Abstract
Objective
To evaluate the feasibility and efficacy of a home-based cognitive-behavioral training program for sleep during late pregnancy.
Methods
A nonrandomized quasi-experimental sample of nulliparous women who received the intervention during pregnancy (n=25) was compared to two control groups (n=76 and n=48) from other intervention studies at similar postintervention time points: approximately 1 month before childbirth and 1–2 months after childbirth. The home-based Sleep Enhancement Training System for Pregnancy consisted of 4 weeks of sound-enhanced audio relaxation programs, readings, and daily sleep diaries. Both control groups received dietary recommendations for improving sleep. Sleep duration (total sleep time) and sleep disruption (wake after sleep onset) were measured using wrist actigraphy for a minimum of 48 hours on consecutive weekdays.
Results
The intervention group had significantly longer sleep duration and less sleep disruption than both control groups, particularly at the postpartum assessment. Intervention participants slept an average of 430 (95% confidence interval [CI] 397–464) minutes during pregnancy, compared to 420 (95% CI 403–438) and 417 (95% CI 395–439) minutes for the two control groups. At the postpartum assessment, the intervention group slept 446 (95% CI 415–478) minutes compared to 390 (95% CI 373–408) and 370 (95% CI 348–393) minutes for controls. In terms of sleep disruption, the intervention group had 16.1% (95% CI 11.9–20.2%) wake after sleep onset during pregnancy, while controls had 13.4% (95% CI 11.2–15.5%) and 20.4% (95% CI 17.7–23.0%). Postpartum, the intervention group had 20.3% (95% CI 16.4–24.1%) wake after sleep onset compared to 26.6% (95% CI 24.5–28.8%) and 28.7% (95% CI 26.0–31.5%) among control. Participant feedback about the intervention was generally positive, although intervention adherence was variable.
Conclusions
This study provides evidence of the feasibility and efficacy of the Sleep Enhancement Training System for Pregnancy intervention for enhancing sleep that typically worsens during late pregnancy and after childbirth.
Précis
A cognitive-behavioral intervention that includes relaxation training demonstrates feasibility and efficacy for minimizing sleep disruption among women in late pregnancy and after childbirth.
Introduction
Sleep disturbance is well-documented in women during the late stages of pregnancy1, 2 and early postpartum period.3, 4 Poor sleep and short sleep duration in mid- or late pregnancy have been associated with longer labor duration, and increased risks of preterm birth, vacuum-assisted delivery, and unplanned cesarean delivery.5–7 It has been hypothesized that chronic sleep loss during pregnancy can be both cause and effect of hyperarousal (i.e., increased physiological and psychological stress and reactivity), leading to stress responses that adversely affect pregnancy outcomes.8 In addition, poor sleep hygiene and hyperarousal have been identified as potential intervention targets for improving sleep among pregnant women.9
Despite the adverse consequences of poor sleep during pregnancy, relatively few interventions exist for improving sleep among pregnant women. The Sleep Enhancement Training System is a cognitive-behavioral intervention with sound-enhanced audio programs to promote relaxation and reduce hyperarousal. It was initially developed to help pilots overcome disturbed sleep related to jetlag and was adapted for individualized, home-based use. The Sleep Enhancement Training System has since been modified to effectively treat chronic insomnia in older adults10 and shift workers,11 but it has not yet been evaluated among pregnant women. Thus, the purpose of this study was to assess the feasibility and efficacy of the Sleep Enhancement Training System intervention modified to enhance sleep among women as their sleep becomes more disrupted during late pregnancy (Sleep Enhancement Training System for Pregnancy) and into the early postpartum period. We hypothesized that the Sleep Enhancement Training System for Pregnancy (SETS-P) study group would have more sleep time and less sleep disruption compared to two control groups at matched timepoints.
Materials and Methods
In this non-randomized controlled study, pregnant women assigned to the SETS-P intervention group were compared to two control groups from our two randomized clinical trials of sleep interventions for childbearing women.12 The primary control group (A) met similar inclusion and exclusion criteria and was demographically similar to the intervention group, while the secondary control group (B) was less socioeconomically advantaged than the intervention group. The current study was approved by the Committee on Human Research at the University of California, San Francisco. Informed consent was obtained from each participant, and all were paid for their time and effort.
All participants included in this analysis were women expecting their first child. To be eligible, all women had to be at least 18 years of age, be able to read and write English, and not be diagnosed with a sleep disorder or working night shift. Because the intervention group and two control groups came from different sleep intervention studies, details about the participants specific to each group are described separately.
All participants assigned to the intervention were recruited in 2006 or 2007. Women were recruited from fee-based childbirth education classes at a large academic hospital. In addition to the eligibility criteria described above, women planning to hire a live-in nanny were excluded. Eligible women were enrolled in the study at 29–31 weeks of gestation to allow sufficient time for completion of the intervention program prior to the last month of pregnancy.
