Abstract
The well-known antiparasitic compound licochalcone A is a potent membrane-active agent that transforms normal erythrocytes into echinocytes in parallel with the inhibition of growth of Plasmodium falciparum cultures, the in vitro antiplasmodial effect apparently being an indirect effect on the host cell. In vitro experiments with synchronous cultures demonstrate that inhibition of invasion is the principal mechanism of growth inhibition. The erythrocyte membrane-modifying effect was also transiently observed in vivo in mice after intravenous administration.
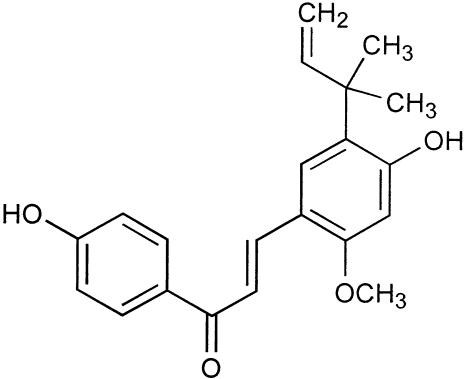
Licochalcone A (Fig. 1) was originally isolated (39, 44) as a constituent of roots and rhizomes of various species of Glycyrrhiza L. (licorice root), and its structure was confirmed by synthesis (20, 22, 44). Subsequent studies resulted in reports of a variety of biological effects of licochalcone A, notably antibacterial (13-15, 17, 18, 36, 43), antileishmanial (3, 4, 8, 34, 45, 46), and antiplasmodial (6, 7, 23) ones. Thus, the compound inhibited growth of Plasmodium falciparum strain 3D7 parasites in vitro with 50% inhibitory concentrations (IC50s) of 5.6 ± 0.6 μM (35) and reduced parasitemia in mice infected with Plasmodium yoelii (7, 23). Compounds related to licochalcone A exhibited comparable antiplasmodial activity in vitro (5, 35).
FIG. 1.
Chemical structure of licochalcone A.
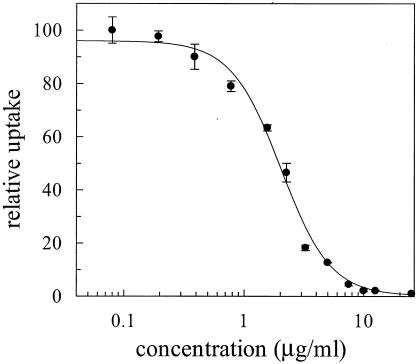
The licochalcone A used in the present work was synthesized as previously described (22, 33, 44). The purity of the compound was assessed by high-performance liquid chromatography (C18 column, acetonitrile gradient in water) and 600-MHz 1H nuclear magnetic resonance and was higher than 99.9%. Determination of the in vitro IC50 (Fig. 2) for P. falciparum strain 3D7 (initial parasitemia, 1.5%) was performed as previously described (49) using 12 concentrations (each used in triplicate) in the range from 0.098 to 25.0 μg/ml (0.29 to 73.9 μM); an average IC50 calculated from three independent determinations was 2.10 ± 0.56 μg/ml (6.21 ± 1.65 μM), which was practically identical to the reported value (35). Microscopic examination of the cultures showed the presence of dead merozoites and ruptured schizonts at concentrations of 1.56 μg/ml and above, and no internal parasites beyond the ring stage were observed.
FIG. 2.
Example of inhibition curve showing licochalcone A inhibition of growth of P. falciparum strain 3D7 cultured in human erythrocytes, as expressed by incorporation of [3H]phenylalanine (49). In this particular determination, an IC50 of 2.02 ± 0.19 μg of licochalcone A/ml (5.97 ± 0.56 μM) is obtained. The IC50 of chloroquine diphosphate determined under identical conditions was 28.3 ± 13.2 ng/ml or 0.055 ± 0.025 μM.
