Abstract
Multiple interactions between the immune system and sleep are known, including the effects of microbial challenge on sleep or the effects of sleep loss on facets of the immune response. Cytokines regulate, in part, sleep and immune responses. Here we examine the role of an anti-inflammatory cytokine, interleukin-37 (IL-37) on sleep in a mouse strain that expresses human IL-37b (IL37tg mice). Constitutive expression of the IL-37 gene in the brains of these mice under resting conditions is low; however, upon an inflammatory stimulus, expression increases dramatically. We measured sleep in three conditions; (a) under baseline conditions and after 6 h of sleep loss, (b) after bolus intraperitoneal administration of lipopolysaccharide (LPS) or IL-1β and (c) after intranasal influenza virus challenge. Under baseline conditions, the IL37tg mice had 7% more spontaneous non-rapid eye movement sleep (NREMS) during the light period than wild-type (WT) mice. After sleep deprivation both WT mice and IL37tg mice slept an extra 21% and 12%, respectively, during the first 6 h of recovery. NREMS responses after sleep deprivation did not significantly differ between WT mice and IL37tg mice. However, in response to either IL-1β or LPS, the increases in time spent in NREMS were about four-fold greater in the WT mice than in the IL37tg mice. In contrast, in response to a low dose of mouse-adapted H1N1 influenza virus, sleep responses developed slowly over the 6 day recording period. By day 6, NREMS increased by 10% and REMS increased by 18% in the IL37tg mice compared to the WT mice. Further, by day 4 IL37tg mice lost less weight, remained more active, and retained their body temperatures closer to baseline values than WT mice. We conclude that conditions that promote IL-37 expression attenuate morbidity to severe inflammatory challenge.
Keywords: Sleep deprivation, Interleukin-1β, IL-37, Influenza virus H1N1, Lipopolysaccharide, IL-1F7, Body weight
Highlights
-
•
Sleep responses to mild acute sleep deprivation are similar in mice transgenic for interleukin-37 (IL37tg) IL37tg and wild type (WT) mice.
-
•
Sleep responses induced by either IL-β or LPS are greatly attenuated in IL37tg mice compared to WT mice.
-
•
After influenza virus challenge, IL37tg mice have reduced morbidities and enhanced sleep responses.
1. Introduction
Cytokine involvement in sleep regulation is extensively characterized (reviewed Imeri and Opp, 2009; Krueger, 2008; Krueger et al., 2008; Mullington et al., 2010; Simpson and Dinges, 2007; Zielinski and Krueger, 2011). Interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα) are pro-inflammatory sleep regulatory cytokines, although several other cytokines, including anti-inflammatory cytokines, are implicated in sleep-wake regulation. Injections of low doses of IL-1β promote sleep; in contrast high doses inhibit sleep (Opp et al., 1991). Similarly, even low doses of TNFα promote a sleep-like state in vitro whereas high doses disrupt neuronal action potential patterns in co-cultures of neurons and glia (Jewett et al., 2015). Sleep deprivation (Taishi et al., 1998, Taishi et al., 1999), stimulation of afferent neurons (Churchill et al., 2008, Hallett et al., 2010) or optogenetic stimulation of neurons in culture (Jewett et al., 2015) up-regulate several brain cytokines including IL-1β and TNFα (reviewed Krueger, 2008). Localized sleep intensity, as determined from the amplitude of electroencephalogram (EEG) slow waves (0.5–4 Hz), is enhanced by local application of IL-1β or TNFα or locally enhanced neuronal activity (Yasuda et al., 2005, Yoshida et al., 2004, Kattler et al., 1994). Conversely, inhibition of cortical TNFα expression inhibits local sleep intensity (Yoshida et al., 2004).
Microbial infections or systemic or central administration of various microbial products enhances sleep and these responses are associated with pro-inflammatory/pro-somnogenic cytokine expressions (reviewed Majde and Krueger, 2005). Influenza virus infections in mice are accompanied by changes in sleep and multiple cytokines (Alt et al., 2007, Chen et al., 2004, Fang et al., 1995, Jhaveri et al., 2006, Kapás et al., 2008, Lupfer et al., 2013). In mice, deficiency of a specific cytokine involved in the responses to influenza virus alters the sleep and morbidity responses to the virus (Alt et al., 2007, Chen et al., 2004, Davis et al., 2015, Traynor et al., 2007). Systemic or central administration of lipopolysaccharide (LPS) also can enhance sleep (Toth and Opp, 2001). Similar to the dose-dependent effects of bolus injections of IL-1β or TNFα, or severe infections, high doses of LPS induce sleep fragmentation suggesting that these effects are the result of stimulating high levels of pro-inflammatory cytokines (Bettis et al., 2006, Lundkvist et al., 2004, Olivadoti and Opp, 2008, Morrow and Opp, 2005).
The dose-dependencies of the direction of effects induced by IL-1β or TNFα suggest negative feedback steps are engaged. Anti-somnogenic cytokines such as IL-4 (Kushikata et al., 1998) and IL-10 (Opp et al., 1995) are less well studied, although their actions on sleep are generally inhibitory. Further, the IL-1 receptor antagonist (Takahashi et al., 1997), as well as cytokine soluble receptors, e.g. the TNF soluble receptor (Takahashi et al., 1995) inhibit sleep. Collectively, the sleep responses to pro- and anti-inflammatory cytokines and those in response to infectious challenge or specific microbial products indicate the involvement of the cytokine network in sleep regulation whether in health or disease.
IL-37b (hereafter called IL-37) is one of 5 isoforms of the IL-1 family of cytokines, formerly called IL-1F7 (Dinarello, 2010, Dinarello et al., 2016, Smith et al., 2000). IL-37 is up-regulated by microbial products, TNFα, IL-1β, IL-18 (Nold et al., 2010). Recombinant IL-37 reduces the production of several pro-inflammatory, pro-somnogenic cytokines including TNFα, IL-1β, and IL-6, while sparing anti-inflammatory/anti-somnogenic cytokines, e.g. IL-10 and the IL-1 receptor antagonist (Nold et al., 2010, Sharma et al., 2008). Such feedback actions result in a dampening of pro-inflammatory responses (the cytokine storm), although the dynamics of such actions are complex and understudied. Humans express IL-37 in various tissues including the brain (Pan et al., 2001). A role for IL-37-driven cytokine regulation is demonstrated in multiple pathologies including microbial infections (Fujita et al., 2013, Imaeda et al., 2013, Li et al., 2013a, Li et al., 2013b, McNamee et al., 2011, Pei et al., 2013, Teng et al., 2014). The mouse IL-37 homologue has not been found (Taylor et al., 2002) and the IL-37 gene is also not present in chimpanzees (Newman et al., 2005). Human cell lines and mouse macrophages transfected with IL-37 have attenuated pro-inflammatory cytokine responses to LPS. A transgenic mouse strain expressing human IL-37 (IL37tg) exhibits attenuated pro-inflammatory cytokine levels in response to LPS (Nold et al., 2010) and dextran sulfate sodium-induced colitis (McNamee et al., 2011). However, in the absence of cytokine or microbial stimulation, IL-37 expression is low in the IL37tg mice (Nold et al., 2010). Similarly, in pilot studies we found that IL-37 mRNA was not detected in WT mice. Further, IL-37 mRNA was detectable in IL37tg mice in the somatosensory cortex and liver following sleep deprivation or LPS treatment.
