Abstract
Knowledge of the amino acids that define recognition of anti-β-lactamase antibodies is critical to the interpretation of sensitivity and specificity of these antibodies when they are used in a clinical or research setting. To this end, we mapped the epitopes of the CMY-2 and SHV-1 β-lactamases by using the SPOT synthesis method. Eight linear epitopes in SHV-1 and seven linear epitopes in CMY-2 were identified by using anti-SHV-1 and anti-CMY-2 polyclonal antibodies, respectively. The epitopes of SHV-1 were mapped to amino acids at the Ambler positions ABL 28 to 38, 42 to 54, 88 to 100, 102 to 114, 170 to 182, 186 to 194, 202 to 210, and 276 to 288. In the epitope spanning amino acids 102 to 114, alanine and X-Scan analysis demonstrated that D104, Y105, P107, and S109 are essential residues for antibody recognition. In the epitope containing amino acids 170 to 182, N170, L173, P174, G175, and D176 were immunodominant. In CMY-2 β-lactamase, amino acids 4 to 16, 70 to 79, 211 to 223, 274 to 286, 289 to 298, 322 to 334, and 343 to 358 of the mature enzyme defined the major linear epitopes. A detailed analysis of the recognition sites that are located in an area analogous to the omega loop of class A β-lactamases (V211 to V223) showed that the amino acids Q215 to E219 are important in antibody binding. Incubation of CMY-2 β-lactamase with a 10-fold molar excess of anti-CMY-2 antibody for 60 min resulted in greater than 80% inhibition of nitrocefin hydrolysis. A 10-fold molar excess of anti-SHV-1 antibody reduced the activity of SHV-1 by 69%. Analysis of the CMY-2 and SHV-1 structures suggest that this reduction of hydrolytic activity may be due in part to the direct binding of antibodies to the omega loop, thereby hindering access of substrate to the active site.
There are multiple ways to identify the linear epitopes of a protein antigen. Protease digestion of antigen proteins, chemical cleavage at specific residues within a protein, and modification of specific amino acids are all methods to identify epitopes and study antigenicity (2, 3, 9, 19). Two of the more widely used and sophisticated methods employed today are the phage display technique and the SPOT synthesis (SPOTs) method. In phage display, peptide libraries are screened by displaying a vast array of peptides on the surface of bacteriophage (18). Complementary binding peptides are then assayed by affinity binding of the antibody. Through sequence comparison, the reactive peptides displayed by the phage can identify essential binding regions. However, disadvantages to this method include limitation of library size and the number of clones that must be processed by sequencing. This technique is used primarily when few details are known about the protein-protein (e.g., antibody-antigen) binding interaction.
In the SPOTs method, antibody-reactive epitopes are mapped on an antigen with known amino acid sequence. SPOTs screens the entire sequence by displaying it as overlapping peptides that are usually 8 to 15 amino acids in length. The simultaneous chemical preparation of these peptides on a membrane support (immobilization) is what is known as SPOT synthesis (6, 7, 15, 17). The term actually refers to the individual peptide spots that are synthesized. These overlapping peptides are assayed for binding reactivity, and the sequence common to the reactive peptides, the consensus sequence, is the epitope (8). We have used the SPOT peptide synthesis method to map the linear epitopes recognized by anti-SHV-1 and anti-CMY-2 polyclonal antibodies to their respective antigens. From the sequence of immunoreactive peptides, we determined which amino acid residues play a role in the binding of the polyclonal rabbit antibodies. Using alanine substitutional analysis, we also identified the amino acids that defined the important epitopes in both β-lactamases.
Hujer et al. used this anti-SHV-1 polyclonal antibody in an enzyme-linked immunosorbent assay (ELISA) format for identification and quantification of SHV β-lactamases produced by clinical isolates and to assess levels of SHV protein expression in SHV mutants created in the laboratory setting (14). In the present study we wanted to discern whether SHV-1 variants are recognized as well as SHV-1 by the anti-SHV-1 antibody or whether SHV recognition due to mutations would be altered. This information could potentially affect screening and quantitation of SHV β-lactamases in clinical isolates and laboratory variants (10, 12-14).
We also wanted to explain observed differences between the polyclonal anti-CMY-2 and anti-SHV-1 antibodies. It was previously reported that the anti-SHV-1 antibody recognized SHV-1 but not TEM-1 β-lactamase, whereas the anti-CMY-2 antibody recognized many AmpC enzymes (14). Also, the anti-CMY-2 antibody could effectively neutralize multiple class C β-lactamases. To understand these observations as well as to further define the selectivity and specificity of both ELISAs, we performed antibody epitope mapping for SHV-1 and CMY-2 β-lactamases via SPOT synthesis analysis. These results validate our use of the SHV ELISA to assess the effects of amino acid substitutions on SHV expression and to screen for quantitate SHV β-lactamase expression in clinical isolates, and they also explain why the anti-CMY-2 antibody recognizes multiple class C β-lactamases (10, 12-14).
MATERIALS AND METHODS
β-Lactamase protein expression and purification.
SHV-1 and CMY-2 β-lactamases were expressed in Escherichia coli DH10B, liberated by periplasmic fractionation, and purified as previously described (12-14). Purity was assessed by Coomassie brilliant blue R250 staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis-electrophoresed samples. Other purified AmpC β-lactamases were a kind gift from Malcolm G. P. Page of Basilea Pharmaceutica AG (Basel, Switzerland). These purified AmpC β-lactamases were isolated from Enterobacter cloacae, Citrobacter freundii, and Pseudomonas aeruginosa. Protein concentrations were determined with a Bio-Rad protein assay (Hercules, Calif.) using bovine serum albumin (Amresco, Solon, Ohio) as a standard. All protein samples were greater than 95% pure. Enzyme homologies were determined by DNA analysis comparisons using DNASIS 2.6 for Windows (Hitachi Software Genetic Systems, South San Francisco, Calif.).
Custom antibody synthesis and antibody purification.
