Abstract
Infection of implanted materials by bacteria constitutes one of the most serious complications following prosthetic surgery. In the present study, we developed a new strategy based on the insertion of an antimicrobial peptide (defensin from Anopheles gambiae mosquitoes) into polyelectrolyte multilayer films built by the alternate deposition of polyanions and polycations. Quartz crystal microbalance and streaming potential measurements were used to follow step by step the construction of the multilayer films and embedding of the defensin within the films. Antimicrobial assays were performed with two strains: Micrococcus luteus (a gram-positive bacterium) and Escherichia coli D22 (a gram-negative bacterium). The inhibition of E. coli D22 growth at the surface of defensin-functionalized films was found to be 98% when 10 antimicrobial peptide layers were inserted in the film architecture. Noticeably, the biofunctionalization could be achieved only when positively charged poly(l-lysine) was the outermost layer of the film. On the basis of the results of bacterial adhesion experiments observed by confocal or electron microscopy, these observations could result from the close interaction of the bacteria with the positively charged ends of the films, which allows defensin to interact with the bacterial membrane structure. These results open new possibilities for the use of such easily built and functionalized architectures onto any type of implantable biomaterial. The modified surfaces are active against microbial infection and represent a novel means of local host protection.
Modern surgery often involves implantable medical devices aimed at replacing altered or missing body parts. Protection of these implants against microorganisms is of particular importance, since infection of the implanted material constitutes one of the most serious complications after surgery. This issue becomes even more problematic for biomaterial implanted in a septic environment, such as laryngeal or oral prostheses. If such infections are left untreated, the formation of a bacterial slime or biofilm at the surfaces of biomaterials (9, 32) can lead to a chronic microbial infection, inflammation, and tissue necrosis (5, 33). These infections are most frequently due to gram-positive bacteria, such as Staphylococcus epidermidis in the case of intravascular catheter-associated infections (31) and Staphylococcus aureus in the case of metallic implants (1). Catheter infections caused by the gram-negative bacterium Escherichia coli have also been reported (10).
The initial step of infection is always linked to the ability of the bacteria to adhere to material surfaces (26). Even if the adhesion is initially reversible, it becomes irreversible later, during the infectious process. In fact, the association of the individual microorganisms and matrix formation lead to an organized and protected structure, the slime, which has a high level of resistance to conventional antibiotic therapy. The increasing problem of bacterial resistance (32, 38) has stimulated growing interest in the development of antimicrobial peptides as new therapeutic agents. Antimicrobial peptides were first discovered in insects and were then reported to exist also in mammals and plants (19). They participate in the innate immune defense against microorganisms. One of the first targets of antimicrobial peptides is the bacterial membrane. Therefore, to develop resistance, a microorganism would have to redesign its membrane structure, changing the composition and/or organization of its lipids, which represents a costly solution for most microbial species. In addition, multicellular organisms fight microorganisms with a large variety of antimicrobial peptides of different structural classes and with different spectra of action.
In the study described here, we have immobilized an antimicrobial peptide, the defensin from Anopheles gambiae mosquitoes, onto a planar substrate by embedding it in multilayer films. Insect defensins are members of a widely distributed family of antibacterial peptides with a typical pattern of six cysteine residues and three disulfide bridges. These peptides are made up of 36 to 46 amino acids and are active against a wide range of gram-positive bacteria and some gram-negative bacteria (such as E. coli D22). Fungi and yeasts were also reported to be affected by defensins (4). Although the detailed mechanism of action of the defensins is not fully understood at present, patch-clamp experiments with giant liposomes support the hypothesis that defensins affect the permeability of the bacterial membrane through the formation of channels in the cytoplasmic membrane (8). This mode of action, with no intracellular target, seemed of interest for the creation of a surface with antimicrobial properties by means of immobilization with defensins.
The main limitation in the development of antimicrobial peptides as therapeutic agents is that although many naturally occurring peptides are active in vitro, they are effective in infected animal models only at very high doses, close to the toxic doses of the peptide (11). Thus, most pharmaceutical strategies have been devoted to the development of agents for topical applications, largely because of the relative safety of topical therapy and the uncertainty surrounding the long-term toxicology of any new class of drug administered systemically (15). An approach to controlling unwanted biofilm formation at the surface of implanted materials is to kill the bacteria during their initial attachment rather than trying to remove them once they have adhered. In this context, we report here on a new approach consisting of simply embedding antimicrobial peptides into multilayer polyelectrolyte films, with the aim being to isolate the peptides while retaining their bioactivities. Interestingly, these films, based on the alternate deposition of oppositely charged polyelectrolyte layers (Fig. 1A), are able to coat any type of surface (e.g., metals and plastics) (12) without any shape limitation (e.g., planar, spherical, or curved) (36). Functionalization of the multilayer films can be accomplished by insertion of peptides or proteins inside the film (Fig. 1B). On the basis of these objectives, the layer-by-layer technique was recently developed with the aim of using it for different biological applications in the fields of biosensors (6), cell signaling control (7), and antiadhesive surfaces (2). To our knowledge, this study is the first one to use the layer-by-layer technique and simple peptide adsorption to provide antimicrobial protection.
