Abstract
Introduction
25-hydroxyvitamin D [25(OH)D] deficiency has been associated with low testosterone levels in men, but there are conflicting reports of its associations with sex hormones in women. Less is known about whether these associations are independent of adiposity and lifestyle factors, and whether they differ by race/ethnicity.
Aim
To examine associations of 25(OH)D concentrations with sex hormone levels.
Methods
Cross-sectional analysis of 3017 men and 2929 women in a multi-ethnic cohort.
Main Outcome Measures
Testosterone, estradiol, dehydroepiandrosterone (DHEA), sex hormone binding globulin (SHBG), and free testosterone.
Results
The mean (SD) levels of 25(OH)D in men and women were 25.7(10.4) and 26.1(12.0) ng/ml, respectively. In men, after adjusting for demographic and lifestyle variables, a 10 ng/ml [25 nmol/L] decrease in 25(OH)D was associated with an average difference of −0.70 nmol/L (95%CI −1.36, −0.05) in SHBG and 0.02 percent (0.01, 0.04) in free testosterone, but was not associated with low total testosterone level (<10.41 nmol/L). In women, a 10 ng/ml decrease in 25(OH)D levels was associated with an average difference of −0.01 nmol/L (−0.01, −0.00) for estradiol, −8.29 nmol/L (−10.13, −6.45) for SHBG, 0.06 percent (0.04, 0.07) for free testosterone, and 0.40 nmol/L (0.19, 0.62) for DHEA. There was no significant interaction by race/ethnicity.
Conclusions
Lower 25(OH)D concentrations were associated with lower SHBG levels and higher free testosterone levels in both men and women, and lower estradiol and higher DHEA levels in women, independent of adiposity and lifestyle. We observed no significant association of 25(OH)D with total testosterone in men. Future studies are needed to determine whether vitamin D supplementation influences sex hormone levels.
Keywords: vitamin D, sex hormones, testosterone, estradiol, sex hormone binding globulin, epidemiology
Graphical Abstract
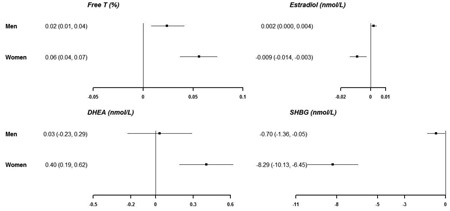
In a cross-sectional analysis of 3017 men and 2931 women in a multi-ethnic cohort, lower vitamin D levels [per 10 ng/ml (25 nmol/L) lower 25(OH)D] were associated with lower SHBG and higher free testosterone in both men and women, and lower estradiol and higher DHEA in women, independent of adiposity and lifestyle factors. Future studies are needed to determine whether vitamin D supplementation influences sex hormone levels
Introduction
Low circulating concentrations of 25-hydroxyvitamin D [25(OH)D], defined as less than 30 ng/ml, are observed in over two-thirds of the US adult population and in an estimated 1 billion individuals worldwide.[1] Deficient 25(OH)D levels are also associated with increased risk for atherosclerotic cardiovascular disease (ASCVD) events.[2–4] Suboptimal vitamin D status is thought to increase ASCVD risk by influencing established vascular risk factors, namely hypertension, diabetes, inflammation, and endothelial dysfunction.[4]
The association of 25(OH)D deficiency with ASCVD may in part be mediated by the sex hormones testosterone (T) and estradiol (E2), as sex hormone levels have also been linked to ASCVD risk and mortality.[5,6] The active metabolite of vitamin D, 1,25-dihydroxyvitamin D, regulates a number of enzymes involved in steroid hormone production, including both adrenal steroid hormones and sex hormones, as well as sex hormone signaling.[7,8]
In men, 25(OH)D levels are positively related to T levels,[9–14] and this association varies by vitamin D receptor genotype[15] and may be attenuated after accounting for adiposity.[9] Treatment with vitamin D supplements may increase T levels.[16] Data from testicular cell cultures suggest that vitamin D, especially 1,25-dihydroxyvitamin D3, has a major role in male steroidogenesis.[7]
In women, studies evaluating the association of vitamin D with sex hormones are inconclusive. In one study of Korean women, 25(OH)D levels were positively associated with higher T but not significantly with E2.[17] In contrast, another study from the Netherlands found that 25(OH)D levels were inversely associated with E2,[18] and a study of women with polycystic ovarian disease found that 25(OH)D levels were inversely associated with free T and DHEA, and positively associated with SHBG.[19] These contrasting studies differed in study populations, race/ethnicity, and adjustment for adiposity measures.
Many of the studies investigating the association of vitamin D with sex hormones were conducted in populations with narrow demographics such as only older adults,[12] in Caucasians,[12,15] in Asian men,[9,10] or in specific conditions such as polycystic ovarian disease.[19] Little is known about potential racial differences in the association between 25(OH)D and sex hormones. Even though U.S white adults have average higher levels of 25(OH)D compared to blacks due to increased skin pigmentation in blacks which blocks UVB-driven synthesis of vitamin D,[20] low 25(OH)D may be a stronger risk factor for diabetes[21] and ASCVD[2,3] in whites compared to blacks.
Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), we sought to evaluate the cross-sectional associations between 25(OH)D levels and sex hormones stratified by sex and determine whether any associations differ by racial/ethnicity. We hypothesized (1) in men, 25(OH)D levels will be positively associated with T levels; (2) in women, 25(OH)D levels will be positively associated with E2 and inversely associated with T; and (3) in both men and women, 25(OH)D will be positively associated with SHBG. We hypothesized that associations would be stronger among whites than blacks.