Participants in the primary control group (A) were recruited from 2001 to 2003 from the same fee-based childbirth education classes as the intervention group. In addition to the eligibility criteria described above, both the woman and her partner had to enroll in this study, and couples were excluded if they planned to hire a live-in nanny. Couples were enrolled at 36 weeks of gestation or later. Only pregnant women assigned to the control group were included for this analysis.
Participants in the secondary control group (B) were recruited from 2004 to 2008 from free prenatal classes and clinics serving low-income women. Women in this group generally had lower socio-economic status (i.e., education and income) than the SETS-P group and control group A, but were included as an additional comparison group. In addition to the eligibility criteria described above, women were excluded if they had a history of diagnosed mood disorder. Women were enrolled at 36 weeks of gestation or later, and only pregnant women assigned to the control group were included for this analysis.
The 4-week Sleep Enhancement Training System for Pregnancy intervention was based on general principles in cognitive-behavioral therapy for insomnia. Included components consist of cognitive restructuring, sleep hygiene, stimulus control, and relaxation training. As summarized in Table 1, materials included a Sleep Enhancement Training System for Pregnancy guidebook with weekly readings and auditory programs provided on an MP3 player. Participants were instructed to listen to the auditory program in bed before going to sleep each night. In the first week, the recordings last 15 min, and in subsequent weeks, the duration was 45 min (or until the participant fell asleep). The auditory program uses 3-Dimensional (3-D) Living Sound™ technology, which allows the listener to perceive the relative distance of sound, thereby enhancing the “reality” of the sound and facilitating the relaxation experience (T. Jackson at 3DAudioMagic.com). The sound-enhanced relaxation training included guided imagery, muscle relaxation, and breathing techniques to reduce arousal and promote sleep. A sleep diary was also included so that participants could document their daily use of the intervention program. In this sample, the 4-week Sleep Enhancement Training System for Pregnancy intervention was started an average of 60 ± 13 days prior to delivery. Once the 4-week intervention was completed, occasional use of the auditory program was recommended, but not required.
Table 1.
Intervention components, adherence, and satisfaction ratings (n=27)
| Week | Intervention component | Adherence* | Satisfaction* | |
|---|---|---|---|---|
| % (n) who completed ‘All’ or ‘Most’ readings or used audio program ‘Every day’ or ‘Most days’ |
% (n) who rated it “Essential” or “Very useful” |
% (n) who would recommend it to others |
||
| Weekly Readings | ||||
| 1 | Change your thinking about sleep | 96% (26) | 59% (16) | 93% (25)† |
| 2 | Lifestyle and environmental factors that affect sleep |
96% (26) | 59% (16) | 96% (26)† |
| 3 | Stress and the relaxation response | 89% (24) | 67% (18)‡ | 89% (23)§ |
| 4 | Establish sleep-promoting habits | 89% (24) | 48% (13)‡ | 78% (21)§ |
| Nightly Audio Programs | ||||
| 1 | Nature Sounds | 89% (24)† | 78% (21) | 100% (27) |
| 2 | Active Relaxation & Sleep | 74% (20)† | 44% (12) | 67% (18) |
| 3 | Relaxation & Sleep | 78% (21)† | 59% (16)† | 74% (20)† |
| 4 | Natural Sleep | 67% (18)† | 52% (14)‡ | 70% (19)‡ |
| 1–4‖ | Daily Audio Program | 22% (6)¶ | 26% (7)† | 63% (17)† |
Adherence and satisfaction with the weekly readings and audio programs were assessed upon completion of the 4-week intervention program at 36 weeks of gestation.
One missing response; percentage reflects the proportion of 27 participants who responded as specified.
Two missing responses; percentage reflects the proportion of 27 participants who responded as specified.
Three missing responses; percentage reflects the proportion of 27 participants who responded as specified.
The Daily Audio Program is prescribed during week 1 and is recommended as an optional intervention component for weeks 2–4.
The remaining 78% reported using the daily audio program ‘Some days’.
Participants in the two control groups were provided with a pamphlet containing recommendations for healthy eating and information about how diet can influence sleep. A research assistant assessed the participants’ current eating habits and referred to the pamphlet for relevant dietary recommendations, if needed. If a participant asked questions about managing sleep or other parenting issues, the research assistant encouraged her to talk with her healthcare provider. For each control group, the control intervention was administered following completion of the sleep assessment in the last month of pregnancy. Thus, the pregnancy sleep assessment for the control groups occurred before exposure to the attention-control intervention.