FIG. 1.
Schematic of polyelectrolyte multilayer film (A) and defensin insertion (B). Adsorption is obtained by adding a layer of the opposite charge; the peptide is embedded under another polyelectrolyte layer.
MATERIALS AND METHODS
Polyelectrolyte multilayer film preparation and defensin insertion.
Polyethyleneimine (PEI; molecular mass, 750 kDa; Aldrich), poly(sodium 4-styrenesulfonate) (PSS; molecular mass,70 kDa, Aldrich), poly(allylamine hydrochloride) (PAH; molecular mass,70 kDa, Aldrich), poly(l-glutamic acid) (PGA; molecular mass,54.8 kDa; Sigma), and poly(l-lysine) (PLL; molecular mass, 23.4 kDa; Sigma) were used to build the films. PEI, PSS, and PAH solutions were each prepared at a concentration of 5 mg/ml, while solutions of PGA and PLL were each prepared at a concentration of 1 mg/ml. All polyelectrolytes were dissolved in a 0.15 M NaCl solution. Multilayer films were prepared either on 12-mm glass coverslips (CML, Nemours, France) pretreated with 10−2 M sodium dodecyl sulfate-0.12 M HCl for 15 min at 100°C and introduced in culture plates or in 96-well plastic plates (Nunc) pretreated with a solution of ethanol-KOH (50%/50%) for 30 min, followed by three rinses with 0.15 M NaCl solution.
Defensin was kindly provided by Entomed SA in dehydrated form and has the following amino acid sequence: ATCDLASGFGVGSSLCAAHCIARRYRGGYCNSKAVCVCRN. This peptide exhibits a high degree of identity and a conserved length compared to those of other insect defensins. The peptide has a pI of about 10, and the protein is positively charged at our working pH (6.5 to 7). The peptide was therefore always adsorbed on top of a PGA layer from a solution of 10 μM in 0.15 M NaCl and was further covered with a PLL layer. Two types of buildup were investigated: PEI-(PSS-PAH)2-PGA-PLL-(PGA-defensin-PLL)n (where n corresponds to the number of PGA-defensin-PLL layers) for films in which the final layer is PLL and PEI-(PSS-PAH)2-PGA-PLL-(PGA-defensin-PLL)n-PGA for films in which the final layer is PGA. Films in which n corresponded to 1, 2, 5, and 10 were investigated by the antimicrobial assays.
Bacterial growth assay.
Two bacterial strains were used for the antimicrobial assays: E. coli D22 (a gift of P. Bocquet, Centre d'Etude Nucléaire, Saclay, France) and Micrococcus luteus (a gift of P. Bulet, UPR CNRS 9022). They were grown aerobically in Luria-Bertani broth (LB) medium at 37°C for E. coli and 30°C for M. luteus. The bacteria were harvested at mid-exponential growth phase and diluted to an optical density at 600 nm (OD600) of 0.001 in a poor broth (PB) medium in order to slow the bacterial growth. Experiments were done in 96-well plates (Nunc), with 100 μl of bacterial growth medium deposited in each well. The peripheral wells were always filled with 100 μl of PB medium in order to decrease the uncertainties in the results due to the high level of evaporation from the wells; they also served as controls for contamination. After 16 h of incubation under agitation (40 rpm) at 37°C for E. coli and 30°C for M. luteus, the bacterial growth was measured with a Metertech Σ 960 spectrometer at 600 nm.
Bacterial adherence assay.
Two E. coli strains were transformed by electroporation with either a plasmid bearing green fluorescent protein (GFP) gene mut3 under the control of the Salmonella enterica serovar Typhimurium ribosomal protein promoter (a gift of B. Lemaitre) or a plasmid bearing the red fluorescent protein (RFP) from a Discosoma sp. (Clontech Laboratories, Inc.). The transformed bacteria expressing GFP or RFP were grown aerobically in LB and selected with ampicillin. For the initial adhesion assays, GFP-expressing bacteria were diluted to an OD600 of 0.01 in LB and plated on the polyelectrolyte multilayers. After 30 min of adhesion, they were rinsed three times with phosphate-buffered saline.
Confocal laser scanning microscopy (CLSM).
PLL conjugated to fluorescein isothiocyanate (PLL-FITC) and PGA conjugated to Texas Red (PGA-TR) were used to image the dye-labeled film in the green and red channels. The 12-mm glass slides were introduced into a homemade chamber and observed by imaging a series of consecutive overlapping optical sections with a Zeiss LSM510 confocal microscope. The thickness of the film was determined by measurement of the width of the green band (which corresponds to PLL-FITC) in computed orthogonal vertical sections through the imaged volumes (29). Both the GFP-expressing and the RFP-expressing E. coli strains were plated at an OD600 of 0.01 in PB medium and were observed after 30 min of incubation.
