Abstract
Combination therapies may be required for long-term management of some patients chronically infected with hepatitis B virus (HBV). Adefovir is a nucleotide analog that has similar activity against wild-type and lamivudine-resistant HBV. In contrast to lamivudine, clinical resistance to the prodrug adefovir dipivoxil emerges infrequently. Based on its clinical efficacy and low frequency of resistance, adefovir dipivoxil may form an important component of combination regimens. We therefore investigated the in vitro antiviral efficacy of combinations of adefovir with other nucleoside analogs (lamivudine, entecavir, emtricitabine [FTC],and telbivudine [L-dT]) and the nucleotide analog tenofovir. Using a novel stable cell line that expresses high levels of wild-type HBV, we assayed the antiviral activity of each drug alone and in combination with adefovir. All two-drug combinations resulted in greater antiviral effects than treatments with single agents and could be characterized as additive by the Bliss independence model. Analysis using the Loewe additivity model indicated that adefovir exerted additive antiviral effects when combined with lamivudine, FTC, or L-dT and moderately synergistic effects when combined with entecavir or tenofovir. There was no evidence of cytotoxicity with any of the drugs when used alone or in combination at the tested doses.
Hepatitis B virus (HBV) is a small hepatotropic DNA virus that is able to establish chronic infection in humans. Chronic HBV infection can last a lifetime, causing persistent hepatitis that frequently progresses to more-severe liver disease. Currently, three agents are approved for the treatment of chronic hepatitis B: alpha interferon (IFN-α), lamivudine, and adefovir dipivoxil. IFN-α, a cytokine with immunomodulatory and antiviral activity, is effective in only one third of indicated patients and is associated with significant side effects. Lamivudine, a cytosine analog in the unnatural levorotary (l) conformation, produces potent reductions in viremia (9) but is hampered by the emergence of viral resistance in approximately 20% of patients per year (23).
Adefovir dipivoxil, an oral prodrug of the AMP analog adefovir, is the most recently approved anti-HBV therapeutic. Adefovir dipivoxil therapy produces a rapid decline in viremia in patients infected with wild-type or lamivudine-resistant HBV (2, 17, 24). Unlike lamivudine, resistance to adefovir dipivoxil emerges infrequently. During clinical trials, the adefovir resistance mutations rtN236T and A181V were observed in samples from <2% of patients who received 96 weeks of therapy and in <4% of patients after 144 weeks of therapy (1, 39; X. Qi, A. Snow, V. Thibault, Y. Zhu, M. Curtis, S. Hadziyannis, C. Brosgart, G. Currie, S. Arterburn, C. Gibbs, M. Miller, and S. Xiong, Abstr. 39th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 57, 2004). Importantly, in vitro and limited clinical data indicate that adefovir-resistant HBV remains sensitive to lamivudine and other developmental nucleosides (39; H. Yang, X. Qi, K. Das, E. Arnold, C. Westland, W. Delaney IV, C. Brosgart, C. Gibbs, M. Miller, and S. Xiong, Abstr. 39th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 383, 2004).
Several other nucleoside and nucleotide analogs are currently in clinical development for the treatment of chronic hepatitis B. The most advanced of these are entecavir (BMS-200,475), emtricitabine (FTC), and telbivudine (L-dT). Entecavir, currently in phase III development, is a deoxyguanosine analog with in vitro activity against both the priming and DNA synthetic activities of HBV polymerase (34). Entecavir appears to have reduced efficacy against lamivudine-resistant HBV in vitro (29); however, preliminary clinical data indicate that high doses of the compound maintain viral suppression in vivo (R. Colonno and R. Rose, Abstr. 53rd Annu. Meet. Am. Assoc. Study Liver Dis., abstr. 1925, 2002). FTC is an l-nucleoside with structural similarity to lamivudine that was recently approved for the treatment of human immunodeficiency virus (HIV) and is in phase III development for chronic hepatitis B. In vitro and clinical studies have indicated that FTC has antiviral activity and a resistance profile similar to that of lamivudine (13, 14); however, preliminary clinical results suggest that resistance to FTC may occur less frequently than with lamivudine (R. Gish, N. Leung, C. Wang, L. Corey, S. Sacks, M. Fried, T. Wright, T. Huy, F. Chan, F. Rousseau, M. Herve, A. Snow, J. Anderson, A. Rigney, and E. Mondou, Abstr. 53rd Annu. Meet. Am. Assoc. Study Liver Dis., abstr. 838, 2002). L-dT is an l-nucleoside analog of thymidine that is in phase III studies. Recent clinical data suggest that L-dT is more efficacious than lamivudine at reducing viremia in patients with wild-type HBV infection (C. Lai, N. Leung, E. Teo, M. Tong, F. Wong, H. Hann, S. Han, T. Poynard, M. Myers, G. Zhao, D. Lloyd, and N. Brown, Abstr. 53rd Annu. Meet. Am. Assoc. Study Liver Dis. 2002, abstr. 554, 2002). The clinical efficacy of L-dT against lamivudine-resistant HBV has not been tested; however, in vitro data suggest that all l-nucleosides, including L-dT and FTC, have decreased efficacy against YMDD mutant HBV (7; W. Delaney IV, H. Yang, M. Miller, C. Gibbs, and S. Xiong, 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 309, 2002). Tenofovir disoproxil fumarate, a prodrug of the AMP analog tenofovir, has activity against both HBV and HIV and is approved for treatment of HIV. Although tenofovir disoproxil fumarate is not approved for use against HBV, it has been used to effectively treat lamivudine-resistant HBV in HIV-coinfected patients (28, 31, 38). Several other compounds, including MCC-478, l-FMAU [1-(2-fluoro-5-methyl-β-l-arabinofuranosyl)uracil], and Val-β-l-2′-deoxycytidine (a valyl prodrug of β-l-2′-deoxycytidine), are also in earlier stages of clinical development for the treatment of chronic hepatitis B (19, 20, 37).
