Abstract
Background
Adolescents are physically, cognitively, socially, and emotionally different than adults in ways that may partially explain why alcohol misuse typically develops during this period. Ample animal-science evidence and nascent ecological evidence points toward developmentally limited differences in sensitivity to alcohol’s stimulatory and sedative effects. Field-based research methods were used to test for such age-related differences in a sample of adolescents through young adults. Potential moderating influences of estimated blood alcohol content (eBAC), as well as typical consumption and level of dependence/consequences were explored.
Methods
Subjective alcohol responses were collected from 1,364 participants, aged 17 to 32 years, recruited outside of venues where drinking takes place in a small metropolitan bar district.
Results
Self-reports of stimulatory response to alcohol were age-related, such that younger participants reported increased subjective stimulation at the time of data collection relative to older participants. Age-related differences in stimulatory responses were more pronounced at lower eBACs and among younger participants who typically drank more heavily. Stimulatory responses generally diminished among older than younger participants, although individuals with greater dependence/consequences consistently reported greater stimulation from drinking. Contrastingly, age, typical consumption, and dependence/consequences were not related to sedation in this sample.
Conclusions
This research provides cross-sectional evidence to support age-, consumption-, and dependence/consequences-related differences in stimulatory alcohol responses among adolescents and young adults assessed within a bar-area context. While cross-sectional, the results of this field-based study provide support for the theory that addiction liability is developmentally linked and associated, in part, with age-related differences in subjective alcohol responses.
Keywords: Adolescent, Alcohol, Field-based Study, Subjective Responses, Alcohol Problems, Stimulation
1. INTRODUCTION
Drinking escalates dramatically during adolescence, and alcohol use disorder (AUD) typically emerges prior to the legal drinking age (Behrendt et al., 2009; Hingson et al., 2006; Johnston, 2015; Windle et al., 2009). Despite the critical importance of the adolescent years for the development of AUD, subjective responses to alcohol’s effects (e.g., stimulation, sedation), which are chief risk factors in contemporary theories of addiction (Kassel et al., 2007; Ray et al., 2010; Sher et al., 2005; Volkow et al., 2016), are rarely directly studied in human adolescents due to restrictions on administration of alcohol to underage drinkers. The present study leveraged field-based data collected in a naturalistic, bar-area setting to test age-related hypotheses regarding alcohol’s effects in a sample spanning adolescence through early adulthood. Because adolescence is a key formative period for AUD, bridging this gap is essential for informing developmental models of alcoholism and advancing early interventions.
1.1. Level of alcohol response
Level of response (LR) to alcohol’s effects is one of the most extensively-studied phenotypes reflecting a genetic predisposition to AUD (Schuckit, 1980, 1984; Schuckit and Smith, 1996, 2011, 2013). Reviews of alcohol-challenge studies in humans support a low LR model, where lower responses to alcohol’s sedative effects confer risk for AUD (Schuckit, 2014), but also a “differentiator” model that distinguishes between rising and falling BrACs (Morean and Corbin, 2010; Newlin and Renton, 2010; Newlin and Thomson, 1990; Quinn and Fromme, 2011). A recent human laboratory study by King and colleagues (2014, 2011) supports a “modified differentiator model”, finding that heavy drinkers who reported greater stimulatory effects and lower sedative effects at peak BrAC experienced more symptoms of alcohol pathology through 6 years of follow-up. Further, re-examination of subjective alcohol responses in this sample demonstrated that initial differences in alcohol sensitivity among heavy drinkers did not diminish over time as AUD symptoms developed (King et al., 2016), which may mark the early stages of addiction in accordance with an allostatic model of AUD development (Volkow et al., 2016).
Regardless of the specific predictions, all LR theories are founded in the idea that early responses to alcohol mark risk for developing AUD. However, current evidence for LR theories (in humans) is primarily based on retrospective reports of early drinking experiences or alcohol challenge studies with adult drinkers. Yet, retrospection may be biased for drinkers with many years of drinking experience (de Wit and Phillips, 2012). For example, in a recent 25-year longitudinal study of male drinkers, retrospective reports of early alcohol effects were more strongly related to problems as participants aged, suggesting that reporting of early drinking effects may be influenced by life experience (Schuckit and Smith, 2013). Thus, subjective effects of alcohol need to be studied as early in the drinking history as possible (Schuckit, 2014).
1.2. Adolescent alcohol responses
Adolescents’ responses to alcohol may differ from adults in ways that contribute to the prototypical heavy drinking during this developmental period (Crabbe et al.,, 2010; de Wit and Phillips, 2012; Schramm-Sapyta et al., 2009; Spear, 2011a). Animal analogues have shown that adolescent rats and mice from outbred strains not only typically drink 2–3 times more alcohol than do adult rats, but are less sensitive to the aversive, sedative and, and motor impairing effects of alcohol while showing greater sensitivity to alcohol’s stimulatory and social-facilitating effects than adults (Quoilin et al., 2010; Spear, 2011b). These alcohol sensitivities often persist into adulthood after chronic alcohol exposure during adolescence in rodents (see Spear and Swartzwelder, 2014, for review), perhaps contributing to the greater propensity for high levels of alcohol use in adulthood after adolescent alcohol exposure (Spear and Varlinskaya, 2010; Windle et al., 2009).
Few studies have directly examined alcohol’s effects in human adolescents. The first and only human laboratory study, published nearly 35 years ago, evaluated risk biomarkers following a moderate dose of alcohol that produced blood alcohol concentrations (BAC) of approximately 0.04 mg/ml among 22 alcohol-naïve boys aged eight to 15 years. These youth reached peak breath alcohol levels at a faster rate than is typical of adults administered a similar alcohol dose, while also showing smaller behavioral changes than anticipated given their BAC (Behar et al., 1983). A recent ecological study assessed dose-related changes in subjective ratings of stimulation and sedation on the ascending limb of intoxication using handheld wireless devices among a small sample of 29 adolescents aged 15 to 19 (Miranda et al., 2014). Youth were instructed to record subjective stimulatory and sedative states just before beginning to drink as well as their subjective responses following each of the first three standard drinks of a drinking episode, which produced an average estimated BAC (eBAC) equivalent to the Behar study (.04 mg/ml). Responses for this adolescent sample were compared to an additional sample of 36 adult drinkers (aged 24 to 64 years) from a separate study implementing a similar protocol. Although adolescents experienced decreases in stimulation as eBAC increased, overall their stimulatory response was greater than that of their adult counterparts at low to moderate eBACs.