Scanning electron microscopy.
After 30 min of incubation on a glass coverslip coated with the polyelectrolyte multilayer, the bacteria were fixed for 2 h at 4°C in 5% glutaraldehyde in 0.05 M cacodylate buffer at pH 7.4, rinsed in cacodylate buffer, postfixed in 1% osmium tetroxide, and then dehydrated in a series of increasing alcohol baths. Cells were observed with a Philips XL30 environmental scanning electron microscope.
Analysis of film growth and surface zeta potential.
The PEI-(PSS-PAH)2-(PGA-PLL)n film construction process (where n corresponds to the number of PGA-PLL bilayers) and the insertion of defensin were monitored by optical waveguide light-mode spectroscopy (OWLS) (27, 34) and analysis with a quartz crystal microbalance (QCM) (20, 30). Briefly, OWLS is sensitive to the penetration depth of an evanescent wave through the film near the waveguide surface and gives access to the optical properties of the films. Details about the experimental setup and the procedure can be found elsewhere (28). The structure of the multilayers was analyzed by using the homogeneous and isotropic monolayer model, which allows the refractive index (nA) and the thickness (dA) to be determined. The amount of peptide adsorbed was calculated by the formula of De Feijter et al. (13): adsorbed mass = (dn/dc)−1× (nA − nC) × dA, where dn/dc is equal to 0.18 cm3/g and nC, which is the refractive index of the buffer, is equal to 1.3342.
At the beginning of the experiment, 100 μl of the PEI solution was manually injected into the measurement cell (37 μl). When a stable adsorption signal was obtained (after about 15 min), the NaCl buffer flow was started and was allowed to continue for about 10 min to rinse the excess material. In the same way, we continued with the alternate adsorption of PSS, PAH, and then PGA and PLL on the waveguide.
QCM (Q-Sense, Göteborg, Sweden) measures the changes in the resonance frequency of a quartz crystal when material is brought from solution. The crystal is excited at its fundamental frequency (about 5 MHz); and observations are made at the third, fifth, and seventh overtones (corresponding to 15, 25, and 35 MHz, respectively). The details of the experimental setup have been presented previously (28), except that the crystal used for the present study was coated with a thick (≈100-nm) SiO2 film. The streaming potential method was also used to characterize the surface. Measurements were made on a homemade apparatus that has been described previously (24). The basic principle is to measure the pressure and the potential differences on both sides of a capillary (radius, 530 μm) made of fused silica by using two flasks containing four electrodes. Details about the procedure are given elsewhere (28). All polyelectrolytes as well as defensin were adsorbed in a 0.15 M NaCl solution, and measurements were performed after the rinsing of each layer.
RESULTS
Polyelectrolyte multilayer film buildup.
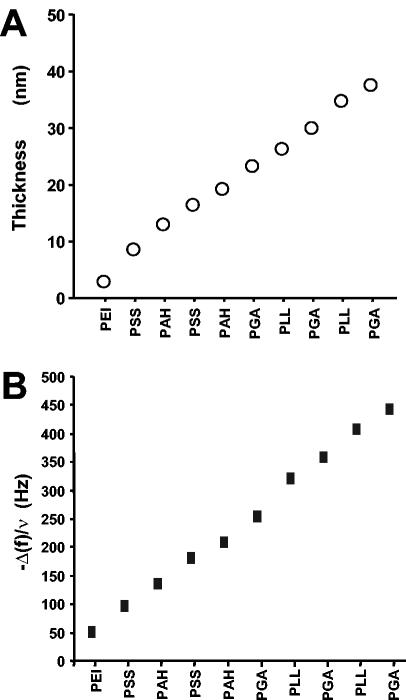
The buildup of the multilayer architectures was monitored step by step by OWLS, which showed an increase in the thickness of the film after each new polyelectrolyte layer was added (Fig. 2A). PEI-(PSS-PAH)2-(PGA-PLL)2-PGA films had a thickness of about 40 nm. The changes in the resonance frequency (Δf) monitored by the QCM technique (Fig. 2B) showed similar increases in thickness, with each layer contributing to an increase in the adsorbed mass on the crystal, thereby modifying the resonance frequency.
FIG. 2.
Construction of the polyelectrolyte multilayer film observed by OWLS (A) and from QCM measurements, with f equal to 15 Hz (B). Both techniques showed linear growth of the polyelectrolyte multilayer films during their formation.
Insertion of defensin in polyelectrolyte multilayer films.