Although the IL-37 gene is absent in WT mice, the IL37tg line has contributed to the mechanistic understanding of immune responses (Bulau et al., 2014, Coll-Miro et al., 2016, Dinarello et al., 2016, McNamee et al., 2011, Luo et al., 2014, Nold et al., 2010, Nold-Petry et al., 2015) and provides a unique model to examine pro- and anti-inflammatory signaling in sleep responses to inflammatory challenges. Here we describe the effects of the presence of the IL-37 gene in mice on sleep. Three distinct conditions were examined. First, we determined spontaneous sleep and sleep responses to mild sleep loss in otherwise unchallenged mice; the presence of IL-37 had little effect on mouse sleep in these conditions. Second, we challenged the IL37tg mice with bolus injections of somnogenic doses of IL-1β or LPS; the anticipated sleep responses to these substances were greatly attenuated. Third, we determined sleep responses to a more pernicious stimulus, influenza virus challenge; in this case the presence of IL-37 enhanced sleep responses and simultaneously reduced morbidity. We conclude that IL-37 plays a central, albeit complex, role in attenuating severe inflammatory challenge.
2. Methods
2.1. Animals and surgery
Male mice (8–10 weeks old) expressing human IL-37 isoform b precursor transgene on a C57BL6 mouse background were locally bred as homozygotes for 8 generations. As previously described (Nold et al., 2010), fertilized eggs of C57BL/6 mice were injected with an IL-37b pIRES plasmid driven by a CMV promoter and contained a GFP expression sequence and a FLAGged C terminus. The eggs were subsequently implanted into C57BL6 females after which male founders mated C57BL6 WT females. A separate colony of C57BL6, WT mice (Jackson Laboratories, Sacramento, CA) were bred in our vivarium and served as controls. Mice were individually housed in sound-attenuated environmental chambers, maintained on a 12:12 h light:dark cycle (light onset=ZT0), at 23±2 °C and provided food and water ad libitum. Mice used for polysomnographic analyses were implanted with electroencephalogram (EEG) electrodes over the right and left parietal cortices (1 mm caudal of bregma and either ±2 mm lateral of bregma) and a ground electrode over the cerebellum (−0.5 mm caudal of lambda; Franklin and Paxinos, 2007). An electromyogram (EMG) electrode was placed in the nuchal muscles. Ketamine-xylazine (87 and 13 mg/kg, respectively) anesthesia was used for surgical procedures as previously described (Davis et al., 2015). Electrodes were mounted onto the skulls with dental cement and the mice were tethered to amplifiers using wire cables. Mice were allowed at least 7 days of recovery from surgery before use. Separate mice were surgically implanted with temperature and activity tracking transponders (TAE-mitters, Mini Mitter, Bend, OR) intraperitoneally under ketamine/xylazine anesthesia and allowed at least 1 week of recovery prior to the experiment. At the conclusion of each experiment mice were euthanized, tails collected and genotypes confirmed by Transnetyx Inc. (Cordova, TN). All experimental protocols were approved by the Washington State University Animal Care and Use Committee and were in compliance with National Institutes of Health Office of Laboratory Animal Welfare guidelines.
2.2. Sleep, temperature and activity recording analyses
Analog EEG (filtered below 0.1 Hz and above 100 Hz with a 60 Hz notch filter) and EMG amplified signals were digitized at 128 Hz and recorded to the local hard drive. Sleep analyses were conducted using Sleep Sign Software (Kissei Comtec Co., Ltd, Japan). EEG wave forms and EMG amplitudes, which reflect brain and muscle activity respectively, are characteristic properties of sleep states in mammals and birds. NREMS, REMS, and waking vigilance states were determined manually off-line in 10 s epochs as previously described (Davis et al., 2015). NREMS was identified by high-amplitude EEG signals and low EMG activity. Regular low-amplitude EEG and minimal EMG activity characterized REMS. Wake periods were recognized by low amplitude fast EEG and high EMG activity. Because mice are mostly active during the dark phase and sleep most of the time during daylight hours, vigilance state durations were calculated in 12 h blocks corresponding to the light (ZT0-12) and dark (ZT12-0) periods. Another measure often used to determine the depth (intensity) of NREMS is the amplitude of EEG slow waves. Although EEG delta wave (0.5–4.0 Hz) amplitudes are regulated independently from duration of sleep, during normal sleep the two measures correlate with each other (Davis et al. 2011). Fast Fourier transformations of Hanning filtered EEG signals (µV2) of each artifact free epoch were used to calculate NREMS EEG delta power [0.5–4 Hz range; also known as slow-wave activity (SWA)] and NREMS EEG power spectra in 1 Hz bins in the frequency range of 0–20 Hz for the 12 h light and/or dark period or the ZT6-12 after sleep deprivation (SD). SWA for each 12 h bin was normalized using the 24 h mean of the state-specific baseline value for each individual mouse (Davis et al., 2015).
For the Mini Mitter implanted mice, telemetric signals were recorded at 6 min intervals using VitalView analysis software (Mini Mitter, Bend, OR), and data points were derived from those values or were averaged over 6 or 24 h bins for body temperature and locomotor activity. Activity counts were normalized for each mouse by dividing the averaged value by its total 24 h baseline activity count and are expressed as a percent of baseline. Baseline was recorded for 24 h beginning at ZT0 and continued for 7 days after infection.
2.3. Virus purification and titration
A mouse-adapted H1N1 A/Puerto Rico/8/34 H1N1 (PR8) influenza virus (lot # 3×010621) was suspended in pyrogen-free allantoic fluid (Specific Pathogen-Free Avian Supply, North Franklin, CT) and isolated by ultracentrifugation sucrose-gradient sedimentation as previously described (Grimm et al., 2007, Majde et al., 2010). The virus was diluted to a protein concentration of 200 µg/ml, aliquoted, frozen on dry ice, and stored at −80°C. A standard hemagglutination assay with two-fold dilutions of purified PR8 (10 µl) mixed with 50 µl chicken red blood cells and incubated at room temperature for 30 min yielded 1×107 virus particles/ml. Samples were tested for endotoxin levels using a Limulus lysate gel-clot assay (Biowhittaker, Walkersville, MD); endotoxin was below detection levels. Additionally, the purified virus was negative for mycoplasma and acholeplasma contamination determined by nested PCR. In Madin–Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, VA), viral titrations were assessed and expressed as median tissue culture infectious doses (TCID50) as previously described (Grimm et al., 2007). The starting titer of purified PR8 virus was 2.5×104 TCID50/ml.
2.4. Experiment 1: Spontaneous sleep and sleep responses to sleep deprivation
WT (n=24) and IL37tg (n=23) mice were acclimated to the experimental chambers and instrumentation for 2 days before recording a 24 h baseline ZT 0-0. A separate group of mice were subjected to gentle handling SD as previously described (Davis et al., 2015). Briefly, mice were kept awake in their home cages by gently disturbing the bedding next to the animal or stroking the mouse with a brush when behavioral indications of sleep were observed. After at least 7 days of recovery from surgery and two days to acclimate to the EEG tethered connection, baseline control recordings (ad libitum sleep) were taken from IL37tg (n=9) and WT (n=7) mice for 24 h. After the baseline day, mice were SD for 6 h after light onset (ZT0-6). EEG and EMG were recorded for the next 18 h beginning immediately after the cessation of SD (ZT 6-0).