Clinical experience with lamivudine and adefovir dipivoxil indicates that most patients will require long-term therapy to maintain suppression of viral replication and remission of liver disease. Patients who do not achieve adequate viral suppression during monotherapy, due either to resistance or to suboptimal primary response, may benefit from regimens containing two or more agents. Indeed, clinical experience from the HIV field indicates that combination therapy is superior to monotherapy in maintaining viral suppression and elevating CD4+ cell counts (11, 12). It is unclear if similar benefits will be realized during combination treatment of chronic hepatitis B, since the investigation of such treatments has only recently been explored in controlled clinical trials. Based on its clinical efficacy against wild-type and lamivudine-resistant HBV, adefovir dipivoxil may form an important component of combination regimens. Accordingly, we have investigated the in vitro antiviral efficacy of combinations of adefovir with other nucleoside and nucleotide analogs.
MATERIALS AND METHODS
Cell culture.
HepG2 cells were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in humidified incubators at 5% CO2 and 37°C. HepG2 cells were grown in minimal essential medium (ATCC) supplemented with 100 U of penicillin/ml, 10 μg of streptomycin/ml, and 10% heat-inactivated fetal bovine serum (Irvine Scientific, Santa Ana, Calif.). The novel HepG2 49-29 cell line (described below) and the HepG2 2.2.15 cell line were maintained under conditions identical to those used for HepG2 cells.
Generation of the HepG2 49-29 cell line.
HepG2 cells were transfected with a plasmid construct encoding two 1.1-unit length HBV genomes (genotype A; GenBank accession no. AF305422) and a neomycin resistance gene. Each HBV genome was under the transcriptional control of a cytomegalovirus promoter, while the neomycin resistance gene was under the control of transcriptional simian virus 40 promoter. Three days after transfection, cells were selected in medium supplemented with 800 μg of G418 (Sigma, St. Louis, Mo.)/ml for approximately 2 weeks. G418-resistant colonies were transferred to 48-well plates and subsequently screened for hepatitis B e antigen (HBeAg) secretion with the colormetric ETI-EBK+ immunoassay kit (DiaSorin, Stillwater, Minn.). HBeAg-positive colonies were further expanded and screened for other markers of HBV replication, including the production of HBsAg (by DiaSorin ETI-MAK-2 immunoassay), extracellular HBV DNA (by PCR analysis of conditioned medium), and intracellular viral replicative intermediates (as described below). To compare HBV expression levels of novel cell lines to that of the reference cell line HepG2 2.2.15, 2 × 106 cells were seeded in 60-mm dishes and cultured for 7 days, at which point all cell lines had reached confluence. Intracellular viral replicative intermediates were then extracted, and all extracts were quantified as described below. Expression levels of HBV DNA for the novel cell lines were then calculated relative to observed replication in HepG2 2.2.15.
Compounds.
Adefovir, tenofovir, FTC, and entecavir were synthesized by Gilead Sciences (Foster City, Calif.). Lamivudine and L-dT were purchased from Moravek Biochemicals (Brea, Calif.).
Determination of IC50s for individual drugs.
HepG2 49-29 cells were seeded at a density of 105 cells/well in 48-well plates and allowed to attach overnight. The following day, cells were treated with medium containing antiviral compounds. Five concentrations of each drug were tested based on antiviral activity data available in the literature (4, 13, 18). Cells were treated every other day for 1 week, after which they were lysed in 200 μl of 0.33% Igepal C-630 in phosphate-buffered saline for 5 min. Cell lysates were transferred to microfuge tubes and spun for 5 min to pellet nuclei. The resulting supernatants were extracted using the Masterpure Total nucleic acid extraction kit (Epicentre, Madison, Wis.), with the modification that proteinase K digestion was extended to 1 h. Purified nucleic acids were resuspended in 20 μl of TE buffer and digested with 5 U of DNase-free RNase. All 20 μl of viral DNA was then fractionated by electrophoresis through 1% agarose gels and transferred to nylon membranes by standard Southern blotting procedures (32). Viral DNA was detected by nucleic acid hybridization with a 33P-labeled HBV probe, and mature forms of the viral genome (relaxed circular and double-stranded replicative intermediates) were quantified with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Regression analyses of antivirus data were performed with TableCurve2D software (SPSS Scientific, Chicago, Ill.), and 50% inhibitory concentration (IC50) values were calculated from the resulting best-fit equations as previously described (8).
Design of combination experiments.
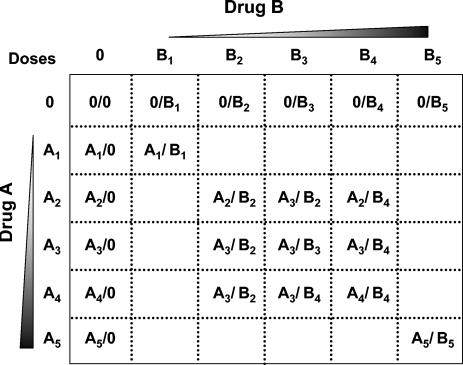
Each combination experiment included 24 samples, 13 of which were controls (five doses of each drug alone plus three doses in three untreated wells) and 11 of which were drug combinations. Of the five concentrations tested for each drug, the middle dose was approximately equal to the IC50; two higher doses (corresponding to approximately three and six times the IC50) and two lower doses (corresponding to approximately 0.16 and 0.33 times the IC50) were also tested. Figure 1 depicts which doses were used in combination for each drug pair. With this assay format, all samples for a single experiment could be contained in the inner 24 wells of a 48-well plate and processed in parallel at the end of the treatment period. Drug treatment, DNA extraction, and viral DNA quantification were performed as described above; all samples from each combination experiment were analyzed on single gels.