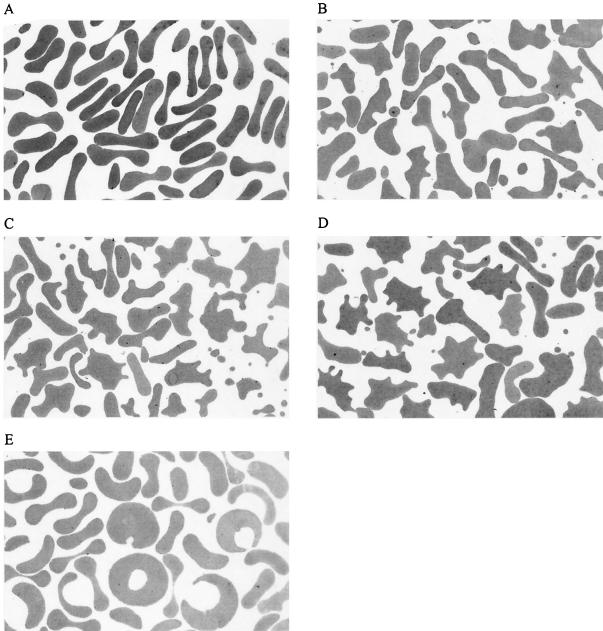
However, simultaneously with the growth inhibition, the exposure of the parasite cultures to licochalcone A caused pronounced membrane perturbations of the erythrocytes in which the parasites grow. The effect of licochalcone A on erythrocytes was the same regardless of whether parasitized or nonparasitized erythrocytes were used and was observed in the same concentration range as that of the in vitro antiplasmodial activity. In order to assess these membrane effects systematically, nonparasitized erythrocytes were incubated with licochalcone A at five different concentrations (0.098, 1.56, 3.13, 5.0, and 25.0 μg/ml) for 48 h as in the growth inhibition assay. The deformations of the cell shape were investigated by light microscopy after fixation to glass slides with methanol followed by staining with Giemsa as well as by the hanging drop technique, by differential interference contrast microscopy after fixation on glass slides with glutaric aldehyde, and by transmission electron microscopy (TEM) using standard methods (49). Control experiments and cultures containing 0.098 μg of licochalcone A/ml showed a normal discocytic shape of erythrocytes. In agreement with previous studies (49), dimethyl sulfoxide (DMSO) present in the medium in concentrations up to 0.5% caused no change of the erythrocyte shape. At licochalcone A concentrations in the range of 1.56 to 5.0 μg/ml, the discocytes were transformed to echinocytes, with type III echinocytes (2) dominating. The shape changes were increasingly pronounced as the concentration increased, with concomitant formation of an increasing number of exovesicles. All microscopic techniques used in this study showed consistent results demonstrating that the observations are not related to sample preparation. The results of the TEM investigations are shown in Fig. 3. Interestingly, the erythrocytes treated with 25.0 μg of licochalcone A/ml (the highest concentration tested) showed more normal cell shapes and even slightly stomatocytic forms (Fig. 3). That the observed membrane shape changes are independent of the presence of P. falciparum parasites was demonstrated in a parallel series of experiments with parasitized erythrocytes (initial parasitemia of 1.5%, incubation time of 48 h, licochalcone A concentration of 0.098 to 25.0 μg/ml).
FIG. 3.
TEMs (magnification, ×2,000) illustrating effects of licochalcone A on erythrocyte membrane. (A) Control erythrocytes incubated for 48 h in medium containing 0.5% DMSO. (B-E) Erythrocytes incubated for 48 h with medium containing 1.56, 3.13, 5.0, and 25.0 μg of licochalcone A/ml, respectively.
In order to investigate the time course of the membrane effects of licochalcone A, nonparasitized erythrocytes were treated with 0, 0.2, 2.0, 10.0, and 25.0 μg/ml of the compound, and the cells were examined by light microscopy after 5, 15, and 30 min and 1, 2, 4, 24, 30, and 48 h. This experiment demonstrated that the uptake of licochalcone A into the erythrocyte membrane and the resulting cell shape deformations take place within 5 min, after which the membrane effects were essentially time independent in the concentration range up to 10.0 μg/ml. By contrast, the erythrocytes treated with 25.0 μg of licochalcone A/ml showed formation of type III echinocytes within 5 min, whereas the above-mentioned return to discocytic or slightly stomatocytic forms was complete only after 24 h. When the erythrocytes were preincubated with 5 μg of licochalcone A/ml for 24 h and concentration of the compound in the medium increased to 25.0 μg/ml, the time course of the membrane shape changes (assessed after 15 and 30 min and 1, 4, 24, 30, and 48 h of additional incubation time) was exactly the same as when the cells were directly exposed to 25.0 μg of licochalcone A/ml. On the other hand, dilution of an erythrocyte culture incubated for 24 h with 25.0 μg of licochalcone A/ml with medium, resulting in a decrease of the concentration to 5.0 μg/ml, did not result in a return to the echinocyte III shape. This shows that the cell shape change induced by 25.0 μg of licochalcone A/ml, and hence the resulting membrane loading, is irreversible under these conditions.