2.5. Experiment 2: Sleep responses to systemic LPS and IL-1β
WT and IL37tg mice were provided with EEG and EMG electrodes as above. After recovery from surgery, baseline data were obtained from both strains for 24 h after an IP injection of saline (0.2 mL/mouse) as a vehicle control. After the control recording period, IL37tg and WT (n=7/strain) mice received IP injections of IL-1β (180 ng in 0.2 mL saline) and EEG and EMG were recorded for the next 24 h. Separate WT and IL37tg mice, also provided with EEG and EMG electrodes and allowed to recover from surgery, received an IP control injection of saline (0.2 mL) at dark onset and baseline EEG and EMG were recorded for 24 h. IL37tg and WT mice (n=8/strain) then received an IP injection of LPS (500 ng in 0.2 mL saline) and EEG and EMG were recorded for 24 h thereafter.
2.6. Experiment 3: EEG responses to influenza challenge
WT (n=12) and IL37tg (n=10) mice received 12.5 TCID50 of purified mouse-adapted PR8 influenza virus. The live virus delivery occurred by intranasal instillation of 50 µL of purified PR8, 25 µL per nostril, under 20% isoflurane/80% polyethylene glycol (isoflurane-PEG) anesthesia (Zielinski et al., 2013). The intranasal virus was given within 1 h from light onset (ZT0-1). After viral challenge, mice were placed back in their recording chambers and EEG and EMG were recorded for the next 6 days. Prior studies (Davis et al., 2015) using the 12.5 TCID50 dose indicate that WT mice show morbidity (e.g. lower weights, activity, temperature and enhanced NREMS amounts) on PI Day 3 and begin dying on PI Day 7. Thus, PI Days 2, 4 and 6 were selected for analyses.
2.7. Experiment 4: Core body temperature, activity and body weight after influenza challenge.
IL37tg and WT mice (n=9/strain) were housed individually in their home cages and placed on receiver plates and acclimated to the recording chamber for at least one week after surgery before recording a 24 h baseline for temperature and activity. Next, mice were infected using the same technique and dose described in Experiment 3, returned to their cages and recordings ensued for the next 7 days. Body weights were taken prior to infection and on subsequent days at ZT0.
3. Statistics
SPSS v.17 was used to analyze time spent in NREMS, REMS, NREMS EEG SWA, locomotor activity, body temperature and weight during the light and dark periods. Mixed two-way ANOVAs with a within-subjects factor of treatment (baseline vs. post sleep deprivation; saline vs. post-injection day 1) and between-subjects factor of strain (WT vs. IL37tg). For the virus experiments a third factor of day (post infection (PI) days 2, 4 and 6 or days 1–7) was added to the model. For the 0–20 Hz power spectra mixed three-way ANOVAs were used to analyze treatment in the 12 h light and dark periods or the 2 h post-IL-1β or LPS injection periods (ZT12-14) with the additional within-subjects factor of spectral frequency in 1 Hz bins. Outliers were excluded based on a>2 standard deviation criterion. A Huynh–Feldt adjustment was used to mitigate sphericity violations of the repeated measures, however the unadjusted degrees of freedom are reported for clarity. Post hoc analyses were performed when main effects or interactions were significant. Additionally, time- or frequency-matched pairwise comparisons were made using independent- or paired-samples t-tests where p<0.05 was considered significant.
4. Results
4.1. Experiment 1: Spontaneous sleep and sleep responses to sleep deprivation
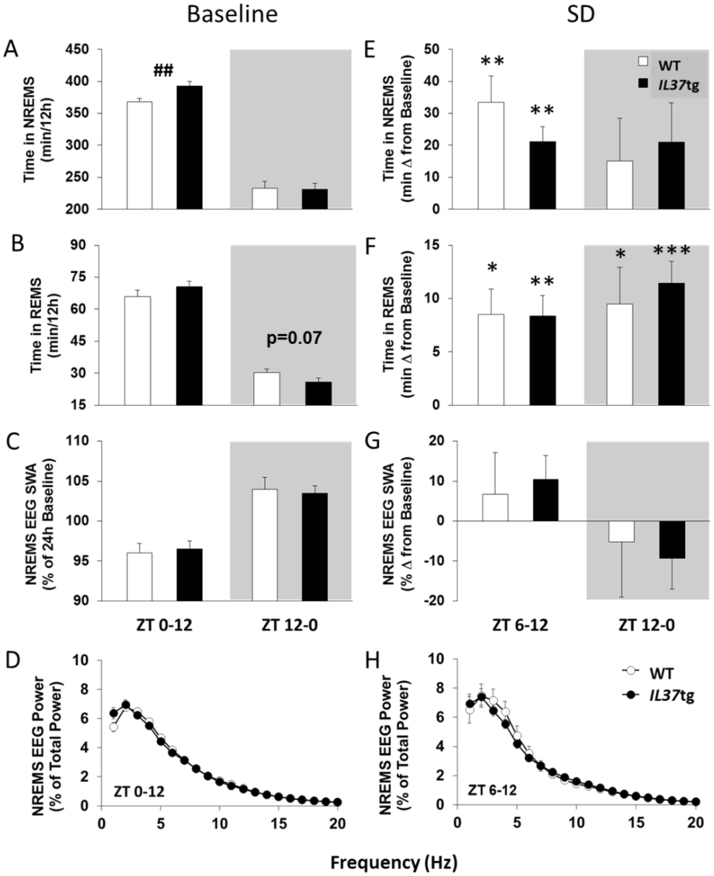
IL37tg mice had 25 min more NREMS during the light period than WT mice [Fig. 1A; t(45)=−2.79, p<0.01]. Most of the increased NREMS in IL37tg mice occurred during ZT4-12 (2 h data not shown). Baseline REMS (Fig. 1B), NREMS EEG SWA (Fig. 1C), or NREMS spectral power (Fig. 1D) were not different between strains during the light or dark periods.
Fig. 1.
IL37tg mice have more spontaneous NREMS and similar sleep rebounds compared to WT mice. NREMS amounts [A], REMS amounts [B] and NREMS EEG SWA [C] in 12 h periods corresponding to the light or dark phase (gray fill) for WT mice (white bars; n=24) and IL37tg mice (black bars; n=23; ## p<0.01, WT vs. IL37tg) are shown. NREMS EEG power [D] during the 12 h light period (ZT0-12) is depicted. After 6 h sleep deprivation, NREMS amounts [E], REMS amounts [F] and NREMS EEG SWA [G] in the subsequent 6 h light period and 12 h dark period (shaded areas) for WT (n=7) and IL37tg mice (n=9) are expressed as change from baseline values. NREMS EEG power [H] during the 6 h light period following sleep deprivation (ZT6-12) is reported (all data expressed as mean±SEM; asterisks indicate within strain, treatment differences,* = p<0.05; ** = p<0.01; *** = p<0.001).
During the 6 h after SD (ZT6-12) WT mice had 33 min more NREMS compared to baseline while IL37tg mice had 21 min more [Fig. 1E; Treatment: F(1,14)=37.97, p<0.001]. NREMS durations were not different between strains and the extra NREMS in the subsequent 12 h dark period (ZT12-0) was not different from baseline in either strain. After SD, REMS rebounds occurred during the light and dark periods [Fig. 1F; Treatment: F(1,14)=31.50, p<0.001 and F(1,14)=30.13, p<0.001, respectively] in both strains, but REMS rebounds were not different between strains. NREMS EEG SWA in ZT6-12 or in ZT12-0 was not significantly different from baseline or between strains nor was NREMS EEG spectral power.
