Abstract
Bacteriophage therapy of bacterial infections has received renewed attention owing to the increasing prevalence of antibiotic-resistant pathogens. A side effect of many antibiotics as well as of phage therapy with lytic phage is the release of cell wall components, e.g., endotoxins of gram-negative bacteria, which mediate the general pathological aspects of septicemia. Here we explored an alternative strategy by using genetically engineered nonreplicating, nonlytic phage to combat an experimental Pseudomonas aeruginosa infection. An export protein gene of the P. aeruginosa filamentous phage Pf3 was replaced with a restriction endonuclease gene. This rendered the Pf3 variant (Pf3R) nonreplicative and concomitantly prevented the release of the therapeutic agent from the target cell. The Pf3R phage efficiently killed a wild-type host in vitro, while endotoxin release was kept to a minimum. Treatment of P. aeruginosa infections of mice with Pf3R or with a replicating lytic phage resulted in comparable survival rates upon challenge with a minimal lethal dose of 3. However, the survival rate after phage therapy with Pf3R was significantly higher than that with the lytic phage upon challenge with a minimal lethal dose of 5. This higher survival rate correlated with a reduced inflammatory response elicited by Pf3R treatment relative to that with the lytic phage. Therefore, this study suggests that the increased survival rate of Pf3R-treated mice could result from reduced endotoxin release. Thus, the use of a nonreplicating modified phage for the delivery of genes encoding proteins toxic to bacterial pathogens may open up a new avenue in antimicrobial therapy.
With the advent of antibiotics, research into phage therapy for bacterial infections was largely abandoned in the West, whereas in the former Soviet Union and in Poland, phage therapy has been used up to the present (33). Animal experiments conducted with scientific rigor showed that phage therapy is effective in treating various bacterial infections (4, 5, 20, 31), and one study (30) indicated that it might even be superior to antibiotics. Several cases in which temperate phage led to increased virulence of the respective pathogens have been reported (23, 34). However, this potential safety risk can be minimized through the use of strictly lytic therapeutic phage and by their detailed molecular characterization. Recent review articles (3, 21) highlight the inherent advantages of phage therapy over antibiotics, such as specific targeting of the pathogen. Nevertheless, phage infections in general culminate in lysis of bacteria and consequently in the release of cell wall components, e.g., endotoxin-release from gram-negative bacteria, which can lead to undesired side effects of phage therapy even when the phage are administered orally (29). Similarly, many β-lactam antibiotics, when used for treatment of systemic gram-negative infections, lead to markedly increased endotoxin levels, which can elicit circulatory shock in patients (15). Hence, there seems to be a need for novel specific and effective bactericidal agents that do not cause this side effect. Toward this goal, it is feasible either to genetically modify lytic phage or to engineer nonlytic phage in such a way that they kill their bacterial target efficiently while leaving the pathogen structurally intact.
In general, large phage possess a dual lysis system consisting of at least a holin and an endolysin (38). At the end of the lytic cycle, the holin compromises the membrane, which allows release of the endolysin to the peptidoglycan. Degradation of this rigid layer is then followed by disintegration of the entire cell envelope, leading to the release of cell wall components. Endotoxin release from gram-negative bacteria can be minimized or avoided through the use or the construction of phage variants with defective endolysin genes. Since the holin function results in dissipation of the membrane potential at the end of the lytic cycle, the bacteria are killed even by endolysin-deficient phage, while the cells retain their structural integrity (25).
Another strategy for avoiding cellular disintegration is the use of nonlytic filamentous phage as a delivery vehicle for genes encoding proteins that are toxic upon their synthesis in the cytoplasm of the host (10, 36). It has recently been demonstrated in a pilot study that in vitro endotoxin release by an M13 variant harboring an endonuclease gene is kept to a minimum relative to that caused by a lytic phage (10). In a conceptually related approach, Westwater et al. (36) have shown that the Escherichia coli load in the blood of infected mice can be significantly reduced by using an M13 variant encoding addiction module toxins. In contrast to antibiotics, the concentration of which decreases after administration, the exponential enrichment of phage after lysis or killing of a given pathogen is generally considered to augment the therapeutic effect (20). However, the use of replicating genetically modified phage can pose a safety risk. The progeny of these phage could be cleared in large quantities from patients to the environment, where they could potentially introduce the toxin genes to otherwise benign bacteria. With this reasoning, in this study we compared the therapeutic efficacy of a nonlytic, nonreplicating phage bearing a toxin gene with that of a lytic phage in treating an experimental Pseudomonas aeruginosa infection of mice.
MATERIALS AND METHODS
Bacterial strains and phage.
P. aeruginosa harboring the Rp1 plasmid (designated PAO1 here) and phage Pf3 were kindly provided by A. Kuhn, University of Hohenheim, Stuttgart, Germany. E. coli strain DM1 (18) was used for all cloning procedures. For use as a lysis-inducing phage control, the PAO1 phage Pt1 was isolated from river water by standard procedures. Phage Pt1 formed clear, large plaques on a lawn of PAO1. Only P. aeruginosa strains harboring the IncP1 plasmid RP1 were susceptible, indicating that Pt1 attaches to the same pili as Pf3. The phage nucleic acid was degraded by RNase, whereas DNase had no effect, showing that Pt1 is an RNA phage (data not shown). All high-titer phage preparations used in this study were purified by CsCl2 density gradient centrifugation to remove cell debris and were then dialyzed against SM buffer (27).