1.3. Field-based Investigations of Alcohol Use
Field-based research methods have historically tested the ecological validity of findings from experimental studies of acute intoxication, and examined phenomena that cannot easily be reproduced in laboratory settings (Clapp et al., 2007; Johnson et al., 2006). For example, field-based alcohol research has provided new insights on individual and environmental predictors of drinking (e.g., Clapp et al., 2008; Clapp et al., 2008; Thombs et al., 2009) and on the effects of alcohol use on cognitive performance among underage drinkers (i.e., age 18–20; Day et al., 2013). Although studies conducted in the natural environment sacrifice some experimental control, this limitation is mitigated by the unique opportunity to observe acute alcohol consumption at levels well above what is permissible in laboratory administration paradigms and, perhaps more importantly, to observe this phenomenon during a pivotal window in the development of AUD among underage drinkers.
1.4. Hypotheses
The present field-based study provides a cross-sectional test of age differences in subjective responses to alcohol, namely stimulatory and sedative effects. We expected that younger age would be associated with greater self-reported stimulation and lesser sedation among adolescents and young adults recruited in an ecologically valid setting and in the context of a natural drinking episode. Previous ecological momentary assessment (EMA) research found that adolescents experience greater stimulation while drinking in the natural environment, relative to adults, but this effect diminished at higher estimated eBACs (Miranda et al., 2014). Therefore, we also tested for interactive effects of age and eBAC, and expected that the relation of age to stimulatory responses to alcohol would be attenuated at higher eBACs. Given the theoretical link between the development of drinking problems and AUD to enhanced stimulatory and blunted sedative subjective responses, we also expected that greater typical alcohol consumption and a higher degree of alcohol dependence/consequences would be associated with greater self-reported stimulatory alcohol responses and lesser sedative responses. We also tested for interactive effects of typical alcohol consumption and alcohol dependence symptoms/consequences with eBAC. Interactive effects of typical alcohol consumption and dependence/consequences with age and gender were also explored.
2. MATERIALS AND METHODS
2.1. Setting and Participants
Data were collected outside of venues where alcohol is served within the downtown bar district in Binghamton, NY, which is a small metropolitan city in the southern tier of New York State. Specifically, this area comprises eight bars within a city block (see Figure 1). Prior studies report on a subset of the current sample for which neuropsychological testing (Celio et al., 2014) and additional online survey data (Usala et al., 2015) were available. The present report utilizes the complete survey sample (N = 1904). Sixty-one cases (3.2%) were removed due to invalid answering, defined as a systematic pattern of marked deviation from survey response options. An additional 64 cases (3.5%) were removed due to missing values on both outcomes. Participants older than 32 years of age were outliers in this sample (> 3 SD above the mean age) and were also removed (2.4%). Finally, samples with estimated BACs of ≤ 0 (see section 2.3) were also eliminated (3.7%).
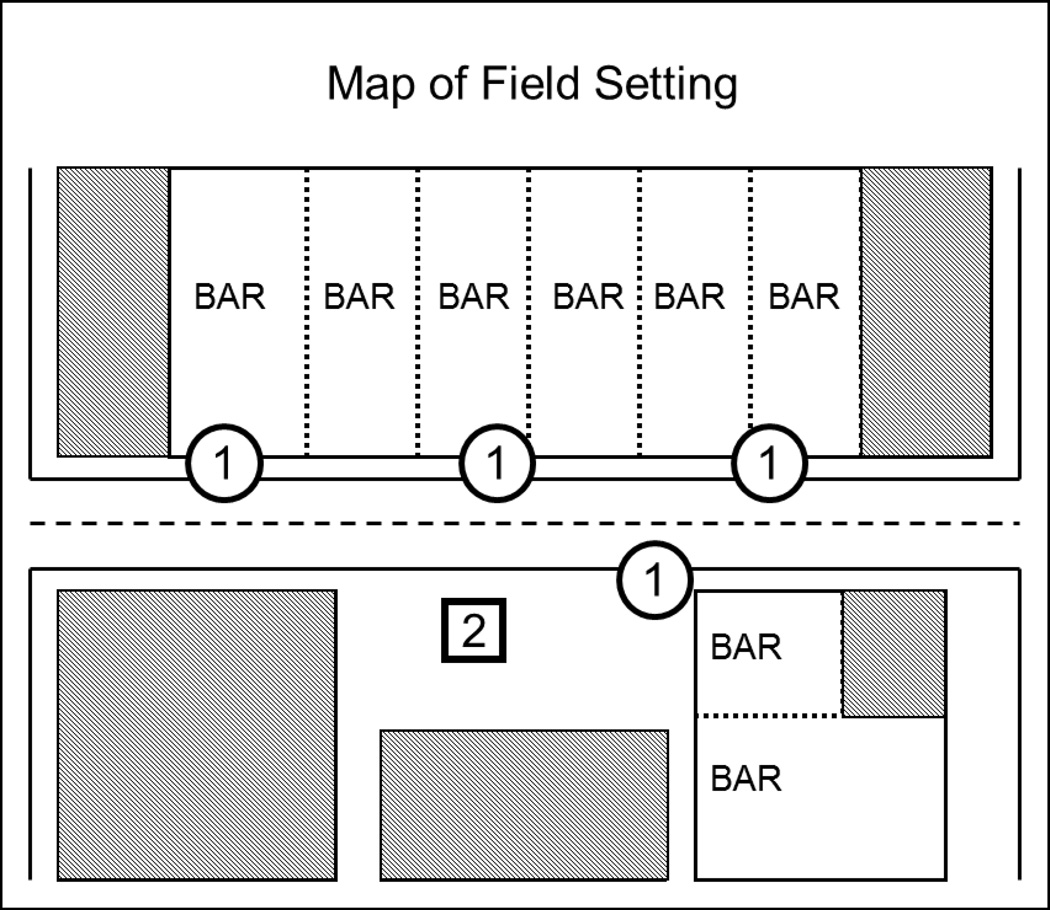
Figure 1.
Overhead map of recruitment area. Circles labeled “1” represent the sidewalk areas where research assistants recruited participants and conducted the consent, interview, and survey. The square labeled “2” represents the research station where neuropsychological testing and other assessments not included in the present analyses were conducted.