4.2. Experiment 2: Sleep responses to systemic LPS and IL-1β
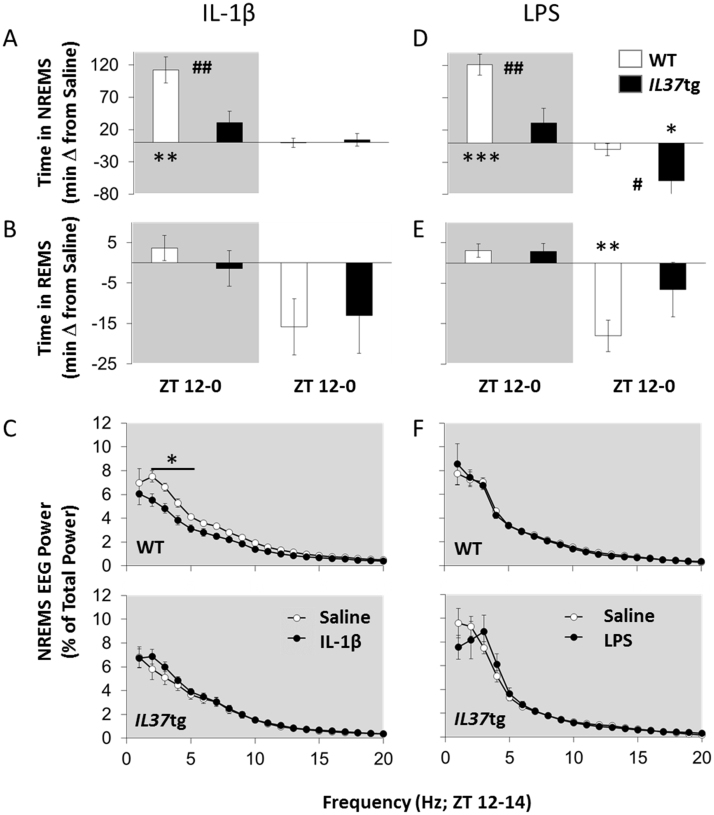
Time in NREMS increased during the first 12 h post-injection (dark period) after IL-1β (180 ng) [Fig. 2A; Treatment: F(1,12)=28.53, p<0.001]. Further, after IL-1β the IL37tg mice spent less time in NREMS compared to WT mice [Strain: F(1,12)=9.23, p<0.01]. Changes in REMS amounts were not statistically significant after IL-1β (Fig. 2B). NREMS spectral power in the 2–5 Hz frequency band during the initial 2 h post-injection period (ZT12-14) was decreased in WT mice, while spectral power in the 1–20 Hz frequency was not different in IL37tg mice [Fig. 2C; Treatment×Strain×Hz: F(19,228)=4.51, p<0.001]. No strain differences on any other sleep response measures were detected during the IL-1β post-injection dark or light periods.
Fig. 2.
IL37tg mice show attenuated sleep responses to IL-1β and LPS. WT mice (white symbols) and IL37tg mice (black symbols) were IP injected with saline (0.2 ml) just prior to dark onset and EEG/EMG was recorded for 24 h. Mice (n=7/strain) then received 180 ng of IL-1β [A, B, C] or 500 ng lipopolysaccharide (LPS; n=8/strain; D, E, F) and recording continued. Duration in state data are presented as differences from saline in NREMS [A,D] and REMS [B, E] averaged over 12 h periods corresponding to dark and light periods. EEG power spectra (1–20 Hz) from artifact-free NREMS-scored epochs over the first 2 h post-injection (ZT12-14) are depicted [C, F; WT top upper panels, IL37tg bottom panels]. Asterisks indicate within strain, treatment differences (* = p<0.05; ** = p<0.01) and pound signs indicate differences between strains (# = p<0.05; ## = p<0.01; gray fill indicates dark period).
In the first 12 h post-injection (dark period), NREMS duration increased in both strains after LPS (500 ng) [Fig. 2D; Treatment: F(1,14)=29.31, p<0.001]. After LPS, however, the NREMS increase in the IL37tg mice was substantially less than in WT mice [Strain: F(1,14)=10.38, p<0.01]. Further, during the light period 12–24 h after LPS injection, IL37tg mice continued to have less NREMS [Strain: F(1,14)=5.57, p<0.05] than WT mice. REMS amounts did not differ during the first 12 h post-injection of LPS, but decreased during the next 12 h (light period) [Fig. 2E; Treatment: F(1,14)=6.69, p<0.05]. No strain differences were detected in REMS amounts after LPS during the post-injection light or dark period. NREMS EEG SWA decreased in both strains following LPS [Treatment: F(1,14)=5.77, p<0.05], in the post-injection dark period (not shown). No strain differences in the power spectrum 0–2 h post-LPS treatment were found (Fig. 2F).
4.3. Experiment 3: EEG responses to influenza challenge
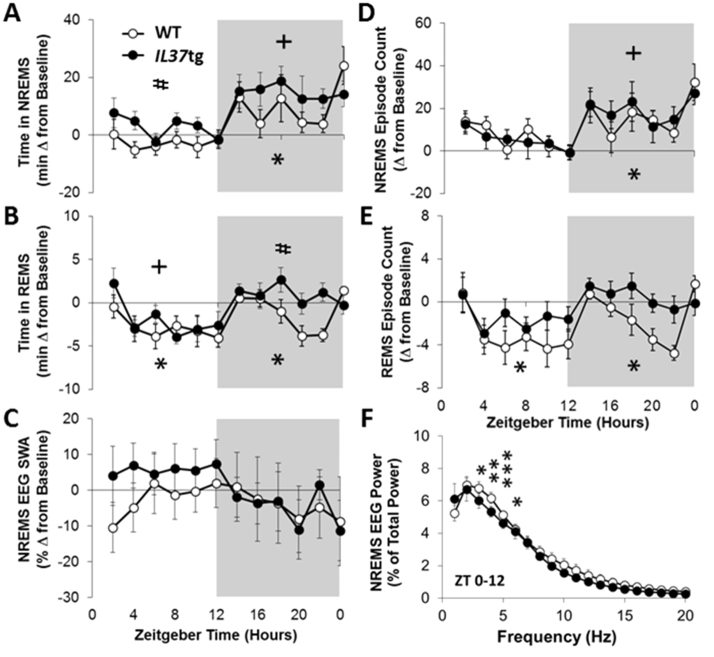
IL37tg mice had more NREMS than WT mice following virus challenge irrespective of the photoperiod. WT mice had on average 8 min less time in NREMS than their baseline value during each of the 12 h light periods on PI days 2, 4 [data not shown; Treatment: F(3,71)=11.12, p<0.001] and 6 (Fig. 3A; 2 h bins displayed). Conversely, during the light phase IL37tg mice exhibited an average of 20 min extra NREMS from baseline, which occurred on each of the PI days 2, 4 and 6 [Strain: F(1,71)=17.25, p<0.001]. Virus infection decreased NREMS episode lengths in WT more than in IL37tg mice [Strain: F(1,71)=9.79, p<0.01] in the light period (data not shown), whereas the number of NREMS bouts slightly increased in both strains [Fig. 3D; Treatment: F(3,71)=3.06, p<0.05].
Fig. 3.
IL37tg mice have enhanced sleep responses to influenza virus challenge on post infection (PI) day 6.IL37tg mice (n=10) had more NREMS [A] during light and/or dark hours (shaded areas representing the 12 h dark period) than WT mice (n=12) and their own baseline values. Both strains manifest an increased number of NREMS episodes [D] during the dark period following viral challenge. REMS amounts [B] during the dark and REMS bout frequency [E] during the light and dark were inhibited in WT mice, but not IL37tg mice after infection. There were no changes in NREMS EEG SWA in on PI day 6 in either strain regardless of the photoperiod [C]. Power spectra [F] decreased with infection in WT mice but not IL37tg mice (PI day 6 ZT0-12 shown; *p<0.05, **p<0.01, ***p<0.001, WT baseline vs. PI day 6; +p<0.05, IL37tg baseline vs. PI day 6 and #p<0.05, WT vs. IL37tg; statistical symbols derived from 12 h data analyses; all data expressed as mean ± SEM).