Participants in the SETS-P intervention group completed a pre-intervention assessment prior to receiving the intervention at 29–31 weeks of gestation. They then had two post-intervention assessments: one at approximately 36 weeks of gestation and the other at 1–2 months postpartum. The two control groups were assessed at 36 weeks of gestation and 1–2 months postpartum. Because the control groups did not have an equivalent assessment at 29–31 weeks of gestation, the pre-intervention assessment for the SETS-P group was not included in this analysis. Given that sleep typically worsens with advancing gestation, changes from pre- to post-intervention without a control group would not be meaningful.
All participants provided demographic information (age, race, ethnicity, education, employment, and income) as part of the initial screening for study eligibility. After the birth, participants were asked questions about the date of birth, labor duration, delivery type, and infant weight.
The timing of the prenatal and postpartum assessments was calculated as the difference between the dates the assessments were initiated and the date of birth.
Participants in the SETS-P group completed a brief self-report assessment of their adherence and satisfaction upon completion of the 4-week Sleep Enhancement Training System for Pregnancy intervention (at 36 weeks of gestation). Adherence was assessed for each weekly reading with questions about how much of each chapter they read (none, some, most, or all) and for each audio program with a question about how often they used it (not at all, a few days, most days, or every day). In addition, for each weekly reading and audio program, participants were asked how useful it was (not at all, somewhat, very, essential) and whether they would recommend it to others (yes or no). They were also asked two questions about how difficult it was to complete the weekly readings and the audio program (not at all, neutral, somewhat, or very difficult). To determine whether participants in the SETS-P group received additional doses of the intervention beyond the initial 4-week training, they answered another set of questions at the postpartum assessment (1–2 months after delivery) about whether and how often they had referred to the weekly readings or listened to the audio programs after completing the 4-week intervention (either prior to or after delivery).
To objectively estimate sleep duration and disruption, each participant was asked to wear a medical-quality wrist actigraph (Ambulatory Monitoring, Inc., Ardsley NY) for 72 consecutive hours as part of each assessment. The wrist-worn actigraph provides continuous data using a microprocessor that senses motion with a piezo-electric linear accelerometer. To minimize the variability due to potential differences in weekday and weekend sleep patterns, data were collected only on weekdays. The same actigraphs were used for the SETS-P group and both control groups.
Actigraph data were analyzed by trained research assistants blinded to intervention group using the autoscoring Cole-Kripke algorithm program available in Action4 software (Ambulatory Monitoring, Inc., Ardsley NY). Two sleep-related outcomes were evaluated in this study: (a) nocturnal sleep duration, or total sleep time in minutes, and (b) nocturnal sleep disruption, or wake after sleep onset, computed as the percentage of time spent awake relative to the time spent in bed after initially falling asleep. For a typical night of 7 hours in bed, wake after sleep onset of 15% represents approximately 1 hour of wake time after initially falling asleep. These outcomes represent standard sleep measures of sleep quantity and quality.13
Congruence between single-night polysomnographic measures and actigraphy measures indicate adequate validity and reliability when sleep is assessed in healthy young adults, including women of childbearing age,13 with strong agreement between the two.14 Although actigraphy has not been specifically validated for use with pregnant women, it is considered to be more acceptable and less burdensome than polysomnography, and more precise than self-report.15 In this study, participants were asked to wear the actigraph for a period of 72 hours to minimize participant burden in these samples of childbearing women. To facilitate interpretation of actigraphy data, participants used 72-hour sleep logs for recording their bed times, wake times, and times when the actigraph was removed. To minimize data loss, actigraphy records with at least 48 hours of data were included in the analysis. Paired t-tests revealed no statistically significant difference between night 1 and night 2 data, and the two nights were significantly correlated for total sleep time (r > .5). Therefore, actigraphy measures were averaged to obtain mean values for each assessment.
Data were analyzed using IBM SPSS Statistics Version 22.0 (IBM Corp, Armonk, NY). Descriptive statistics were used to summarize the characteristics of the sample. Sample characteristics for the three groups were compared using chi-square tests for categorical variables and analysis of variance for continuous variables, with Dunnet’s post-hoc tests for comparison of the intervention to each control group. Multilevel regression models (MLM) were used to compare the three groups on the post-intervention sleep outcomes of total sleep time and wake after sleep onset over time. MLMs were specified using the SPSS Mixed Models – Linear module, with full maximum likelihood. MLM analyses use all available data for each participant, even those with missing pregnancy or postpartum data. Missingness accounted for 5% of the data in the multilevel analyses. To evaluate the impact of mood disorder on the findings, a sensitivity analysis was conducted by excluding women with a history of mood disorder. A two-tailed alpha level of .05 was used for all statistical tests, and 95% confidence intervals (CI) are reported for all mean estimates. Effect sizes (Cohen’s d) were calculated for each post-intervention assessment. Power analyses indicated that 25 participants were needed for 80% power to detect a medium sized effect (i.e., Cohen’s d of 0.50).