2.2. Procedures
All study procedures were approved by the university institutional review board, and additional permission was obtained from the city government and local law enforcement. Recruitment and data collection took place between 11:00 pm and 2:30 am on Thursday and Friday nights. Each night, the research team comprised 8 to 12 research assistants (RAs) supervised by a project director. Recruitment was conducted in groups, with three or four RAs per group. All RAs were trained to approach potential participants outside of drinking venues and deliver a brief informational statement about the purpose of the study and the procedures involved. During this process, RAs observed whether individuals displayed overt symptoms of severe intoxication (e.g., unsteady gait, balance impairment, markedly slurred and/or incoherent speech). As is common in field-based alcohol research (e.g., Thombs et al., 2009), such individuals were not invited to participate due to concerns about ability to provide informed consent and to complete the basic elements of the protocol (e.g., answering questions in interview format, completing a paper and pencil survey while standing). Further, individuals were asked if they had completed our study on a previous night, and those who had were not invited to participate. After providing informed consent [we obtained a waiver of written consent to protect participant anonymity; one’s signature would have constituted the only personally identifiable information gathered during this protocol. Questions specifically related to protocol development or IRB review for this study may be directed to Mark Celio, (mark_celio@brown.edu).] participants completed a semi-structured interview and a paper-and-pencil survey that collectively took six to eight minutes to complete. Participants were not compensated for taking part in this brief survey study.
2.3. Measures
During the semi-structured interview, participants indicated what time they started drinking to determine the duration of the most recent drinking episode. Participants also indicated the number of standard drinks they consumed. A standard drink was explicitly defined as a 12-ounce serving of beer, 5-ounce serving of wine, or a 1.5-ounce serving of liquor (National Institute on Alcohol Abuse and Alcoholism, 2016). Demographics (i.e., age, gender, weight) were also assessed and used to calculate eBACs using the following equation developed by Mathews and Miller (1979):
eBAC = [c/2)*(GC/w)]−(β60*t)
Specifically, t represents time (in hours) from the start of the drinking episode until the time of assessment, c represents the number of standard drinks consumed, GC represents a gender constant (9.0 for females, 7.5 for males), w represents weight in pounds, and β60 represents the metabolism rate of alcohol per hour (0.017 g/dl). This equation outperforms other approaches in terms of accuracy in estimating actual breath alcohol concentration after an uncontrolled episode of drinking (Hustad and Carey, 2005). Depending on the values for time spent drinking and drinks consumed, this calculation strategy can return negative estimates of BAC. Negative values may be recoded as 0.000 or removed from analyses. Given our focus on subjective responses to alcohol, participants with eBAC values that were less than or equal to 0.000 were removed from all analyses. Additionally, atypically high eBAC values were windsorized at the 95th percentile.
The Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) assessed the degree to which participants engaged in harmful/problematic drinking during the past 12 months. Research supports the AUDIT as a valid means of screening for alcohol dependence/consequences (for review see Reinert and Allen, 2007) under test circumstances where a structured interview or other formal diagnostic measure is not feasible due to time constraints. This questionnaire consists of 10 items, with higher scores indicative of more problematic drinking. Although the total score is often used as an index of harmful/problematic drinking, research suggests the AUDIT can be divided into two distinct subscales (Doyle et al., 2007; Maisto et al., 2000; von der Pahlen et al., 2008). The first subscale (termed “AUDIT consumption”) includes AUDIT items 1–3 assessing frequency of drinking, typical quantity, and frequency of heavy drinking. The second subscale (termed “dependence/consequences”) provides an index of alcohol dependence symptoms and related consequences and includes AUDIT items 4–10 assessing impaired control over drinking, increased salience of drinking, morning drinking, guilt after drinking, blackouts, alcohol-related injuries, and others’ concerns about drinking. Subjective ratings of alcohol-induced stimulant and sedative effects were assessed using a modified version of the 14-item Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993). Due to the time limitations inherent in field-based research, we administered three stimulant items (“excited,” “talkative,” and “up”) and three sedative items (“inactive,” “sedated,” and “sluggish”). These items were derived through pilot testing using the 14-item BAES in the same target setting and population. Specifically, principal component factor analysis identified the three stimulant and three sedative items with the highest factor loadings. Although the brief BAES was not available at the time our study was designed, four out of the six resulting items overlap with those items included in this measure (Rueger et al., 2009). Participants were asked “to what extent has drinking produced each of these feelings right now?” Each item was scored on an 11-point scale anchored by 0 indicating “not at all” and 10 indicating “extremely.” A mean score was calculated separately for stimulant and sedative effects.
2.4. Analytic Plan
Multiple linear regression analyses with simultaneous entry and listwise deletion were used to test the hypotheses that younger age, AUDIT consumption scores, and AUDIT alcohol dependence/consequences scores would be associated with greater self-reported stimulation and lesser sedation. Gender was included as an a priori covariate. Interactive effects with eBAC were explored using a backward model-building approach, with nonsignificant higher order interactive effects removed until a final model was reached. Age was treated as a continuous variable, but explored as a three-category grouping variable for descriptive and graphical purposes. To facilitate tests of interactive effects, all continuous variables were centered at the grand mean prior to entry. To correct for tests of three focal independent variables (i.e., age, AUDIT consumption, AUDIT dependence/consequences), the significance level was specified as p < .017.
3. RESULTS
The final sample with data for all focal variables included 1364 participants. Participants were ages 17 to 32 years (M = 21.0, SD =2.2), 40.2% women, and the majority were White, non-Hispanic (79.5%) college students (85.0%). Overall, this was a heavy drinking sample, with the average eBAC at the time of testing well above the legal limit (i.e., 0.124 mg/ml; range = .001 to .288) and average AUDIT total score of 13.28 (SD = 6.73), which is more than 50% higher than the recommended cutoff for hazardous drinking (i.e., AUDIT total score ≥8). The majority of participants reported consumption of alcohol within the past hour (96.3%).
Descriptive analyses stratified by three age groupings are shown in Table 1. The first group reflects underage drinkers (ages 17 to 20 years), the second extends into the mid-twenties (ages 21 to 24 years), and the third into the early thirties (ages 25 to 32 years). Linear, univariate associations of study variables were explored continuously through zero-order correlation analyses shown in Table 2. Finally, Table 3 shows results of multiple linear regression analyses with age, AUDIT typical consumption and dependence/consequences subscales, gender, and eBAC entered simultaneously, with multiple measures predicting (often differentially) stimulatory (left panel) versus sedative (right panel) responses to alcohol.
Table 1.