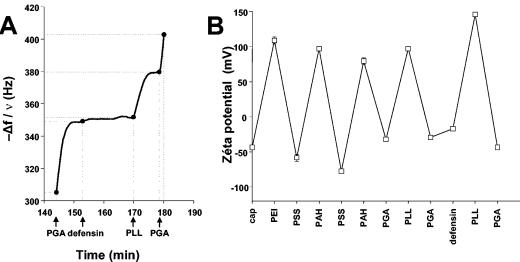
Film stability results from electrostatic interactions between polyelectrolyte chains. We embedded defensin peptides in the multilayer structure by adsorption during film construction. Because the defensin is positively charged (pHi = 4.1) at our working pH of 6.5 to 7, it was adsorbed over a negative PGA layer. The QCM measurements (Fig. 3A) allowed us to detect the adsorption of defensin, although the variations in frequency (5 Hz) (Fig. 3A) were very low. This could be explained by the relatively low molecular mass of the defensin protein (about 4 kDa) compared to those of the polyelectrolytes (40 kDa for PGA and 60 kDa for PLL) or by the lower global charge of the peptide compared to the overall charges of PGA and PLL. The amount of peptide adsorbed could be estimated by OWLS to be about 0.016 μg/cm2 (as determined from the formula of De Feijter et al. [13]).
FIG. 3.
Insertion of the defensin, followed by QCM measurements (A). The augmentation of −Δf/ν (where ν = 3 and f = 15 Hz) at the end of defensin deposition as well as the increase in the thickness of the film suggest protein adsorption. (B) Insertion of the defensin followed by streaming potential measurements. The surface charge stays anionic after the adsorption of the defensin.
Peptide adsorption was also assessed by determination of the change in the surface zeta potential observed by the streaming potential experiment (Fig. 3B). The zeta potential alternates between positive and negative values for the PEI-(PSS-PAH)2-(PGA-PLL)-PGA film, confirming the buildup of the film. After the deposition of the second PGA layer, defensin was adsorbed and the absolute value of the zeta potential increased by 15 mV; however, the potential remained negative. The peptide deposition thus did not seem to be sufficient to reverse the zeta potential. The subsequent PLL layer deposition led to a strong reversal of the zeta potential toward a positive value (≈150 mV). The buildup could then be further pursued.
Taken together, the QCM, zeta potential, and OWLS measurements suggest the effective adsorption of defensin between the PGA and the PLL layers.
Antimicrobial activity of functionalized multilayer films.
The activities of the defensin in both films in which the final layer is PLL and films in which the final layer is PGA were evaluated by use of defensin in the following architectures: PEI-(PSS-PAH)2-PGA-PLL-PGA-defensin-PLL for films in which the final layer is PLL or PEI-(PSS-PAH)2-PGA-PLL-PGA-defensin-PLL-PGA for films in which the final layer is PGA. The functionalized multilayer films were tested with two bacterial strains: M. luteus (a gram-positive organism) and E. coli (strain D22, a gram-negative organism). The activity of defensin in solution against these two strains is well described (37), with activities over ranges of concentrations from 0.75 to 1.5 μM against E. coli and 0.2 to 0.4 μM against M. luteus. In the layer buildup, defensin was adsorbed from a 10 μM concentrated solution, which was chosen to be 10 times higher than the MIC of the peptide in solution. Defensin retained its antimicrobial activity when it was inserted in the polyelectrolyte multilayer films, reducing the numbers of bacteria by 86.4% ± 7.97% for M. luteus (Fig. 4A) and 78.55% ± 11.55% for E. coli (Fig. 4B). As a control, the nonfunctionalized films in which the final layer was PLL reduced the number of bacteria by only 43.28% ± 15.31% for M. luteus and 8.23% ± 7.84% for E. coli, whereas films in which the final layer is PGA had no effect on E. coli (102.76% ± 13.39%) and very few effects on M. luteus (decrease in the number of bacteria, 18.44% ± 16.40%).
FIG. 4.
Antimicrobial assays. The results are expressed as a percentage of the results for the control ± standard deviation. The functionalized films showed a large capacity to reduce bacterial growth: 86.4% for M. luteus and 78.5% for E. coli D22 (***, P > 0.001). No effects were observed when the final layer was negatively charged with PGA. Def, defensin.
Interestingly, the antimicrobial effect of the defensin-loaded films was achieved only when the final polyelectrolyte layer was PLL, whereas no effects could be observed with films in which the final layer was PGA. The reduction in the growth of bacteria on nonfunctionalized positively charged surfaces has already been described (17), but with large discrepancies between bacterial strains. In our experiments, the reduction was never comparable to the one achieved with defensin-functionalized, positively charged surfaces. Moreover, this important difference in the effects of the peptide between films in which the final layer was PLL or PGA proves that the efficacy of the peptide was not linked to its release into the culture medium but that it retains its activity when it is embedded into the film. The only difference between films in which PLL and PGA were the final layers was the outermost surface charge of the film (PLL is a polycation and PGA is a polyanion). The observed discrepancy may therefore be explained by differences in the adhesions of the bacteria onto the two film types.
Modification of defensin concentration in multilayer films.
In order to obtain higher levels of antimicrobial activity in the film, we tried to increase the amount of peptide adsorbed in the polyelectrolyte multilayer films. We therefore first increased the defensin concentration in solution during the construction of the film. No significant differences in the activities of films constructed with a peptide solution of 5, 10, 50, or 100 μM could be seen. In all cases, the effect measured against E. coli cultures was about 80%. This result could be attributed to peptide adsorption saturation on the polyelectrolyte layer. The increase in the peptide concentration in solution does not seem to modify the saturation level of the protein adsorption and therefore does not increase the antimicrobial efficiency of the film.