Anti-CMY-2 and anti-SHV-1 polyclonal rabbit antibodies were produced by SIGMA-Genosys (The Woodlands, Tex.), and Protein G column purification (SIGMA-Genosys) was used to isolate polyclonal immunoglobulin G antibodies from rabbit serum as previously described (14). In brief, 5 ml of rabbit serum was passed through a 5-ml Hi-Trap Protein G column. The bound rabbit antibodies were eluted with 0.1 M glycine (pH 2.7) and were neutralized with 1 M Tris HCl (pH 8.8). The antibodies were then dialyzed against PBS (pH 7.4), the concentrations were measured by spectrophotometric determination of the optical density at 280 nm, and the samples were aliquoted and frozen at −20°C for long-term storage. The sensitivity of the antibodies to the intact antigens has been demonstrated by Western blot analysis and in the ELISA format as previously reported (14).
SPOT membrane synthesis.
Overlapping 13-mer peptides, offset by two amino acids derived from the sequence of SHV-1 β-lactamase (GenBank accession number AF124984), were designed and synthesized by SIGMA-Genosys as outlined in their manual (SIGMA-Genosys Custom SPOTs Technical Manual). A total of 127 peptides were synthesized and immobilized for this assay. These peptides are covalently bound at the C terminus to a Whatman 50 cellulose membrane, while the N terminus remains unbound. Five to 10 nmol per peptide was synthesized on the cellulose support by Fmoc-L amino acid chemistry. N-terminal acetylation of the peptides was also performed for increased stability (SIGMA-Genosys Custom SPOTs Technical Manual). These bound peptides were then directly probed with anti-SHV-1 polyclonal antibody to perform the epitope-mapping studies. The same protocol was used to synthesize the linear overlapping 13-mer peptides for the mature CMY-2 β-lactamase (GenBank accession number X91840), with the exception that 117 peptides, offset by three amino acids, were synthesized.
Probing of the SPOTs membranes with anti-CMY-2 or anti-SHV-1 antibody.
The cellulose membranes containing the overlapping peptides were washed for 2 min with 100% methanol to wet the membranes. This was followed by two 10-min washes at room temperature (RT) on an undulating rocker with 25 ml of 20 mM Tris HCl-buffered 150 mM saline (pH 7.4) (TBS). The SPOTs membranes were probed in the same manner as immunoblots after protein transfer to the membrane and were incubated overnight at RT in blocking buffer (5% bovine serum albumin in TBS) (12, 14). The following day the membranes were incubated for 4 h at RT with either anti-CMY-2 or anti-SHV-1 polyclonal antibodies at a concentration of 1 μg/ml in blocking buffer. The membranes were washed four times for 10 min each in T-TBS (TBS containing 0.05% Tween 20) followed by incubation with a 1:3,000 dilution of horseradish peroxidase-conjugated Protein G (Bio-Rad, Hercules, Calif.) in blocking buffer. The cellulose supports, after four more washes, were processed for film development (chemiluminescent detection) with an Amersham Pharmacia Biotech ECL kit (Piscataway, N.J.).
Analysis of SPOTs membranes.
TIF images of the films were generated with the Bio-Rad Gel Doc 2000 Imaging System. From the TIF images the reactivity pattern of the SPOTs membrane synthetic peptides was assessed using Scion Image densitometry software, a Windows-compatible version of NIH Image (www.scioncorp.com). Densitometric assessments were performed three times for each experiment. The most intense spot per blot was designated 100% signal intensity, and all other values were expressed as a percentage of this value. Only spots with a densitometric value of greater than 20% were reported.
Model construction.
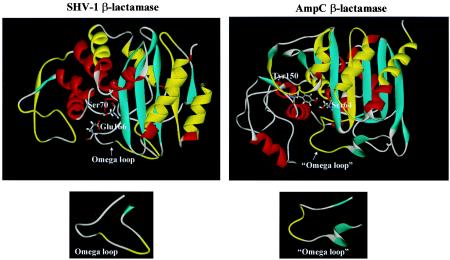
Molecular representation of the crystal structure of SHV-1 and AmpC of C. freundii (Protein Data Bank entries 1SHV and 1FR1, respectively) were used to demonstrate the amino acids recognized by the polyclonal antibodies. Representations were generated using the AccelRys ViewerLite 4.2 program (www.accelrys.com/products/). Antibody-reactive epitopes are highlighted in yellow.
Identification of key residues for antibody binding by alanine scanning.
Alanine scanning and SPOTs epitope mapping of peptides -Q100DLVDYSPVSEKH112- and -E168LNEALPGDARDT180- in the SHV-1 β-lactamase were performed to specifically determine which amino acids were critical for anti-SHV-1 antibody binding in two important domains. Thirteen Ala-substituted synthetic peptides per epitope were generated to test the contribution of each amino acid in these regions to anti-SHV-1 antibody binding. For the -Q100DLVDYSPVSEKH112- epitope, the first synthetic peptide is listed above and served as the wild-type comparator; the 13 additional peptides had one amino acid change each, thereby changing every amino acid in the above sequence to an alanine in one representative peptide. The same was done for the second SHV-1 epitope, -E168LNEALPGDARDT180-, and the CMY-2 β-lactamase epitope defining the antibody-reactive omega loop region, -V211SPGQLDAEAYGV223-, with the exception that substituted peptides containing naturally occurring alanine residues served as wild-type comparators (16). The key antibody binding epitopes were identified on the basis of at least a 50% reduction in binding by the antibody compared to that of the native peptide. All peptides were cellulose bound, probed, and analyzed as previously described.
X-Scan of amino acid position D104 in SHV-1.
The peptide -R98QQDLVDYSPVSE110- (with amino acid 104 in boldface) was synthesized. An X-Scan experiment was performed on position 104 in the SHV-1 β-lactamase by replacing all other 19 amino acids at this position within the framework of the peptide sequence listed above (4). The assay was carried out on a SPOTs membrane as described above.
Antibody inactivation studies of SHV-1 and AmpC β-lactamases.