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) study design has previously been reported.[22] Briefly, MESA is a prospective cohort study from six sites across the U.S. investigating risk factors and progression of subclinical ASCVD. The baseline information was collected between 2000 and 2002 from 6,814 individuals aged 45–84 years, from four race/ethnic groups (White, Black, Hispanic, and Chinese), who were free from clinically known ASCVD. The present analysis included all study participants at the baseline exam, excluding 641 participants who did not have sex hormone measurement and 225 participants who did not have 25(OH)D measurement, for a total analytic sample of 5,948 (49% women), [Supplemental Figure 1]. The study was approved by the institutional review board of each participating institution, and all participants provided informed consent.
Baseline Factors
Demographic characteristics, smoking status, physical activity, medical history, self-reported health status, and medication use including blood pressure and cholesterol-lowering medications were collected through standardized questionnaires. Level of education was defined as <high school, high school/technical school/ associate degree, and college/graduate/ professional school. Physical activity was estimated as the total amount of intentional exercise performed in a usual week and measured in metabolic equivalent task–minutes. Smoking status was categorized into never, former, or current smoker. Self-reported health status was classified into poor/fair, good, and very good/excellent.
Height, weight, waist circumference, hip circumference, and blood pressure were measured by trained staff. Body mass index (BMI) was calculated as weight (kg)/ height (m2). Waist-hip ratio (WHR) was calculated as waist circumference (cm)/ hip circumference (cm). Resting blood pressure was measured three times in the seated position using a Dinamap automated sphygmomanometer, and the average of the 2nd and 3rd readings was used as the baseline blood pressure.
At the MESA baseline exam, blood samples were obtained in the morning between 7:30 am and 10:30 am after a 12-hour fast to measure glucose, lipids and high-sensitivity C-reactive protein (hsCRP). Additional serum was stored at −70 °C, and 25(OH)D and sex hormones were later measured from stored frozen samples (see details below). Diabetes was classified as having a fasting blood glucose ≥126 mg/dl (7 mmol/l) and/or the self-reported history of a physician-diagnosis of diabetes, or the use of diabetes medications. Estimated glomerular filtration rate (eGFR) was calculated based on the combination of serum creatinine and cystatin C concentrations using the CKD-EPI equation.[23]
Measurement of 25-Hydroxyvitamin D Concentrations
25(OH)D concentrations were measured in frozen serum samples collected at the MESA baseline exam by high-performance HPLC–tandem mass spectrometry at the University of Washington,[24] and calibrated to National Institute of Standards and Technology standards.[25] Interassay coefficients of variation were 8.5% for 25(OH)D3 and 11.8% for 25(OH)D2. Since 25(OH)D and sex hormone levels were measured from samples obtained at the same visit, we did not adjust for seasonal variation. 25(OH)D levels were used to categorize vitamin D status into three clinically relevant groups as endorsed by the Endocrinology Society Clinical Practice Guidelines:[20] ≥30 ng/ml (≥75 nmol/L, optimal), 20–<30 ng/ml (50–<75 nmol/l, intermediate) and <20 ng/ml (<50 nmol/L, deficient). To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
Measurement of Sex hormones
Using frozen serum samples that were collected in the morning of the MESA baseline exam, sex hormone concentrations were measured at the University of Massachusetts Medical Center Sex Hormone Laboratory (Worcester, MA). Total T and dehydroepiandrosterone (DHEA) were measured using radioimmunoassay kits, SHBG by a chemiluminescent enzyme immunometric assay using Immulite kits (Diagnostic Products Corporation, Los Angeles, CA), and E2 by an ultra-sensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX).[26] Concentrations of free T percent was calculated from total T, SHBG, and albumin levels using a method described by calculated according to the method of Södergård et al,[27] and bioavailable T was calculated as the sum of SHBG-bound T and albumin-bound T. Bioavailable T, calculated from T and SHBG, has been shown to be comparable to apparent free T concentration obtained by equilibrium dialysis.[28] Testosterone deficiency was defined as total T<10.41 nmoI/L (300 ng/dl). The quality control serum was obtained from a large pool that was aliquoted into storage vials and labeled identically to MESA participant samples. The coefficient of variation for total T, SHBG, DHEA and E2 were 12.3%, 9.0%, 11.2% and 10.5%, respectively.
Statistical Analysis
All analyses were stratified by sex. Multivariable linear regression was used to assess the cross-sectional associations of 25(OH)D and sex hormones levels at the MESA baseline exam. Logistic regression was used to assess the association of 25(OH)D with the odds of testosterone deficiency among men. Multivariable-adjusted average differences and 95% confidence intervals were calculated per a 10 ng/ml (25 nmol/L) decrease in 25(OH)D concentrations assuming a linear relationship between 25(OH)D and hormone levels. In addition, we compared those with 25(OH)D deficiency (<20 ng/ml [50 nmol/L]) to those with optimal levels (≥30 ng/ml [75 nmol/L]).
To account for potential confounders, we used models with increasing degrees of adjustment. Model 1 adjusted for age, race/ethnicity, and study site. Model 2, our primary analytic model, further adjusted for lifestyle variables including BMI, smoking, education, intentional physical exercise, and self-reported health status. Model 3 further adjusted for potential vascular risk factors including diabetes, systolic blood pressure, use of antihypertensive medications, eGFR, total cholesterol, HDL cholesterol, use of lipid lowering medication usage, and hsCRP. These vascular risk factors may be affected by vitamin D, but they are not known to directly influence the association of vitamin D with sex hormones, so this was an exploratory model. We also performed 2 additional sensitivity analyses: (1) excluding women who were on hormone replacement therapy and (2) using WHR instead of BMI as our measure of adiposity.
We used Wald tests for cross-product terms to test for interactions between 25(OHD) and race/ethnicity. All reported P values were two-sided and the significance level was set at 0.05. All analyses were performed using STATA version 12 (StataCorp LP, College Station, Texas).