Several defensin layers were then deposited in the film architecture (Fig. 5). In fact, one of the major advantages of the multilayer technique is precisely that it allows the deposition of multiple layers of the proteins or the peptides tested. The number of peptide layers was increased by embedding the peptide in the following architecture: PEI-(PSS-PAH)2-(PGA-defensin-PLL)n, with n equal 1, 2, 5, or 10. In these experiments, the defensin concentration in solution was kept constant at 10 μM. As seen in Fig. 5, no significant differences could be observed between one or two defensin layers, but five layers of defensin significantly increased the antimicrobial activity (up to 90%). Even more interestingly, 10 layers of defensin brought the antimicrobial activity of the functionalized films to 98.75% (OD600 measurements with the values for the controls normalized to 100: control, 100 ± 5.4; 10 layers of defensin, 1.25 ± 0.31), which is the detection limit of our system. This clearly shows that a maximum antimicrobial effect can be reached simply by increasing the number of defensin layers deposited in the film.
FIG. 5.
Potential antimicrobial effect of the functionalized multilayer films, according to the number of peptide layers. No effect could be observed on films constructed with a defensin solution of 5, 10, 50, or 100 μM (data not shown). Nevertheless, the increase in the number of layers of defensin (in a 10 μM solution) increases the antimicrobial effect of the film up to 98.75% with 10 layers of defensin. *, P > 0.1; ***, P > 0.001; CTRL, control.
Bacterial adhesion onto multilayer films.
In order to understand the precise role of the outermost layer of the polyelectrolyte multilayer film, bacterial adhesion observations were achieved with both the films in which the final layer was PGA and the films in which the final layer was PLL. The zeta potentials for the two bacterial strains were measured and were found to be negative for both species (for M. luteus, −20 ± 2.7 mV; for E. coli, −18.6 ± 0.8 mV). As the streaming potential measurements clearly show a strong positive zeta potential for the films in which the final layer is PLL (+58.1 ± 0.9 mV) (Fig. 3C), it may be possible for the surface to dock to the bacteria if they come into contact with the films in which the final layer is PLL.
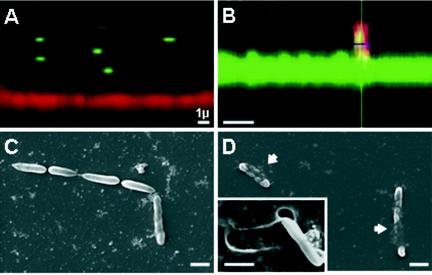
An observation confirming this hypothesis was obtained from confocal microscopy images of sections of the films in contact with the bacteria (Fig. 6A and B). From these confocal microscopy observations, it was found that the number of bilayers in the film had to be increased to about 20 (thickness at this stage, ≈1.6 μm). Moreover, E. coli strains were chosen to express RFP or GFP when they were placed on PLL-FITC- and PGA-TR-labeled films, respectively. The choice of two distinct colors allows observation of the films and the bacteria in two separate channels (green and red) and for the bacteria to be clearly distinguished over the films. The fact that PLL and PGA are both able to diffuse within the whole (PGA-PLL) film architecture (22) allows a clear visualization of the z sections of the films (either in the green channel, when PLL-FITC is used, or in the red channel, when PGA-TR is used). The lateral views of the films and the bacteria obtained by confocal laser scanning microscopy reveals that over a short period of time, the bacteria rarely come into contact with the films in which the final layer is PGA (Fig. 6A). On the other hand, the bacteria were often found to be in close contact with the film when PLL was the outermost layer and even seemed to partially penetrate the film architecture (Fig. 6B).
FIG. 6.
Bacterial adhesion as function of the final layer charge. (A and B) Confocal microscopy observations of relations between E. coli GFP and a film in which PGA-TR is the final layer (A) and E. coli RFP and a film in which PLL-FITC is the final layer (B). Note that the bacteria rarely come into contact with the film in which PGA is the final layer, while they were found to be in close contact with the film when PLL was the outermost layer. (C and D) Scanning electron microscopy observations of E. coli on a film in which PGA is the final layer (C) and a film in which PLL is the final layer (D). Note that on the film in which PLL is the final layer, the bacteria seem to be embedded in the multilayer structure (arrows). The removal of one bacterium (inset, panel D) reveals a clear impression in the multilayer film. Bars, 1 μm.