To perform the inactivation studies, 3.5 and 18 nM (unless otherwise stated) SHV-1 or AmpC β-lactamase was mixed with various molar ratios of either anti-SHV-1 or anti-CMY-2 (anti-AmpC) antibodies in 20 mM PBS (pH 7.4), as stated in Table 3. Following a 1-h incubation of antibody and β-lactamase at RT, 100 μM nitrocefin (λ = 482 nm, Δɛ = 17,400 M−1cm−1; Becton Dickinson, Cockeysville, Md.), 500 μM ampicillin (λ = 235 nm, Δɛ = −900 M−1cm−1; Sigma, St. Louis, Mo.), or 50 μM cefazolin (λ = 260 nm, Δɛ = −7,400 M−1cm−1; GlaxoSmithKline, Research Triangle Park, N.C.) was added, and initial velocity measurements (first 5 s of hydrolysis) were obtained. Control experiments were performed in the manner described above using anti-CMY-2 antibody for SHV-1 inactivation studies and anti-SHV-1 antibody for CMY-2 inactivation studies.
TABLE 3.
Antibody inactivation studies of SHV-1 and CMY-2 β-lactamasesa
| β-Lactamase/substrate | Antibody:β-lactamase molar ratio | Velocity (μM−1 s−1) | % Decrease |
|---|---|---|---|
| Anti-SHV-1:SHV-1 | |||
| 3.5 nM SHV-1/Amp | 0:1 | 13.2 ± 1.1 | |
| 3.5 nM SHV-1/Amp | 5:1 | 3.2 ± 0.2 | 76 |
| 3.5 nM SHV-1/Amp | 10:1 | 1.5 ± 0.1 | 89 |
| 3.5 nM SHV-1/Amp | 20:1 | 1.2 ± 0.1 | 91 |
| Anti-CMY-2:SHV-1 | |||
| 3.5 nM SHV-1/Amp | 10:1 | 11.3 ± 0.4 | 14 |
| 3.5 nM SHV-1/Amp | 20:1 | 11.2 ± 0.2 | 15 |
| Anti-SHV-1:SHV-1 | |||
| 18 nM SHV-1/NCF | 0:1 | 2.7 ± 0.2 | |
| 18 nM SHV-1/NCF | 5:1 | 1.5 ± 0.1 | 42 |
| 18 nM SHV-1/NCF | 10:1 | 0.8 ± 0.1 | 69 |
| 18 nM SHV-1/NCF | 20:1 | 0.7 ± 0.1 | 76 |
| Anti-CMY-2:SHV-1 | |||
| 18 nM SHV-1/NCF | 5:1 | 2.6 ± 0.1 | 0 |
| 18 nM SHV-1/NCF | 10:1 | 2.8 ± 0.1 | 0 |
| 18 nM SHV-1/NCF | 20:1 | 2.6 ± 0.1 | 0 |
| Anti-CMY-2:CMY-2 | |||
| 3.5 nM CMY-2/Cefaz | 0:1 | 1.2 ± 0.1 | |
| 3.5 nM CMY-2/Cefaz | 5:1 | 0.7 ± 0.1 | 42 |
| 3.5 nM CMY-2/Cefaz | 10:1 | 0.4 ± 0.01 | 67 |
| 3.5 nM CMY-2/Cefaz | 20:1 | 0.4 ± 0.03 | 67 |
| Anti-SHV-1:CMY-2 | |||
| 3.5 nM CMY-2/Cefaz | 5:1 | 1.2 ± 0.03 | 0 |
| 3.5 nM CMY-2/Cefaz | 10:1 | 1.2 ± 0.01 | 0 |
| 3.5 nM CMY-2/Cefaz | 20:1 | 1.1 ± 0.03 | 0 |
| Anti-CMY-2:CMY-2 | |||
| 18 nM CMY-2/NCF | 0:1 | 3.6 ± 0.03 | |
| 18 nM CMY-2/NCF | 5:1 | 2.1 ± 0.03 | 42 |
| 18 nM CMY-2/NCF | 10:1 | 0.5 ± 0.1 | 86 |
| 18 nM CMY-2/NCF | 20:1 | 0.7 ± 0.01 | 81 |
| Anti-SHV-1:CMY-2 | |||
| 18 nM CMY-2/NCF | 5:1 | 3.6 ± 0.1 | 0 |
| 18 nM CMY-2/NCF | 10:1 | 3.6 ± 0.1 | 0 |
| 18 nM CMY-2/NCF | 20:1 | 3.5 ± 0.1 | 0 |
| Anti-CMY-2:E. cloacae | |||
| 18 nM E. cloacae/NCF | 0:1 | 1.7 ± 0.02 | |
| 18 nM E. cloacae/NCF | 10:1 | 0.2 ± 0.03 | 88 |
| Anti-CMY-2:C. freundii | |||
| 5 nM C. freundii/NCF | 0:1 | 2.1 ± 0.1 | |
| 5 nM C. freundii/NCF | 10:1 | 0.5 ± 0.03 | 76 |
| Anti-CMY-2:P. aeruginosa | |||
| 18 nM P. aeruginosa/NCF | 0:1 | 5.2 ± 0.3 | |
| 18 nM P. aeruginosa/NCF | 10:1 | 4.4 ± 0.3 | 16 |
β-Lactamase was mixed with various molar ratios of either anti-SHV-1 or anti-CMY-2 antibody, as described in Materials and Methods. Each velocity determination was repeated in triplicate, and standard deviations were calculated. NCF, 100 μM nitrocefin; Amp, 500 μM ampicillin; Cefaz, 50 μM cefazolin. Percent decrease is relative to the velocity determined when no antibody was added.
Peptide competition assay.
In order to assess the specificity of anti-CMY-2 antibody binding to amino acids 211 to 223, we performed a peptide competition assay. The soluble peptide, -V211SPGQLDAEAYGV223-, corresponding to amino acids 211 to 223 in the CMY-2 β-lactamase, was synthesized by SIGMA-Genosys. A 1 mM stock solution was made in deionized filtered water, with subsequent dilutions being made from this stock. Peptide:antibody:β-lactamase mixtures were prepared in 20 mM PBS (pH 7.4). The soluble peptide was allowed to preincubate for an hour at RT with the anti-CMY-2 antibody at a ratio of 500:1, respectively, after which the mixture was incubated for another hour at RT with the CMY-2 β-lactamase. When cefazolin was the indicator substrate, 17.5 μM peptide, 35 nM anti-CMY-2 antibody, and 3.5 nM CMY-2 β-lactamase were the concentrations that composed the 5,000:10:1 ratio. When nitrocefin was the indicator substrate, 90 μM peptide, 180 nM anti-CMY-2 antibody, and 18 nM CMY-2 β-lactamase were the concentrations that composed the 5,000:10:1 ratio. After 2 h of total preincubation, substrate was added and initial velocity measurements of cefazolin or nitrocefin hydrolysis were obtained as described above. Control experiments were performed without adding antibody or peptide, or the combination of both, for preincubation with CMY-2 β-lactamase.