FIG. 1.
Matrix of drug concentrations used during two-drug antiviral combination experiments. During each two-drug combination assay, individual drugs (A and B) were tested alone at five concentrations that spanned the linear region of their experimentally determined dose responses (data not shown). For drugs A and B, dose 1 was 0.16 times the IC50, dose 2 was 0.33 times the IC50, dose 3 equaled the IC50, dose 4 was 3 times the IC50, and dose 5 was 6 times the IC50 (dose numbers are indicated by subscripts). To test the combination activity of drugs A and B, 11 combination doses were administered to cells, as indicated by the matrix.
Analysis of combination data.
Antivirus data were analyzed using both the Loewe additivity and Bliss independence drug interaction models (15, 16). The Loewe additivity model is defined by the equation dx/Dx + dy/Dy = 1, where Dx and Dy are the doses of individual drugs required to exert the same effect as doses dX and dy used in combination. If the experimental product of this equation (termed the Loewe combination index) is equal to 1, the data are considered additive; indices of <1 or >1 indicate synergy or antagonism, respectively. The CombiTool program (version 2.001) was used to quantify differences between observed effects and those predicted by the Loewe additivity equation as well as to calculate combination indices (10). Single-drug control data from each triplicate experiment were first fit to the equation z = 100 ×{1/[1+(x/a)b]} × {1/[1+(y/c)d]}, which defines a three-dimensional surface as the product of two logistic dose equations (one derived from each drug), using the TableCurve3D program (SPSS Science). x and y are the concentrations of drugs A and B, respectively; z is the antiviral response. Parameters a and b are the dose response of drug A, while parameters c and d are the dose responses of drug B. Antiviral parameters for each drug were then entered into the logistic function of CombiTool to generate the expected effect of drug combinations, based on an additive Loewe interaction. Three-dimensional plots depicting the differences between the experimental (observed) and predicted data were generated using Sigmaplot 8.0 (SPSS Science).
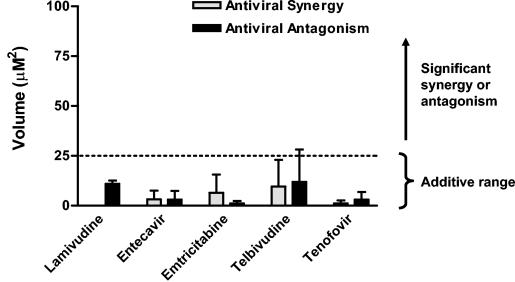
The Bliss independence model is defined by the equation Exy = Ex + Ey − (ExEy), where (Exy) is the additive effect of drugs x and y as predicted by their individual effects (Ex and Ey). The MacSynergy II program, version 1.0 (M. N. Prichard, K. R. Aseltine, and C. Shipman, Jr., University of Michigan) was used to evaluate antivirus data according to the Bliss independence model MacSynergy II uses a nonparametric three-dimensional approach to quantify areas where observed effects are significantly greater (synergy) or less (antagonism) than those predicted from single-drug control data. As suggested by Prichard et al., triplicate data sets were assessed at the 95% confidence level and should be interpreted as follows: volumes of synergy or antagonism at values of <25 μM2 are considered insignificant, those at values of >25 but <50 μM2 are considered minor but significant, those at values of >50 but <100 μM2 are considered moderate and potentially important in vivo, and those at values of >100 μM2 are considered strong and likely to be important in vivo.
Cytotoxicity testing.
To assess cytostatic or cytotoxic effects of drug combinations, HepG2 49-29 cells were seeded into 96-well plates at a density of 2 × 104 cells/well and exposed to compounds for 1 week with a treatment schedule identical to that described above for the antivirus assays. Each drug was tested alone and in combination with adefovir at the highest doses used for antiviral combination testing. Following the drug treatment, cell viability was assessed by sodium 3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) cleavage with a commercially available assay (Roche). One-way analysis of variance (ANOVA) was used to compare cell viability among the treated and untreated cultures with GraphPad Prism (version 3.03; GraphPad Software, San Diego, Calif.). P values of <0.05 were considered significant.
RESULTS
Generation of a novel cell line expressing high levels of HBV.
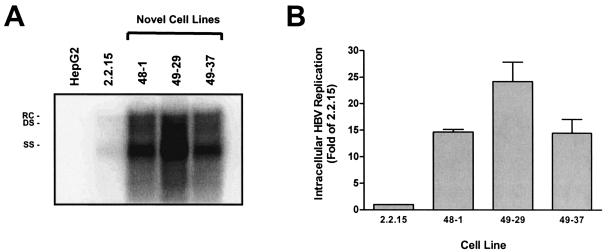
Previous combination studies have utilized the HepG2 2.2.15 cell line, which expresses modest amounts of HBV (21). To facilitate combination experiments, a novel stably transfected cell line (HepG2 49-29) that expresses high levels of HBV replicative intermediates was generated. HepG2 cells were transfected with a plasmid construct encoding two 1.1-unit length HBV genomes (genotype A) and a neomycin resistance gene. After selection in G418, clones that tested positive for HBeAg, HBsAg, and extracellular HBV DNA were further expanded and screened for intracellular replicating HBV. Several positive clones were obtained; however, clone 49-29 was selected for further studies, since it produced approximately 25-fold-more intracellular replicative intermediates than the reference cell line HepG2 2.2.15 (Fig. 2).
