Abstract
This study employs time-kill techniques to examine the most common drug combinations used in the therapy of methicillin-resistant Staphylococcus aureus (MRSA) infections, vancomycin plus either gentamicin or rifampin. Community-associated MRSA were more likely to be synergistically inhibited by combinations of vancomycin and gentamicin versus vancomycin alone compared to inhibition associated with hospital-acquired strains.
Despite being considered the drug of choice for serious methicillin-resistant Staphylococcus aureus (MRSA) infections, vancomycin therapy for MRSA often yields less than ideal results compared to that of β-lactam treatment of methicillin-susceptible S. aureus (MSSA) (6, 7, 19, 22, 28). The suboptimal response of MRSA infections to vancomycin often leads clinicians to add a second or even third antimicrobial, the most widely used of which have been rifampin and aminoglycosides (5, 15), despite discordant in vitro studies and the lack of supporting clinical data (13, 21, 27). Over the past several years, there has been a dramatic rise in the isolation of MRSA from patients who have no recognized link to the hospital (2, 10, 24). Community-associated (CA)-MRSA strains are often sensitive to a much broader array of antimicrobials other than β-lactams than are hospital-acquired (HA)-MRSA strains (23, 29).
We hypothesized that the greater susceptibility of CA-MRSA to gentamicin and rifampin would result in increased bactericidal activity, compared to that of HA-MRSA, when these drugs were combined with vancomycin. The purposes of this investigation were (i) to study the differential effects of combination therapy of vancomycin plus gentamicin or vancomycin plus rifampin versus vancomycin alone in CA-MRSA in comparison with HA-MRSA and MSSA and (ii) to evaluate whether altering the dose of rifampin changed the in vitro results of the combination of vancomycin and rifampin.
Unique patient isolates of S. aureus were selected to obtain 25 CA-MRSA, 10 HA-MRSA, and 11 MSSA. Designation of isolates as CA versus HA was made in accordance with Centers for Disease Control and Prevention guidelines (14). MICs were determined by disk diffusion methods and by broth macrodilution technique. Time-kill studies were done following the methods of Eliopoulos and Mollering (11), with a starting inoculum of 5 × 106 CFU/ml. Vancomycin was studied at 10 μg/ml, gentamicin at 1 μg/ml, and rifampin at 0.5 μg/ml; selected strains were also studied at rifampin concentrations of 0.016 and 3 μg/ml. For the purposes of this study, synergy was said to be present if, after a 24-h incubation, the number of CFU was ≥2 log10 lower in the presence of the combination than with the single, more active, agent. Antagonism was said to be present if CFU were ≥2 log10 higher after incubation with the combination than with the single, more active, agent.
All strains of S. aureus used in this study were susceptible to vancomycin, with no differences among CA-MRSA, HA-MRSA,or MSSA. CA-MRSA were more likely to be susceptible to gentamicin (90%) than were HA-MRSA (50%) (P = 0.016). The MIC of rifampin ranged from ≤0.016 to 0.25 μg/ml, with a minimum 50% inhibitory concentration of 0.31 μg/ml with no differences among the three groups of organisms.
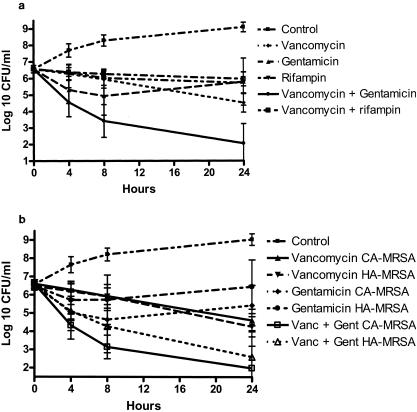
The mean rate of killing for vancomycin alone was similar among HA-MRSA, CA-MRSA, and MSSA (at 24 h, P = 0.877; Table 1). In the presence of 1 μg of gentamicin/ml alone, CFU for all isolates declined by a mean of 0.79 log10 at 24 h. When the two drugs were added together, killing was nearly 100-fold greater or more at each time point than it had been for vancomycin alone (Table 1, Fig. 1a). Synergy was observed for 23 out of 25 (92%) CA-MRSA, 5 out of 10 (50%) HA-MRSA, and 8 out of 11 (73%) MSSA (synergy was more frequent for CA-MRSA and MSSA than for HA-MRSA [P = 0.022]; CA-MRSA were not different from MSSA [P = 0.123]). At 24 h, the mean decrease with the combination of vancomycin and gentamicin compared to that with vancomycin alone was 2.78 log10 for CA-MRSA, 2.84 log10 for MSSA, and 1.49 log10 for HA-MRSA (P = 0.025 for CA-MRSA and P = 0.023 for MSSA, having greater decreases versus HA-MRSA; Fig. 1b). Synergy was noted for 41% of gentamicin-resistant isolates and 91% of susceptible isolates (P < 0.001).