In order to determine which developmental stage of the parasite is susceptible to the presence of licochalcone A in the erythrocyte membrane, cultures synchronized by use of magnetic cell sorting were used (42). Thus, the late-stage parasites were separated, transferred to flasks with the growth medium, and allowed to develop to ring stages or to trophozoites. The synchronous cultures were then treated with 0.098, 1.56, 3.13, 5.0, and 25.0 μg of licochalcone A/ml, and the effect on the growth was monitored by light microscopy.
The control culture starting with ring stages showed late trophozoites (and a few schizonts) after 24 h and rings (and a few merozoites) after 48 h. The cultures treated with 5.0 or 25.0 μg of licochalcone A/ml showed only a few early trophozoites after 24 h, and those treated with 0.098 to 3.13 μg/ml contained trophozoites and schizonts. After 48 h the culture treated with 25.0 μg of licochalcone A/ml showed only a few early trophozoites and internal undeveloped merozoites. The culture treated with 3.13 and 5.0 μg of licochalcone A/ml showed a few late trophozoites, schizonts, and merozoites. The cultures treated with the lowest concentrations of the compound contained a lot of ring stages. The control culture starting with trophozoites contained ring stages (and a few ruptured schizonts) after 24 h and rings and trophozoites after 48 h. Treatment with 5.0 or 25.0 μg of licochalcone A/ml resulted in the presence of mainly trophozoites and a few undeveloped internal parasites after 24 h. The culture treated with 3.13 μg/ml showed the presence of equal amounts of trophozoites and rings. The cultures treated with 0.098 or 1.56 μg of licochalcone A/ml were similar to the control. After 48 h, in all the cultures the number of internal parasite stages was strongly diminished (in a concentration-dependent manner), and external and internal undeveloped merozoites were present. These experiments demonstrate that the most significant growth inhibition is observed when the culture goes through the erythrocyte invasion stage and that the extent of inhibition parallels the extent of erythrocyte membrane modification, as in the experiments with asynchronous cultures (Fig. 2).
Since licochalcone A was reported to decrease parasitemia in mice infected with P. yoelii (7), it was of interest to assess possible effects of the compound on the erythrocyte morphology in vivo. Three administration routes were tested: oral, intraperitoneal, and intravenous. For oral administration, mice (20 to 25 g, three mice per dose) were administered 0, 20, or 100 mg of licochalcone A/kg of body weight in olive oil (5 μl/g of body weight of a solution containing 0, 4, or 20 mg of licochalcone A/ml, obtained by 3:17 dilution of stock solutions in DMSO with olive oil). For intraperitoneal administration, mice (25 to 30 g, three mice per dose) were administered 0, 10, or 50 mg of licochalcone A/kg in saline containing Brij 35 (10 μl/g of body weight of a solution containing 0, 1, or 5 mg of licochalcone A/ml, obtained by 3:37 dilution of stock solutions in DMSO with 5% Brij 35 in saline). For intravenous administration, mice (25 to 30 g, three mice per dose) were administered 0, 10, or 25 mg of licochalcone A/kg; the compound was injected into a tail vein either as 0.75 μl/g of body weight of a solution in DMSO (0, 13, or 33 mg/ml) or as 5 μl/g of body weight of a solution in 5% Brij 35 in saline (0, 2, or 5 mg/ml). Control mice were administered the vehicles alone. Solutions for the intraperitoneal and intravenous administrations were subjected to sterile filtration prior to use. Blood samples were withdrawn from the eye 1 h and 24 h after the administration, smears were prepared, and the erythrocyte shapes were assessed microscopically.