The characteristic virus-induced NREMS elevations during the dark were detected in both strains [Treatment: F(3,71)=5.17, p<0.01]. During the dark period both strains increased duration of NREMS; WT mice had on average 41 min extra NREMS and IL37tg mice had 74 min extra NREMS on each of the PI days 2, 4 and 6. The NREMS increases in the dark period were statistically significant from their respective baseline values on PI days 2 and 6 in WT mice and PI days 4 and 6 in IL37tg mice (baseline and PI day 6 data shown in Fig. 3). Overall, NREMS episode lengths were longer in IL37tg mice, than in WT mice [Strain: F(1,71)=8.81, p<0.01] in the dark. After infection, the frequency of NREMS bouts in the dark period increased in both strains, and was significant on PI day 6 [Fig. 3D; Treatment: F(3,71)=5.47, p<0.01].
After influenza challenge of WT mice, REMS duration decreased in the light and dark periods (Fig. 3B) compared to their baseline REMS. In IL37tg mice, attenuated REMS occurred in the light period; whereas, during the dark period, elevated REMS amounts occurred in response to virus infection [Treatment: F(3,71)=7.02, p<0.001]. The REMS increases during the dark period in IL37tg mice were statistically significant on PI day 4 (not shown). In summary, there were significant decreases from baseline on PI day 6 (light and dark) in WT mice. IL37tg mice manifested REMS decreases on PI days 4 and 6 in the light period and increases during PI day 4 in the dark period. In IL37tg mice, the length of REMS episodes in the light period were longer after infection on PI day 4 [data not shown; Treatment: F(3,71)=4.42, p<0.01] and did not change in WT mice. After viral challenge the number of REMS bouts decreased in both strains (Fig. 3E), during the light [Treatment: F(3,71)=5.19, p<0.01], but only decreased in WT mice during the dark [Treatment: F(3,71)=3.58, p<0.05] which was different between strains on PI day 6.
During the light period, NREMS EEG SWA post-infection responses were higher in IL37tg mice whereas they did not change in WT mice [Fig. 3C; Strain: F(1,71)=17.25, p<0.001]. The overall strain differences for NREMS SWA during the light were statistically significant, but these effects for any single PI day were not significant. NREMS spectral content during the light phase showed slight decreases from baseline in WT mice [Fig. 3F; Treatment×Hz: F(19,380)=4.41, p<0.05], but not in IL37tg mice. In WT mice, the differences were significant in the 3–6 Hz window. During the dark period, no significant strain or treatment differences in NREMS EEG SWA or NREMS power spectra (not shown) were detected.
4.4. Experiment 4: Activity, core body temperature and body weight after influenza challenge.
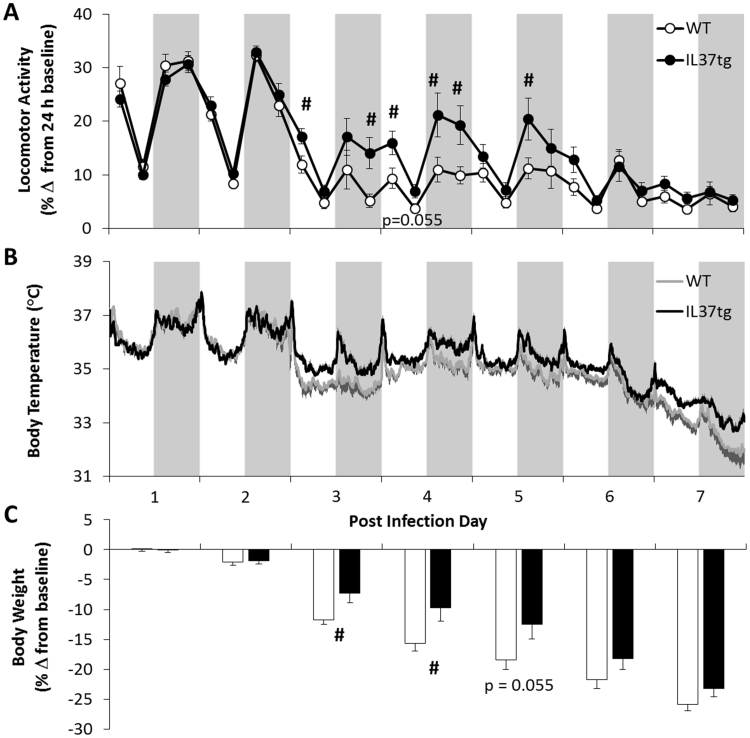
There was attenuated virus-induced morbidity in IL37tg mice compared to WT mice. The decreased locomotor activity that follows influenza infection in WT mice was less pronounced in IL37tg mice during PI days 3, 4 and 5 [Fig. 4A; Strain×Day: F(6,96)=4.53, p<0.05]. A hypothermic response to infection was observed on PI days 3–7 [Fig. 4B; Day: F(6,96)=88.27, p<0.001] in both strains. Although WT mice temperatures were lower than those of IL37tg mice on post-inoculation days 3–7, these temperature differences between strains were not statistically significant after the Huynh–Feldt adjustment [Fig. 4B; Strain×Day: F(6,96)=2.52, p=0.067]. Influenza induced decreases in body weight were less prominent in IL37tg mice than WT mice [Fig. 4C; Strain×Day: F(6,96)=4.85, p<0.05]. Pairwise comparisons indicated that WT mice lost 3.28±0.2 and 4.4±0.4 g from baseline, which was significantly different from the 2.1±0.5 and 2.8±0.6 g lost in IL37tg mice, on PI days 3 and 4, respectively.
Fig. 4.
IL37tg mice are more active, less hypothermic, and have less weight loss than WT mice following viral challenge. Strain differences in [A] locomotor activity (6 h means±SEM), [B] temperature (6 min means and positive SEM for IL37tg and negative SEM for WT) and [C] body weights (daily means and negative SEM) are most apparent on post-infection days 3–5 (#p<0.05, WT vs. IL37tg; n=9/strain).
5. Discussion
Sleep responses after the three different inflammatory challenge conditions were distinct from each other and in each case the presence of the IL-37 gene in mice had an effect. In baseline conditions, IL-37 expression in IL37tg mice is low (McNamee et al., 2011, Luo et al., 2014, Coll-Miro et al., 2016) and baseline sleep was only slightly increased in IL37tg mice above that of WT mice; the biological significance of the small change is questionable. The reason for the failure to express IL-37 in the resting state is likely due to the instability of the sequence as described by Bufler et al. (2004), so this is not unexpected. Similarly, after the short acute 6 h SD, both strains had NREMS and REMS rebounds and there were no strain differences. Several pro-inflammatory cytokines, including IL-1β and TNFα, play a role in sleep responses to SD (reviewed in Besedovsky, Lange and Born, 2012; Davis and Krueger, 2012; Imeri and Opp, 2009; Jewett and Krueger, 2012). Thus in a variety of species, including humans, chronic SD is associated with an inflammatory response and increases in IL-1β and TNF expression (Irwin et al., 2016, Vgontzas et al., 2004, Zielinski et al., 2014). In mice, although chronic prolonged SD enhances sleep-promoting pro-inflammatory cytokines, acute 6 h of SD is associated with small increases in pro-inflammatory cytokine mRNA expression in some parts of the brain, but not in the sleep regulatory hypothalamus (Dumaine and Ashley, 2015, Zielinski et al., 2012). Regardless, because the presence of IL-37 attenuates inflammation-induced pro-inflammatory cytokine expression in IL37tg mice, it is possible that the smaller NREMS responses in the IL37tg mice compared to WT mice, albeit not significantly different, were the result of attenuated pro-inflammatory responses.
