2057506. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJM. F-19 MRI for Quantitative in Vivo Cell Tracking. Trends Biotechnol. 2010;28:363–370. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tirotta I, Dichiarante V, Pigliacelli C, Cavallo G, Terraneo G, Bombelli FB, Metrangolo P, Resnati G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem Rev. 2015;115:1106–1129. doi: 10.1021/cr500286d. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens ET, Flores R, Xu HY, Morel PA. In Vivo Imaging Platform for Tracking Immunotherapeutic Cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 7.George C, Chandrakumar N. Chemical-Shift-Resolved F-19 NMR Spectroscopy between 13.5 and 135 MHz: Overhauser-DNP-Enhanced Diagonal Suppressed Correlation Spectroscopy. Angew Chem, Int Ed. 2014;53:8441–8444. doi: 10.1002/anie.201402320. [DOI] [PubMed] [Google Scholar]
- 8.Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T. In Vivo Tracking of Human Neural Stem Cells with F-19 Magnetic Resonance Imaging. PLoS One. 2011;6:e29040. doi: 10.1371/journal.pone.0029040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langereis S, Keupp J, van Velthoven JLJ, de Roos IHC, Burdinski D, Pikkemaat JA, Grull H. A Temperature-Sensitive Liposomal H-1 CEST and F-19 Contrast Agent for MR Image-Guided Drug Delivery. J Am Chem Soc. 2009;131:1380–1381. doi: 10.1021/ja8087532. [DOI] [PubMed] [Google Scholar]
- 10.Takaoka Y, Kiminami K, Mizusawa K, Matsuo K, Narazaki M, Matsuda T, Hamachi I. Systematic Study of Protein Detection Mechanism of Self-Assembling F-19 NMR/MRI Nanoprobes toward Rational Design and Improved Sensitivity. J Am Chem Soc. 2011;133:11725–11731. doi: 10.1021/ja203996c. [DOI] [PubMed] [Google Scholar]
- 11.Mizukami S, Takikawa R, Sugihara F, Hori Y, Tochio H, Walchli M, Shirakawa M, Kikuchi K. Paramagnetic Relaxation-Based F-19 MRI Probe to Detect Protease Activity. J Am Chem Soc. 2008;130:794–795. doi: 10.1021/ja077058z. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita H, Mizukami S, Sugihara F, Nakanishi Y, Yoshioka Y, Kikuchi K. Multifunctional Core-Shell Silica Nanoparticles for Highly Sensitive F-19 Magnetic Resonance Imaging. Angew Chem, Int Ed. 2014;53:1008–1011. doi: 10.1002/anie.201308500. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa D, Taguchi H, Ibrahim NF, Morikawa S, Shiino A, Inubushi T, Hirao K, Shirai N, Sogabe T, Tooyama I. Preferred Features of a Fluorine-19 MRI Probe for Amyloid Detection in the Brain. J Alzheimers Dis. 2014;39:617–631. doi: 10.3233/JAD-131025. [DOI] [PubMed] [Google Scholar]
- 14.Janjic JM, Srinivas M, Kadayakkara DKK, Ahrens ET. Self-Delivering Nanoemulsions for Dual Fluorine-19 MRI and Fluorescence Detection. J Am Chem Soc. 2008;130:2832–2841. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]
- 15.Patrick MJ, Janjic JM, Teng HB, O’Hear MR, Brown CW, Stokum JA, Schmidt BF, Ahrens ET, Waggoner AS. Intracellular pH Measurements Using Perfluorocarbon Nanoemulsions. J Am Chem Soc. 2013;135:18445–18457. doi: 10.1021/ja407573m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu LZ, Chen JJ, Yang XX, Caruthers SD, Lanza GM, Wickline SA. Rapid Quantification of Oxygen Tension in Blood Flow with A Fluorine Nanoparticle Reporter and A Novel Blood Flow-Enhanced-Saturation-Recovery Sequence. Magn Reson Med. 2013;70:176–183. doi: 10.1002/mrm.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Hanlon CE, Amede KG, O’Hear MR, Janjic JM. NIR-Labeled Perfluoropolyether Nanoemulsions for Drug Delivery and Imaging. J Fluorine Chem. 2012;137:27–33. doi: 10.1016/j.jfluchem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurecht KJ, Blakey I, Peng H, Squires O, Hsu S, Alexander C, Whittaker AK. Functional Hyperbranched Polymers: Toward Targeted in Vivo F-19 Magnetic Resonance Imaging Using Designed Macromolecules. J Am Chem Soc. 2010;132:5336–5337. doi: 10.1021/ja100252y. [DOI] [PubMed] [Google Scholar]