Results
The mean(SD) ages of men and women were 62.1(10.2) and 64.6(9.1) years, and the mean(SD) 25(OH)D levels were 25.7(10.4) and 26.1(12.0) ng/ml [corresponds to 64.1(26.0) and 65.1(30.0) nmol/L), respectively. Vitamin D deficiency was present in 31% of men and 32% of women. Vitamin D deficient participants were younger, more likely to be black, smokers, and high school graduates, and more likely to have higher DHEA, free T, BMI, and blood pressure, and lower SHBG, triglycerides and HDL cholesterol compared with participants with optimal 25(OH)D (Table 1).
Table 1.
Characteristics of participants by 25(OH)D categories (N=5,946) at MESA baseline exam (2000–2002)*
| Men | Women | |||||
|---|---|---|---|---|---|---|
| 25(OH)D Categories | ≥30 ng/ml | 20–<30 ng/ml | <20 ng/ml | ≥30 ng/ml | 20–<30 ng/ml | <20 ng/ml |
| N | 950 | 1077 | 990 | 1028 | 903 | 998 |
| Demographics & Behaviors | ||||||
| Age, years | 63.1 ± 10.1 | 62.4 ± 10.3 | 61.0 ± 10.1 | 65.4 ± 9.1 | 64.9 ± 9.1 | 63.5 ± 9.0 |
| Race/Ethnicity | ||||||
| White | 550 (57.9) | 430 (39.9) | 213 (21.5) | 598 (58.2) | 300 (33.2) | 217 (21.7) |
| Chinese-American | 110 (11.6) | 162 (15.0) | 108 (10.9) | 134 (13.0) | 138 (15.3) | 72 (7.2) |
| Black | 84 (8.8) | 243 (22.6) | 445 (44.9) | 132 (12.8) | 218 (24.1) | 484 (48.5) |
| Hispanic | 206 (21.7) | 242 (22.5) | 224 (22.6) | 164 (16.0) | 247 (27.4) | 225 (22.5) |
| Education | ||||||
| <High school | 139 (14.7) | 171 (15.9) | 171 (17.4) | 171 (16.7) | 226 (25.1) | 227 (22.9) |
| High school, technical school, or associate degree |
388 (41.0) | 452 (42.0) | 437 (44.4) | 505 (49.2) | 431 (47.9) | 539 (54.3) |
| College, graduate or professional school |
420 (44.4) | 453 (42.1) | 376 (38.2) | 350 (34.1) | 242 (26.9) | 227 (22.9) |
| Smoking | ||||||
| Never | 371 (39.2) | 462 (42.9) | 400 (40.6) | 597 (58.2) | 559 (62.2) | 561 (56.5) |
| Former | 462 (48.8) | 470 (43.7) | 415 (42.1) | 356 (34.7) | 245 (27.3) | 275 (27.7) |
| Current | 114 (12.0) | 144 (13.4) | 170 (17.3) | 73 (7.1) | 95 (10.6) | 157 (15.8) |
| Total intentional exercise, met-min/week† | 1320.0 (427.5 – 2730.0) |
1050.0 (247.5 – 2235.0) |
735.0 (0.0 – 1905.0) |
915.0 (262.5 – 2070.0) |
660.0 (105.0 – 1650.0) |
502.5 (0.0 – 1470.0) |
| Sex Hormone Biomarkers | ||||||
| Total testosterone (nmoI/L) | 15.3 ± 5.8 | 14.7 ± 5.1 | 14.7 ± 5.7 | 1.0 ± 1.1 | 1.1 ± 1.1 | 1.1 ± 0.8 |
| Low total testosterone (<10.41 nmoI/L)‡ | 152 (16.0) | 191 (17.8) | 213 (21.5) | |||
| Bioavailable Testosterone (nmoI/L) | 5.5 ± 2.6 | 5.4 ± 1.8 | 5.4 ± 1.9 | 0.3 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.3 |
| Estradiol (nmoI/L) | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.2 |
| Dehydroepiandrosterone (nmoI/L) | 13.5 ± 7.1 | 13.9 ± 7.2 | 14.8 ± 8.6 | 10.5 ± 5.9 | 11.4 ± 6.2 | 12.4 ± 6.6 |
| Free Testosterone (Percent) | 1.9 ± 0.5 | 2.0 ± 0.5 | 2.0 ± 0.5 | 1.2 ± 0.6 | 1.4 ± 0.6 | 1.4 ± 0.5 |
| SHBG (nmoI/L) | 46.3 ± 18.4 | 43.2 ± 18.9 | 43.8 ± 20.2 | 93.9 ± 66.9 | 73.5 ± 51.1 | 64.1 ± 40.2 |
| Physiologic Characteristics | ||||||
| Health status | ||||||
| Poor/fair | 47 (5.0) | 64 (6.0) | 104 (10.6) | 71 (6.9) | 113 (12.6) | 146 (14.7) |
| Good | 350 (37.1) | 430 (40.5) | 396 (40.3) | 397 (38.8) | 414 (46.2) | 472 (47.5) |
| Very good/excellent | 546 (57.9) | 569 (53.5) | 482 (49.1) | 554 (54.2) | 370 (41.2) | 375 (37.8) |
| BMI, kg/m2 | 27.1 ± 3.9 | 27.8 ± 4.3 | 28.6 ± 4.8 | 26.6 ± 5.2 | 28.6 ± 5.4 | 30.8 ± 6.6 |
| Waist circumference, cm | 97.7 ± 10.8 | 99.0 ± 11.9 | 100.7 ± 13.4 | 92.7 ± 14.2 | 98.0 ± 14.7 | 101.9 ± 16.3 |
| Systolic BP, mm Hg | 124.8 ± 18.9 | 125.8 ± 19.0 | 127.1 ± 20.1 | 126.7 ± 22.8 | 129.4 ± 23.3 | 132.0 ± 23.9 |
| Diastolic BP, mm Hg | 74.1 ± 9.1 | 74.8 ± 9.3 | 76.1 ± 9.8 | 67.6 ± 9.6 | 69.2 ± 9.8 | 70.7 ± 11.0 |
| Total cholesterol, mg/dl | 189.5 ± 32.6 | 188.3 ± 35.7 | 186.5 ± 35.8 | 201.2 ± 34.2 | 201.3 ± 34.6 | 201.1 ± 38.2 |
| HDL cholesterol, mg/dl | 46.1 ± 11.8 | 44.1 ± 11.6 | 45.2 ± 12.2 | 60.0 ± 16.0 | 55.5 ± 14.6 | 54.2 ± 15.0 |
| LDL cholesterol, mg/dl | 117.3 ± 29.4 | 116.3 ± 30.4 | 115.9 ± 32.6 | 114.5 ± 30.7 | 118.9 ± 31.7 | 121.5 ± 33.9 |
| Triglyceride, mg/dl | 133.2 ± 81.7 | 143.3 ± 120.1 | 129.8 ± 79.5 | 131.9 ± 73.1 | 134.4 ± 74.3 | 127.7 ± 88.3 |
| eGFR, mL/min/1.73 m2 | 75.0 ± 15.0 | 78.4 ± 15.4 | 82.1 ± 16.8 | 71.9 ± 14.7 | 76.8 ± 15.8 | 78.7 ± 16.4 |