In contrast to the baseline and SD-induced sleep responses, there were clear and large attenuations of IL-1β- and LPS-induced NREMS responses. The doses of IL-1β and LPS used in the current experiments are pro-somnogenic (Davis et al., 2015, Opp et al., 1991, Taishi et al., 2012). Because IL-1β can induce itself and LPS induces IL-1β and other pro-inflammatory cytokines, one mechanism responsible for the attenuated IL-1β- or LPS-induced sleep responses is IL-37-mediated inhibition of pro-inflammatory cytokines. That said, higher doses of either IL-1β (Opp et al., 1991) or LPS (Taishi et al., 2012, Toth and Opp, 2001) inhibit and/or fragment sleep suggesting that more severe inflammatory conditions can be sleep inhibitory. After the influenza virus challenge, although increases in NREMS durations were observed in both WT and IL37tg mice, it seems likely that the larger increases in NREMS in IL37tg mice were the result of an attenuation of an excessive upregulation of pro-inflammatory cytokines associated with the severe infection. This interpretation is consistent with the attenuation of locomotor, weight loss and temperature responses in the IL37tg mice, although the effects manifest on PI days 3–5 after which the mice succumbed to the infection and morbidity measures more closely approximated those of WT mice. Still the transgene seems to confer some degree of host protection to the extent that measures of morbidity after virus challenge were of lower magnitude in the IL37tg mice than in WT mice; this observation is also consistent with prior reports (Bulau et al., 2014, McNamee et al., 2011, Nold et al., 2010, Slimani et al., 2014).
Although it has been known for over 10 years that IL-37 binds to the alpha chain of the IL-18 receptor (IL-18Rα) (Kumar et al., 2002, Pan et al., 2001), the affinity is markedly low (Kumar et al., 2002) such that IL-37 is not a receptor antagonist for IL-18Rα (Bufler et al., 2002, Kumar et al., 2002) and therefore cannot account for the anti-inflammatory properties of IL-37. IL-37 also binds to the IL-18 binding protein (Bufler et al., 2002), but this complex also does not function as a receptor antagonist for IL-18. In the IL37tg mice, the anti-inflammatory effects on sleep responses to IL-1β, LPS, and influenza infection are likely due to the formation of a complex of IL-37 with IL-18Rα and the co-receptor IL-1R8 (formerly TIR8) (Nold-Petry et al., 2015). However, the signal is not that of IL-18 but rather an attenuated signal due to the decoy nature of IL-1R8 (Garlanda et al., 2013a, Garlanda et al., 2013b). The IL37tg mice are protected against a systemic response to LPS (Nold et al., 2010), but IL37tg mice deficient in IL-1R8 are not protected (Nold-Petry et al., 2015). In WT mice, recombinant IL-37 is effective in suppressing inflammation in several mouse models of inflammation, but not in mice deficient in IL-1R8 (Li et al., 2015, Lunding et al., 2015, Moretti et al., 2014). Although speculative, the observed responses could occur via the activation of other nonspecific pathways in IL37tg mice.
In general, the presence of IL-37 enhances anti-inflammatory cytokine expression and inhibits pro-inflammatory cytokines (Sharma et al., 2008). There are however many nuances to cytokine expression dynamics and actions. Thus, cytokine regulation and expression are complex processes occurring over multiple time scales lasting seconds to hours or even days. These events are mediated, in part, by various microbial stimuli, cell signaling mechanisms, cleavage enzymes, post-transcriptional regulation, feedback inhibition, receptors and their components, and inhibitory cytokines. Further, many cytokines, including IL-1β and TNFα have diurnal variations in their expression influencing the expression levels of other cytokines. For example, the cytokine expression of TNFα has an expression pattern that typically occurs earlier than IL-1β in response to most stimuli, although it is shorter in duration of expression (Jewett et al., 2015). This is, in part, reflective in the sleep effects found in IL-1β-and TNFα-KO mice or their respective receptor KO mice where the inhibition of NREMS and SWA responses in these mice across the day or to somnogenic stimuli corresponds with these expression timing and duration patterns (Kapás et al., 2008, Fang et al., 1998, Taishi et al., 1998, Taishi et al., 1999). Moreover, anti-inflammatory cytokines such as IL-10 and TGFβ tend to occur hours after IL-1β and TNFα, in a compensatory action (Toth and Opp, 2001, Kubota et al., 2000, Opp et al., 1995).
Data presented herein also have bearing on the issue of whether sleep is beneficial for recuperation from microbial challenge. The current findings are consistent with earlier work correlating robust sleep responses to infectious challenge to lower morbidity (Toth et al., 1993). Similar findings were reported in bacteria-infected drosophila that had less sleep rebound after sleep deprivation also had increased mortality rates (Kuo and Williams, 2014). Similarly, we recently showed that mice lacking a component of the IL-1 receptor signaling complex, brain-specific IL-1 receptor accessory protein, slept less and had higher mortality after influenza virus challenge using the same virus and dose as that used in this report (Davis et al., 2015). The antithesis also holds in that disrupted sleep, impedes recuperation (Faraut et al., 2012, Friese et al., 2009, Tang et al., 2009). Regardless, current data suggesting the larger NREMS responses to viral challenge occurring in the IL37tg mice aids in recuperation remain correlative, not causative.
In conclusion, there is a robust literature linking sleep and sleep loss to host defenses (reviewed Besedovsky et al., 2012; Imeri and Opp, 2009; Toth and Jhaveri, 2003; Zielinski and Krueger, 2011). Indeed, chronic sleep loss is associated with chronic inflammation and acute sleep loss induces multiple cytokines in brain and systemically and they are involved in the inflammatory processes. Similarly, sleep pathologies such as insomnia and sleep apnea are accompanied by chronic low-grade inflammation. A separate literature describes the relationships between severe inflammation, induced by microbial products such as LPS, muramyl peptides, and various viral products such as viral double stranded RNA to sleep responses (reviewed Majde and Krueger, 2005; Imeri and Opp, 2009). Current results are consistent with and expand that literature by suggesting that IL-37 has a role in host protection, including sleep responses, in response to inflammatory challenge.
Conflicts of interest
The authors declare there are no Conflicts of Interest associated with this publication.
Acknowledgments
This work was supported in part by National Institutes of Health, grant numbers NS025378 and HD036520 to JMK and AI15614 and CA046934 to CAD.