TABLE 1.
Time-kill responses for S. aureus
| Organisms and drug(s)a | Mean CFU log10 decrease at:
|
Mean CFU log10 difference compared to that of vancomycin alone at 24 h | P value for combination compared to that of vancomycin alone at 24 h | ||
|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | |||
| All S. aureus isolates | |||||
| Vancomycin alone | −0.28 | −0.65 | −2.04 | ||
| Vancomycin + rifampin | −0.20 | −0.31 | −0.62 | +1.42 | <0.001 |
| Vancomycin + gentamicin | −2.09 | −3.15 | −4.54 | −2.49 | <0.001 |
| All MRSA | |||||
| Vancomycin alone | −0.33 | −0.64 | −2.06 | ||
| Vancomycin + rifampin | −0.25 | −0.32 | −0.64 | +1.42 | <0.001 |
| Vancomycin + gentamicin | −1.95 | −3.03 | −4.47 | −2.41 | <0.001 |
| HA-MRSA | |||||
| Vancomycin alone | −0.26 | −0.54 | −2.21 | ||
| Vancomycin + rifampin | −0.02 | −0.20 | −0.53 | +1.68 | <0.001 |
| Vancomycin + gentamicin | −1.44 | −2.24 | −3.71 | −1.49 | 0.005 |
| CA-MRSA | |||||
| Vancomycin alone | −0.31 | −0.68 | −1.95 | ||
| Vancomycin + rifampin | −0.35 | −0.38 | −0.69 | +1.26 | <0.001 |
| Vancomycin + gentamicin | −2.24 | −3.47 | −4.73 | −2.78 | <0.001 |
| MSSA | |||||
| Vancomycin alone | −0.30 | −0.75 | −2.00 | ||
| Vancomycin + rifampin | −0.04 | −0.28 | −0.54 | +1.45 | <0.001 |
| Vancomycin + gentamicin | −2.11 | −3.22 | −4.84 | −2.84 | <0.001 |
Rifampin dose, 0.5 μg/ml; gentamicin dose, 1 μg/ml.
FIG. 1.
(a) Time-kill curves for all isolates of S. aureus. Antibiotic concentrations: vancomycin, 10 μg/ml; gentamicin, 1 μg/ml; rifampin, 0.625 μg/ml. Errors bars indicate ±1 standard deviation. (b) Time-kill curves for isolates of MRSA. Error bars indicate ±1 standard deviation.
In contrast, the addition of rifampin at 0.5 μg/ml to vancomycin resulted in decreased bactericidal activity at 4, 8, and 24 h compared to the activity of vancomycin alone (Table 1, Fig. 1a), an effect that was similar among the three groups of isolates (P = 0.734). No evidence of synergistic effect was noted for any of the isolates, whereas the presence of rifampin was antagonistic in nine strains (four CA-MRSA strains, three HA-MRSA strains, and two MSSA strains). Similar results were obtained when rifampin was studied at either low dose (0.16 μg/ml) or high dose (3.0 μg/ml) (data not shown).