| CRP, mg/l† | 1.2 (0.6 – 2.8) | 1.4 (0.7 – 2.9) | 1.6 (0.7 – 3.4) | 2.4 (1.0 – 5.0) | 2.5 (1.0 – 5.5) | 3.2 (1.5 – 6.7) |
| Antihypertension medication | 321 (33.8) | 389 (36.1) | 355 (35.9) | 379 (36.9) | 382 (42.3) | 455 (45.6) |
| Lipid lowering medication usage | 176 (18.6) | 185 (17.2) | 137 (13.9) | 194 (18.9) | 169 (18.8) | 178 (17.9) |
| Hormone replacement therapy | NA | NA | NA | 426 (42.3) | 287 (32.4) | 229 (23.8) |
data are mean ± SD or number (%)
data are median (IQR)
low testosterone was only defined in men
To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496. To convert TC, HDL-C, and LDL-C to mmol/L from mg/dL, divide by 38.67. To convert TG to mmol/L from mg/dL, divide by 88.57.
Abbreviations: 25-hydroxyvitamin D, 25(OH)D; Multiethnic study of Atherosclerosis, MESA; Sex Hormone Binding Globulin, SHBG; blood pressure, BP; High density lipoprotein, HDL; Low density lipoprotein, LDL; estimated Glomerular Filtration Rate, eGFR; C-Reactive Protein, CRP
The association of 25(OH)D concentrations with sex hormone levels in men is shown in Table 2 (and stratified by race/ethnicity in Supplemental Table 1). Among men, in models adjusted for demographics and lifestyle factors, a 10 ng/ml decrease in 25(OH)D was associated with an average difference of −0.70 nmol/L (−1.36, −0.05) for SHBG, 0.0002 nmol/L (0.000, 0.004) for E2, and 0.02 percent (0.01, 0.04) for free T (Model 2), which remained statistically significant after further adjustment for vascular risk factors (Model 3). Patterns of association were similar when comparing vitamin D deficiency with optimal levels, but the association of 25(OH)D deficiency with lower SHBG was only statistically significant in Model 1.
Table 2.
| Adjusted difference (95% CI) | |||
|---|---|---|---|
| Model 1† | Model 2‡,# | Model 3‖ | |
| 25(OH)D, per 10 ng/ml lower | |||
| Total T (nmoI/L) | −0.39 (−0.59, −0.19) | −0.18 (−0.38, 0.01) | −0.14 (−0.34, 0.05) |
| Bioavailable T (nmoI/L) | −0.05 (−0.12, 0.02) | −0.01 (−0.08, 0.07) | 0.01 (−0.06, 0.09) |
| Estradiol (nmoI/L) | 0.002 (0.001, 0.004) | 0.002 (0.000, 0.004) | 0.002 (0.000, 0.004) |
| DHEA (nmoI/L) | 0.02 (−0.23, 0.27) | 0.03 (−0.23, 0.29) | 0.01 (−0.25, 0.27) |
| Free T (Percent) | 0.03 (0.02, 0.05) | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.04) |
| SHBG (nmoI/L) | −1.19 (−1.85, −0.53) | −0.70 (−1.36, −0.05) | −0.73 (−1.37, −0.09) |
| Total T (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −0.74 (−1.23, −0.25) | −0.45 (−0.93, 0.03) | −0.33 (−0.81, 0.14) |
| 25(OH)D <20 ng/ml | −0.95 (−1.49, −0.41) | −0.46 (−0.99, 0.07) | −0.39 (−0.92, 0.14) |
| Bioavailable T (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −0.06 (−0.23, 0.12) | −0.00 (−0.18, 0.17) | 0.02 (−0.16, 0.20) |
| 25(OH)D <20 ng/ml | −0.19 (−0.39, 0.01) | −0.09 (−0.28, 0.11) | −0.04 (−0.24, 0.17) |
| Estradiol (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.001 (−0.003, 0.006) | 0.001 (−0.004, 0.005) | 0.001 (−0.004, 0.005) |
| 25(OH)D <20 ng/ml | 0.003 (−0.002, 0.008) | 0.002 (−0.003, 0.007) | 0.003 (−0.002, 0.007) |
| DHEA (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −0.08 (−0.70, 0.53) | −0.06 (−0.68, 0.56) | −0.05 (−0.68, 0.57) |
| 25(OH)D <20 ng/ml | 0.06 (−0.62, 0.74) | 0.08 (−0.61, 0.77) | −0.03 (−0.72, 0.67) |
| Free T (Percent) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.09 (0.05, 0.13) | 0.07 (0.03, 0.11) | 0.06 (0.02, 0.10) |
| 25(OH)D <20 ng/ml | 0.07 (0.03, 0.12) | 0.05 (0.00, 0.09) | 0.05 (0.01, 0.10) |
| SHBG (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −3.03 (−4.64, −1.41) | −2.21 (−3.79, −0.63) | −1.87 (−3.41, −0.32) |
| 25(OH)D <20 ng/ml | −2.39 (−4.17, −0.61) | −1.24 (−2.99, 0.51) | −1.46 (−3.17, 0.25) |
Results are shown as beta coefficients (95% CI) from linear regression. Statistically significant results are bolded. To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
25(OH)D = 25-hydroxyvitamin D; T= Testosterone; DHEA = Dehydroepiandrosterone; SHBG = Sex hormone binding globulin
Model 1: age, race/ethnicity, study site
Model 2: Model 1 plus additional potential confounding lifestyle variables (BMI categories, smoking, education, self-reported good health status, intentional physical activity)
Model 3: Model 2 plus potential vascular risk factor mediators (diabetes, systolic blood pressure, use of antihypertensive medications, eGFR, total cholesterol, HDL cholesterol, use of lipid lowering medication usage, C-reactive protein.)