Results
Of the 29 women enrolled in the Sleep Enhancement Training System for Pregnancy intervention study, 25 had post-intervention actigraphy data (20 prenatal and 25 postpartum) and were included in the analysis. The four women who were excluded had no post-intervention actigraphy data: one withdrew due to fetal demise, another no longer had time to participate, and two others delivered before completing the intervention and also missed the postpartum assessment. Control group A consisted of 76 women after excluding one participant expecting twins. Control group B consisted of 48 women after excluding two women with no prenatal or postpartum actigraphy data.
Sample characteristics for the three groups are presented in Table 2. As expected, the SETS-P intervention group and control group A were similarly more socioeconomically advantaged, and control group B was more racially and ethnically diverse. The groups did not differ significantly on any childbirth variables or on timing of the prenatal and postpartum assessments.
Table 2.
Sample characteristics for the experimental Sleep Enhancement Training System for Pregnancy group and the two control groups
| Sample characteristics | SETS-P (S) (n=25) |
Primary Control (A)* (n=76) |
Secondary Control (B)* (n=48) |
Statistics† |
|---|---|---|---|---|
| Demographics | ||||
| Maternal age (years), mean ± SD | 32.7 ± 4.4 | 32.1 ± 5.1 | 27.1 ± 6.5 | S>B, p<.001 |
| Race or Ethnicity, % (n) | p<.001 | |||
| Asian | 20% (5) | 17% (13) | 31% (15) | |
| African-American | 4% (1) | 3% (2) | 19% (9) | |
| Caucasian | 72% (18) | 63% (48) | 25% (12) | S>B, p<.001 |
| Latina | 0% (0) | 12% (9) | 23% (11) | S<B, p=.009 |
| Other | 4% (1) | 5% (4) | 2% (1) | |
| Completed college, % (n) | 88% (22) | 80% (61) | 33% (16) | S>B, p<.001 |
| Employed, % (n) | 80% (20) | 72% (55) | 27% (13) | S>B, p<.001 |
| Income ≥$3000/month, % (n) | 83% (20) | 92% (70) | 12% (5) | S>B, p<.001 |
| Childbirth characteristics | ||||
| Labor (hrs), mean ± SD‡ | 19.9 ± 11.1 | 17.3 ± 10.3 | 16.8 ± 10.8 | p=.58 |
| Cesarean rate, % (n) | 24% (6) | 25% (19 of 75) | 35% (16 of 46) | p=.47 |
| Infant birthweight (kg), mean ± SD§ | 3.46 ± 0.47 | 3.53 ± 0.44 | 3.36 ± 0.60 | p=.19 |
| Timing of Assessments | ||||
| Prenatal (days before birth)‖ | 21.2 ± 10.8 | 23.4 ± 10.2 | 18.3 ± 11.7 | p=.045¶ |
| Postpartum (days after birth)# | 30.7 ± 12.0 | 30.9 ± 14.8 | 28.4 ± 9.0 | p=.57 |
SETS-P, Sleep Enhancement Training System for Pregnancy; SD, standard deviation
The primary control group (A) had similar enrollment criteria and was demographically similar to the SETS-P group, while the secondary control group (B) was less socio-economically advantaged than the other two.
If omnibus ANOVA or chi-square test was significant, Dunnet’s post-hoc tests or post-hoc chi-square tests compared the SETS-P group to each of the control groups.
Labor duration is based on women with vaginal births only: SETS-P n=19; control A n=56; control B n=30.
Due to missing birthweight for 4 infants, n=74 for control A and n=46 for control B.
Due to missing prenatal data for 11 women, n=20 for SETS-P, n=71 for control A, and n=47 for control B.
The control groups differed from each other (p=.045), but neither differed from the SETS group in post-hoc tests.
Due to missing postpartum data for 6 women, n=73 for control A and n=45 for control B.