- 19.Huang XN, Huang G, Zhang SR, Sagiyama K, Togao O, Ma XP, Wang YG, Li Y, Soesbe TC, Sumer BD, Takahashi M, Sherry AD, Gao JM. Multi-Chromatic pH-Activatable F-19-MRI Nanoprobes with Binary ON/OFF pH Transitions and Chemical-Shift Barcodes. Angew Chem, Int Ed. 2013;52:8074–8078. doi: 10.1002/anie.201301135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Aburto R, Narayanan TN, Nagaoka Y, Hasumura T, Mitcham TM, Fukuda T, Cox PJ, Bouchard RR, Maekawa T, Kumar DS, Torti SV, Mani SA, Ajayan PM. Fluorinated Graphene Oxide; a New Multimodal Material for Biological Applications. Adv Mater. 2013;25:5632–5637. doi: 10.1002/adma201301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu YH. The First Magnetic-Nanoparticle-Free Carbon-Based Contrast Agent of Magnetic-Resonance Imaging-Fluorinated Graphene Oxide. Small. 2014;10:1451–1452. doi: 10.1002/smll.201303644. [DOI] [PubMed] [Google Scholar]
- 22.Boccalon M, Franchi P, Lucarini M, Delgado JJ, Sousa F, Stellacci F, Zucca I, Scotti A, Spreafico R, Pengo P, Pasquato L. Gold Nanoparticles Protected by Fluorinated Ligands for F-19 MRI. Chem Commun. 2013;49:8794–8796. doi: 10.1039/c3cc44572k. [DOI] [PubMed] [Google Scholar]
- 23.Chen SZ, Yang YQ, Li HD, Zhou X, Liu ML. pH-Triggered Au-Fluorescent Mesoporous Silica Nanoparticles for F-19 MR/Fluorescent Multimodal Cancer Cellular Imaging. Chem Commun. 2014;50:283–285. doi: 10.1039/c3cc47324d. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Yue XY, Wang Y, Qian CQ, Huang P, Lizak M, Niu G, Wang F, Rong PF, Kiesewetter DO, Ma Y, Chen XY. A Symmetrical Fluorous Dendron-Cyanine Dye-Conjugated Bimodal Nanoprobe for Quantitative F-19 MRI and NIR Fluorescence Bioimaging. Adv Healthcare Mater. 2014;3:1326–1333. doi: 10.1002/adhm.201400088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolfe BE, Blakey I, Squires O, Peng H, Boase NRB, Alexander C, Parsons PG, Boyle GM, Whittaker AK, Thurecht KJ. Multimodal Polymer Nanoparticles with Combined F-19 Magnetic Resonance and Optical Detection for Tunable, Targeted, Multimodal Imaging in Vivo. J Am Chem Soc. 2014;136:2413–2419. doi: 10.1021/ja410351h. [DOI] [PubMed] [Google Scholar]
- 26.Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Dual-Function Probe to Detect Protease Activity for Fluorescence Measurement and F-19 MRI. Angew Chem, Int Ed. 2009;48:3641–3643. doi: 10.1002/anie.200806328. [DOI] [PubMed] [Google Scholar]
- 27.Lim YT, Noh YW, Cho JH, Han JH, Choi BS, Kwon J, Hong KS, Gokarna A, Cho YH, Chung BH. Multiplexed Imaging of Therapeutic Cells with Multispectrally Encoded Magneto-fluorescent Nanocomposite Emulsions. J Am Chem Soc. 2009;131:17145–17154. doi: 10.1021/ja904472z. [DOI] [PubMed] [Google Scholar]
- 28.Liang C, Diao S, Wang C, Gong H, Liu T, Hong GS, Shi XZ, Dai HJ, Liu Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv Mater. 2014;26:5646–5652. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 29.Song XJ, Gong H, Yin SN, Cheng L, Wang C, Li ZW, Li YG, Wang XY, Liu G, Liu Z. Ultra- Small Iron Oxide Doped Polypyrrole Nanoparticles for In Vivo Multimodal Imaging Guided Photothermal Therapy. Adv Funct Mater. 2014;24:1194–1201. [Google Scholar]