p-interactions for race/ethnicity used Model 2 and were 0.69, 0.88, 0.62, 0.47, 0.80, 0.61 for Total T, Bioavailable T, Estradiol, DHEA, Free T, and SHBG, respectively.
The association of 25(OH)D concentrations with the odds of low T levels (<10.41 nmol/L) in men is shown in Table 3 (and stratified by race/ethnicity in Supplemental Table 2). Men with 25(OH)D deficiency had a higher prevalence of total T deficiency in models adjusted for demographic factors only (Model 1), but this association was attenuated and no longer statistically significant after further adjustment for adiposity and lifestyle factors.
Table 3.
Associations of serum 25(OH)D concentrations with low total testosterone (<10.41 nmoI/L) in men*
| Adjusted Odds Ratios (95% CI) | |||
|---|---|---|---|
| Model 1† | Model 2‡ | Model 3‖ | |
| 25(OH)D, per 10 ng/ml lower | 1.19 (1.08, 1.32) | 1.11 (0.99, 1.23) | 1.08 (0.96, 1.20) |
| 25(OH)D categories | |||
| 25(OH)D ≥30 ng/ml | Reference (1) | Reference (1) | Reference (1) |
| 25(OH)D 20–<30 ng/ml | 1.12 (0.88, 1.43) | 1.02 (0.79, 1.30) | 0.96 (0.75, 1.24) |
| 25(OH)D <20 ng/ml | 1.46 (1.13, 1.89) | 1.23 (0.94, 1.60) | 1.18 (0.90, 1.55) |
Results presented as Odds Ratios (95% CI) from logistic regression. Statistically significant results are bolded. To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
Model 1: age, race/ethnicity, study site
Model 2: Model 1 plus additional potential confounding lifestyle variables (BMI categories, smoking, education, self-reported good health status, intentional physical activity)
Model 3: Model 2 plus potential vascular risk factor mediators (diabetes, systolic blood pressure, use of antihypertensive medications, eGFR categories, total cholesterol, HDL cholesterol, use of lipid lowering medication usage, C-reactive protein.)
The association of 25(OH)D concentrations with sex hormone levels in women is shown in Table 4 (and stratified by race/ethnicity in Supplemental Table 3). Among women, in models adjusted for demographics and lifestyle factors, a 10 ng/ml decrease in 25(OH)D was associated with an average difference of −0.01 nmol/L (−0.01, −0.00)] for E2, −8.29 nmol/L (−10.13, −6.45) for SHBG, 0.06 percent (0.04, 0.07) for free T, and 0.40 nmol/L (0.19, 0.62) for DHEA (Model 2), which remained similar and statistically significant after further adjustment for vascular risk factors except for E2 (Model 3). Patterns of associations were similar when comparing vitamin D deficient to optimal levels.
Table 4.