FIG. 2.
Intracellular HBV replication levels in novel stably transfected cell lines. Several novel HBV-expressing cell lines were generated by the stable transfection of HepG2 cells with a plasmid encoding two 1.1-unit length HBV genomes. (A) Three HBsAg- and HBeAg-positive cell lines (48-1, 49-29, and 49-37) and the reference cell line HepG2 2.2.15 were each seeded at a density of 2 × 106 cells in 60-mm dishes and were allowed to grow to confluence over 1 week. Southern blot analysis was used to quantify the total amount of intracellular HBV replication present in each culture. Relaxed circular (RC), double-stranded (DS), and single-stranded (SS) forms of the HBV genome are shown. (B) The relative amount of double-stranded replicative intermediates present in the three cell lines compared to that of HepG2 2.2.15 is presented (values are the mean of two experiments; error bars indicate standard deviation).
Dose selection for antiviral combination assays.
Five clinically relevant compounds were chosen to test in combination with adefovir: lamivudine, FTC, entecavir, L-dT, and tenofovir. To select appropriate doses for combination testing, the IC50 of each drug against wild-type HBV in HepG2 49-29 cells was determined (Table 1). IC50 data obtained with the HepG2 49-29 cell line agreed well with previously reported data generated with other stable or transient HBV expression systems (4, 13, 18, 40). In addition to the IC50s, two higher doses (approximately three and six times the IC50) and two lower doses (approximately 0.16 and 0.33 times the IC50) were chosen for combination testing, since these concentrations spanned the linear region of the dose-response curves.
TABLE 1.
Antiviral activity of compounds in HepG2 49-29 cells
| Compound | IC50a |
|---|---|
| Adefovir | 0.215 ± 0.003 |
| Lamivudine | 0.023 ± 0.008 |
| Entecavir | 0.0013 ± 0.0003 |
| FTC | 0.033 ± 0.007 |
| L-dT | 0.335 ± 0.042 |
| Tenofovir | 0.172 ± 0.059 |
50% inhibitory concentration. All values are micromolar concentrations and represent the average of two or more experiments ± the standard deviation.
Antiviral efficacy of drug combinations.
For each drug pair, two independent combination experiments were run in triplicate, and viral replication was quantified by Southern blotting and nucleic acid hybridization. Sample results for an individual combination experiment are presented in Fig. 3. The resulting antivirus data were then analyzed with the two predominant models for defining drug interaction: Loewe additivity and Bliss independence. For analysis with the Loewe additivity model, the single-drug and untreated-control data from each experiment were first fit to a three-dimensional dose-response surface; the resulting parameters were then used to calculate expected additive values and Loewe combination indices with the CombiTool program. During these analyses, the IC50s derived from single-drug controls were in close agreement with the initial data generated (Tables 1 and 2). The average Loewe combination index of the combination data points was calculated for each drug (Table 2). Combinations of adefovir with lamivudine and L-dT produced combination indices of approximately 1, indicating an additive antiviral interaction. The combinations of adefovir with entecavir, FTC, or tenofovir produced average combination indices of <1, which suggests antiviral synergy. With respect to the combination of adefovir and FTC, the average combination index of 0.87 ± 0.08 is not significantly outside the 0.9-to-1.1 range, which is considered to be nearly additive (T. C. Chou and M. P. Hayball, CalcuSyn: Windows software for dose effect analysis program manual, Biosoft, Ferguson, Mo.). Combinations of adefovir with either entecavir or tenofovir produced average combination indices of approximately 0.7, which can be interpreted as moderate synergy according to the Loewe additivity model.
FIG. 3.
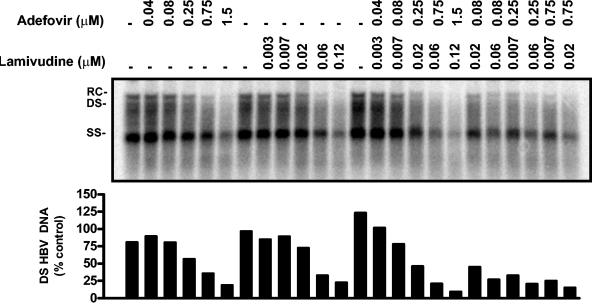
Sample primary data for an antiviral combination experiment. Following drug treatment, HBV replicative intermediates were extracted from HepG2 49-29 cells and analyzed by Southern blotting with a 33P-labeled HBV probe. (A) The results of a combination experiment assaying the antiviral effects of adefovir plus lamivudine are presented. The micromolar doses of adefovir and lamivudine are indicated above each lane (−, no treatment was given). Relaxed circular (RC), double-stranded (DS), and single-stranded (SS) forms of the HBV genome are shown. (B) Quantification of double-stranded viral replicative intermediates (DS) obtained by PhosphorImager analysis.
TABLE 2.
IC50 and R2 values derived from three-dimensional surface plots and average Loewe combination indices.
| Drug pair | IC50a
|
R2b | Loewe CIc | |
|---|---|---|---|---|
| Drug 1 (adefovir) | Drug 2 | |||
| Adefovir + lamivudine | 0.27 ± 0.04 | 0.023 ± 0.001 | 0.92 ± 0.03 | 1.03 ± 0.24 |
| Adefovir + entecavir | 0.22 ± 0.06 | 0.001 ± 0.0001 | 0.93 ± 0.04 | 0.72 ± 0.25 |
| Adefovir + FTC | 0.28 ± 0.01 | 0.049 ± 0.006 | 0.95 ± 0.01 | 0.87 ± 0.08 |
| Adefovir + L-dT | 0.19 ± 0.01 | 0.35 ± 0.04 | 0.96 ± 0.01 | 1.00 ± 0.04 |
| Adefovir + tenofovir | 0.23 ± 0.01 | 0.20 ± 0.07 | 0.89 ± 0.06 | 0.72 ± 0.03 |
All values are micromolar concentrations and represent the average of two experiments performed in triplicate ± standard deviation.