One of the characteristics of CA-MRSA are their propensity to carry the basis for methicillin resistance, the mec gene, in a novel type of staphylococcal chromosomal cassette (SCC) known as type IV SCCmec (9, 17). The SCCmec type of each MRSA isolates was determined by PCR using the method of Okuma et al. (26). Positive controls NCTC 10492, (SCCmec type I), N315 (SCCmec type II), 85/2082 (SCCmec type III), and CA 05 (SCCmec type IVa) were kindly provided by Keiichi Hiramatsu and Teruyo Ito, Department of Bacteriology, Juntendo University, Tokyo, Japan. All of the isolates studied here that were classified as CA by epidemiologic guidelines published by the Centers for Disease Control and Prevention carried type IVa SCCmec. Five of the HA-MRSA had type II SCCmec while the other five contained type IVa. No strains carried type I or type III SCCmec. The CA-MRSA were more likely to carry type IV SCCmec than were hospital isolates (P = 0.001). The MRSA isolates containing type IVa SCCmec were more likely to be synergistically affected by the combination of vancomycin and gentamicin than were those having type II SCCmec (P = 0.044).
Treatment of serious MRSA infections with vancomycin is associated with persistent bacteremia and a substantial rate of complication and relapse (7, 19). Until recently, MRSA infections were nearly always HA, and the known tendency of these organisms to exhibit aminoglycoside resistance reduced interest in adding gentamicin to vancomycin (3). The finding that nearly all CA-MRSA studied here were sensitive to gentamicin is in accord with previously published reports (1, 10). An unexpected finding, however, was that 5 of 10 HA-MRSA strains also were sensitive to gentamicin. Aminoglycoside testing of MRSA in the past has suggested much higher rates of resistance (3). The results of this study suggest that aminoglycoside resistance among MRSA at our center is decreasing in a manner similar to that recently reported in France and in Minnesota (18, 23).
The decreased killing activity of S. aureus when rifampin was added to vancomycin is in accordance with previous reports that employed time-kill methods (16, 31, 32). The significance of in vitro studies with this combination has been questioned by some authors, who have noted indifference or antagonism when test tube methods were used but appear to have shown synergy in animal models (4, 25, 27). The clinical evidence for the use of this combination is equally discordant, with one trial and a few case reports reporting efficacy in treating MRSA infections while in a randomized trial the addition of rifampin to vancomycin for MRSA bacteremia had no effect (12, 19, 20, 30). However, previous in vitro data has suggested that the addition of rifampin to cell wall-active agents may facilitate bactericidal activity, particularly against organisms sequestered within foci that are not readily accessible to antibiotics or immune mechanisms (8). Another previous study noted that rifampin was synergistic with ciprofloxacin at sub-MIC doses but was antagonistic at higher doses (31). When varying the concentration of rifampin between 0.0156 μg/ml (sub-MIC) up to 3.0 μg/ml, we found no difference in the killing effects of the combination of vancomycin and rifampin. Thus, the antagonistic interaction noted in vitro between vancomycin and rifampin is not concentration dependent.
The slow response of some MRSA infections to vancomycin is likely to prompt clinicians to continue to employ additional agents in combination with vancomycin in an attempt to improve the response to therapy. There appears to be changing resistance patterns to aminoglycosides among MRSA isolates that might be expected to make these organisms more responsive to combination therapy. At the present time, the high rates of gentamicin susceptibility among CA-MRSA suggest that combination therapy may be particularly effective for patients with infections due to these isolates. Clinical studies evaluating this regimen are needed more than ever given the rising rates of methicillin resistance among community S. aureus isolates.
REFERENCES
- 1.Almer, L. S., V. D. Shortridge, A. M. Nilius, J. M. Beyer, N. B. Soni, M. H. Bui, G. G. Stone, and R. K. Flamm. 2002. Antimicrobial susceptibility and molecular characterization of community-acquired methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 43:225-232. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus: Minnesota and North Dakota, 1997-1999. Morb. Mortal. Wkly. Rep. 48:707-710. [PubMed] [Google Scholar]
- 3.Ayliffe, G. A. 1997. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 24(Suppl. 1):S74-S79. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, A. S., and K. Lam. 1985. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J. Infect. Dis. 151:157-165. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, H. F. 1988. Methicillin-resistant staphylococci. Clin. Microbiol. Rev. 1:173-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, F. Y., B. B. MacDonald, J. E. Peacock, Jr., D. M. Musher, P. Triplett, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 82:322-332. [DOI] [PubMed] [Google Scholar]
- 7.Chang, F. Y., J. E. Peacock, Jr., D. M. Musher, P. Triplett, B. B. MacDonald, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82:333-339. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche, R. O., and R. J. Hamill. 1994. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob. Agents Chemother. 38:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daum, R. S., T. Ito, K. Hiramatsu, F. Hussain, K. Mongkolrattanothai, M. Jamklang, and S. Boyle-Vavra. 2002. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J. Infect. Dis. 186:1344-1347. [DOI] [PubMed] [Google Scholar]
- 10.Eady, E. A., and J. H. Cove. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16:103-124. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulous, G., and R. J. Mollering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams and Wilkins Co., Baltimore, Md.