| Adjusted differences (95% CI) | |||
|---|---|---|---|
| Model 1† | Model 2‡,# | Model 3‖ | |
| 25(OH)D, per 10 ng/ml lower | |||
| Total T (nmoI/L) | 0.01 (−0.03, 0.04) | −0.01 (−0.05, 0.02) | −0.02 (−0.06, 0.02) |
| Bioavailable T (nmoI/L) | 0.01 (0.00, 0.02) | 0.00 (−0.01, 0.01) | −0.00 (−0.01, 0.01) |
| Estradiol (nmoI/L) | −0.01 (−0.01, −0.00) | −0.01 (−0.01, −0.00) | −0.00 (−0.01, 0.00) |
| DHEA (nmoI/L) | 0.49 (0.28, 0.70) | 0.40 (0.19, 0.62) | 0.29 (0.07, 0.50) |
| Free T (Percent) | 0.09 (0.07, 0.11) | 0.06 (0.04, 0.07) | 0.03 (0.01, 0.05) |
| SHBG (nmoI/L) | -11.21 (-13.03, -9.39) | -8.29 (-10.13, -6.45) | -6.20 (-7.94, -4.46) |
| Total T (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.04 (−0.06, 0.13) | 0.01 (−0.09, 0.10) | −0.00 (−0.10, 0.09) |
| 25(OH)D <20 ng/ml | 0.01 (−0.09, 0.11) | −0.04 (−0.14, 0.06) | −0.05 (−0.15, 0.05) |
| Bioavailable T (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.02 (−0.01, 0.05) | 0.00 (−0.03, 0.03) | −0.01 (−0.04, 0.02) |
| 25(OH)D <20 ng/ml | 0.03 (−0.00, 0.06) | −0.00 (−0.03, 0.03) | −0.02 (−0.05, 0.01) |
| Estradiol (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.01) | −0.00 (−0.02, 0.01) |
| 25(OH)D <20 ng/ml | −0.02 (−0.04, −0.01) | −0.02 (−0.04, −0.01) | −0.01 (−0.03, 0.01) |
| DHEA (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.61 (0.06, 1.16) | 0.42 (−0.14, 0.98) | 0.19 (−0.37, 0.75) |
| 25(OH)D <20 ng/ml | 1.40 (0.83, 1.98) | 1.18 (0.59, 1.77) | 0.86 (0.26, 1.45) |
| Free T (Percent) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | 0.14 (0.09, 0.19) | 0.08 (0.03, 0.13) | 0.03 (−0.01, 0.08) |
| 25(OH)D <20 ng/ml | 0.22 (0.17, 0.28) | 0.14 (0.09, 0.19) | 0.08 (0.03, 0.13) |
| SHBG (nmoI/L) | |||
| 25(OH)D ≥ 30 ng/ml | Reference (0) | Reference (0) | Reference (0) |
| 25(OH)D 20–<30 ng/ml | −15.76 (−20.63, −10.88) | −9.72 (−14.53, −4.90) | −5.89 (−10.41, −1.37) |
| 25(OH)D <20 ng/ml | −25.44 (−30.49, −20.39) | −17.54 (−22.61, −12.47) | −12.22 (−16.99, −7.44) |
Results are shown as beta coefficients (95% CI) from linear regression. Statistically significant results are bolded. To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
25(OH)D = 25-hydroxyvitamin D; T= Testosterone; DHEA = Dehydroepiandrosterone; SHBG = Sex hormone binding globulin
Model 1: age, race/ethnicity, study site
Model 2: Model 1 plus additional potential confounding lifestyle variables (BMI categories, smoking, education, self-reported good health status, intentional physical activity)
Model 3: Model 2 plus potential vascular risk factor mediators (diabetes, systolic blood pressure, use of antihypertensive medications, eGFR, total cholesterol, HDL cholesterol, use of lipid lowering medication usage, C-reactive protein.)
p-interactions for race/ethnicity used Model 2 and were 0.40, 0.64, 0.72, 0.97, 0.78, 0.60 for Total T, Bioavailable T, Estradiol, DHEA, Free T, and SHBG, respectively.
There were no statistically significant interactions by race/ethnicity for any of the associations of 25(OH)D concentrations with sex hormone levels.
Sensitivity analysis restricted to women not taking hormone replacement therapy (n=1,916) also showed that a 10 ng/ml (25 nmol/L) decrease in 25(OH)D was associated with lower SHBG and higher free T percent similar to our primary analysis in all women (Supplemental Table 4, Model 2). However, the associations of 25(OH)D with DHEA and E2, while similar to the analyses of all women, were attenuated so that they were not longer statistically significant.
Discussion
In this large multi-ethnic study of men and women, we found that lower 25(OH)D concentrations were associated with lower SHBG and higher free T in both men and women, and lower E2 and higher DHEA in women independent of adiposity and lifestyle. We did not find any heterogeneity by race/ethnicity for any of these associations.
We were not able to confirm any previously-reported associations of lower 25(OH)D concentrations with lower total T or T deficiency, after adjustment for adiposity and lifestyle factors. In a prior study from Amsterdam of older men, low vitamin D concentrations (<30 ng/ml) were associated with lower total T independent of BMI and lifestyle factors, but only levels <10 ng/ml were associated with lower bioavailable T.[12] Many other studies[9–14] (but not all[29]), have also linked low vitamin D levels with low T in men. Surprisingly, we found that lower 25(OH)D concentrations were associated with higher free T in men. This is due predominantly to lower SHBG levels. T binds very strongly to SHBG, and total T may not be indicative of tissue level T deficiency.[30] In our study, we explored the association of 25(OH)D with total T, bioavailable T, and free T, and we only found association of 25(OH)D with free T in men in the more fully adjusted models. One question that arises is which measure of T is most important for health. The 2010 Endocrine Society Guidelines recommend that for men with suspected hypogonadism that morning total T assessed by a reliable assay should be the initial diagnostic test, but measurement of free or bioavailable T levels, using validated assays, should be considered for men who have total T near the lower limit of normal or suspected to have abnormalities in SHBG concentrations.[31]
Although both low 25(OH)D and low total T concentrations can be markers of poor health status, vitamin D is thought to play a causal role in T production. 1,25-dihydroxyvitamin D has both genomic and non-genomic effects on Sertoli cells in the testes leading to increased testosterone steroidogenesis in cell culture models.[7,8] However, supplemental trials in adult men have shown inconsistent findings with one study showing vitamin D supplementation modestly increased serum T levels[16] and another study finding null results.[13] Therefore, whether treating vitamin D deficiency can improve a low T state directly is inconclusive at this time, and our data does not support a relationship beyond shared risk factors.
In women, we found that low 25(OH)D was associated with a more androgenic pattern of higher DHEA levels and higher free T. We also found a very modest – albeit statistically significant - association of lower 25(OH)D with lower E2. However, in the MESA cohort, women were almost exclusively post-menopausal (96% of our sample) with extremely low E2 levels as appropriate for their age. A prior study found that pre-menopausal women had higher levels of total 25(OH)D, vitamin D binding protein, and calculated free and bioavailable 25(OH)D compared to postmenopausal women, and that serum E2 levels correlated with vitamin D binding protein.[32] Thus, associations between vitamin D and E2 might be more clinically meaningful in pre-menopausal women, but we were unable to test for that in our cohort.