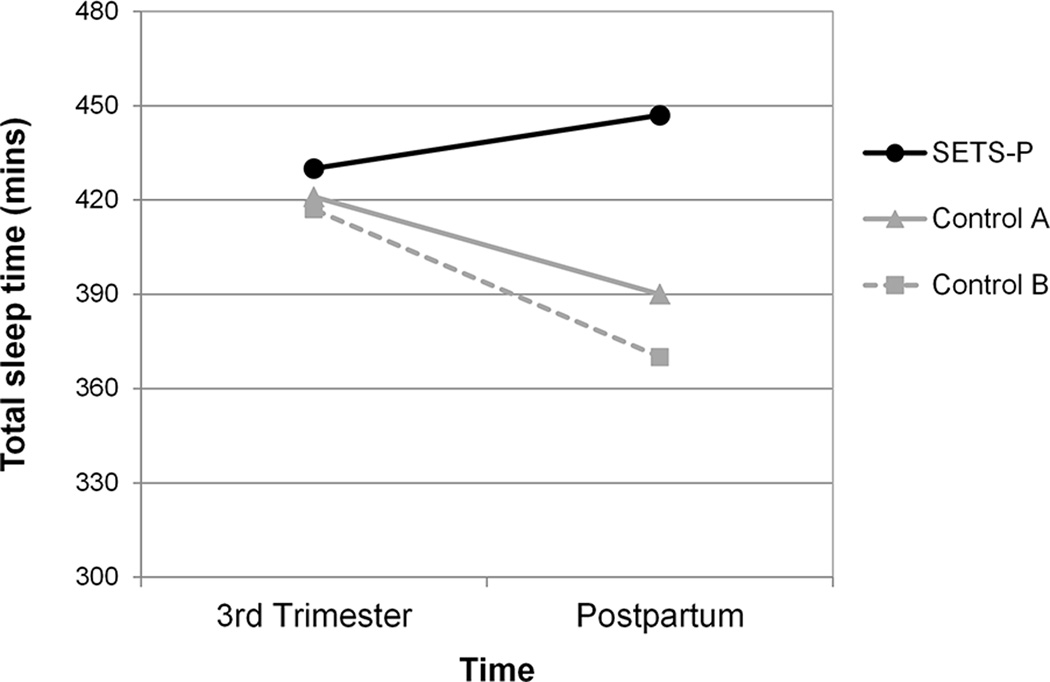
Participants in the SETS-P intervention group had significantly longer sleep duration and less sleep disruption compared to participants in either control group (Table 3). For sleep duration, there was a significant main effect for group (p=.020), as well as a significant group-by-time interaction (p=.032), reflecting that the effect of group was stronger at the postpartum assessment than at the third trimester assessment (Figure 1). At the pregnancy post-intervention assessment, the SETS-P group had a mean total sleep time of 430 minutes (CI 397–464), which was 10 and 14 minutes longer than control groups A and B, respectively). The effect sizes for these group comparisons (d = 0.10 and 0.13, respectively) indicate minimal intervention effects during the third trimester. At the postpartum assessment, mean total sleep time for the SETS-P group increased to 446 minutes (CI 415–478), while mean total sleep time for control groups A and B decreased over time and were 56 and 76 minutes lower than the SETS-P group, respectively). The effect sizes for these group comparisons (d = 0.77 and 0.87, respectively) indicated large intervention effects at the postpartum assessment.
Table 3.
Post-intervention group comparison of sleep characteristics
| Mean (95% CI) | ||||
|---|---|---|---|---|
| Sleep characteristic | SETS-P (n=25) |
Primary control (A) (n=76) |
Secondary control (B) (n=48) |
P-values for main and group × time interaction effects |
| Sleep duration (total sleep time, minutes) |
Group: p=.020 Interaction: p=.032 |
|||
| Pregnancy (36 weeks) | 430 (397–464) | 420 (403–438) | 417 (395–439) | |
| Postpartum (1–2 months) | 446 (415–478) | 390 (373–408) | 370 (348–393) | |
| Sleep disruption (wake after sleep onset, %) |
Group: p=.001 Interaction: p=.005 |
|||
| Pregnancy (36 weeks) | 16.1 (11.9–20.2) | 13.4 (11.2–15.5) | 20.4 (17.7–23.0) | |
| Postpartum (1–2 months) | 20.3 (16.4–24.1) | 26.6 (24.5–28.8) | 28.7 (26.0–31.5) | |
CI, confidence interval; SETS-P, Sleep Enhancement Training System for Pregnancy.
Figure 1. Sleep duration over time for the SETS-P group and two control groups.
Women who received the SETS-P intervention had more total sleep time than women in the control groups, particularly at the postpartum assessment.