Participant Characteristics Stratified by Age
| Ages 17 to 20 n = 558 |
Ages 21 to 24 n = 710 |
Ages 25 to 32 n = 96 |
Full Sample n = 1364 |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable |
Mean or % |
SD |
Mean or % |
SD |
Mean or % |
SD |
Mean or % |
SD |
| Age | 19.26 | 0.76 | 21.56 | 0.84 | 27.08 | 2.17 | 21.01 | 2.22 |
| % female | 46.6 | 36.3 | 32.3 | 40.2 | ||||
| % White, non-Hispanic | 79.9 | 78.0 | 87.5 | 79.5 | ||||
| % Current student | 96.4 | 83.1 | 33.3 | 85.0 | ||||
| Estimated BAC | .135 | .076 | .120 | .076 | .099 | .074 | .124 | .077 |
| Time spent drinking (in hours) | 2.79 | 1.62 | 3.09 | 1.97 | 3.23 | 1.90 | 2.98 | 1.84 |
| AUDIT Consumption | 7.57 | 2.31 | 7.40 | 2.43 | 6.46 | 3.52 | 7.40 | 2.49 |
| AUDIT Dependence/consequences | 6.19 | 5.52 | 5.79 | 4.93 | 4.74 | 4.93 | 5.88 | 5.19 |
| Stimulatory effects | 7.22 | 2.19 | 7.19 | 2.16 | 5.94 | 2.75 | 7.12 | 2.24 |
| Sedative effects | 2.50 | 2.21 | 2.29 | 2.21 | 2.40 | 2.63 | 2.385 | 2.24 |
Note. SD = standard deviation.
Table 2.
Zero-order Correlations of Putative Predictors with Stimulatory and Sedative Effects
| Variable | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|
| 1. Age | ||||||
| 2. Female gender | − .12*** | |||||
| 3. Estimated BAC | − .14*** | .05* | ||||
| 4. Consumption | − .10*** | − .31*** | .32*** | |||
| 5. Dependence/consequences | − .06* | − .12*** | .19*** | .47*** | ||
| 6. Stimulatory effects | − .14*** | .05 | .28*** | .23*** | .18*** | |
| 7. Sedative effects | − .02 | − .08** | − .02 | .01 | .10*** | .00 |
Note. Consumption and Dependence/consequences reflect subscale scores of the AUDIT.
p < .05,
p < .001,
p < .001.
Table 3.
Final Multiple Linear Regression Models for Responses to Alcohol
| Stimulatory effects | Sedative effects | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | β | b | SE | p | β | b | SE | p |
| Age | − .06 | − .06 | .03 | .019 | − .03 | − .03 | .03 | .259 |
| Female gender | .09 | .40 | .13 | .002 | − .09 | − .40 | .13 | .003 |
| Estimated BAC | .20 | 5.77 | .80 | < .001 | − .02 | − .69 | .85 | .416 |
| AUDIT Consumption | .17 | .15 | .03 | < .001 | − .07 | − .06 | .03 | .037 |
| AUDIT Dependence/consequences | .08 | .04 | .01 | .004 | .13 | .06 | .01 | < .001 |
| Age × eBAC | .08 | 1.06 | .36 | .003 | ― | ― | ― | ― |
| Age × Consumption | − .12 | − .03 | .01 | < .001 | ― | ― | ― | ― |
| Age × Dependence/consequences | .08 | .02 | .01 | .009 | ― | ― | ― | ― |
Note. b = unstandardized beta coefficient. eBAC = estimated blood alcohol concentration. All continuous predictors were centered prior to entry. The pattern of significant results was not altered with the addition of race or current student status. Interactive effects with gender were not significant and were removed from final models. For sedative effects, all interactive effects were non-significant and were removed from final models. Bonferroni correction for multiple tests (3 focal independent variables) would require p < .017.
Age
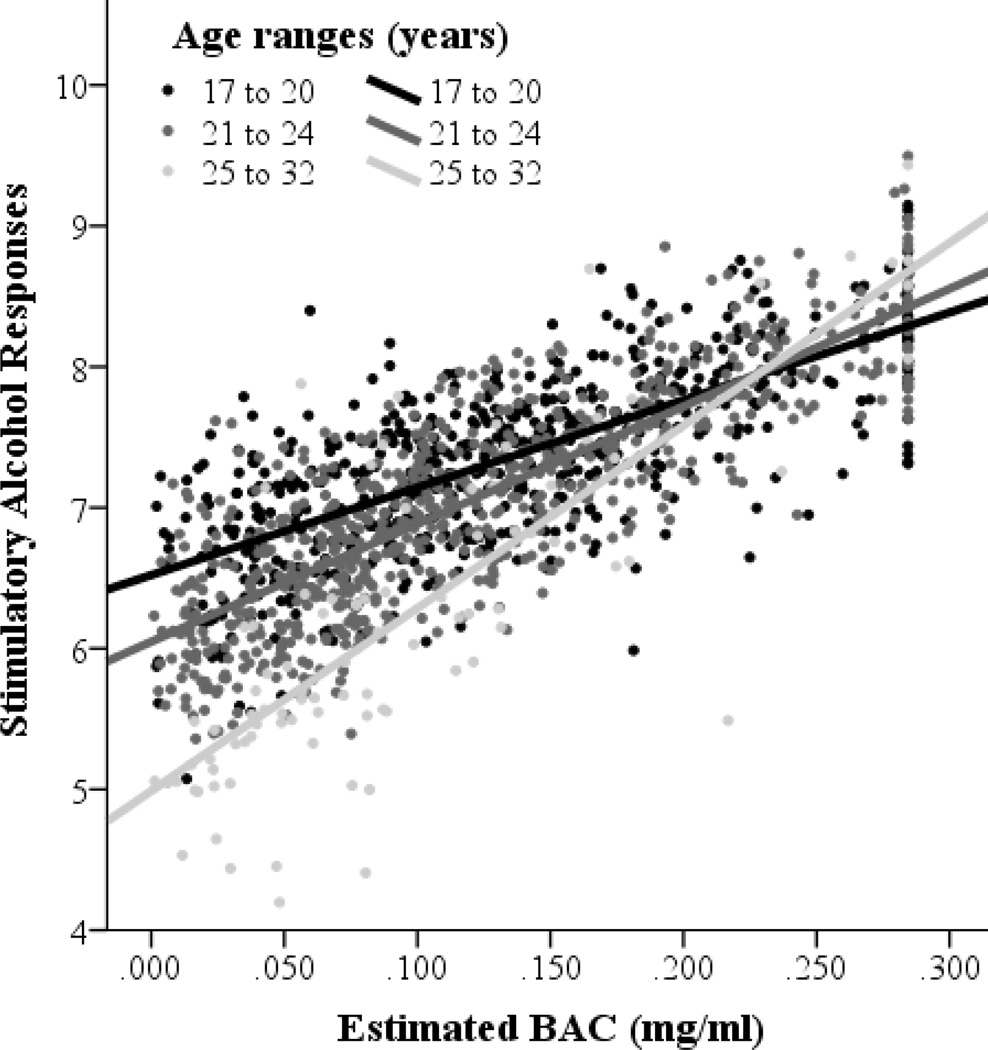
As expected, younger age was significantly associated with higher ratings of stimulatory alcohol responses in univariate analyses, r = − .14, p < .001 (see Table 2). However, in multivariate analyses, this age relation was qualified by an interaction with eBAC, suggesting that younger age is primarily associated with higher ratings of stimulatory alcohol responses at lower eBACs, p = .08, p = .003 (see Table 3). These results are depicted graphically with age as a three-category grouping variable in Figure 2. Contrary to our expectations, younger age was not significantly associated with lesser alcohol-induced sedation in a natural environment, and interactive effects of age with eBAC on sedative responses were also not significant.