R2 refers to dose-response fit of both drugs on a three-dimensional surface plot (mean ± standard deviation).
Average Loewe combination indices (CI) for all combination data points are shown ± standard deviations.
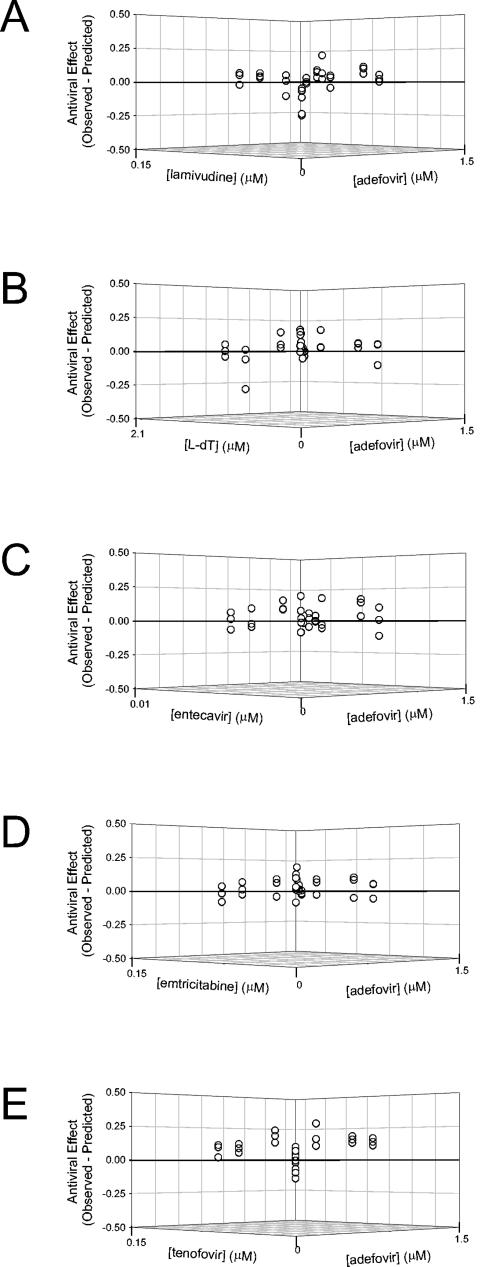
Residual plots, depicting the difference between the observed and the expected antiviral effects (based on the Loewe additivity equation), are presented in Fig. 4. For the adefovir plus lamivudine, L-dT, or FTC combinations, most residuals were within 1 standard deviation of the zero-interaction plane and were distributed approximately evenly above and below the plane, consistent with an interpretation of Loewe additivity. Combinations of adefovir with either entecavir or tenofovir tended to produce residuals that were distributed above the zero interaction surface. For these combinations, a greater number of combination points lay beyond 1 standard deviation but within 2 standard deviations of the zero-interaction surface, suggesting a moderate synergistic drug interaction.
FIG. 4.
Three-dimensional residual plots comparing experimental (observed) and predicted levels of HBV replication for antiviral combination data. The results of each antiviral combination experiment were analyzed by graphing the difference between observed antiviral effects and effects predicted from the single drug controls based on the Loewe additivity model (see Materials and Methods). The antiviral effect is presented in the z plane as a fraction of total viral replication (derived from untreated controls). The concentration of adefovir (in micromoles) is indicated on the y axes, and concentrations of the second drugs (in micromoles) are graphed on the x axes. Points that fall above the additive zero-interaction plane (z = 0) can be considered synergistic, since the observed antiviral effect was greater than predicted; conversely, points falling below the zero-interaction plane can be considered antagonistic. Each graph presents the results of one triplicate experiment where adefovir was assayed in combination with lamivudine (A), entecavir (B), FTC (C), L-dT (D), or tenofovir (E).
For analysis with the Bliss independence model, antiviral combination data were analyzed with the MacSynergy II program. Volumes of statistically significant antiviral synergy and antagonism quantified by MacSynergy II for each pair of compounds are presented in Fig. 5. Results of this analysis indicated that minor regions of synergy and antagonism were identified for all compounds tested in combination with adefovir. The interaction volumes identified by MacSynergy II were <25 μM2 for all drugs and are predicted to be insignificant in vivo. Thus, the antiviral interaction between adefovir and the other tested compounds did not appear to deviate significantly from additivity as defined by the Bliss independence model.
FIG. 5.
MacSynergy II analysis of antiviral combination data. Primary data from Southern blot analyses were analyzed according to the Bliss independence model of drug interaction with MacSynergy II. Volumes of statistically significant synergy and antagonism quantified at the 95% confidence level are indicated. Volumes shown are the means of two independent experiments performed in triplicate (error bars indicate standard deviation). Per Prichard et al., volumes of <25 μM2 are considered insignificant, while those of >25 μM2 are considered significant (see Materials and Methods).
Cytotoxic and cytostatic effects of drug treatment.