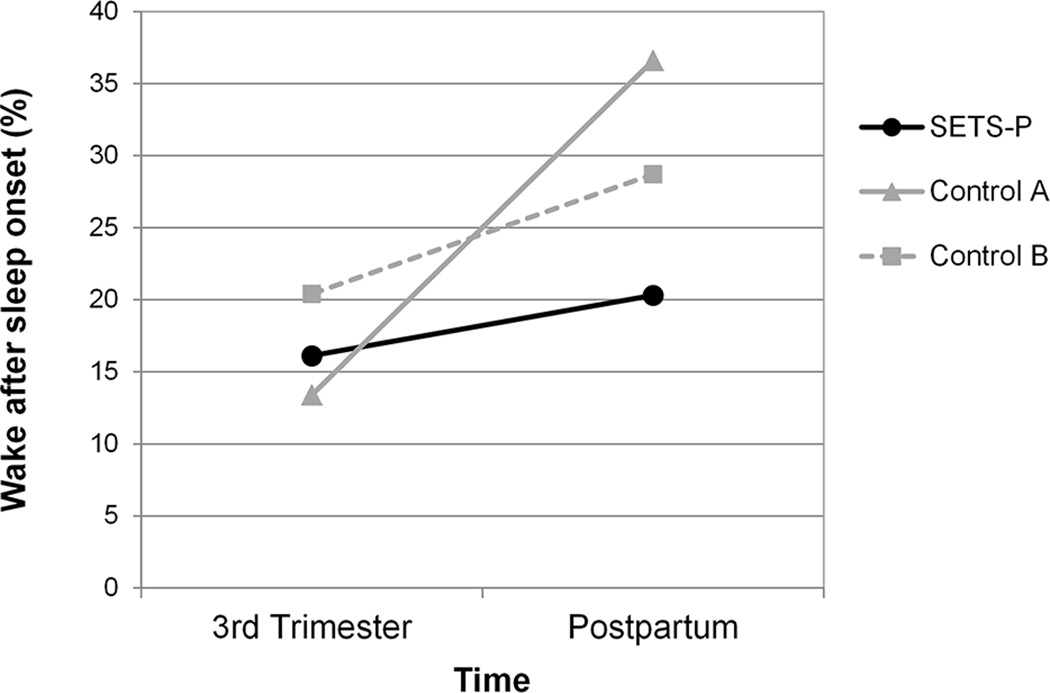
Similarly, for sleep disruption (i.e., wake after sleep onset), there was a significant main effect for group (p=.001), and again, there was a significant group-by-time interaction (p=.005), reflecting that the effect of group was only evident at the postpartum assessment (Figure 2). At the third trimester post-intervention assessment, the SETS-P group had mean wake after sleep onset (i.e., percentage of time spent awake relative to the time spent in bed after initially falling asleep) of 16.1%, which was 2.7 percentage points higher than control group A and 4.3 percentage points lower than control group B, respectively. The effect sizes for these third trimester pregnancy group comparisons (d = 0.35 and −0.39, respectively) indicated small intervention effects that differ in opposite directions from control groups A and B. At the postpartum assessment, infants were 4–8 weeks of age, and mean wake after sleep onset of the SETS-P group of mothers increased to 20.3%, while mean wake after sleep onset for control groups A and B mothers increased more sharply to 6.4 and 8.5 percentage points higher than the SETS-P group, respectively). The effect sizes for these group comparisons (d = −0.73 and −0.79, respectively) indicated moderate intervention effects at the postpartum assessment and in the same direction compared to both control groups A and B.
Figure 2. Sleep disruption over time for the SETS-P group and two control groups.
Women who received the SETS-P intervention had less sleep disruption (i.e., wake after sleep onset) than women in the control groups at the postpartum assessment. At the third trimester assessment, the SETS-P group had more sleep disruption than control A, but less than control B.
For the sensitivity analysis, we excluded the 10 women with a history of mood disorder from the SETS-P group (n=1) and control group A (n=9). There were no exclusions from control group B. Excluding these ten women did not alter the significance of the intervention effects.
Of the 29 participants enrolled in the Sleep Enhancement Training System for Pregnancy intervention, 27 completed the intervention evaluation at the third trimester pregnancy assessment and 25 completed an additional evaluation at the postpartum assessment. As shown in Table 1, most participants (89 – 96%, or an average of 93%) completed the weekly readings, and most (78 – 96%) would recommend them to others. Depending on the chapter, 48 – 67% found the information in the readings ‘Essential’ or ‘Very useful’. A majority of the participants (67 – 89%, or an average of 77%) reported using the 45-minute nightly audio programs on all or most nights, and 44 – 78% rated them as ‘Essential’ or ‘Very useful’. Of the four nightly audio programs, ‘Nature sounds’ was used mostly consistently, and 100% of the participants would recommend it to others. In contrast, only 22% reported consistent use of the daytime audio program, although it is presented as optional after the first intervention week. When asked about difficulty completing the intervention, 30% reported at least a little difficulty with the readings and 78% reported at least a little difficulty consistently listening to the audio programs. In the postpartum evaluation, 56% of the participants reported continuing to use the audio programs (mostly ‘Nature sounds’) after the 4-week intervention and prior to delivery, but none used it after delivery.