- 30.Cheng L, Yang K, Li YG, Chen JH, Wang C, Shao MW, Lee ST, Liu Z. Facile Preparation of Multifunctional Upconversion Nanoprobes for Multimodal Imaging and Dual-Targeted Photothermal Therapy. Angew Chem, Int Ed. 2011;50:7385–7390. doi: 10.1002/anie.201101447. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Liu J, He Q, Chen HL, Cui JB, Xu SY, Zhao YL, Chen CY, Wang LY. Smart Cu1.75S Nanocapsules with High and Stable Photothermal Efficiency for NIR Photo-Triggered Drug Release. Nano Res. 2015;8:4038–4047. [Google Scholar]
- 32.Cui JB, Xu SY, Guo C, Jiang R, James TD, Wang LY. Highly Efficient Photothermal Semiconductor Nanocomposites for Photothermal Imaging of Latent Fingerprints. Anal Chem. 2015;87:11592–11598. doi: 10.1021/acs.analchem.5b03652. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Peng S, Li YB, Cui JB, Chen HL, Wang LY. Development of NIR-II Fluorescence Image-Guided and pH-Responsive Nanocapsules for Cocktail Drug Delivery. Nano Res. 2015;8:1932–1943. [Google Scholar]
- 34.Roper DK, Ahn W, Hoepfner M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J Phys Chem C. 2007;111:3636–3641. doi: 10.1021/jp064341w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou SS, Kaehr B, Kim J, Foley BM, De M, Hopkins PE, Huang J, Brinker CJ, Dravid VP. Chemically Exfoliated MoS2 as Near-Infrared Photothermal Agents. Angew Chem, Int Ed. 2013;52:4160–4164. doi: 10.1002/anie.201209229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, Korgel BA. Copper Selenide Nanocrystals for Photothermal Therapy. Nano Lett. 2011;11:2560–2566. doi: 10.1021/nl201400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu HY, Chen D, Li LL, Liu TL, Tan LF, Wu XL, Tang FQ. Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angew Chem, Int Ed. 2011;50:891–895. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- 38.Lv RC, Yang PP, He F, Gai SL, Yang GX, Lin J. Hollow Structured Y2O3:Yb/Er-CuxS Nanospheres with Controllable Size for Simultaneous Chemo/Photothermal Therapy and Bioimaging. Chem Mater. 2015;27:483–496. [Google Scholar]
- 39.Tian QW, Hu JQ, Zhu YH, Zou RJ, Chen ZG, Yang SP, Li RW, Su QQ, Han Y, Liu XG. Sub-10 nm Fe3O4@Cu2-xS Core-Shell Nanoparticles for Dual-Modal Imaging and Photothermal Therapy. J Am Chem Soc. 2013;135:8571–8577. doi: 10.1021/ja4013497. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZJ, Wang J, Nie X, Wen T, Ji YL, Wu XC, Zhao YL, Chen CY. Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. J Am Chem Soc. 2014;136:7317–7326. doi: 10.1021/ja412735p. [DOI] [PubMed] [Google Scholar]
- 41.Cheng L, Yang K, Chen Q, Liu Z. Organic Stealth Nanoparticles for Highly Effective in Vivo Near-Infrared Photothermal Therapy of Cancer. ACS Nano. 2012;6:5605–5613. doi: 10.1021/nn301539m. [DOI] [PubMed] [Google Scholar]
- 42.Tian QW, Jiang FR, Zou RJ, Liu Q, Chen ZG, Zhu MF, Yang SP, Wang JL, Wang JH, Hu JQ. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25. 7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano. 2011;5:9761–9771. doi: 10.1021/nn203293t. [DOI] [PubMed] [Google Scholar]
- 43.Huang S, Bai M, Wang LY. General and Facile Surface Functionalization of Hydrophobic Nanocrystals with Poly(amino acid) for Cell Luminescence Imaging. Sci Rep. 2013;3:2023–2027. doi: 10.1038/srep02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng ML, Wang LY. Unexpected Luminescence Enhancement of Upconverting Nanocrystals by Cation Exchange with Well Retained Small Particle Size. Nano Res. 2014;7:782–793. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.