This suggests that the adhesion mechanism by which the bacteria come into contact with the film is also needed for the peptide to be active when it is embedded in the film. If the bacteria do not come into contact with the film and remain in close contact with the film for a sufficient period of time, as may be the case for films in which the final layer is PGA, the peptide will not be able to interact with and disrupt the bacteria cell membrane. Furthermore, the observations of bacteria adhering to films in which the final layer was PGA (Fig. 6C) or PLL (Fig. 6D) obtained with a scanning electron microscope suggest that bacteria are embedded into the multilayer films in which PLL is the final layer, while the bacteria only seem to lie on the tops of the films in which PGA is the final layer. These observations support the hypothesis that the activity of the antimicrobial peptide is due to close contact with the bacterial membrane.
DISCUSSION
In this work the physicochemical properties of polyelectrolyte multilayer films were used in order to easily embed antimicrobial peptides in the film architecture and therefore build biofunctionalized interfaces protective against infections. Antimicrobial protection for implanted materials can be achieved by various approaches. The first one is obtained by preventing or reducing bacterial adhesion at the material surface. This approach was tested by the use of various physical modifications (39) or monolayer surface coatings (21). It was also evaluated by the use of the polyelectrolyte multilayer technique in which PLL or PGA was grafted with polyethylene glycol (PEG) and inserted into the film several times (2, 23). Those experiments showed that films containing PEG are able to reduce bacterial adhesion by more than 90% and that the number of layers containing PEG correlated with an increase in the bacterial antiadhesion properties (2). These results underline the advantages of multilayer films in comparison with monolayer deposits. However, the antiadhesion properties of such films are not specific against microbial agents, since such surfaces exhibit also protein- and cell-repellent properties. Therefore, the use of such a technique to provide protection can become a real solution for temporary nonabsorbable materials such as sutures, meshes, and drainage tubes; but it may prevent wound healing around any permanently implanted material, such as cardiologic stents, dental implants, and orthopedic materials. Furthermore, in many potential biomedical applications, a simple reduction of bacterial adhesion would not be sufficient to protect the implanted material.
Alternatively, another method, which we have developed in the present study, is to protect these materials by attempting to kill the bacteria after they have adhered to the protected surface. Different techniques for the immobilization of an antimicrobial peptide at the surface have been described in the literature. For instance, Haynie et al. (18) covalently linked the carboxy-terminal amino acid of magainin with an ethylenediamine-modified polyamide resin. They demonstrated that immobilized peptides derived from the magainins eliminated all cells in growing cultures of gram-positive and gram-negative bacteria. Another technique was used for nisin, a small (3.3-kDa) peptide that is produced by the common milk bacterium Lactococcus lactis (25) and that is a member of a group of ribosomally produced peptides called “lantibiotics.” These lantibiotics have been adsorbed onto polyvinyl chloride on silica surfaces and have maintained their activity, thereby killing microorganisms that adhered to the surface in vitro (3). Unfortunately, this method is based on the attachment of only one protein and suffers from this limitation. The mechanisms of resistance to nisin have already been described for E. coli, Salmonella, and Saccharomyces cerevisiae (14, 16).
An antimicrobial process based on a single protein will always be limited either by the antimicrobial spectrum of this peptide or by any new bacterial resistance. Insertion of proteins into polyelectrolyte multilayer films, however, is a technique that offers several major advantages. (i) It is a simple method. (ii) The method is not limited to a single type of peptide. Because no chemical modification is needed to embed the peptide, any antimicrobial peptide can be inserted. (iii) The method permits the peptide concentration to be increased by multiplication of the number of peptide layers. (iv) Antimicrobial peptide combinations can be used to enlarge the antimicrobial spectrum or to improve the efficiency against the same bacterial strain. The embedding of multiple peptides could constitute a way to increase the spectrum of protection and to prevent possible microbial resistance. From that perspective, other antimicrobial peptides, instead of the defensin used in this study, could be tested by the same approach, alone or in combination, in the polyelectrolyte multilayer films. For instance, cationic peptide antibiotics (such as polymyxin B, gramicidin D, gramicidin S, and the cathelicidins) could be good candidates for insertion in the films. Also, one may potentially multifunctionalize the film by inserting additional proteins or peptides that have other biological activities, such as anti-inflammatory molecules and adhesion peptides.
The biomedical applications of such antimicrobial interfaces are extremely diverse and are mainly linked to two factors: the biocompatibility of the multilayer films and the stability of the films in vivo. The cytotoxicity of the multiple layers has already been tested (35). In those experiments, the investigators evaluated the biocompatibility of the multilayers using human periodontal ligament cells or osteoblast-like cells (SaOS-2 cells). Different parameters, such as cell adherence, viability, growth, maintenance of the cellular phenotype, and the inflammatory response, were investigated. Good biocompatibility was found for films in which the final layer was PSS, PGA, or PLL. With respect to the stability of the films in vivo, preliminary fluorimetry and QCM experiments done in the laboratory have shown that these multilayer films are stable for more than 2 weeks in the presence of body fluids (e.g., blood or saliva). Moreover, the presence of fetal bovine serum (5% [vol/vol]) in the bacterial growth medium did not modify the antimicrobial activity of the functionalized films.