Similar to other studies[10,14,19] (although not all[12]), we found an association between lower 25(OH)D concentrations and lower SHBG in both men and women. The association was robust even after further adjustment for vascular risk factors, with a stronger association in women. SHBG is a glycoprotein synthesized in the liver that binds 17-β-hydroxysteroids such as T (with high affinity) and E2 (with lower affinity).[33] SHBG levels are strongly correlated with T levels but inversely correlated with glucose and insulin levels independently of T levels.[34] Low SHBG levels have been shown to be strongly and consistently related to elevated ASCVD risk factors of higher insulin, glucose, hemostatic and inflammatory markers, and adverse lipids even after controlling for BMI.[35] Similarly, vitamin D deficiency is also associated with impaired β cell function (insulin secretion), insulin resistance, glucose intolerance, incident type 2 diabetes,[21] and inflammatory markers.[4] However, we found that vitamin D and SHBG were still associated with each other even after adjustment for diabetes and inflammation.
Vitamin D is a fat soluble vitamin; vitamin D deficiency is also associated with obesity as adipose cells are thought to “sequester” vitamin D and thus reduce circulating serum levels.[36] SHBG levels are also low in obesity states.[37] But we still found in women that vitamin D deficiency was strongly associated with lower SHBG levels even after adjusting for measure of adiposity using BMI. Waist-hip-ratio may be a better marker of central adiposity and diabetes/ASCVD risk compared to BMI,[38] but our results were similar in sensitivity analyses where we alternatively adjusted for WHR instead of BMI.
Racial/ethnic differences have been reported in the association of SHBG with adverse metabolic profile,[39] and in the association of vitamin D levels with metabolic syndrome and diabetes.[21] We hypothesized that there would be differences by race/ethnicity in the association of vitamin D with SHBG and other sex hormones; however our study did not find any significant racial/ethnic interactions.
It is important to address some limitations of our findings. First, this was a cross-sectional study and contains the inherent limitation of providing associations without establishing temporality or causality. While we adjusted for numerous potential confounding and lifestyle factors, residual confounding may still underlie the observed associations seen. Low 25(OH)D concentrations may simply be a potent marker of a poorer health state, although our findings were robust even after adjustment for self-reported health status. Also we measured 25(OH)D levels, not the activated form 1,25-dihydroxyvitamin D. However 1,25-dihydroxyvitamin D deficiency is tightly regulated by parathyroid hormone and concentrations may be in the normal range even in the setting of 25(OH)D deficiency. As a consequence, 25(OH)D is recognized as the best biomarker for assessing sufficiency/deficiency status.[20] Finally, we only had single measurements of 25(OH)D and sex hormones, which are subject to within person variability and measurement error, and may not reflect long-term levels of these biomarkers.
Our study has several strengths. We investigated this question in MESA, a large well-characterized cohort representative of men and women of a broad age range (45–85 years) and of 4 race/ethnicities, which provides additional insight as prior studies were limited to only narrow age, sex, or race demographic groups. To our knowledge, this is the first study to evaluate adjusted race/ethnic differences in the association of 25(OH)D and sex hormone levels; although we did not find any evidence for heterogeneity by race/ethnicity.
In conclusion, we found that lower 25(OH)D concentrations were associated with lower SHBG and higher free T in both men and women, and lower E2 and higher DHEA in women independent of adiposity and lifestyle factors. However, with the exception of the association of 25(OH)D with SHBG in women, most of the adjusted differences in mean levels of sex hormones between vitamin D deficient and optimal individuals were modest. Further studies are needed to explore if such differences are clinically meaningful despite the statistical significance. Prospective studies with serial measures of vitamin D and sex hormones are needed to determine any temporal relationships, and clinical trials are needed to evaluate whether treating a low vitamin D state can alter sex hormone levels.
Supplementary Material
Highlights.
Both vitamin D and sex hormone levels have been linked with cardiovascular risk.
We examined the relationship between vitamin D and sex hormone levels among men and women.
Low vitamin D levels were associated with lower levels of sex hormone binding globulin and higher levels of free testosterone in both men and women.
Low vitamin D levels are associated with lower estradiol and higher dehydroepiandrosterone levels in women.
These associations between vitamin D and sex hormones were independent of adiposity and lifestyle.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
Dr Michos was supported by NIH/NINDS grant R01NS072243 and by the Blumenthal Scholars Fund for Preventive Cardiology Research. The MESA study was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by R01 HL074406, R01 HL074338, and R01 HL096875.
Abbreviations
- MESA
Multi-ethnic Study of Atherosclerosis
- 25(OH)D
25-hydroxyvitamin D
- ASCVD
Atherosclerotic cardiovascular disease
- DHEA
Dehydroepiandrosterone
- E2
Estradiol
- SHBG
Sex Hormone Binding Globulin
- BMI
Body Mass Index
- WHR
Waist-Hip Ratio
- eGFR
Estimated Glomerular Filtration Rate
- hsCRP
High-sensitivity C-Reactive Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
DZ designed the research, analyzed the data (under the supervision of EG and EDM), wrote the first draft of the paper, and had primary responsibility for its final content..
PO reviewed the manuscript and provided critical scientific input.
IHdB reviewed the manuscript and provided critical scientific input.
PLL reviewed the manuscript and provided critical scientific input.
YMKF reviewed the manuscript and provided critical scientific input.
EG reviewed the manuscript and provided critical scientific input.
DSS reviewed the manuscript and provided critical scientific input.
WSP reviewed the manuscript and provided critical scientific input.
RRK reviewed the manuscript and provided critical scientific input.
KLB reviewed the manuscript and provided critical scientific input.
EDM designed the research, reviewed the manuscript and provided critical scientific input and had primary responsibility for the paper’s final content.
The manuscript was approved by the MESA Publication committee.
Conflict of interest
The authors report no conflict of interest with this work.