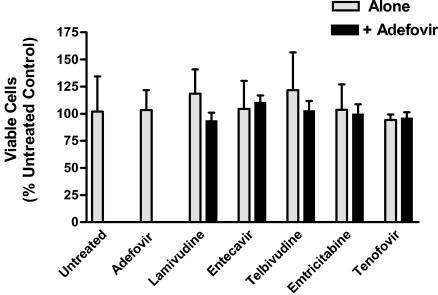
Since the doses used for all antiviral compounds were at least 100-fold less than their respective 50% cytotoxic concentrations, cellular toxicity was not expected, based on additive interactions. However, to ensure that unpredicted synergistic toxicities did not contribute to the antiviral effects observed during combination assays, we assayed the cytotoxic and cytostatic effects of each drug combination. Three independent assays were performed in triplicate with each drug tested alone and in combination with adefovir at the highest doses used for the antivirus assays (Fig. 6) No significant cytotoxic effects were observed for any of the cultures treated with one or two drugs (P = 0.67; one-way ANOVA).
FIG. 6.
Cytotoxic and cytostatic effects of drug combinations on HepG2 49-29 cells. HepG2 49-29 cells were seeded into 96-well plates at a density of 2 × 104 cells/well and exposed to the indicated compounds for 1 week. Cell viability was subsequently assayed by XTT cleavage. Three independent assays were performed in triplicate with each drug tested alone and in combination with adefovir at the highest doses used for the antivirus assays (1.5 μM adefovir, 0.12 μM lamivudine, 0.009 μM entecavir, 2.04 μM L-dT, 0.24 μM FTC, and 1.5 μM tenofovir). The mean number of viable cells for each treatment condition (as a percentage of the untreated controls) is shown; error bars indicate standard deviations. No significant cytotoxicity was observed for any of the cultures treated with one or two drugs (P = 0.67; one-way ANOVA).
DISCUSSION
Combination therapy is crucial to the management of HIV infection. Although there are important viral and pathogenic differences between HBV and HIV infections, it is hoped that combination therapy will provide similar benefits for patients with chronic hepatitis B infection. Ideally, combination therapy for chronic hepatitis B would significantly enhance HBeAg seroconversion rates. Unfortunately, seroconversion may not be an achievable endpoint for all chronic hepatitis B patients, particularly those who lack significant immune activity against the virus. However, even in the absence of seroconversion, antiviral therapy could aim to achieve long-term suppression of viral load, which has proven to translate into a remission of liver disease. Thus, for patients that do not undergo HBeAg seroconversion, the combination of two or more antiviral agents with additive and/or synergistic antiviral activity and distinct resistance profiles may represent the best option for long-term suppression of viral load and disease management.
Few data on the in vitro activity of drug combinations against HBV are currently available. Korba et al. previously showed that combinations of lamivudine and penciclovir had synergistic activity against HBV in HepG2 2.2.15 cells (21) and that the combination of lamivudine and famciclovir produced additive to synergistic effects against woodchuck hepatitis virus in vivo (22). Colledge et al. reported additive or synergistic effects of adefovir, lamivudine, and penciclovir when used in combination against the duck HBV in primary duck hepatocytes (5, 6). Seigneres et al. provided enzymatic, cell culture, and in vivo data indicating that combinations of emtricitabine, amdoxovir, and clevudine (l-FMAU) provided efficacy superior to treatment with any of the drugs as a single agent (36).
Our results, which were generated with a novel stable cell line expressing high levels of HBV, indicate that combinations of adefovir with other anti-HBV drugs produce greater in vitro antiviral effects than any of the agents used alone. We did not observe any evidence of cytotoxicity during these assays at the highest tested combination doses. Our observations are in agreement with the reported 50% cytotoxic concentrations for all compounds, which are orders of magnitude higher than the antiviral doses used here. Further experiments will be needed to determine whether additive, synergistic, or antagonistic in vitro cytotoxicity is observed when cells are treated with high doses of these drugs.
Interpretation of the antiviral results with the Bliss independence model indicated that none of the tested combinations deviated significantly from additivity. Analysis using the Loewe additivity model indicated that the activity of adefovir in combination with l-nucleosides (lamivudine, FTC, and L-dT) was additive while combinations of adefovir with entecavir or tenofovir were synergistic. As noted previously, it is not uncommon for the Bliss independence and Loewe additivity models to disagree (10). The underlying basis for the discrepancy between these two models is generally due to the shape of the individual drugs dose-response curves. When both drugs have identical exponential dose responses, both models will be in agreement (3). However, for compounds with steeper dose responses, greater combination effects may be observed with the Loewe additivity model. For compounds with flatter dose responses, greater combination effects may be observed with the Bliss model (10).
It is unclear why combinations of adefovir with entecavir or tenofovir resulted in Loewe synergy, while the l-nucleoside combinations produced Loewe additivity. Interestingly, adefovir, tenofovir, and entecavir are purine analogs, whereas all of the tested l-nucleosides are pyrimidines. One explanation is that combinations of adefovir with entecavir or tenofovir affect purine metabolism in a way that enhances the anabolic and antiviral efficacy of these drugs. Alternatively, tenofovir and entecavir may have better in vitro activity in a subpopulation of cells (e.g., those in a distinct stage of the cell cycle and with differential nucleoside and/or nucleotide kinase expression) than the tested lnucleosides. It should be noted that differential activation of nucleoside and nucleotide analogs in distinct cell types has previously been observed in vivo with the duck HBV model (26, 27). Activity of different agents in distinct cell compartments provides another theoretical argument in favor of using combination therapy in a clinical setting.
The observed Loewe synergy could also theoretically result from combining multiple agents that act as inhibitors of the HBV-priming reaction. Indeed, previous in vitro studies have demonstrated that entecavir inhibits HBV priming in addition to DNA synthesis (34); similar studies performed with the duck HBV system have indicated that adefovir can also inhibit hepadnaviral priming (35). In contrast, deoxycytidine analogs (including lamivudine and FTC) are theoretically unable to act as substrates for the HBV priming reaction, since the priming sequence (UUC or UUCA) does not allow for base pairing with these nucleotides (25). It is currently unclear if L-dT can act as an inhibitor of HBV priming. Base pairing with adenosine does not appear to be required, and there are no in vitro studies addressing L-dT's ability to inhibit the hepadnaviral priming reaction.