Figure 2.
Age-related effects on stimulatory alcohol responses depicted graphically across estimated BACs with age as a three-category grouping variable.
AUDIT consumption
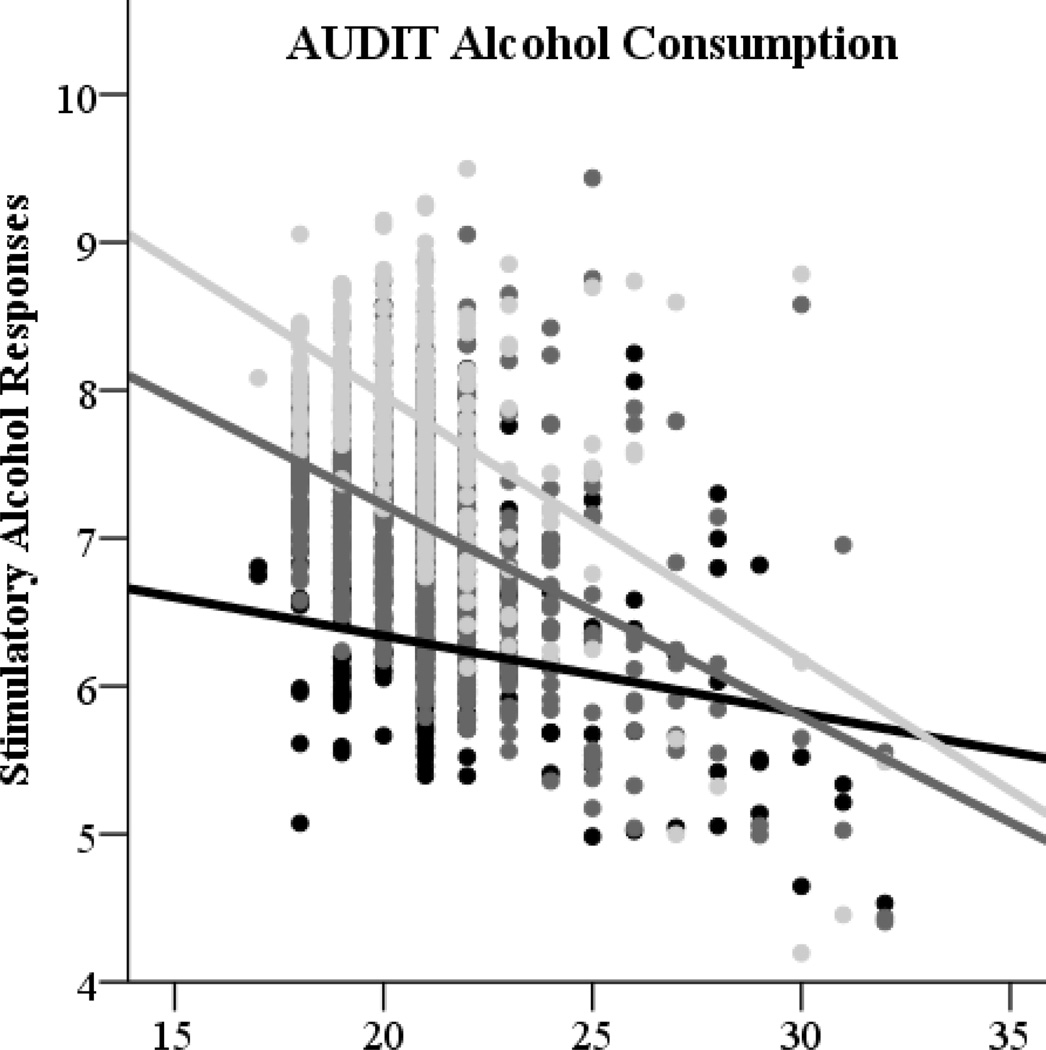
As expected, AUDIT consumption scores were associated with greater stimulatory effects in univariate analyses, r = .23, p < .001. Greater AUDIT consumption scores were also significantly associated with greater stimulatory responses to alcohol in multivariate analyses accounting for eBAC, age, gender, and AUDIT dependence/consequences (see Table 3). A nonsignificant interactive effect suggested that the relation of AUDIT consumption to stimulation did not depend on eBAC or gender, but was related to age, β = − .12, p < .001. These results are depicted graphically at +/− 1 standard deviation for AUDIT consumption subscale scores (see Figure 3, left panel). AUDIT consumption scores were not significantly associated with sedative effects in univariate analyses, but were marginally associated with decreased sedative responses in a multivariate test controlling for age, gender, eBAC, and AUDIT dependence/consequences, β = − .07, p = .037. (This effect was marginal when correcting for multiple tests (3 focal independent variables: .05/3 = p < .017). Contrary to expectations, the association of AUDIT consumption scores with sedative responses did not depend on eBAC, as indicated by a nonsignificant interactive effect. This association also did not depend on age or gender.
Figure 3.
Effects of AUDIT alcohol consumption (left panel) and AUDIT dependence/consequences (right panel) subscale scores on stimulatory alcohol responses depicted graphically across participant ages (17 to 32 years).
AUDIT dependence/consequences
As expected, greater dependence/consequences scores were associated with greater stimulatory responses to drinking in a natural environment, r = .18, p < .001. This effect did not depend on eBAC, but did depend on age, β = .08, p = .003. Age relations to stimulatory responses are depicted graphically at +/− 1 standard deviation for AUDIT dependence/consequences subscale scores (see Figure 3, right panel). Contrary to expectations, higher AUDIT dependence/consequences scores were associated with increased sedative effects of alcohol in univariate analyses, r = .10, p < .001, rather than decreased sedative responses. This effect remained significant when accounting for age, gender, eBAC, and AUDIT consumption scores in multivariate analyses (see Table 3). Nonsignificant interactive effects suggested that the association of AUDIT dependence/consequences scores and increased sedative effects did not depend on eBAC, age, or gender.