Neither of the above experiments demonstrated deviations from the normal discocytic erythrocyte shape in the eye blood. However, while the mice subjected to peroral or intraperitoneal administration showed normal behavior, those subjected to the intravenous administration were clearly disturbed by the administration of licochalcone A (but not of the vehicles alone), especially at the highest doses (symptoms included fatigue, increased heartbeat rate, and abnormal walking). Therefore, additional experiments using the intravenous administration route were performed. Thus, two mice (20 to 25 g) received 25-mg/kg licochalcone A in DMSO (0.75 μl/g), two mice received 25-mg/kg licochalcone A in 5% Brij 35 in saline (5 μl/g), and two groups of two control mice received the vehicles alone via the tail vein. Blood was withdrawn from the eye and the tail immediately after the administration and from the eye 5 min after the administration. The circulated blood, sampled from the eye immediately after administration of licochalcone A in either of the two vehicles, contained type II and III echinocytes (2) as the dominating cell form, with only a few discocytes present. The blood sampled locally from the tail showed more pronounced alterations and the presence of spheroechinocytes (2). No erythrocyte shape changes occurred after intravenous administration of the vehicles alone (0.75 μl of DMSO or 5 μl of 5% Brij 35 in saline per g of body weight). In the circulated blood sampled from the eye 5 min after the administration of licochalcone A, only mild erythrocyte shape alterations were apparent, with discocytes and type I echinocytes (2) as the dominating cell shapes.
The present experiments demonstrate that licochalcone A is a potent membrane-active agent, causing rapid and concentration-dependent transformation of discocytes into echinocytes (Fig. 3) paralleling the antiplasmodial activity (Fig. 2). The effect could also be transiently observed in vivo after intravenous administration of the compound, but the cells returned quickly to the normal shape, presumably as the result of redistribution of licochalcone A in lipophilic compartments of the body or removal of damaged erythrocytes. It is well documented that the discocyte-to-echinocyte transformations are caused by incorporation of chemicals into the outer layer of the lipid bilayer of the erythrocyte membrane (2, 19, 28, 41). Thus, rapid and concentration-dependent transformation of discocytes to echinocytes accompanied by exovesiculation has been observed with a number of amphiphiles and other compounds (16, 21, 47). The partial return of the cell shape to normal at very high licochalcone A concentrations (25.0 μg/ml) can be explained by redistribution of the compound into the inner membrane layer (21, 47); although the cells acquire a more normal shape, it is obvious that also at this concentration the erythrocyte membrane is loaded with licochalcone A. While the initial uptake of licochalcone A into the erythrocyte membrane is relatively fast (up to 5 min), the redistribution observed at high concentrations is slow (up to 24 h).
At the same time, the experiments with synchronous cultures strongly suggest that the major effect of licochalcone A is inhibition of erythrocyte invasion by merozoites and/or inhibition of initial growth of internalized merozoites. The invasion and growth of P. falciparum in erythrocytes depend on the normal function and integrity of the erythrocyte membrane; as it grows inside the erythrocyte, the parasite causes extensive modifications of the host cell, including rearrangement of the host cell's macromolecules, expression of large numbers of its own proteins, and establishment of new permeation pathways (9, 10, 25). Modifications of the erythrocyte membrane composition, expressed as the echinocyte formation, are thus expected to cause conditions unfavorable for the in vitro proliferation of the parasite. There is indeed a lot of evidence emphasizing the importance of the erythrocyte membrane state and composition for the parasite's growth (11, 24, 26, 31, 32, 37, 40). We have recently demonstrated that a variety of compounds inhibit in vitro growth of P. falciparum by virtue of their incorporation into the erythrocyte membrane (1, 47-49). The described correlation between the echinocytogenic and in vitro antiplasmodial effects of licochalcone A provides a novel example of this effect. We believe that the observations reported herein are of profound interest in relation to the current efforts to develop chalcones as novel antimalarial drugs (5, 6, 12, 23, 27, 29, 30, 35, 38), in part because the in vitro assay with chalcones presumably provides information about structure-activity relationships for the erythrocyte membrane-modifying effect rather than for a direct antiparasitic effect, and in part because of expected problems with intravenous administration of the drug. With respect to the reported in vivo antimalarial activities of chalcones, we note the prevalent lack of correlation between IC50s for chalcones and chloroquine in vitro (P. falciparum) and their relative effects in vivo (mouse models employing Plasmodium berghei and P. yoelii) (7, 12, 30, 38). While the latter discrepancy has yet to be explained, the erythrocyte membrane changes described for the first time in this work are a convenient means of identification of possible artifacts due to host-cell effects of chalcones during in vitro antiplasmodial screens. In addition, the membrane activity of licochalcone A may be a significant factor in other in vitro assays employing whole cells.