To date, there are relatively few controlled clinical studies that have examined the use of two drugs to treat chronic hepatitis B. The majority of these have been combinations of IFN-α and lamivudine (reviewed by Schalm) (33); however, the use of adefovir in combination with lamivudine and other agents is now being explored. Preliminary clinical studies using IFN-α plus lamivudine have provided evidence of greater antiviral effect and a delay in lamivudine resistance; however, these studies have not demonstrated a conclusive improvement in serocoversion. Furthermore, the drawbacks of IFN-α therapy (side effects, the difficulties of parenteral administration, and contraindications to using the drug with several patient populations) limit its utility as a combination agent, especially for long-term patient management. The treatment of lamivudine-resistant HBV with the combination of lamivudine and adefovir dipivoxil was shown to suppress viremia in patients but more than with adefovir dipivoxil alone (30). Preliminary results from a study comparing the combination of adefovir dipivoxil plus lamivudine versus lamivudine monotherapy in naïve patients have recently been presented (J. Sung, Lai, J. S. Zeuzem, W. Chow, E. Heathcote, R. Perrillo, C. Brosgart, M. Woessner, S. Scott, and E. Campbell, Abstr. 38th Annu. Meet. Eur. Assoc. Study Liver Dis. 2003, abstr. 69, 2003). These results indicated that the two therapies produced similar initial viral load reductions, but the combination reduced the emergence of lamivudine resistance and produced better virologic response late in the study. Seroconversion rates were not significantly different between patients receiving combination therapy and those receiving lamivudine monotherapy. Analyses from the second year of this study are ongoing and may reveal a greater difference between the monotherapy and combination arms; these data will have strong implications for the treatment of chronic infection.
In conclusion, we used a novel HBV-expressing cell line to investigate the antiviral activity of activity of adefovir in combination with other approved or phase III investigational anti-HBV drugs. Our results indicated that two-drug combinations that included adefovir produced greater in vitro antiviral effects than those of single agents. The observed antiviral combination effects were characterized as additive with the Bliss independence model or additive to synergistic with the Loewe additivity model. Importantly, there was no evidence of in vitro cytotoxicity and no evidence of antiviral antagonism with any of the drug combinations at the tested doses. Combination therapy should be explored further clinically, especially for patients who are unable to achieve full serum HBV DNA suppression during monotherapy. The activity of adefovir against lamivudine-resistant HBV, the infrequent emergence of adefovir resistance, and the data provided here suggest that adefovir dipivoxil may form an important component of future combination regimens.
Acknowledgments
We thank Jurgen Suhnel (Institute of Molecular Biotechnology, Jena, Germany) and Mark Prichard (Aviron, Mountain View, Calif.) for providing the CombiTool and MacSynergy II programs, respectively. We thank Tim Shaw and Stephen Locarnini (VIDRL, N. Melbourne, Australia) and Arnold Fridland (Gilead, Foster City, Calif.) for scientific discussions.
REFERENCES
- 1.Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou, Y., M. Bochet, V. Thibault, V. Calvez, M. H. Fievet, P. Vig, C. S. Gibbs, C. Brosgart, J. Fry, H. Namini, C. Katlama, and T. Poynard. 2001. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet 358:718-723. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1989. What is synergy? Pharmacol. Rev. 41:93-141. [PubMed] [Google Scholar]
- 4.Bryant, M. L., E. G. Bridges, L. Placidi, A. Faraj, A.-G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J.-L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J.-P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colledge, D., G. Civitico, S. Locarnini, and T. Shaw. 2000. In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob. Agents Chemother. 44:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colledge, D., S. Locarnini, and T. Shaw. 1997. Synergistic inhibition of hepadnaviral replication by lamivudine in combination with penciclovir in vitro. Hepatology 26:216-225. [DOI] [PubMed] [Google Scholar]
- 7.Das, K., X. Xiong, H. Yang, C. E. Westland, C. S. Gibbs, S. G. Sarafianos, and E. Arnold. 2001. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC). J. Virol. 75:4771-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney, W. E., IV, R. Edwards, D. Colledge, T. Shaw, J. Torresi, T. G. Miller, H. C. Isom, C. T. Bock, M. P. Manns, C. Trautwein, and S. Locarnini. 2001. Cross-resistance testing of antihepadnaviral compounds using novel recombinant baculoviruses which encode drug-resistant strains of hepatitis B virus. Antimicrob. Agents Chemother. 45:1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dienstag, J. L., E. R. Schiff, T. L. Wright, R. P. Perrillo, H. W. Hann, Z. Goodman, L. Crowther, L. D. Condreay, M. Woessner, M. Rubin, and N. A. Brown. 1999. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 341:1256-1263. [DOI] [PubMed] [Google Scholar]
- 10.Dressler, V., G. Muller, and J. Suhnel. 1999. CombiTool—a new computer program for analyzing combination experiments with biologically active agents. Comput. Biomed. Res. 32:145-160. [DOI] [PubMed] [Google Scholar]
- 11.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, M. Rubin, et al. 1995. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N. Engl. J. Med. 333:1662-1669. [DOI] [PubMed] [Google Scholar]