RESULTS
Mapping the anti-SHV-1 antibody-reactive epitopes of SHV-1 β-lactamase.
A SPOTs membrane, probed with anti-SHV-1 antibody, revealed eight linear epitopes. These epitopes consist of the SHV-1 amino acids ABL 28 to 38, 42 to 54, 88 to 100, 102 to 114, 170 to 182, 186 to 194, 202 to 210, and 276 to 288 (Table 1, Fig. 1a) (1). Mapping the epitopes that are recognized by the polyclonal anti-SHV-1 antibody to a model generated from the SHV-1 crystal structure coordinates reveals that the amino acids corresponding to the external surface of SHV-1 are the epitopes recognized (Fig. 2).
TABLE 1.
Peptide sequences of SHV-1 β-lactamase recognized by the anti-SHV-1 antibodya
| Recognized amino acids in SHV-1 | Sequence | % Signal intensity |
|---|---|---|
| 26-38 | S P Q P L E Q I K L S E S | 34 |
| 28-40 | Q P L E Q I K L S E S Q L | 32 |
| Consensus 28-38 | Q P L E Q I K L S E S | |
| TEM sequence | E T L V K V K D A E D | |
| 36-48 | S E S Q L S G R V G M I E | 14 |
| 38-50 | S Q L S G R V G M I E M D | 28 |
| 40-52 | L S G R V G M I E M D L A | 62 |
| 42-54 | G R V G M I E M D L A S G | 65 |
| 44-56 | V G M I E M D L A S G R T | 50 |
| 46-58 | M I E M D L A S G R T L T | 34 |
| Consensus 42-54 | G R V G M I E M D L A S G | |
| TEM sequence | A R V G Y I E L D L N S G | |
| 88-100 | D E Q L E R K I H Y R Q Q | 80 |
| 90-102 | Q L E R K I H Y R Q Q D L | 56 |
| 92-104 | E R K I H Y R Q Q D L V D | 29 |
| 94-106 | K I H Y R Q Q D L V D Y S | 21 |
| Consensus 88-100 | D E Q L E R K I H Y R Q Q | |
| TEM sequence | Q E Q L G R R I H Y S Q N | |
| 98-110 | R Q Q D L V D Y S P V S E | 48 |
| 100-112 | Q D L V D Y S P V S E K H | 55 |
| 102-114 | L V D Y S P V S E K H L A | 61 |
| 104-116 | D Y S P V S E K H L A D G | 59 |
| Consensus 102-114 | L V D Y S P V S E K H L A | |
| TEM sequence | L V E Y S P V T E K H L T | |
| 164-176 | R W E T E L N E A L P G D | 64 |
| 166-178 | E T E L N E A L P G D A R | 81 |
| 168-180 | E L N E A L P G D A R D T | 96 |
| 170-182 | N E A L P G D A R D T T T | 100 |
| 172-184 | A L P G D A R D T T T P A | 89 |
| 174-186 | P G D A R D T T T P A S M | 80 |
| 176-188 | D A R D T T T P A S M A A | 35 |
| Consensus 170-182 | N E A L P G D A R D T T T | |
| TEM sequence | N E A I P N D E R D T T M | |
| 182-194 | T P A S M A A T L R K L L | 19 |
| 184-196 | A S M A A T L R K L L T S | 20 |
| 186-198 | M A A T L R K L L T S Q R | 22 |
| 188-200 | A T L R K L L T S Q R L S | 8 |
| 190-202 | L R K L L T S Q R L S A R | 9 |
| Consensus 186-194 | M A A T L R K L L | |
| TEM sequence | M A T T L R K L L | |
| 198-210 | R L S A R S Q R Q L L Q W | 28 |
| 200-212 | S A R S Q R Q L L Q W M V | 31 |
| 202-214 | R S Q R Q L L Q W M V D D | 31 |
| Consensus 202-210 | R S Q R Q L L Q W | |
| TEM sequence | A S R Q Q L I D W | |
| 276-288 | N Q Q I A G I G A A L I E | 21 |
| Consensus 276-288 | N Q Q I A G I G A A L I E | |
| TEM sequence | N R Q I A E I G A S L I K |
The percent signal intensity for each of the recognized peptide spots, regions of consensus in each of the recognized peptides of SHV-1 β-lactamase, and the corresponding regions for TEM-1 β-lactamase are shown. Standard deviations for signal intensity percentages never exceeded 8% of the reported values. Boldface indicates region of identical amino acids between peptides. Boldface italics indicates peptide consensus region of SHV-1 β-lactamase and corresponding identical amino acids in TEM-1 β-lactamase.
FIG. 1.
SPOT synthesis membranes probed with designated antibodies. (a) SHV-1 SPOTs membrane probed with 1 μg of anti-SHV-1 antibody/ml. Spot 1 represents amino acids 26 to 38; Spot 2, amino acids 28 to 40; etc. Reactivity of overlapping synthetic peptides that correspond to the entire sequence of SHV-1 β-lactamase. (b) Peptide 37 (-R98QQDLVDYSPVSE110-) from the above membrane was used for substitutional analysis of this antibody-reactive epitope in SHV-1 β-lactamase. Spot 1 corresponds to the wild-type sequence. Spots 2 to 20 correspond to the substitution of all 19 amino acids at position 104 in SHV-1 β-lactamase. (c) CMY-2 SPOTs membrane probed with 1 μg of anti-CMY-2 antibody/ml. Reactivity of overlapping synthetic peptides that correspond to the entire sequence of CMY-2 β-lactamase.
FIG. 2.
SHV-1 and AmpC β-lactamases. Antibody binding epitopes are highlighted in yellow.
SPOTs membrane percent signal intensity values (calculated from densitometry measurements) are reported in Table 1. The peptide sequences spanning residues 170 to 182 show the greatest recognition by the antibody. All subsequent signal intensities are reported relative to this maximal signal. The peptides with second and third highest signal intensity are those areas that contained amino acids 88 to 100 (80% intensity) and 42 to 54 (65% intensity). The TEM-1 β-lactamase amino acid sequence corresponding to these mapped epitopes is also listed in Table 1.