4. DISCUSSION
This cross-sectional study examined whether the subjective stimulatory and sedative effects of alcohol vary across adolescence and young adulthood, and tested whether the amount of typical alcohol consumption or level of alcohol problems predicted differential effects across development. In terms of the stimulant effects of alcohol, these findings extend animal models that show adolescent rodents are more sensitive to ethanol stimulation than adults, build on a recent human study that compared the subjective effects of alcohol among adolescents and adults in the natural environment, and underscore the potential clinical relevance of early alcohol exposure for the pathogenesis of addiction. Our results showed that younger participants experienced greater stimulation from alcohol at lower, but not higher, blood alcohol levels. We also found significant interactions of age with alcohol dependence/consequences and typical alcohol consumption. It appeared that participants with the highest levels of alcohol dependence/consequences reported heightened alcohol-induced stimulation overall, whereas this relation was age dependent for participants with fewer symptoms of alcohol dependence/consequences. Greater alcohol consumption over the past 12 months was also significantly associated with greater stimulatory responses to alcohol, even after controlling for eBAC, and this effect also related to age. Youth who consumed greater volumes of alcohol reported greater alcohol-induced stimulation, but this effect diminished for older participants. Contrary to our expectations, we did not find an association between age and self-reported sedation, and the interactive effects of age and alcohol dependence/consequences on the sedative effects of alcohol were also not significant.
A longitudinal study is needed to understand whether our cross-sectional findings correspond to decreasing stimulatory responses to alcohol over the developmental transition from adolescence to adulthood. Nonetheless, we provide some of the first human research to coincide with evidence from animal models finding adolescent rodents to be more sensitive to alcohol’s stimulatory effects than their adult counterparts (Spear, 2011b). In addition, our findings are consistent with results of a recent study by Miranda and colleagues (2014) that used EMA methods to characterize subjective responses to alcohol among adolescents, aged 15 to 19 years, and found that youth experienced greater stimulation from alcohol than adults, aged 24 to 64 years, especially at lower blood alcohol levels. On the whole, these findings implicate early to mid-adolescence as a period of heightened sensitivity to the stimulatory effects of alcohol. Such elevations in alcohol stimulation during adolescence may be in part related to the heightened novelty-seeking and elevations in risk-taking evident at this time (Geier, 2013), with the novelty, risks, and excitement associated with drinking perhaps contributing to greater positive expectancies (Vilenne and Quertemont, 2015) and greater stimulatory responses to alcohol at this age. This heightened sensitivity to alcohol’s stimulatory effects, paired with adolescents’ underdeveloped neurocognitive ability to adaptively problem-solve and inhibit potentially harmful behavior, appears to confer risk for addiction. Moreover, repeated drinking during adolescence may compromise the ability to modulate craving or desire to drink, which may explain why early alcohol use predicts lifelong struggles with addiction.
Another central finding of this study was that age-related stimulatory responses to alcohol appeared particularly pronounced at younger ages for those with higher levels of typical alcohol consumption or greater alcohol dependence/consequences. Although the neurobiological pathways that underlie alcohol dependence are multifaceted and complex, the observed age-related sensitivity to alcohol’s stimulatory effects is consistent with an allostatic model of addiction where repeated alcohol consumption alters brain reward circuits (Koob and Le Moal, 2001; Volkow and Li, 2005). The fact that these positive relations of alcohol use, dependence symptoms and related consequences, and stimulatory responses to alcohol were less pronounced at older ages could reflect a desensitization of the brain’s reward system, which is purported to develop as individuals progress from social drinking toward pathological alcohol use (Berridge and Robinson, 2003; Lubman et al., 2007). Indeed, research with both animal models and humans shows that alcohol-induced increases in dopamine levels, a critical component of the brain’s neural circuitry underlying the pleasurable effects of alcohol and other drug use, were attenuated among addicted individuals relative to non-addicted controls (Volkow et al., 2016). It should be noted, however, that stimulation might not be synonymous with the concept of “reward.” This distintintion has important implications. Incentive sensitization theory posits that as individuals progress in the pathogenesis of addiction, neural systems that mediate motivational process of incentive salience (i.e., wanting) become sensitized (Berridge and Robinson, 2003; Lubman et al., 2007). Within this framework, however, incentive sensitization is exclusive to motivational processes (i.e., wanting) and does not encompass corresponding changes in the subjective pleasurable effects of alcohol. This disproportionately heightened desire to drink alcohol relative to the pleasurable effects of drinking is theorized to be the catalyst for addiction (Robinson and Berridge, 2008).
In terms of alcohol’s sedative effects, we did not find a main effect of age or eBAC on sedation. The lack of association between age and sedation is inconsistent with most preclinical studies, which show adolescent rodents are less sensitive than adults to alcohol’s sedative effects. This finding is consistent, however, with the only other human field-based study (Miranda et al., 2014), which indicated that alcohol increased sedation among adolescents in a dose-dependent fashion, and that this effect did not differ as a function of age. A notable difference between Miranda et al. (2014) and the present study, however, is that the present study did not find an association between eBAC and sedation. There are several possible explanations for this null finding. First, and perhaps most importantly, sedative effects of alcohol are most prominent while blood alcohol levels decline. The protocols employed in both studies were not designed to capture the descending limb of the biphasic blood alcohol curve, and, thus, neither project may be well-suited to examine subjective responses that are most prevalent during the descending limb. Further, participants in this field-based study will have consumed variable amounts of alcohol over variable time intervals, and thus, BACs will not follow a traditional rising, peak, and descending BAC curve. This level of control—traditionally achieved in lab-based studies—was compromised in the present study to achieve a higher degree of ecological validity and include a larger age range of adolescent drinkers (under 21 years of age). Second, subjective responses to alcohol vary considerably across persons based on myriad individual difference characteristics (Bujarski and Ray, 2014). It is possible that the heterogeneous nature of the sample compromised our ability to capture certain effects of alcohol. Third, we relied on single episode drinking data from each participant, with information about recent alcohol use collected based on participants’ retrospective reports, albeit within the same day. More fine-grained EMA approaches that capture multiple drinking episodes within each participant may improve power to test effects.