Acknowledgments
We thank Anne Corfitz and Maiken Christensen (Centre for Medicinal Parasitology, Copenhagen University Hospital) for cultivation and synchronization of P. falciparum; Dorte Brix, Bettina Jensen, and Birgitte Simonsen (The Danish University of Pharmaceutical Sciences) for in vitro assays, in vivo assays, and high-performance liquid chromatography analyses, respectively; and Gunilla Henriksson and Esa Nummelin (Åbo Akademi University) for assistance with the TEM work.
REFERENCES
- 1.Asili, J., M. Lambert, H. L. Ziegler, D. Stœrk, M. Sairafianpour, M. Witt, G. Asghari, I. S. Ibrahimi, and J. W. Jaroszewski. 2004. Labdanes and isopimaranes from Platycladus orientalis and their effects on erythrocyte membrane and on Plasmodium falciparum growth in the erythrocyte host cells. J. Nat. Prod. 67:631-637. [DOI] [PubMed] [Google Scholar]
- 2.Bessis, M. 1972. Living blood cells and their ultrastructure. Springer-Verlag, Heidelberg, Germany.
- 3.Chen, M., S. B. Christensen, J. Blom, E. Lemmich, L. Nadelmann, K. Fich, T. G. Theander, and A. Kharazmi. 1993. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 37:2550-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., S. B. Christensen, T. G. Theander, and A. Kharazmi. 1994. Antileishmanial activity of licochalcone A in mice infected with Leishmania major and in hamsters infected with Leishmania donovani. Antimicrob. Agents Chemother. 38:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M., S. B. Christensen, L. Zhai, M. H. Rasmussen, T. G. Theander, S. Frøkjœr, B. Steffansen, J. Davidsen, and A. Kharazmi. 1997. The novel oxygenated chalcone, 2,4-dimethoxy-4′-butoxychalcone, exhibits potent activity against human malaria parasite Plasmodium falciparum in vitro and rodent parasites Plasmodium berghei and Plasmodium yoelii in vivo. J. Infect. Dis. 176:1327-1333. [DOI] [PubMed] [Google Scholar]
- 6.Chen, M., J. A. Klaumünzner, L. Zhai, A. Kharazmi, and J. Jensen. 2002. Effect of antimalarial licochalcone A on the electron transport chain of Plasmodium falciparum and Plasmodium berghei, p. 493-498. In Parasitology—ICOPA X: symposia, workshops and contributed papers. Proceedings of the 10th International Congress on Parasitology, Vancouver, Canada. Monduzzi Editore, Bologna, Italy.