Substitutional and X-Scan analyses of the Q100-H112 and E168-T180 epitopes of the SHV-1 β-lactamase.
From analysis of the SPOTs synthetic membrane used for substitutional analysis, we determined that residues D104, Y105, P107, and S109 are essential for recognition by the polyclonal anti-SHV antibody to SHV-1 (Fig. 3). In contrast, residues 100 to 103, 106, 108, and 110 all tolerate alanine substitutions. All 19 naturally occurring amino acid variations were replaced and compared to the wild-type Asp at position 104 (X-Scan analysis). All amino acid substitutions at this position reduce recognition by the antibody to below detectable levels in the peptide -R98QQDLVDYSPVSE110- (Fig. 1b). Substitutional analysis was also carried out for amino acid residues 168 to 180 in the SHV-1 β-lactamase. Residues N170, L173, P174, G175, and D176 were determined to be essential for antibody binding in this linear epitope (Fig. 2 and 4). The antigenicity of amino acids 172 and 177 could not be determined by this method, because they are naturally occurring alanine residues (served as controls).
FIG. 3.
Identification by alanine scanning of essential antibody reactive residues in the peptide -Q100DLVDYSPVSEKH112- of SHV-1 β-lactamase. Densitometric values obtained from analysis of the SPOTsmembrane are expressed in terms of percent signal intensity, with the wild-type (WT) peptide being assigned a value of 100%. Data for each individually alanine replaced amino acid is represented relative to the wild-type peptide. Standard deviation of each determination is ≤5% of the reported value.
FIG. 4.
Identification by alanine scanning of essential antibody-reactive residues in the peptide -E168LNEALPGDARDT180- of SHV-1 β-lactamase. Densitometric values obtained from analysis of the SPOTs membrane are expressed in terms of percent signal intensity, with the wild-type (WT) peptide A172A being assigned a value of 100%. Data for each individually alanine replaced amino acid is represented relative to the wild-type peptide. Standard deviation of each determination is ≤5% of the reported value.
Mapping the anti-CMY-2 antibody-reactive epitopes of CMY-2 β-lactamase.
Likewise, we mapped the epitopes of CMY-2 that were recognized by the anti-CMY-2 polyclonal rabbit antibody. The SPOTs membrane that was probed with the anti-CMY-2 antibody revealed seven linear epitopes. These epitopes are composed of the CMY-2 amino acids 4 to 16, 70 to 79, 211 to 223, 274 to 286, 289 to 298, 322 to 334, and 343 to 358 (Fig. 1c and Table 2). There are two highly reactive amino acid sequences, those residues spanning amino acids 4 to 16 (98% signal intensity) and 211 to 223 (97% signal intensity). The third most highly recognized region spans amino acids 289 to 301 (60% signal intensity). All other regions have less than a 40% signal intensity (Fig. 1c and Table 2).
TABLE 2.
Peptide sequences of CMY-2 β-lactamase recognized by the anti-CMY-2 (AmpC) antibodya
| Recognized amino acids in CMY-2 | Sequence | % Signal intensity |
|---|---|---|
| 1-13 | A A K T E Q Q I A D I V N | 100 |
| 4-16 | T E Q Q I A D I V N R T I | 98 |
| 7-19 | Q I A D I V N R T I T P L | 87 |
| 10-22 | D I V N R T I T P L M Q E | 73 |
| CMY-2 aa 4-16 | T E Q Q I A D I V N R T I | |
| AmpC C. freundii | T E Q Q I A D I V N R T I | |
| P99 E. cloacae | S E K Q L A E V V A N T I | |
| AmpC M. morganii | A A D N V A A V V D S T I | |
| 67-79 | K T F N G V L G G D A I A | 25 |
| 70-82 | N G V L G G D A I A R G E | 23 |
| CMY-2 aa 70-79 | N G V L G G D A I A | |
| AmpC C. freundii | N G V L G G D A I A | |
| P99 E. cloacae | T G V L G G D A I A | |
| AmpC M. morganii | T G V L G A V S V A | |
| 208-220 | P V H V S P G Q L D A E A | 83 |
| 211-223 | V S P G Q L D A E A Y G V | 97 |
| 214-226 | G Q L D A E A Y G V K S S | 81 |
| CMY-2 aa 211-223 | V S P G Q L D A E A Y G V | |
| AmpC C. freundii | V S P G Q L D A E A Y G V | |
| P99 E. cloacae | V S P G M L D A Q A Y G V | |
| AmpC M. morganii | V S P G Q L D A E S Y G V | |
| 271-283 | W E M L N W P L K A D S I | 24 |
| 274-286 | L N W P L K A D S I I N G | 29 |
| 277-289 | P L K A D S I I N G S D S | 32 |
| CMY-2 aa 274-286 | L N W P L K A D S I I N G | |
| AmpC C. freundii | L N W P V K A D S I I S G | |
| P99 E. cloacae | L N W P V E A N T V V E G | |
| AmpC M. morganii | Y D W P Q Q L D M I I N G | |
| 286-298 | G S D S K V A L A A L P A | 50 |
| 289-301 | S K V A L A A L P A V E V | 60 |
| CMY-2 aa 289-298 | S K V A L A A L P A | |
| AmpC C. freundii | S K V A L A A L P A | |
| P99 E. cloacae | S K V A L A P L P V | |
| AmpC M. morganii | N E V A L Q P H P V | |
| 319-331 | T G G F G S Y V A F V P E | 25 |
| 322-334 | F G S Y V A F V P E K N L | 32 |
| 325-337 | Y V A F V P E K N L G I V | 33 |
| 328-340 | F V P E K N L G I V M L A | 26 |
| CMY-2 aa 322-334 | F G S Y V A F V P E K N L | |
| AmpC C. freundii | F G S Y V A F V P E K N L | |
| P99 E. cloacae | F G S Y V A F I P E K N I | |
| AmpC M. morganii | F G A Y V A F I P E K N V | |
| 340-352 | A N K S Y P N P V R V E A | 17 |
| 343-355 | S Y P N P V R V E A A W R | 18 |
| 346-358 | N P V R V E A A W R I L E | 38 |
| CMY-2 aa 343-358 | S Y P N P V R V E A A W R I L E | |
| AmpC C. freundii | S Y P N P V R V E A A W R I L E | |
| P99 E. cloacae | S Y P N P A R V E A A Y H I L E | |
| AmpC M. morganii | N T P N T E R V K A A Q A I L S |
CMY-2 immobilized synthetic peptide sequences recognized by the antibody and their corresponding amino acid positions in CMY-2. The percent signal intensity for each of the recognized peptide spots, regions of consensus in each of the recognized peptides of CMY-2 β-lactamase, and the corresponding consensus region for the AmpC β-lactamase of C. freundii, P99 E. cloacae, and AmpC of M. morganii are shown. Standard deviations for signal intensity percentages never exceeded 8% of the reported values. Boldface indicates region of identical amino acids between peptides. Boldface italics indicates peptide consensus region of CMY-2 β-lactamase and corresponding identical amino acids in related AmpC β-lactamases.