References
- Alt J.A., Bohnet S., Taishi P., Duricka D., Obal F., Jr, Traynor T., Majde J.A., Krueger J.M. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav. Immun. 2007;21:60–67. doi: 10.1016/j.bbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettis R., Iacuzio D., Jung T., Fuchs R., Aultman R., Gyldmark M. Impact of influenza treatment with oseltamivir on health, sleep and daily activities of otherwise healthy adults and adolescents. Clin. Drug Investig. 2006;26:329–340. doi: 10.2165/00044011-200626060-00004. [DOI] [PubMed] [Google Scholar]
- Bufler P., Azam T., Gamboni-Robertson F., Reznifov L.L., Kumar S., Dinarello C.A., Kim S.H. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl. Acad. Sci. USA. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufler P., Gamboni-Robertson F., Azam T., Kim S.H., Dinarello C.A. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem. J. 2004;381:503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulau A.M., Nold M.F., Li S., Nold-Petry C.A., Fink M., Mansell A., Schwerd T., Hong J., Rubartelli A., Dinarello C.A., Bufler P. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. USA. 2014;111(7):2650–2655. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Duricka D., Nelson S., Mukherjee S., Bohnet S.G., Taishi P., Majde J.A., Krueger J.M. Influenza virus-induced sleep responses in mice with targeted disruptions in neuronal or inducible nitric oxide synthases. J. Appl. Physiol. 2004;97:17–28. doi: 10.1152/japplphysiol.01355.2003. [DOI] [PubMed] [Google Scholar]
- Churchill L., Rector D.M., Yasuda K., Fix C., Rojas M.J., Yasuda T., Krueger J.M. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Miro M., Francos-Quijorna I., Santos-Nogueira E., Torres-Espin A., Bufler P., Dinarello C.A., Lopez-Vales R. Beneficial effects of IL-37 after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA. 2016;113(5):1411–1416. doi: 10.1073/pnas.1523212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.J., Clinton J.M., Jewett K.A., Zielinski M.R., Krueger J.M. Delta wave power: an independent sleep phenotype or epiphenomenon? J. Clin. Sleep. Med. 2011;7:S16–S18. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.J., Dunbrasky D., Oonk M., Taishi P., Opp M.R., Krueger J.M. The neuron-specific interleukin-1 receptor accessory protein is required for homeostatic sleep and sleep responses to influenza viral challenge in mice. Brain Behav. Immun. 2015;47:35–43. doi: 10.1016/j.bbi.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.J., Krueger J.M. Sleep and Cytokines. Sleep. Med. Clin.; Biol. Sleep. 2012;7:517–527. doi: 10.1016/j.jsmc.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. IL-1 family nomenclature. Nat. Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Nold-Petry C., Nold M., Fujita M., Li S., Kim S., Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 2016;46(5):1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaine J.E., Ashley N.T. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R1062–R1069. doi: 10.1152/ajpregu.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Sanborn C.K., Renegar K.B., Majde J.A., Krueger J.M. Influenza viral infections enhance sleep in mice. Proc. Soc. Exp. Biol. Med. 1995;210:242–252. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- Fang J., Wang Y., Krueger J.M. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am. J. Physiol. 1998;274:R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Faraut B., Boudjeltia K.Z., Vanhamme L., Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep. Med. Rev. 2012;16(2):137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd Edn. Academic Press; San Diego: 2007. [Google Scholar]
- Friese R.S., Bruns B., Sinton C.M. Sleep deprivation after septic insult increases mortality independent of age. J. Trauma. 2009;66:50–54. doi: 10.1097/TA.0b013e318190c3a1. [DOI] [PubMed] [Google Scholar]
- Fujita H., Inoue Y., Seto K., Komitsu N., Aihara M. Interleukin-37 is elevated in subjects with atopic dermatitis. J. Dermatol. Sci. 2013;69:173–175. doi: 10.1016/j.jdermsci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Garlanda C., Riva F., Bonavita E., Mantovani A. Negative regulatory receptors of the IL-1 family. Semin Immunol. 2013;25:408–415. doi: 10.1016/j.smim.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Garlanda C., Riva F., Bonavita E., Gentile S., Mantovani A. Decoys and regulatory “receptors” of the IL-1/toll-like receptor superfamily. Front Immunol. 2013;4:180. doi: 10.3389/fimmu.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Staeheli P., Hufbauer M., Koerner I., Martínez-Sobrido L., Solórzano A., García-Sastre A., Haller O., Kochs G. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc. Natl. Acad. Sci. USA. 2007;104:6806–6811. doi: 10.1073/pnas.0701849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett H., Churchill L., Taishi P., De A., Krueger J.M. Whisker stimulation increases expression of nerve growth factor- and interleukin-1beta-immunoreactivity in the rat somatosensory cortex. Brain Res. 2010;1333:48–56. doi: 10.1016/j.brainres.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda H., Takahashi K., Fujimoto T., Kasumi E., Ban H., Bamba S., Sonoda H., Shimizu T., Fujiyama Y., Andoh A. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin. Exp. Immunol. 2013;172(3):410–416. doi: 10.1111/cei.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L., Opp M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett K.A., Krueger J.M. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam. Horm. 2012;89:241–257. doi: 10.1016/B978-0-12-394623-2.00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett K.A., Taishi P., Sengupta P., Roy S., Davis C.J., Krueger J.M. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur. J. Neurosci. 2015;42:2078–2090. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri K.A., Ramkumar V., Trammell R.A., Toth L.A. Spontaneous, homeostatic, and inflammation-induced sleep in NF-kappaB p50 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1516–R1526. doi: 10.1152/ajpregu.00262.2006. [DOI] [PubMed] [Google Scholar]
- Kapás L., Bohnet S.G., Traynor T.R., Majde J.A., Szentirmai E., Magrath P., Taishi P., Krueger J.M. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. J. Appl. Physiol. 2008;105:1187–1198. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattler H., Dijk D.J., Borbély A.A. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep. Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Krueger J.M., Rector D.M., Roy S., HPA Van Dongen, Belenky G., Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Fang J., Kushikata T., Krueger J.M. Interleukin-13 and transforming growth factor-beta1 inhibit spontaneous sleep in rabbits. Am. J. Physiol. 2000;279:R786–R792. doi: 10.1152/ajpregu.2000.279.3.R786. [DOI] [PubMed] [Google Scholar]
- Kumar S., Hanning C.R., Brigham-Burke M.R., Rieman D.J., Lehr R., Khandekar S., Kirkpatrick R.B., Scott G.F., Lee J.C., Lynch F.J., Gao W., Gambotto A., Lotze M.T. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- Kuo T.H., Williams J.A. Acute sleep deprivation enhances post-infection sleep and promotes survival during bacterial infection in drosophila. Sleep. 2014;37:859–869. doi: 10.5665/sleep.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushikata T., Fang J., Wang Y., Krueger J.M. Interleukin-4 inhibits spontaneous sleep in rabbits. Am. J. Physiol. 1998:R1185–R1191. doi: 10.1152/ajpregu.1998.275.4.R1185. [DOI] [PubMed] [Google Scholar]
- Li C., Zhao P., Sun X., Che Y., Jiang Y. Elevated levels of cerebrospinal fluid and plasma interleukin-37 in patients with Guillain-Barré syndrome. Mediat. Inflamm. 2013:639712. doi: 10.1155/2013/639712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ji H., Cai Y., Ayana D.A., Lv P., Liu M., Jiang Y. Serum interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. J. Interferon Cytokine Res. 2013;33(10):612–618. doi: 10.1089/jir.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Neff C.P., Barber K., Hong J., Luo Y., Azam T. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA. 2015;112(8):2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Cai X., Liu S., Wang S., Nold-Petry C.A. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl. Acad. Sci. USA. 2014;111:15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunding L., Webering S., Vock C., Schroder A., Raedler D., Schaub B. IL-37 requires IL-18Ralpha and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy. 2015;70(4):366–373. doi: 10.1111/all.12566. [DOI] [PubMed] [Google Scholar]