- 7.Chen, M., T. G. Theander, S. B. Christensen, L. Hviid, L. Zhai, and A. Kharazmi. 1994. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob. Agents Chemother. 38:1470-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, M., L. Zhai, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke, B. M., N. Mohandas, and R. L. Coppel. 2001. The malaria-infected red blood cell: structural and functional changes. Adv. Parasitol. 50:1-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai, A. S., S. M. Bezrukov, and J. Zimmerberg. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005. [DOI] [PubMed] [Google Scholar]
- 11.Dluzewski, A. R., K. Rangachari, W. B. Gratzer, and R. J. M. Wilson. 1983. Inhibition of malarial invasion of red cells by chemical and immunochemical linking of spectrin molecules. Br. J. Haematol. 55:629-637. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez, J. N., J. E. Charris, G. Lobo, N. G. de Dominguez, M. M. Moreno, F. Riggione, E. Sanchez, J. Olson, and P. J. Rosenthal. 2001. Synthesis of quinolinyl chalcones and evaluation of their antimalarial activity. Eur. J. Med. Chem. 36:555-560. [DOI] [PubMed] [Google Scholar]
- 13.Friis-Møller, A., M. Chen, K. Fuursted, S. B. Christensen, and A. Kharazmi. 2002. In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med. 68:416-419. [DOI] [PubMed] [Google Scholar]
- 14.Fukai, T., A. Marumo, K. Kaitou, T. Kanda, S. Terada, and T. Nomura. 2002. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 73:536-539. [DOI] [PubMed] [Google Scholar]
- 15.Fukai, T., A. Marumo, K. Kaitou, T. Kanda, S. Terada, and T. Nomura. 2002. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 71:1449-1463. [DOI] [PubMed] [Google Scholar]
- 16.Hägerstrand, H., and B. Isomaa. 1989. Vesiculation induced by amphiphiles in erythrocytes. Biochim. Biophys. Acta 982:179-186. [DOI] [PubMed] [Google Scholar]
- 17.Haraguchi, H., K. Tanimoto, Y. Tamura, K. Mizutani, and T. Kinoshita. 1998. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 48:125-129. [DOI] [PubMed] [Google Scholar]
- 18.Hatano, T., Y. Shintani, Y. Aga, S. Shiota, T. Tsuchiya, and T. Yoshida. 2000. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 48:1286-1292. [DOI] [PubMed] [Google Scholar]
- 19.Iglic, A., V. Kralj-Iglic, and H. Hägerstrand. 1998. Amphiphile induced echinocyte-spherostomatocyte transformation of red blood cell shape. Eur. J. Biophys. 27:335-339. [DOI] [PubMed] [Google Scholar]
- 20.Islam, A., and M. A. Hossain. 1993. Synthesis of licochalcone-A. Indian J. Chem. Sect. B 32B:713-715. [Google Scholar]
- 21.Isomaa, B., H. Hägerstrand, and G. Paatero. 1987. Shape transformations induced by amphiphiles in erythrocytes. Biochim. Biophys. Acta 899:93-103. [DOI] [PubMed] [Google Scholar]
- 22.Khan, S. A., and M. Krishnamurti. 1983. Synthesis of licochalcone A. Indian J. Chem. Sect. B 22B:276-277. [Google Scholar]
- 23.Kharazmi, A., M. Chen, T. Theander, and S. B. Christensen. 1997. Discovery of oxygenated chalcones as novel antimalarial agents. Ann. Trop. Med. Parasitol. 91:S91-S95. [Google Scholar]
- 24.Kidson, C., G. Lamont, A. Saul, and G. T. Nurse. 1981. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc. Natl. Acad. Sci. USA 78:5829-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk, K. 2001. Membrane transport in the malaria-infected erythrocyte. Physiol. Rev. 81:495-537. [DOI] [PubMed] [Google Scholar]
- 26.Lauer, S., J. Van Wye, T. Harrison, H. McManus, B. U. Samuel, N. L. Hiller, N. Mohandas, and K. Haldar. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, R., G. L. Kenyon, F. E. Cohen, X. Chen, B. Gong, J. N. Dominguez, E. Davidson, G. Kurzban, R. E. Miller, E. O. Nuzum, P. J. Rosenthal, and J. H. McKerrow. 1995. In vitro antimalarial activity of chalcones and their derivatives. J. Med. Chem. 38:5031-5037. [DOI] [PubMed] [Google Scholar]
- 28.Lim, G. H. W., M. Wortis, and R. Mukhopadhyay. 2002. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer-couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA 99:16766-16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M., P. Wilairat, S. L. Croft, A. L.-C. Tan, and M.-L. Go. 2003. Structure-activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem. 11:2729-2738. [DOI] [PubMed] [Google Scholar]