Figure 2 shows these immunoreactive linear epitopes (in yellow) mapped onto the crystal structure of the AmpC of C. freundii (97% amino acid homology) as a model enzyme. Table 2 illustrates consensus regions for the AmpC β-lactamases of C. freundii, P99 β-lactamase of E. cloacae, and the AmpC β-lactamase of Morganella morganii. Six of these seven recognized epitopes are 100% homologous to AmpC of C. freundii. Comparison of the CMY-2 and P99 amino acid sequences, which share 71% DNA homology, reveals that five out of the seven antibody-recognized epitopes are homologous. Even in the AmpC β-lactamase of M. morganii, which has only 54% DNA homology, one out of the seven epitopes is 92% identical (epitope V211-V223) (14).
Alanine substitutional analysis of the linear epitope V211-V223 in the CMY-2 β-lactamase.
The epitope -V211SPGQLDAEAYGV223- was further studied using alanine substitution of every amino acid spanning this peptide region. Amino acids Q215, L216, D217, and E219 are critical for antibody recognition (Fig. 2 and 5). The antigenicity of amino acids 218 and 220 could not be determined in this assay, because these are naturally occurring alanine residues and their antibody reactivity corresponds to what is seen in the wild-type peptide epitope.
FIG. 5.
Identification by alanine scanning of essential antibody-reactive residues in the peptide -V211SPGQLDAEAYGV223- of CMY-2 β-lactamase. Densitometric values obtained from analysis of the SPOTs membrane are expressed in terms of percent signal intensity, with the wild-type (WT) peptide A218A being assigned a value of 100%. Data for each individually alanine replaced amino acid is represented relative to the wild-type peptide. Standard deviation of each determination is ≤5% of the reported value.
Anti-SHV-1 and anti-CMY-2 antibody inactivation studies.
We next investigated whether anti-SHV-1 or anti-CMY-2 antibodies interfered with the hydrolytic activity of SHV-1 or AmpC β-lactamases. With regard to SHV-1 β-lactamase, inhibition was most significant for the substrate ampicillin. Preincubation of the anti-SHV-1 antibody with SHV-1 for 1 h at a 10:1 ratio inhibited ampicillin hydrolysis by 89%, and at a 20:1 ratio 91% inhibition was observed (Table 3). Nitrocefin hydrolysis could also be inhibited by addition of the anti-SHV-1 antibody. A ratio of 10:1, anti-SHV-1 antibody to SHV-1, inhibited nitrocefin hydrolysis by 69%, and it inhibited hydrolysis by 76% at a 20:1 ratio. Control experiments were done with anti-CMY-2 antibody incubated with SHV-1. No reduction in catalytic activity was observed at a 5:1, 10:1, or 20:1 antibody-to-enzyme ratio with nitrocefin as the substrate; however, a 15% nonspecific reduction was noted for ampicillin hydrolysis at 10:1 and 20:1 ratios (Table 3).
The inhibitory property of the anti-CMY-2 antibody against CMY-2 was also tested with nitrocefin and cefazolin as substrates. Both 10:1 and 20:1 ratios of anti-CMY-2 antibody to CMY-2 β-lactamase (1-h incubation) produced 67% reduction in hydrolytic activity with cefazolin as the substrate and a greater than 80% reduction with nitrocefin as the substrate. When the incubation time for the antibody-β-lactamase mixtures was extended to 2 h, only an additional 0 to 4% decrease in hydrolytic activity was noted. We reported the 1-h value. The anti-CMY-2 antibody also interfered with hydrolysis of the AmpC β-lactamases of E. cloacae and C. freundii but not with that of P. aeruginosa (Table 3). For the CMY-2 inactivation studies, control experiments were done with anti-SHV-1 antibody incubated with CMY-2. No reduction in catalytic activity was observed at a 5:1, 10:1, or 20:1 antibody-to-enzyme ratios (Table 3). If the polyclonal antibodies had been affinity purified by using their respective antigens, the ratio required for inactivation would be significantly reduced.
Peptide competition assay.
To assess if the inhibition of catalytic activity by anti-CMY-2 antibodies could be overcome, we designed a synthetic peptide containing amino acids V211 to V223 to try and interfere with the antibody-neutralizing activity. With either cefazolin or nitrocefin as the substrate, we were not able to demonstrate restoration of CMY-2 hydrolytic activity with peptide/anti-CMY-2 antibody preincubation followed by inactivation experiments as performed above.
DISCUSSION
Antibodies produced against bacterial β-lactamases have a variety of applications (14). Small soluble fragments derived from heavy-chain dromedary antibodies, which are potent, tight binding inhibitors of TEM-1 and BcII metallo-β-lactamase, were described recently (5). In this study, we have characterized the linear epitopes of the SHV-1 and CMY-2 β-lactamases recognized by two polyclonal rabbit antibodies raised against these two β-lactamases.