Drinking levels over the past 12 months and severity of alcohol problems were both related to alcohol’s sedating effects but in different ways. Higher typical consumption predicted lesser sedation, whereas greater alcohol problems predicted greater sedation. The negative association between past 12-month drinking levels and sedation is consistent with human studies with adults (King et al., 2011, 2014; Roche et al., 2014) and may reflect greater tolerance among heavier drinkers. Although age did not influence this association, preclinical studies suggest that adolescents, as compared to adults, show distinct neuroadaptations to repeated alcohol exposure that may influence the development of tolerance (Morales et al., 2011). It is noteworthy that Miranda and colleagues (2014) did not find an association between drinking histories, including percent drinking and heavy drinking days in the past 90 days, and alcohol-induced sedation. Further human research is needed to better understand these associations in youth.
4.1. Limitations
Several limitations must be considered when interpreting these results. First, our data are cross-sectional and hence the directionality of the relationship between alcohol dependence/consequences and the experience of the stimulant effects of alcohol is speculative. Specifically, based on leading theoretical models of addiction (Volkow et al., 2016), we interpret our findings to suggest that individuals’ subjective effects of alcohol change as they transition from nonpathological drinking patterns to dependence. By contrast, it is possible that – given the cross-sectional nature of this study – youths with more drinking problems had distinct subjective responses to alcohol that predated the development of pathological drinking patterns. Second, eBAC was estimated only once at the time of assessment, and we could not ascertain where this may fall in the participant’s entire drinking trajectory for the evening. Participants started drinking approximately three hours prior to the assessment, and the average eBAC of .119 mg/ml exceeded typical target peak BACs in human laboratory studies. Although our approach provides a more externally valid drinking picture, this is only a snapshot of the participant’s full drinking episode. Future ecological research with repeated assessments and more fine-grained temporal resolution would build meaningfully on the present findings. Last, this initial, cross-sectional study utilizes a sample that is primarily non-Hispanic, White college students, which limits generalizability to more diverse groups. Generalizability may be further limited by our field-based methodology involving data collection outside of venues within a bar district. Our sample of 17–20-year-olds is likely to reflect those underage drinkers who consume alcohol illegally in a bar setting, which may limit generalizability to other younger drinkers who do not drink in this context.
4.2. Conclusions
This study provided some of the first in vivo human evidence for age-related changes in stimulatory responses to alcohol among underage drinkers (ages 17 to 20 years). Inclusion of legal drinkers (ages 21 to 32 years) allowed for age comparisons over the developmentally critical period of adolescence through young adulthood. Findings were consistent with the only other ecological study to compare adolescent and adult alcohol responses (Miranda et al., 2014). Despite methodological differences, both studies found that adolescents experience heightened sensitivity to alcohol’s stimulatory effects, with age-related discrepancies becoming less pronounced at higher eBACs. The present study added to this work by also evaluating the relation of dependence/consequences to alcohol responses. Our findings raise the question of whether age-related discrepancies are a function of the pathogenesis of AUD or whether differences reflect a biological predisposition toward AUD development. Thus, our work encourages future research on alcohol responses among younger drinkers through natural observation, particularly prospective studies.
Highlights.
A large field study of age-related differences in the acute effects of alcohol.
Stimulation and sedation were reported by 1737 participants, aged 17 to 32 years.
Younger participants reported greater stimulation, but no differences in sedation.
Findings support the theory that addiction liability is developmentally linked.
Acknowledgments
Role of Funding Source
Funding for this study was provided by the Binghamton University Professional Employees Council (PEC) Individual Development Award and The Center for Development and Behavioral Neuroscience (CDBN), as well as National Institute of Alcohol Abuse and Alcoholism grants (AA07459, AA07850). The sponsors of the study had no role in the study design, data collection, data analyses, data interpretation, or writing of this report.
This paper is dedicated to the memory of Gerard E. Johansen, whose collaborative spirit and inquisitive mind made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors conceptualized the present study and contributed to the editing of the manuscript. HT, RM, and LS conducted the background literature review and contributed to the interpretation of the findings. HT, MC, and RM wrote the first draft of the manuscript. HT and MC conducted statistical analyses. MC and LS co-directed the study and provided oversight of data collection. All authors contributed to and approved the final manuscript.
Conflict of Interest
No conflict declared.
Contributor Information
Hayley Treloar, Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, United States.
Mark A. Celio, Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, United States
Stephen A. Lisman, Department of Psychology, Binghamton University (SUNY), Binghamton, NY 13902-6000, United States
Robert Miranda, Jr., Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, United States
Linda P. Spear, Department of Psychology, Binghamton University (SUNY), Binghamton, NY 13902-6000, United States
REFERENCES
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2nd. Geneva: WHO; 2001. [Google Scholar]
- Behar D, Berg C, L RJ, Nelson W, Linnoila M, Cohen M, Bozevich Marshall T. Behavioral and physiological effects of ethanol in high-risk and control children: a pilot study. Alcohol. Clin. Exp. Res. 1983;7:404–410. doi: 10.1111/j.1530-0277.1983.tb05495.x. http://doi.org/10.1111/j.1530-0277.1983.tb05495.x. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. http://doi.org/10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. http://doi.org/10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend. 2014;140:161–167. doi: 10.1016/j.drugalcdep.2014.04.015. http://doi.org/10.1016/j.drugalcdep.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MA, Usala JM, Lisman SA, Johansen GE, Vetter-O’Hagen CS, Spear LP. Are we drunk yet? Motor versus cognitive cues of subjective intoxication. Alcohol. Clin. Exp. Res. 2014;38:538–544. doi: 10.1111/acer.12276. http://doi.org/10.1111/acer.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JD, Holmes MR, Reed MB, Shillington AM, Freisthler B, Lange JE. Measuring college students’ alcohol consumption in natural drinking environments: field methodologies for bars and parties. Eval. Rev. 2007;31:469–489. doi: 10.1177/0193841X07303582. http://doi.org/10.1177/0193841X07303582. [DOI] [PubMed] [Google Scholar]
- Clapp JD, Johnson MB, Shillington AM, Lange JE, Voas RB. Breath alcohol concentrations of college students in field settings: seasonal, temporal, and contextual patterns. J. Stud. Alcohol Drugs. 2008;69:323–331. doi: 10.15288/jsad.2008.69.323. [DOI] [PubMed] [Google Scholar]