- 30.Liu, M., P. Wilairat, and M.-L. Go. 2001. Antimalarial alkoxylated and hydroxylated chalcones: structure-activity relationship analysis. J. Med. Chem. 44:4443-4452. [DOI] [PubMed] [Google Scholar]
- 31.McColm, A. A., M. Hommel, and P. I. Trigg. 1980. Inhibition of malaria parasite invasion into erythrocytes pre-treated with membrane-active drugs. Mol. Biochem. Parasitol. 1:119-127. [DOI] [PubMed] [Google Scholar]
- 32.Mons, B. 1990. Preferential invasion of malarial merozoites into young red blood cells. Blood Cells 16:299-312. [PubMed] [Google Scholar]
- 33.Nielsen, S. F., M. Chen, T. G. Theander, A. Kharazmi, and S. B. Christensen. 1995. Synthesis of antiparasitic licorice chalcones. Bioorg. Med. Chem. Lett. 5:449-452. [Google Scholar]
- 34.Nielsen, S. F., S. B. Christensen, G. Cruciani, A. Kharazmi, and T. Liljefors. 1998. Antileishmanial chalcones: statistical design, synthesis, and three-dimensional quantitative structure-activity relationship analysis. J. Med. Chem. 41:4819-4832. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen, S. F., A. Kharazmi, and S. B. Christensen. 1998. Modifications of the α,β-double bond in chalcones only marginally affect the antiprotozoal activity. Bioorg. Med. Chem. 6:937-945. [DOI] [PubMed] [Google Scholar]
- 36.Okada, K., Y. Tamura, M. Yamamoto, Y. Inoue, R. Takagaki, K. Takahashi, S. Demizu, K. Kajiyama, Y. Hiraga, and T. Kinoshita. 1989. Identification of antimicrobial and antioxidant constituents from licorice of Russian and Xinjiang origin. Chem. Pharm. Bull. 37:2528-2530. [DOI] [PubMed] [Google Scholar]
- 37.Pasvol, G., and R. J. M. Wilson. 1989. Red cell deformability and invasion by malaria parasites. Parasitol. Today 5:218-221. [DOI] [PubMed] [Google Scholar]
- 38.Ram, V. J., A. S. Saxena, S. Srivastava, and S. Chandra. 2000. Oxygenated chalcones and bischalcones as potential antimalarial agents. Bioorg. Med. Chem. Lett. 10:2159-2161. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh, T., and S. Shibata. 1975. New type of chalcones from licorice root. Tetrahedron Lett. 16:4461-4462. [Google Scholar]
- 40.Samuel, B. U., N. Mohandas, T. Harrison, H. McManus, W. Rosse, M. Reid, and K. Haldar. 2001. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 276:29319-29329. [DOI] [PubMed] [Google Scholar]
- 41.Sheetz, M. P., and S. J. Singer. 1974. Biological membranes as bilayer couples, a molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA 71:4457-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 43.Tsukiyama, R.-I., H. Katsura, N. Tokuriki, and M. Kobayashi. 2002. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother. 46:1226-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, R.-S., K.-L. Wen, S.-F. Jiang, C.-G. Wang, F.-X. Jiang, Y.-Y. Xie, and Y.-S. Gao. 1978. Isolation, structure and total synthesis of licochalcone. Huaxue Xuebao 37:289-297. [Google Scholar]
- 45.Zhai, L., J. Blom, M. Chen, S. B. Christensen, and A. Kharazmi. 1995. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 39:2742-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai, L., M. Chen, J. Blom, T. G. Theander, S. B. Christensen, and A. Kharazmi. 1999. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J. Antimicrob. Chemother. 43:793-803. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler, H. L., H. Franzyk, M. Sairafianpour, M. Tabatabai, M. D. Tehrani, K. Bagherzadeh, H. Hägerstrand, D. Stœrk, and J. W. Jaroszewski. 2004. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure-activity relationships for betulinic acid analogues. Bioorg. Med. Chem. 12:119-127. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler, H. L., T. H. Jensen, J. Christensen, D. Stœrk, H. Hägerstrand, A. A. Sittie, C. E. Olsen, T. Staalsø, P. Ekpe, and J. W. Jaroszewski. 2002. Possible artifacts in the in vitro determination of antimalarial activity of natural products that incorporate into lipid bilayer: apparent antiplasmodial activity of dehydroabietinol, a constituent of Hyptis suaveolens. Planta Med. 68:547-549. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler, H. L., D. Stœrk, J. Christensen, L. Hviid, H. Hägerstrand, and J. W. Jaroszewski. 2002. In vitro Plasmodium falciparum drug sensitivity assay: inhibition of parasite growth by incorporation of stomatocytogenic amphiphiles into the erythrocyte membrane. Antimicrob. Agents Chemother. 46:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]