- 12.Faville, R. J., Jr., D. E. Zaske, E. L. Kaplan, K. Crossley, L. D. Sabath, and P. G. Quie. 1978. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA 240:1963-1965. [DOI] [PubMed] [Google Scholar]
- 13.Fortun, J., E. Navas, J. Martinez-Beltran, J. Perez-Molina, P. Martin-Davila, A. Guerrero, and S. Moreno. 2001. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: cloxacillin versus glycopeptides in combination with gentamicin. Clin. Infect. Dis. 33:120-125. [DOI] [PubMed] [Google Scholar]
- 14.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 15.Hackbarth, C. J., and H. F. Chambers. 1989. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob. Agents Chemother. 33:995-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackbarth, C. J., H. F. Chambers, and M. A. Sande. 1986. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob. Agents Chemother. 29:611-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama, Y., T. Ito, and K. Hiramatsu. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent, F., H. Lelievre, M. Cornu, F. Vandenesch, G. Carret, J. Etienne, and J. P. Flandrois. 2001. Fitness and competitive growth advantage of new gentamicin-susceptible MRSA clones spreading in French hospitals. J. Antimicrob. Chemother. 47:277-283. [DOI] [PubMed] [Google Scholar]
- 19.Levine, D. P., B. S. Fromm, and B. R. Reddy. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674-680. [DOI] [PubMed] [Google Scholar]
- 20.Massanari, R. M., and S. T. Donta. 1978. The efficacy of rifampin as adjunctive therapy in selected cases of staphylococcal endocarditis. Chest 73:371-375. [DOI] [PubMed] [Google Scholar]
- 21.Mulazimoglu, L., S. D. Drenning, and R. R. Muder. 1996. Vancomycin-gentamicin synergism revisited: effect of gentamicin susceptibility of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1534-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulligan, M. E., K. A. Murray-Leisure, B. S. Ribner, H. C. Standiford, J. F. John, J. A. Korvick, C. A. Kauffman, and V. L. Yu. 1993. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am. J. Med. 94:313-328. [DOI] [PubMed] [Google Scholar]
- 23.Naimi, T. S., K. H. LeDell, D. J. Boxrud, A. V. Groom, C. D. Steward, S. K. Johnson, J. M. Besser, C. O'Boyle, R. N. Danila, J. E. Cheek, M. T. Osterholm, K. A. Moore, and K. E. Smith. 2001. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin. Infect. Dis. 33:990-996. [DOI] [PubMed] [Google Scholar]
- 24.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 25.Norden, C. W., and M. Shaffer. 1983. Treatment of experimental chronic osteomyelitis due to Staphylococcus aureus with vancomycin and rifampin. J. Infect. Dis. 147:352-357. [DOI] [PubMed] [Google Scholar]
- 26.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer, S. M., and M. J. Rybak. 1996. Pharmacodynamics of once- or twice-daily levofloxacin versus vancomycin, with or without rifampin, against Staphylococcus aureus in an in vitro model with infected platelet-fibrin clots. Antimicrob. Agents Chemother. 40:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Auwera, P., F. Meunier-Carpentier, and J. Klastersky. 1983. Clinical study of combination therapy with oxacillin and rifampin for staphylococcal infections. Rev. Infect. Dis. 5(Suppl. 3):S515-S522. [DOI] [PubMed] [Google Scholar]
- 31.Van der Auwera, P., and P. Joly. 1987. Comparative in-vitro activities of teicoplanin, vancomycin, coumermycin and ciprofloxacin, alone and in combination with rifampicin or LM 427, against Staphylococcus aureus. J. Antimicrob. Chemother. 19:313-320. [DOI] [PubMed] [Google Scholar]
- 32.Watanakunakorn, C., and J. C. Guerriero. 1981. Interaction between vancomycin and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 19:1089-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]