- Lundkvist G.B., Kristensson K., Bentivoglio M. Why trypanosomes cause sleeping sickness. Physiol. (Bethesda) 2004;19:198–206. doi: 10.1152/physiol.00006.2004. [DOI] [PubMed] [Google Scholar]
- Lupfer C., Thomas P.G., Anand P.K., Vogel P., Milasta S., Martinez J., Huang G., Green M., Kundu M., Chi H., Xavier R.J., Green D.R., Lamkanfi M., Dinarello C.A., Doherty P.C., Kanneganti T.D. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde J.A., Krueger J.M. Links between the innate immune system and sleep. J. Allergy Clin. Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Majde J.A., Kapás L., Bohnet S.G., De A., Krueger J.M. Attenuation of the influenza virus sickness behavior in mice deficient in Toll-like receptor 3. Brain Behav. Immun. 2010;24:306–315. doi: 10.1016/j.bbi.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee E.N., Masterson J.C., Jedlicka P., McManus M., Grenz A., Collins C.B., Nold M.F., Bufler P., Dinarello C.A., Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc. Natl. Acad. Sci. USA. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S., Bozza S., Oikonomou V., Renga G., Casagrande A., Iannitti R.G. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10(11):e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington J.M., Simpson N.S., Meier-Ewert H.K., Haack M. Sleep loss and inflammation. Best. Pract. Res Clin. Endocrinol. Metab. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J.D., Opp M.R. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav. Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Newman T.L., Tuzun E., Morrison V.A., Hayden K.E., Ventura M., McGrath S.D., Rocchi M., Eichler E.E. A genome-wide survey of structural variation between human and chimpanzee. Genome Res. 2005;15:1344–1356. doi: 10.1101/gr.4338005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold M.F., Nold-Petry C.A., Zepp J.A., Palmer B.E., Bufler P., Dinarello C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold-Petry C.A., Lo C.Y., Rudloff I., Elgass K.D., Li S., Gantier M.P. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- Olivadoti M.D., Opp M.R. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–348. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp M.R., Smith E.M., Hughes T.K., Jr Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. Neuroimmunol. 1995;60:165–168. doi: 10.1016/0165-5728(95)00066-b. [DOI] [PubMed] [Google Scholar]
- Opp M.R., Obál F., Krueger J.M. Interleukin-1 alters rat sleep: temporal and dose-related effects. Am. J. Physiol. 1991;260:R52–R58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- Pan G., Risser P., Mao W., Baldwin D.T., Zhong A.W., Filvaroff E., Yansura D., Lewis L., Eigenbrot C., Henzel W.J., Vandlen R. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13:1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- Pei B., Xu S., Liu T., Pan F., Xu J., Ding C. Associations of the IL-1F7 gene polymorphisms with rheumatoid arthritis in Chinese Han population. Int. J. Immunogenet. 2013;40:199–203. doi: 10.1111/iji.12007. [DOI] [PubMed] [Google Scholar]
- Simpson N., Dinges D.F. Sleep and inflammation. Nutr. Rev. 2007;65:S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Smith D.E., Renshaw B.R., Ketchem R.R., Kubin M., Garka K.E., Sims J.E. Four new members expend the interleukin-1 superfamily. J. Biol. Chem. 2000;275:1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- Sharma S., Kulk N., Nold M.F., Gräf R., Kim S.H., Reinhardt D., Dinarello C.A., Bufler P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J. Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- Slimani H., Yufeng Z., Yousif N., Ao L., Fullerton D., Dinarello C., Meng X. Critical role IL-37 to ameliorate endotoxemic cardiac depression in aging mice: a critical role of suppression cardiodepressant cytokines. Cardiovasc Res. 2014;103(Suppl. 1):S92. [Google Scholar]
- Taishi P., Chen Z., Obál F., Jr, Hansen M.K., Zhang J., Fang J., Krueger J.M. Sleep-associated changes in interleukin-1beta mRNA in the brain. J. Interferon Cytokine Res. 1998;18:793–798. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- Taishi P., Davis C.J., Bayomy O., Zielinski M.R., Liao F., Clinton J.M., Smith D.E., Krueger J.M. Brain-specific interleukin-1 receptor accessory protein in sleep regulation. J. Appl. Physiol. 2012;112:1015–1022. doi: 10.1152/japplphysiol.01307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P., Gardi J., Chen Z., Fang J., Krueger J.M. Sleep deprivation increases the expression of TNF alpha mRNA and TNF 55kD receptor mRNA in rat brain. Physiologist. 1999;42:A4. [Google Scholar]
- Takahashi S., Tooley D.D., Kapás L., Fang J., Seyer J.M., Krueger J.M. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflugers Arch. 1995;431:155–160. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Fang J., Kapás L., Wang Y., Krueger J.M. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am. J. Physiol. 1997;273:R677–R682. doi: 10.1152/ajpregu.1997.273.2.R677. [DOI] [PubMed] [Google Scholar]
- Tang Y., Preuss F., Turek F.W., Jakate S., Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep. Med. 2009;10(6):597–603. doi: 10.1016/j.sleep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.L., Renshaw B.R., Garka K.E., Smith D.E., Sims J.E. Genomic organization of the interleukin-1 locus. Genomics. 2002;79:726–733. doi: 10.1006/geno.2002.6752. [DOI] [PubMed] [Google Scholar]
- Teng X., Hu Z., Wei X., Wang Z., Guan T., Liu N., Liu X., Ye N., Deng G., Luo C., Huang N., Sun C., Xu M., Zhou X., Deng H., Edwards C.K., 3rd, Chen X., Wang X., Cui K., Wei Y., Li J. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J. Immunol. 2014;192(4):1815–1823. doi: 10.4049/jimmunol.1300047. [DOI] [PubMed] [Google Scholar]
- Toth L.A., Tolley E.A., Krueger J.M. Sleep as a prognostic indicator during infectious disease in rabbits. Proc. Soc. Exp. Biol. Med. 1993;203:179–192. doi: 10.3181/00379727-203-43590. [DOI] [PubMed] [Google Scholar]
- Toth L.A., Opp M.R. Cytokine- and microbially induced sleep responses of interleukin-10 deficient mice. Am. J. Physiol. 2001:R1806–R1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- Toth L.A., Jhaveri K. Sleep mechanisms in health and disease. Comp. Med. 2003;53:473–486. [PubMed] [Google Scholar]
- Traynor T.R., Majde J.A., Bohnet S.G., Krueger J.M. Interferon type I receptor-deficient mice have altered disease symptoms in response to influenza virus. Brain Behav. Immun. 2007;21:311–322. doi: 10.1016/j.bbi.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A., Chrousos G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Yoshida H., Garcia-Garcia F., Kay D., Krueger J.M. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Peterfi Z., García-García F., Kirkpatrick R., Yasuda T., Krueger J.M. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Zielinski M.R., Kim Y., Karpova S.A., McCarley R.W., Strecker R.E., Gerashchenko D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014;580:27–31. doi: 10.1016/j.neulet.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski, M.R., Krueger, J.M., 2011. Sleep and innate immunity. In: Miller, M.A., (Ed.), Encyclopedia of Bioscience, Front. Biosciences. (Schol. Ed.) 3, pp. 632–642. [DOI] [PMC free article] [PubMed]
- Zielinski M.R., Souza G., Taishi P., Bohnet S.G., Krueger J.M. Olfactory bulb and hypothalamic acute-phase responses to influenza virus: effects of immunization. Neuroimmunomodulation. 2013;20:323–333. doi: 10.1159/000351716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski M.R., Taishi P., Clinton J.M., Krueger J.M. 5′-ectonucleotidase knockout mice lack non-REM sleep responses to sleep deprivation. Eur. J. Neurosci. 2012;35:1789–1798. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]