All of the epitopes recognized by the anti-SHV-1 antibody are located on the outer surface of the SHV-1 β-lactamase (Fig. 2). Identification of these epitopes is important in determining which amino acids affect antibody recognition when assessing the effects on β-lactamase production after site-directed or site-saturation mutagenesis. Amino acid positions in SHV-1 that we have altered for structure-function studies include M69, D104, S130, R164, E166, D179, T235, G238, E240, and R244 (10, 12, 13). From our analysis it is clear which amino acid changes could potentially affect antibody recognition and assessment of β-lactamase steady-state production. In SHV-1 these sites include the D104 and D179 amino acid residues. All other aforementioned substituted sites (e.g., M69, G238, E240) (10, 12, 13) are not involved in anti-SHV-1 antibody binding. Therefore, it is appropriate to use the ELISA format to assess levels of protein expression in these variants.
Because of the importance of these sites in TEM extended-spectrum β-lactamases, we chose to further examine the role that D104 and D179 play in recognition by the anti-SHV-1 antibody. Substitutional and X-Scan analysis revealed that D104 is critical for antibody recognition. Amino acid substitutions are not permitted at this site; when substitution occurs, antibody recognition is greatly diminished (Fig. 1b and 3). The epitope containing D179 was analyzed in a similar manner. Alanine substitution at position 179 (D179A) did not significantly alter antibody binding to this peptide (Fig. 4). However, alteration of N170 and neighboring amino acids does decrease antibody binding by 50% or more.
Taken together these results validate the ELISA format as a method for screening clinical isolates for the expression of SHV-1 and SHV extended-spectrum β-lactamases. All relevant sites found in the predominant naturally occurring SHV variants (SHV-2 and SHV-5) do not influence antibody binding. Epitope analysis of the SHV-1 β-lactamase with the anti-SHV-1 antibody also explains why the anti-SHV-1 antibody does not recognize TEM-1 β-lactamase (14). Included in Table 1 are TEM-1 amino acids (consensus sequence numbering) that correspond to the mapped residues in SHV-1. With the exception of only one of the eight mapped amino acid sequences, no other consensus regions in TEM-1 have more than four contiguous homologous amino acids. The TEM-1 peptide that has five contiguous homologous amino acids has only a 22% signal intensity when the corresponding SHV-1 peptide is probed with anti-SHV-1 polyclonal antibody. Recognition by anti-SHV-1 polyclonal antibody of SHV β-lactamases occurs through the contribution of eight linear epitopes, each contributing a certain percentage to that recognition. TEM β-lactamases do not possess these epitopes with high enough identity.
CMY-2 epitopes mapped to the outer surface of the CMY-2 β-lactamase and to a region corresponding to the omega loop of class A enzymes. In class C β-lactamases this omega loop region is more exposed to solvent than in class A enzymes (Fig. 2). This region is one of the major epitopes of CMY-2 that the anti-CMY-2 antibody recognized, and it is a highly conserved region in more than 30 AmpC β-lactamases. This, in combination with other conserved epitopes in AmpC enzymes, allows for various degrees of antibody recognition. Other CMY-2 epitopes recognized by the anti-CMY-2 polyclonal antibody are also highly conserved among AmpC β-lactamases (Table 2). Table 2 lists the sequences of purified AmpCs that we used in our analysis (14).
We wondered if the highly homologous omega loop region in the majority of class C β-lactamases is one of the main reasons that the anti-CMY-2 antibody, also termed the anti-AmpC antibody, is capable of recognizing and effectively inhibiting multiple AmpC enzymes (Table 3) (14). This concept is central to designing a monoclonal antibody for AmpC β-lactamase detection and inhibition. The highly homologous nature of this region would allow for detection of many AmpC enzymes and potentially would have the ability to inhibit these β-lactamases as well.
To test this notion, a soluble peptide was synthesized identical to the omega loop region of the CMY-2 β-lactamase amino acids V211 to V223. In competition experiments, the peptide was unable to inhibit the ability of anti-CMY-2 β-lactamase antibody to neutralize activity (Table 4). Multiple explanations exist for this observation. First, the CMY-2 antibody is polyclonal, and we are only using a single, albeit important, epitope (soluble peptide) to block its binding. Second, antibody affinity for the epitope may vary, with the antibody having lower binding affinity for the peptide than the intact antigen (11). This could be due to the lack of secondary and tertiary structure in the soluble peptide that the same sequence would otherwise possess in the native antigen. Third, the omega loop epitope may be part of a discontinuous epitope recognized by the anti-CMY-2 antibody, and therefore the soluble peptide is not recognized with as great an affinity as with the intact CMY-2 β-lactamase. A linear peptide antagonist may not be adequate to restore CMY-2 β-lactamase activity. Experiments are under way to explore these affinity issues.
TABLE 4.
Antibody inactivation studies of CMY-2 β-lactamase with peptidea
| β-lactamase/substrate | Peptide:antibody:β-lactamase molar ratio | Velocity (μM−1 s−1) | % Decrease | |||
|---|---|---|---|---|---|---|
| Pep:anti-CMY-2:CMY-2
|
||||||
| 3.5 nM CMY-2/Cefaz | 5,000:0:1 | 1.0 ± 0.02 | ||||
| 3.5 nM CMY-2/Cefaz | 0:0:1 | 1.1 ± 0.03 | 0 | |||
| 3.5 nM CMY-2/Cefaz | 0:10:1 | 0.4 ± 0.05 | 60 | |||
| 3.5 nM CMY-2/Cefaz | 5,000:10:1 | 0.3 ± 0.07 | 70 | |||
| Pep:anti-CMY-2:CMY-2
|
||||||
| 18 nM CMY-2/NCF | 5,000:0:1 | 4.0 ± 0.1 | ||||
| 18 nM CMY-2/NCF | 0:0:1 | 3.6 ± 0.03 | 10 | |||
| 18 nM CMY-2/NCF | 0:10:1 | 1.5 ± 0.01 | 63 | |||
| 18 nM CMY-2/NCF | 5,000:10:1 | 1.4 ± 0.04 | 65 | |||
β-Lactamase was mixed with various molar ratios of anti-CMY-2 antibody, as described in Materials and Methods. Each velocity determination was repeated in triplicate, and standard deviations were calculated. Pep, synthetic peptide V211-V223 of CMY-2 β-lactamase. NCF, 100 μM nitrocefin; Cefaz, 50 μM cefazolin. Percent decrease is relative to the velocity determined when no antibody was added.