Therefore, the conjunction of good biocompatibility and the high degree of stability of polyelectrolyte multilayer films, together with the possibility of varying the number of adsorbed active proteins or peptides and their amounts, could lead to biomedical applications ranging from the protection of all the tools used for medical applications, such as catheter, needles, surgical tools, and tubes, to all types of materials that come into contact with wounds for a restricted period of time.
Acknowledgments
We thank our colleagues from INSERM Unit 595 for support and advice, especially Vincent Ball for measurement of the zeta potentials of the bacteria, Philippe Lavalle for the QCM experiments, and Philippe Mezzini for the PGA-TR. We are grateful to Geraldine Koenig and Christine Affolter for technical help. We also thank Anne Braun and J. Meinwald for helpful comments about the manuscript, Jerome Mutterer (IBMP, Strasbourg, France) for access to the confocal laser scanning microscope, and J. H. Lignot for scanning electron microscopy.
C.E. is grateful to the Faculty of Odontology of Strasbourg for financial support.
REFERENCES
- 1.Barth, E., Q. M. Myrvik, W. Wagner, and A. G. Gristina. 1989. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials 10:325-328. [DOI] [PubMed] [Google Scholar]
- 2.Boulmedais, F., B. Frisch, O. Etienne, P. Lavalle, C. Picart, J. Ogier, J. C. Voegel, P. Schaaf, and C. Egles. 2004. Polyelectrolyte multilayer films with pegylated polypeptides as a new type of anti-microbial protection for biomaterials. Biomaterials 25:2003-2011. [DOI] [PubMed] [Google Scholar]
- 3.Bower, C. K., J. E. Parker, A. Z. Higgins, M. E. Oest, J. T. Wilson, B. A. Valentine, M. K. Bothwell, and J. McGuire. 2002. Protein antimicrobial barriers to bacterial adhesion: in vitro and in vivo evaluation of nisin-treated implantable materials. Colloids Surfaces B Biointerfaces 25:81-90. [Google Scholar]
- 4.Bulet, P., C. Hetru, J. L. Dimarcq, and D. Hoffmann. 1999. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23:329-344. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. L., B. W. Ramsey, and A. L. Smith. 1993. Clinical manifestations and treatment of pulmonary infections in cystic fibrosis. Adv. Pediatr. Infect. Dis. 8:53-66. [PubMed] [Google Scholar]
- 6.Caruso, F., K. Niikura, D. N. Furlong, and Y. Okahata. 1997. 2. Assembly of alternating polyelectrolyte and protein multilayer films for immunosensing. Langmuir 13:3427-3433. [Google Scholar]
- 7.Chluba, J., J. C. Voegel, G. Decher, P. Erbacher, P. Schaaf, and J. Ogier. 2001. Peptide hormone covalently bound to polyelectrolytes and embedded into multilayer architectures conserving full biological activity. Biomacromolecules 2:800-805. [DOI] [PubMed] [Google Scholar]
- 8.Cociancich, S., A. Ghazi, C. Hetru, J. A. Hoffmann, and L. Letellier. 1993. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J. Biol. Chem. 268:19239-19245. [PubMed] [Google Scholar]
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Dankert, J., A. H. Hogt, and J. Feijen. 1986. Biomedical polymers: bacterial adhesion, colonization, and infection. Crit. Rev. Biocompatibility 2:219-301. [Google Scholar]
- 11.Darveau, R. P., M. D. Cunningham, C. L. Seachord, L. Cassiano-Clough, W. L. Cosand, J. Blake, and C. S. Watkins. 1991. Beta-lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob. Agents Chemother. 35:1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decher, G. 1997. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 29:1232-1237. [Google Scholar]
- 13.De Feijter, J. A., J. Benjamins, and F. A. Veer. 1978. Ellipsometry as a tool to study the adsorption behavior of synthetic and biopolymers at the air-water interface. Biopolymers 17:1759-1772. [Google Scholar]
- 14.Dielbandhoesing, S. K., H. Zhang, L. H. Caro, J. M. van der Vaart, F. M. Klis, C. T. Verrips, and S. Brul. 1998. Specific cell wall proteins confer resistance to nisin upon yeast cells. Appl. Environ. Microbiol. 64:4047-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimarcq, J. L., and I. Hunneyball. 2003. Pharma-entomology: when bugs become drugs. Drug Discov. Today 8:107-110. [DOI] [PubMed] [Google Scholar]
- 16.Ganzle, M. G., C. Hertel, and W. P. Hammes. 1999. Resistance of Escherichia coli and Salmonella against nisin and curvacin A. Int. J. Food Microbiol. 48:37-50. [DOI] [PubMed] [Google Scholar]