- Clapp JD, Min JW, Shillington AM, Reed MB, Ketchie Croff J. Person and environment predictors of blood alcohol concentrations: a multi-level study of college parties. Alcohol. Clin. Exp. Res. 2008;32:100–107. doi: 10.1111/j.1530-0277.2007.00547.x. http://doi.org/10.1111/j.1530-0277.2007.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict. Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. http://doi.org/10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Celio Ma, Lisman Sa, Johansen GE, Spear LP. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. J. Stud. Alcohol Drugs. 2013;74:635–641. doi: 10.15288/jsad.2013.74.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci. Biobehav. Rev. 2012;36:1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. http://doi.org/10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SR, Donovan DM, Kivlahan DR. The factor structure of the Alcohol Use Disorders Identification Test (AUDIT) J. Stud. Alcohol Drugs. 2007;68:474–479. doi: 10.15288/jsad.2007.68.474. [DOI] [PubMed] [Google Scholar]
- Geier CF. Hormones and Behavior Adolescent cognitive control and reward processing: implications for risk taking and substance use. Hormones Behav. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. http://doi.org/10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR, Edition F. Age at drinking onset and alcohol dependence. Arch. Pediatric Adolesc. Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hustad JTP, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. J. Stud. Alcohol. 2005;66:130–138. doi: 10.15288/jsa.2005.66.130. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15830913. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Lange JE, Voas RB, Clapp JD, Lauer E, Snowden CB. The Sidewalk Survey: a field methodology to measure late-night college drinking. Eval. Rev. 2006;30:27–43. doi: 10.1177/0193841X04273255. http://doi.org/10.1177/0193841X04273255. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring The Future Results On Drug Use: 1975–2014: Overview, Key Findings On Adolescent Drug Use. Ann Arbor: University of Michigan; 2015. [Google Scholar]
- Kassel JD, Veilleux JC, Wardle MC, Yates MC, Greenstein JE, Evatt DP, Roesch LL. Negative affect and addiction. In: Al-Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Amsterdam: Elsevier, Ltd.; 2007. pp. 171–189. [Google Scholar]
- King AC, Hasin D, Connor SJO, Mcnamara PJ, Cao D. Archival report a prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol. Psychiatry. 2016;79:489–498. doi: 10.1016/j.biopsych.2015.05.007. http://doi.org/10.1016/j.biopsych.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol. Psychiatry. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. http://doi.org/10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Wit De H, Mcnamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch. Gen. Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. http://doi.org/10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Hall WD. Substance use and the adolescent brain: a toxic combination? J. Psychopharmacol. 2007;21:792–794. doi: 10.1177/0269881107078309. http://doi.org/10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Conigliaro J, McNeil M, Kraemer K, Kelley ME. An empirical investigation of the factor structure of the AUDIT. Psychol. Assess. 2000;12:346–353. doi: 10.1037//1040-3590.12.3.346. http://doi.org/10.1037/1040-3590.12.3.346. [DOI] [PubMed] [Google Scholar]
- Miranda R, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, Chun T, Gwaltnery CJ, Justus A, Tidey J, Blanchard A, Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. J. Abnorm. Psychol. 2014;123:117–129. doi: 10.1037/a0035328. http://doi.org/10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol. Clin. Exp. Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. http://doi.org/10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) What Is A Standard Drink? 2016 Retrieved April 18, 2016, from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink.
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol. Clin. Exp. Res. 2010;34:199–205. doi: 10.1111/j.1530-0277.2009.01081.x. http://doi.org/10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol. Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. http://doi.org/10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol. Clin. Exp. Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. http://doi.org/10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology. 2010;212:501–512. doi: 10.1007/s00213-010-1971-z. http://doi.org/10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Subst. Use Misuse. 2010;45:1742–1765. doi: 10.3109/10826084.2010.482427. http://doi.org/10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. http://doi.org/10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. http://doi.org/10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J. Stud. Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch. Gen. Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. http://doi.org/10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. The answer you get depends on the question you ask. Biol. Psychiatry. 2014;75:754–755. doi: 10.1016/j.biopsych.2014.03.018. http://doi.org/10.1016/j.biopsych.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch. Gen. Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Onset and course of alcoholism over 25 years in middle class men. Drug Alcohol Depend. 2011;113:21–28. doi: 10.1016/j.drugalcdep.2010.06.017. http://doi.org/10.1016/j.drugalcdep.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Stability of scores and correlations with drinking behaviors over 15 years for the Self-Report of the Effects of Alcohol Questionnaire. Drug Alcohol Depend. 2013;128:194–199. doi: 10.1016/j.drugalcdep.2012.08.022. http://doi.org/10.1016/j.drugalcdep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Ann. Rev. Clin. Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. http://doi.org/10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: setting the stage for alcohol use disorders? Child Dev. Perspect. 2011a;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. http://doi.org/10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev. Cogn. Neurosci. 2011b;1:390–403. doi: 10.1016/j.dcn.2011.08.001. http://doi.org/10.1016/j.dcn.2011.08.001.Rewards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci. Biobehav. Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. http://doi.org/10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev. Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. http://doi.org/10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs DL, O’Mara R, Dodd MJ, Hou W, Merves ML, Weiler RM, Pokomy SB, Goldberger BA, Reingle J, Werch CC. A field study of bar-sponsored drink specials and their associations with patron intoxication. J. Stud. Alcohol Drugs. 2009;70:206–214. doi: 10.15288/jsad.2009.70.206. [DOI] [PubMed] [Google Scholar]
- Usala JM, Celio MA, Lisman SA, Day AM, Spear LP. A field investigation of the effects of drinking consequences on young adults’ readiness to change. Addict. Behav. 2015;41:162–168. doi: 10.1016/j.addbeh.2014.10.016. http://doi.org/10.1016/j.addbeh.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilenne A, Quertemont E. Explicit and implicit positive alcohol expectancies in problem and non-problem drinkers: differences across age groups from young adolescence to adulthood. Frontiers Psychol. 2015;6:1–9. doi: 10.3389/fpsyg.2015.01773. http://doi.org/10.3389/fpsyg.2015.01773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. http://doi.org/10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li T-K. The neuroscience of addiction. Nat. Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- von der Pahlen B, Santtila P, Witting K, Varjonen M, Jern P, Johansson A, Sandnabba NK. Factor structure of the Alcohol Use Disorders Identification Test (AUDIT) for men and women in different age groups. J. Stud. Alcohol Drugs. 2008;69:616–621. doi: 10.15288/jsad.2008.69.616. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear L, Fuligni A, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. Transitions into underage and problem drinking: summary of developmental processes and mechanisms: Ages 10–15. Alcohol Res. Health. 2009;32:30–40. [PMC free article] [PubMed] [Google Scholar]