Ethical approval
The study was approved by the institutional review board of each participating institution, and all participants provided informed consent.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, Lutsey PL. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241(1):12–17. doi: 10.1016/j.atherosclerosis.2015.04.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, Sharrett AR, Melamed ML. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28(4):367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15(1):293. doi: 10.1007/s11883-012-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C, Cushman M, Kleindorfer D, Lisabeth L, Redberg RF, Safford MM. A review of the relationships between endogenous sex steroids and incident ischemic stroke and coronary heart disease events. Curr Cardiol Rev. 2015;11(3):252–260. doi: 10.2174/1573403X1103150515110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, Feinleib M, Michos ED, Dobs A, Platz EA. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171(5):583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofer D, Munzker J, Schwetz V, Ulbing M, Hutz K, Stiegler P, Zigeuner R, Pieber TR, Muller H, Obermayer-Pietsch B. Testicular synthesis and vitamin D action. J Clin Endocrinol Metab. 2014;99(10):3766–3773. doi: 10.1210/jc.2014-1690. [DOI] [PubMed] [Google Scholar]
- 8.Zanatta L, Zamoner A, Zanatta AP, Bouraima-Lelong H, Delalande C, Bois C, Carreau S, Silva FR. Nongenomic and genomic effects of 1alpha,25(OH)2 vitamin D3 in rat testis. Life Sci. 2011;89(15–16):515–523. doi: 10.1016/j.lfs.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Chin KY, Ima-Nirwana S, Wan Ngah WZ. Vitamin D is significantly associated with total testosterone and sex hormone-binding globulin in Malaysian men. Aging Male. 2015;18(3):175–179. doi: 10.3109/13685538.2015.1034686. [DOI] [PubMed] [Google Scholar]
- 10.Wang N, Han B, Li Q, Chen Y, Chen Y, Xia F, Lin D, Jensen MD, Lu Y. Vitamin D is associated with testosterone and hypogonadism in Chinese men: Results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol. 2015;13:74. doi: 10.1186/s12958-015-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 2012;77(1):106–112. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafiq R, van Schoor NM, Sohl E, Zillikens MC, Oosterwerff MM, Schaap L, Lips P, de Jongh RT. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Jorde R, Grimnes G, Hutchinson MS, Kjaergaard M, Kamycheva E, Svartberg J. Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm Metab Res. 2013;45(9):675–681. doi: 10.1055/s-0033-1345139. [DOI] [PubMed] [Google Scholar]
- 14.Anic GM, Albanes D, Rohrmann S, Kanarek N, Nelson WG, Bradwin G, Rifai N, McGlynn KA, Platz EA, Mondul AM. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol (Oxf) 2016;85(2):258–266. doi: 10.1111/cen.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laczmanski L, Lwow F, Mossakowska M, Puzianowska-Kuznicka M, Szwed M, Kolackov K, Krzyzanowska-Swiniarska B, Bar-Andziak E, Chudek J, Sloka N, Milewicz A. Association between vitamin D concentration and levels of sex hormones in an elderly Polish population with different genotypes of VDR polymorphisms (rs10735810, rs1544410, rs7975232, rs731236) Gene. 2015;559(1):73–76. doi: 10.1016/j.gene.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43(3):223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 17.Chang EM, Kim YS, Won HJ, Yoon TK, Lee WS. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J Clin Endocrinol Metab. 2014;99(7):2526–2532. doi: 10.1210/jc.2013-3873. [DOI] [PubMed] [Google Scholar]
- 18.Janssen HC, Emmelot-Vonk MH, Verhaar HJ, van der Schouw YT. Determinants of vitamin D status in healthy men and women aged 40–80 years. Maturitas. 2013;74(1):79–83. doi: 10.1016/j.maturitas.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.He C, Lin Z, Robb SW, Ezeamama AE. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2015;7(6):4555–4577. doi: 10.3390/nu7064555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. doi: 10.3945/ajcn.115.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS C.-E. Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, Kestenbaum B, de Boer IH. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243–1251. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84(2):956–962. doi: 10.1021/ac202047n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 29.Lerchbaum E, Pilz S, Trummer C, Rabe T, Schenk M, Heijboer AC, Obermayer-Pietsch B. Serum vitamin D levels and hypogonadism in men. Andrology. 2014;2(5):748–754. doi: 10.1111/j.2047-2927.2014.00247.x. [DOI] [PubMed] [Google Scholar]
- 30.Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010;64(6):682–696. doi: 10.1111/j.1742-1241.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM E.S. Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 32.Pop LC, Shapses SA, Chang B, Sun W, Wang X. Vitamin D-Binding Protein in Healthy Pre- and Postmenopausal Women: Relationship with Estradiol Concentrations. Endocr Pract. 2015;21(8):936–942. doi: 10.4158/EP15623.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27(Pt 6):532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 34.Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 35.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI S. Investigators. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111(10):1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 36.Mutt SJ, Hypponen E, Saarnio J, Jarvelin MR, Herzig KH. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;5:228. doi: 10.3389/fphys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haffner SM, Katz MS, Stern MP, Dunn JF. Relationship of sex hormone binding globulin to overall adiposity and body fat distribution in a biethnic population. Int J Obes. 1989;13(1):1–9. [PubMed] [Google Scholar]
- 38.Cheng CH, Ho CC, Yang CF, Huang YC, Lai CH, Liaw YP. Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr Res. 2010;30(9):585–593. doi: 10.1016/j.nutres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Berman DM, Rodrigues LM, Nicklas BJ, Ryan AS, Dennis KE, Goldberg AP. Racial disparities in metabolism, central obesity, and sex hormone-binding globulin in postmenopausal women. J Clin Endocrinol Metab. 2001;86(1):97–103. doi: 10.1210/jcem.86.1.7147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.