- 12.Eron, J. J., Jr. 1996. The treatment of antiretroviral-naive subjects with the 3TC/zidovudine combination: a review of North American (NUCA 3001) and European (NUCB 3001) trials. AIDS 10(Suppl. 5):S11-S19. [DOI] [PubMed] [Google Scholar]
- 13.Furman, P. A., M. Davis, D. C. Liotta, M. Paff, L. W. Frick, D. J. Nelson, R. E. Dornsife, J. A. Wurster, L. J. Wilson, and J. A. Fyfe. 1992. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob. Agents Chemother. 36:2686-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gish, R. G., N. W. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco, W., H. D. Unkelback, G. Pöch, J. Sühnel, M. Kundi, and W. Bödeker. 1992. Consensus on concepts and terminology for combined-action assessment: the Saariselkä agreement. Arch. Complex Environ. Stud. 4:65-69. [Google Scholar]
- 16.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 17.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 18.Innaimo, S. F., M. Seifer, G. S. Bisacchi, D. N. Standring, R. Zahler, and R. J. Colonno. 1997. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob. Agents Chemother. 41:1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiya, N., A. Kubota, Y. Iwase, K. Sekiya, M. Ubasawa, and S. Yuasa. 2002. Antiviral activities of MCC-478, a novel and specific inhibitor of hepatitis B virus. Antimicrob. Agents Chemother. 46:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocic, I. 2000. Clevudine University of Georgia/Abbott/Bukwang/Triangle/Yale University. Curr. Opin. Investig. Drugs 1:308-313. [PubMed] [Google Scholar]
- 21.Korba, B. E. 1996. In vitro evaluation of combination therapies against hepatitis B virus replication. Antiviral Res. 29:49-51. [DOI] [PubMed] [Google Scholar]
- 22.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. L. Gerin, and B. C. Tennant. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and famciclovir against WHV replication in chronic WHV carrier woodchucks. Antiviral Res. 45:19-32. [DOI] [PubMed] [Google Scholar]
- 23.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 24.Marcellin, P., T.-T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, C. L. Brosgart, and the Adefovir Dipivoxil 437 Study Group. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 25.Nassal, M., and A. Rieger. 1996. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J. Virol. 70:2764-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoll, A., S. Locarnini, S. T. Chou, R. Smallwood, and P. Angus. 2000. Effect of nucleoside analogue therapy on duck hepatitis B viral replication in hepatocytes and bile duct epithelial cells in vivo. J. Gastroenterol. Hepatol. 15:304-310. [DOI] [PubMed] [Google Scholar]
- 27.Nicoll, A. J., D. L. Colledge, J. J. Toole, P. W. Angus, R. A. Smallwood, and S. A. Locarnini. 1998. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue. Antimicrob. Agents Chemother. 42:3130-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunez, M., M. Perez-Olmeda, B. Diaz, P. Rios, J. Gonzalez-Lahoz, and V. Soriano. 2002. Activity of tenofovir on hepatitis B virus replication in HIV-co-infected patients failing or partially responding to lamivudine. AIDS 16:2352-2354. [DOI] [PubMed] [Google Scholar]
- 29.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters, M. G., H. W. Hann, P. Martin, E. J. Heathcote, P. Buggisch, R. Rubin, M. Bourliere, K. Kowdley, C. Trepo, D. F. Gray, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and C. L. Brosgart. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91-101. [DOI] [PubMed] [Google Scholar]
- 31.Ristig, M. B., J. Crippin, J. A. Aberg, W. G. Powderly, M. Lisker-Melman, L. Kessels, and P. Tebas. 2002. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. J. Infect. Dis. 186:1844-1847. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Analysis and cloning of eukaryotic genomic DNA, p. 9.1-9.62. In N. Ford, C. Nolan, and M. Ferguson (ed.), Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Schalm, S. W. 2003. Combination therapy for chronic hepatitis B. J. Hepatol. 39:S146-S150. [DOI] [PubMed] [Google Scholar]
- 34.Seifer, M., R. K. Hamatake, R. J. Colonno, and D. N. Standring. 1998. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob. Agents Chemother. 42:3200-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seigneres, B., S. Aguesse-Germon, C. Pichoud, I. Vuillermoz, C. Jamard, C. Trepo, and F. Zoulim. 2001. Duck hepatitis B virus polymerase gene mutants associated with resistance to lamivudine have a decreased replication capacity in vitro and in vivo. J. Hepatol. 34:114-122. [DOI] [PubMed] [Google Scholar]
- 36.Seigneres, B., P. Martin, B. Werle, O. Schorr, C. Jamard, L. Rimsky, C. Trepo, and F. Zoulim. 2003. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 47:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Standring, D. N., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, E. Cretton-Scott, R. F. Schinazi, M. Myers, M. L. Bryant, and J. P. Sommadossi. 2001. Antiviral β-l-nucleosides specific for hepatitis B virus infection. Antivir. Chem. Chemother. 12(Suppl. 1):119-129. [PubMed] [Google Scholar]
- 38.van Bommel, F., T. Wunsche, D. Schurmann, and T. Berg. 2002. Tenofovir treatment in patients with lamivudine-resistant hepatitis B mutants strongly affects viral replication. Hepatology 36:507-508. [DOI] [PubMed] [Google Scholar]
- 39.Villeneuve, J. P., D. Durantel, S. Durantel, C. Westland, S. Xiong, C. L. Brosgart, C. S. Gibbs, P. Parvaz, B. Werle, C. Trepo, and F. Zoulim. 2003. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 39:1085-1089. [DOI] [PubMed] [Google Scholar]
- 40.Yang, H., C. E. Westland, W. E. Delaney, I. V., E. J. Heathcote, V. Ho, J. Fry, C. Brosgart, C. S. Gibbs, M. D. Miller, and S. Xiong. 2002. Resistance surveillance in chronic hepatitis B patients treated with adefovir dipivoxil for up to 60 weeks. Hepatology 36:464-473. [DOI] [PubMed] [Google Scholar]