- 17.Gottenbos, B., H. C. van der Mei, F. Klatter, D. W. Grijpma, J. Feijen, P. Nieuwenhuis, and H. J. Busscher. 2003. Positively charged biomaterials exert antimicrobial effects on gram-negative bacilli in rats. Biomaterials 24:2707-2710. [DOI] [PubMed] [Google Scholar]
- 18.Haynie, S. L., G. A. Crum, and B. A. Doele. 1995. Antimicrobial activities of amphiphilic peptides covalently bonded to a water-insoluble resin. Antimicrob. Agents Chemother. 39:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 20.Hook, F., M. Rodahl, P. Brzezinski, and B. Kasemo. 1998. Measurements using the quartz crystal microbalance technique of ferritin monolayers on methyl-thiolated gold: dependence of energy dissipation and saturation coverage on salt concentration. J. Colloid Interface Sci. 208:63-67. [DOI] [PubMed] [Google Scholar]
- 21.Huang, N.-P., R. Michel, J. Voros, M. Textor, R. Hofer, A. Rossi, D. L. Elbert, J.A. Hubbell, and N. D. Spencer. 2001. Poly(l-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir 17:489-498. [Google Scholar]
- 22.Jessel, N., F. Atalar, P. Lavalle, J. Mutterer, G. Decher, P. Schaaf, J. C. Voegel, and J. Ogier. 2003. Bioactive coatings based on polyelectrolyte multilayer architecture functionalised by embedded proteins. Adv. Materials 15:692-695. [Google Scholar]
- 23.Kenausis, G. L., J. Vörös, D. L. Elbert, N. Huang, R. Hofer, L. Ruiz-Taylor, M. Textor, J. A. Hubbell, and N. D. Spencer. 2000. Poly(l-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J. Phys. Chem. B 104:3298-3309. [Google Scholar]
- 24.Ladam, G., P. Schaaf, G. Decher, J. Voegel, and F. J. Cuisinier. 2002. Protein adsorption onto auto-assembled polyelectrolyte films. Biomol. Eng. 19:273-280. [DOI] [PubMed] [Google Scholar]
- 25.Martin, I., J. M. Ruysschaert, D. Sanders, and C. J. Giffard. 1996. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur. J. Biochem. 239:156-164. [DOI] [PubMed] [Google Scholar]
- 26.Ofek, I., and R. Doyle. 1994. Bacterial adhesion to cells and tissues. Chapman & Hall, New York, N.Y.
- 27.Picart, C., G. Ladam, B. Senger, J.-C. Voegel, P. Schaaf, F. J. G. Cuisinier, and C. Gergely. 2001. Determination of structural parameters characterizing thin films by optical methods: a comparison between scanning angle reflectometry and optical waveguide lightmode spectroscopy. J. Chem. Phys. 115:1086-1094. [Google Scholar]
- 28.Picart, C., P. Lavalle, P. Hubert, F. J. G. Cuisinier, G. Decher, P. Schaaf, and J.-C. Voegel. 2001. Buildup mechanism for poly(l-lysine)/hyaluronic acid films onto a solid surface. Langmuir 17:7414-7424. [Google Scholar]
- 29.Picart, C., J. Mutterer, L. Richert, Y. Luo, G. D. Prestwich, P. Schaaf, J.-C. Voegel, and P. Lavalle. 2002. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 99:12531-12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodahl, M., and B. Kasemo. 1996. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sensors Actuators B (Chemical) 37:111-116. [Google Scholar]
- 31.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231-243. [DOI] [PubMed] [Google Scholar]
- 32.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 33.Stickler, D., and R. McLean. 1995. Biomaterials associated infections: the scale of the problem. Cells Materials 5:167-182. [Google Scholar]
- 34.Tiefenthaler, K., and W. Lukosz. 1989. Sensitivity of grating couplers as integrated optical chemical sensors. J. Optical Soc. Am. B (Optical Physics) 6:209-220. [Google Scholar]
- 35.Tryoen-Toth, P., D. Vautier, Y. Haikel, J.-C. Voegel, P. Schaaf, J. Chluba, and J. Ogier. 2002. Viability, adhesion, and bone phenotype of osteoblast-like cells on polyelectrolyte multilayer films. J. Biomed. Materials Res. 60:657-667. [DOI] [PubMed] [Google Scholar]
- 36.Vautier, D., J. Hemmerle, C. Vodouhe, G. Koenig, L. Richert, C. Picart, J. C. Voegel, C. Debry, J. Chluba, and J. Ogier. 2003. 3-D surface charges modulate protrusive and contractile contacts of chondrosarcoma cells. Cell Motil. Cytoskeleton 56:147-158. [DOI] [PubMed] [Google Scholar]
- 37.Vizioli, J., A. M. Richman, S. Uttenweiler-Joseph, C. Blass, and P. Bulet. 2001. The defensin peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes. Insect Biochem. Mol. Biol. 31:241-248. [DOI] [PubMed] [Google Scholar]
- 38.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-781. [DOI] [PubMed] [Google Scholar]
- 39.Wang, I. W., J. M. Anderson, M. R. Jacobs, and R. E. Marchant. 1995. Adhesion of Staphylococcus epidermidis to biomedical polymers: contributions of surface thermodynamics and hemodynamic shear conditions. J. Biomed. Materials Res. 29:485-493. [DOI] [PubMed] [Google Scholar]