Discussion
This study demonstrated protocol feasibility and efficacy of a 4-week home-based, cognitive-behavioral intervention for enhancing maternal sleep, which typically worsens during late pregnancy and postpartum. Adherence was acceptable, with 93% of participants completing most or all of the weekly readings and 77% using the nightly audio programs most days or every day. The intervention did not significantly impact sleep during late pregnancy when sleep is continuing to worsen before labor begins. However, at the postpartum assessment, these new mothers slept about an hour longer and had less sleep disruption than both control groups. This finding was particularly surprising given that the intervention does not include content focused on night-time newborn care and none of the women reported using the auditory programs postpartum. Explanations for this delayed intervention response include the possibility that the stimulus control and relaxation training effects may improve over time or that sleep disturbance during pregnancy is influenced by physiological factors (e.g., increased urinary frequency, positional discomfort, restless legs) less amenable to a cognitive-behavioral intervention than postpartum sleep disturbance. Facilitating postpartum sleep may have additional benefits, such as reducing postpartum depression16 and improving maternal-infant bonding.17
Prior studies evaluating sleep interventions for childbearing women have yielded inconsistent results.18–20 Several interventions that may reduce stress and hyperarousal (e.g., yoga) have shown promise, but few used objective sleep measures.21, 22 Because postpartum sleep disturbance is often attributed to night-time infant care, most interventions aim to improve maternal sleep and well-being by improving infant sleep.18, 20 However, while these interventions may modestly increase infant sleep, they generally have limited effects on maternal and other infant outcomes.23 An alternative paradigm24 normalizes nocturnal infant waking and promotes restructuring of maternal cognitions, as well as stimulus control and relaxation to help mothers and infants fall back to sleep more easily. Consistent with this paradigm, the Sleep Enhancement Training System for Pregnancy intervention focuses on hyperarousal and counter-productive thoughts that can interfere with sleep. Although it does not include specific night-time infant care strategies, they could be incorporated into future versions.
The Sleep Enhancement Training System for Pregnancy audio programs utilize 3-D recording technology called 3-Dimensional Living Sound™. This sound technology has a life-like quality hypothesized to facilitate neurocognitive processes involved in relaxation and has been shown to improve outcomes in several clinical10, 25 and non-clinical11 populations. Our results support these prior findings and suggest that 3-D sound may also be a convenient and cost-effective option for promoting relaxation and improving sleep for childbearing women.
In this study, participants started the 4-week intervention at approximately 30 weeks of gestation in anticipation of worsening sleep by 40 weeks of gestation. Since most women did not benefit from the intervention at this stage of pregnancy and several delivered before finishing it, future studies should consider initiating the intervention earlier in pregnancy. This would allow women more time to develop relaxation skills prior to the onset of third-trimester sleep problems.
Results from this study need to be interpreted in consideration of its strengths and limitations. The sample is small, which precluded analysis of potentially confounding variables, such as infant feeding type or help with night-time infant care. The study design did not involve random assignment, but instead made use of existing control groups from other studies, and thus observed sleep differences may be due to other factors. Controls were enrolled and received the intervention at a later gestational age than the intervention group, and the lack of a 31-week assessment for controls precluded group comparisons of pre- to post-intervention changes. History of mood disorder was an exclusion only for control group B, although a sensitivity analysis suggests this did not impact study findings. Use of an objective sleep measure was a strength, but to minimize participant burden and missing data, outcomes were based on 48 hours of actigraphy rather than the recommended 7 days. Additionally, actigraphy has not been validated for use with pregnant women, and it is possible that some physiological effects of pregnancy could impact an actigraph’s ability to accurately distinguish sleep from wake. Finally, the intervention was designed to reduce hyperarousal that interferes with sleep, but hyperarousal was not specifically assessed in this initial study and therefore could not be evaluated as a potential mechanism for the observed postpartum sleep improvements.
In conclusion, this home-based cognitive-behavioral intervention demonstrates promise for promoting sleep in late pregnancy and early postpartum. It has high potential as a convenient, low-cost alternative to more resource-intensive group-based or professionally-administered interventions. Further evaluation of home-based, relaxation-focused, cognitive-behavioral interventions is warranted as a strategy for optimizing the sleep, health, and well-being of childbearing women.
Acknowledgments
Finanical Support: This research was supported by a grant from the National Institutes of Health (# R43 HD051243). The study was conducted at the University of California, San Francisco.
Christopher Alsten was President of Inner Health, Inc. and developed the experimental intervention evaluated in this study. He is included as a posthumous co-author based on his substantial contributions to the development of the study and his role as its Principal Investigator.
Supported by a grant from the National Institutes of Health (# R43 HD051243).
The authors thank Dr. Tom Jackson, MD, for developing the 3D Living Sound© audio programs included in the SETS intervention and granting permission for use in this research, Annelise Gardiner for her contributions to data collection and actigraphy analysis, and the research participants for generously giving their time.