It is our view that steric hindrance of the active site by the antibody bound to the omega loop region of CMY-2 and SHV-1 β-lactamases contributes to its reduction of hydrolytic activity. Figure 2 locates the catalytic Ser70 for SHV-1 and catalytic Ser64 for the AmpC β-lactamase of C. freundii. The distances measured in the crystal structure of C. freundii (1FR1) between residues Ala220, an antibody-bound residue in the omega loop of this AmpC enzyme, and the catalytic Ser64 are known. The distance between the Ala 220 αC and the Ser64 αC is 6.36 Å, and the distance between the αC of Ala220 and the γO of Ser64 is 6.42 Å. Based on this proximity, we propose that the antibody either blocks the active site or interferes with movement of the omega loop of the β-lactamase (Fig. 2). Hence, the nitrocefin substrate cannot access the binding cavity. These notions are consistent with other studies that postulate that peptides inhibiting β-lactamase activity bind near or block the active site (5, 11). The major challenge in our case is determining the sequence and molecular interactions of the complementarity-determining region that inhibits β-lactamase activity. It has not escaped our notice that a combination of binding interactions may be responsible for the inhibitory activity.
In summary, we have defined the major epitopes of SHV-1 and CMY-2 β-lactamases, and we have demonstrated the validity of screening clinical isolates and laboratory strains with our ELISA. Our findings suggest that anti-CMY-2 and anti-SHV-1 antibodies may interfere with catalytic activity by recognizing a site in the omega loop region. Critical binding to solvent-exposed areas may also promote significant displacements at other sites that are crucial in catalysis. The recognition of the omega loop may be extremely useful in the design of monoclonal antibodies for detection purposes. Furthermore, this relationship may serve as a first step in the design of effective peptidomimetic agents to inactivate β-lactamases.
Acknowledgments
This work was supported by grants from the Veterans Affairs Medical Center Merit Review Program (R.A.B.).
We thank Marion S. Helfand for critical reading of the manuscript.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J.-M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atassi, M. Z. 1967. Specific cleavage of tryptophyl peptide bonds with periodate in sperm whale myoglobin. Arch. Biochem. Biophys. 120:56-59. [DOI] [PubMed] [Google Scholar]
- 3.Atassi, M. Z., and B. J. Saplin. 1968. Immunochemistry of sperm whale myoglobin: I. The specific interaction of some tryptic peptides obtained by cleavage at proline peptide bonds. Biochemistry 9:3854-3861. [DOI] [PubMed] [Google Scholar]
- 4.Choulier, L., D. Laune, G. Orfanoudakis, H. Wlad, J. Janson, C. Granier, and D. Altschuh. 2001. Delineation of a linear epitope by multiple peptide synthesis and phage display. J. Immunol. Methods 249:253-264. [DOI] [PubMed] [Google Scholar]
- 5.Conrath, K. E., M. Lauwereys, M. Galleni, A. Matagne, J.-M. Frere, J. Kinne, L. Wyns, and S. Muyldermans. 2001. β-Lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob. Agents Chemother. 45:2807-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank, R. 2002. The SPOT-synthesis technique synthetic peptide arrays on membrane supports-principles and applications. J. Immunol. Methods 267:13-26. [DOI] [PubMed] [Google Scholar]
- 7.Frank, R. 1992. Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48:9217. [Google Scholar]
- 8.Geysen, H. M., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habeeb, A. F., and M. Z. Atassi. 1971. Enzymic and immunochemical properties of lysozyme: Derivatives modified at lysine residues by guanidination, acetylaion, succinylation or maleylation. Immunochemistry 8:1047-1059. [DOI] [PubMed] [Google Scholar]
- 10.Helfand, M. S., A. M. Hujer, F. D. Sonnichsen, and R. A. Bonomo. 2002. Unexpected advanced generation cephalosporinase activity of the M69F variant of SHV β-lactamase. J. Biol. Chem. 277:47719-47723. [DOI] [PubMed] [Google Scholar]
- 11.Huang, W., Z. Beharry, Z. Zhang, and T. Palzkill. 2003. A broad-spectrum peptide inhibitor of β-lactamase identified using phage display and peptide arrays. Protein Eng. 16:853-860. [DOI] [PubMed] [Google Scholar]
- 12.Hujer, A. M., K. M. Hujer, and R. A. Bonomo. 2001. Mutagenesis of amino acid residues in the SHV-1 beta-lactamase: the premier role of Gly238Ser in penicillin and cephalosporin resistance. Biochim. Biophys. Acta 1547:37-50. [DOI] [PubMed] [Google Scholar]
- 13.Hujer, A. M., K. M. Hujer, M. S. Helfand, V. E. Anderson, and R. A. Bonomo. 2002. Amino acid substitutions at Ambler position Gly238 in the SHV-1 β-lactamase: exploring sequence requirements for resistance to penicillins and cephalosporins. Antimicrob. Agents Chemother. 46:3971-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hujer, A. M., M. G. Page, M. S. Helfand, B. Yeiser, and R. A. Bonomo. 2002. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV B-lactamases. J. Clin. Microbiol. 40:1947-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer, A., and J. Schneider-Mergener. 1998. Synthesis and application of peptide libraries bound to continuous cellulose membranes. Methods Mol. Biol. 87:25-39. [DOI] [PubMed] [Google Scholar]
- 16.Lobkovsky, E., P. C. Moews, H. Liu, H. Zhao, J. M. Frere, and J. R. Knox. 1993. Evolution of an enzyme activity:crystallographic structure at 2-A resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 90:11257-11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reineke, U., C. Ivascu, M. Schlief, C. Landgraf, S. Gericke, G. Zahn, H. Herzel, R. Volkmer-Engert, and J. Schneider-Mergener. 2002. Identification of distinct antibody epitopes and mimotopes from a peptide array of 5520 randomly generated sequences. J. Immunol. Methods 267:37-51. [DOI] [PubMed] [Google Scholar]
- 18.Smith, G. P., and J. K. Scott. 1993. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 217:228-257. [DOI] [PubMed] [Google Scholar]
- 19.Tribbick, G. 2002. Multipin peptide libraries for antibody and receptor epitope screening and characterization. J. Immunol. Methods 267:27-35. [DOI] [PubMed] [Google Scholar]