Footnotes
Financial Disclosure: The other authors did not report any potential conflicts of interest.
References
- 1.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 2.Hutchison BL, Stone PR, McCowan LM, Stewart AW, Thompson JM, Mitchell EA. A postal survey of maternal sleep in late pregnancy. BMC Pregnancy Childbirth. 2012;12:144. doi: 10.1186/1471-2393-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivertsen B, Hysing M, Dorheim SK, Eberhard-Gran M. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth. 2015;15:129. doi: 10.1186/s12884-015-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203:465 e1–465 e7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191:2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 6.Blair LM, Porter K, Leblebicioglu B, Christian LM. Poor sleep quality and associated inflammation predict preterm birth: heightened risk among African Americans. Sleep. 2015;38:1259–1267. doi: 10.5665/sleep.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung HM, Ko SH, Chen CH. The association between prenatal sleep quality and obstetric outcome. J Nurs Res. 2014;22:147–154. doi: 10.1097/jnr.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 8.Palagini L, Gemignani A, Banti S, Manconi M, Mauri M, Riemann D. Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 2014;15:853–859. doi: 10.1016/j.sleep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Tsai SY, Lee CN, Wu WW, Landis CA. Sleep hygiene and sleep quality of third-trimester pregnant women. Res Nurs Health. 2016;39:57–65. doi: 10.1002/nur.21705. [DOI] [PubMed] [Google Scholar]
- 10.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17:288–298. [PubMed] [Google Scholar]
- 11.Lee KA, Gay CL, Alsten CR. Home-based behavioral sleep training for shift workers: a pilot study. Behav Sleep Med. 2014;12:455–468. doi: 10.1080/15402002.2013.825840. [DOI] [PubMed] [Google Scholar]
- 12.Lee KA, Gay CL. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Res Nurs Health. 2011;34:7–19. doi: 10.1002/nur.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 14.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Herring SJ, Foster GD, Pien GW, Massa K, Nelson DB, Gehrman PR, et al. Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breath. 2013;17:1323–1327. doi: 10.1007/s11325-013-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson A, Murphy KE, Sloan E, Uleryk E, Dalfen A. The relationship between sleep and postpartum mental disorders: A systematic review. J Affect Disord. 2015;176:65–77. doi: 10.1016/j.jad.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Tikotzky L. Postpartum maternal sleep, maternal depressive symptoms and self-perceived mother-infant emotional relationship. Behav Sleep Med. 2016;14:5–22. doi: 10.1080/15402002.2014.940111. [DOI] [PubMed] [Google Scholar]
- 18.Stremler R, Hodnett E, Lee K, MacMillan S, Mill C, Ongcangco L, et al. A behavioral-educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006;29:1609–1615. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]
- 19.Stremler R, Hodnett E, Kenton L, Lee K, Weiss S, Weston J, et al. Effect of behavioural-educational intervention on sleep for primiparous women and their infants in early postpartum: multisite randomised controlled trial. BMJ. 2013;346:f1164. doi: 10.1136/bmj.f1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall WA, Hutton E, Brant RF, Collet JP, Gregg K, Saunders R, et al. A randomized controlled trial of an intervention for infants' behavioral sleep problems. BMC Pediatr. 2015;15:181. doi: 10.1186/s12887-015-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beddoe AE, Lee KA, Weiss SJ, Kennedy HP, Yang CP. Effects of mindful yoga on sleep in pregnant women: a pilot study. Biol Res Nurs. 2010;11:363–370. doi: 10.1177/1099800409356320. [DOI] [PubMed] [Google Scholar]
- 22.Ko YL, Lee HJ. Randomised controlled trial of the effectiveness of using back massage to improve sleep quality among Taiwanese insomnia postpartum women. Midwifery. 2014;30:60–64. doi: 10.1016/j.midw.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Douglas PS, Hill PS. Behavioral sleep interventions in the first six months of life do not improve outcomes for mothers or infants: a systematic review. J Dev Behav Pediatr. 2013;34:497–507. doi: 10.1097/DBP.0b013e31829cafa6. [DOI] [PubMed] [Google Scholar]
- 24.Whittingham K, Douglas P. Optimizing parent-infant sleep from birth to 6 months: a new paradigm. Infant Ment Health J. 2014;35:614–623. doi: 10.1002/imhj.21455. [DOI] [PubMed] [Google Scholar]
- 25.Ondrasik S, Russo M, Alsten C. The use of "virtual reality" sound technology in psychophysiological sleep enhancement training. Aviat Space Environ Med. 2010;81:525–526. doi: 10.3357/asem.2764.2010. [DOI] [PubMed] [Google Scholar]