Construction of plasmids pU430 and pUM430.
Based on the known nucleotide sequence of phage Pf3 (17), oligonucleotides F18 (5′-TTTTTTTTTTGAGCTCGCCTTTGTAGGAGTCCTGACATGATCCGTTC-3′) and G18 (5′-TTTTTTTTTTGGTACCCTAAAAAAGATCACCAAGG-3′) were designed. By using these primers and Pf3 Rf DNA as a template, a fragment containing ORF430 was generated by PCR. A SacI site and a KpnI site were introduced 5′ and 3′ of ORF430, respectively. Primer F18 also changed the ribosome binding site of ORF430 from GGGAG to AGGAG. The resulting PCR fragment was cleaved with SacI and KpnI and was inserted into the corresponding sites of pUCP24 (35), resulting in plasmid pU430.
Next, a PCR fragment containing the methylase gene BglIIM was inserted downstream of ORF430 in pU430. Plasmid pMBN2 (2) containing the BglIIM gene was used as a template together with oligonucleotides V14 and W14 in a PCR. V14 (5′-AAAAAAAAAAGGTACCAGACGTCGG-3′) introduced a KpnI site 5′ of the ribosome binding site, and W14 (5′-AAAAAAAAAACTGCAGGC-GTAAGAG-3′) introduced a PstI site 3′ of the stop codon of the BglIIM gene. The KpnI-PstI fragment was inserted into plasmid pU430, resulting in plasmid pUM430 (Fig. 1).
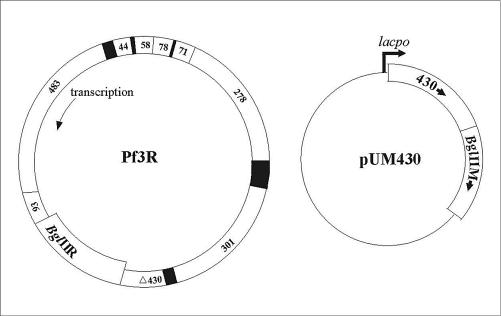
FIG. 1.
Schematic representations of the genetic organization of the modified phage Pf3R, encoding the BglII endonuclease gene, and of plasmid pUM430, encoding ORF430 and the BglII methyltransferase gene.
Construction of Pf3R.
First, a 2,593-bp fragment containing ORF430 and a 3,240-bp fragment were obtained by digestion of Pf3 Rf DNA with AgeI and BclI. The fragment, containing ORF430, was first ligated into plasmid pSL1180 (8) and then reisolated as a BamHI and HindIII fragment and inserted into the corresponding sites of plasmid pK194 (12). The ATG start codon of ORF430 was modified by introducing an NdeI site, allowing in-frame insertion of the BglII restriction endonuclease gene (BglIIR) while leaving the TGA stop codon of the preceding Pf3 ORF93 intact. This was done by PCR-mediated site-directed mutagenesis using oligonucleotides A20 (5′-GCCTTTGTGGGAGTCCTCATATGATCCGTTCAATTATTGC-3′) and A21 (5′-GCAATAATTGAACGGATCATATGAGGACTCCCACAAAGGC-3′). The resulting plasmid was designated pK194ORF430. Plasmid pMBR1 (2) containing BglIIR was used as a template in a PCR together with oligonucleotides A22 and A23. Oligonucleotide A22 (5′-TTTTTTTTTTCATATGAAGAT-TGATATAACGGACT-3′) introduced an NdeI site in the BglIIR ATG start codon, and oligonucleotide A23 (5′-TTTTTTTTTTGCATGCGTCTAATAAAATTTAATATGTCACG-3′) introduced an SphI site 3′ of the stop codon. Next, most of ORF430 was excised from plasmid pK194ORF430 by digestion with NdeI and SphI and was replaced with the PCR fragment containing the BglIIR gene, resulting in plasmid pK430BglIIR. After the modified Pf3 region was excised from pK430BglIIR with AgeI and BclI, it was ligated to the 3,240-bp fragment of Pf3 obtained by AgeI/BclI digestion in the first step. The ligation mixture was transformed into PAO1 carrying plasmid pUM430 by electroporation. Candidate recombinant phage were isolated from areas of growth inhibition (“pseudoplaques”). Those unable to replicate on PAO1 were further analyzed by PCR and restriction analysis. A schematic representation of the genetic organization of Pf3R is shown in Fig. 1.
Endotoxin assay.
Samples (1 ml)of uninfected bacteria and of cultures infected with Pf3R and Pt1 were taken at an optical density at 600 nm (OD600) of 0.2 prior to infection and 90, 240, and 420 min after infection. A multiplicity of infection (MOI) of 50 was used for both phage in this experiment. Supernatant endotoxin levels were determined in triplicate with the QCL-1000 test kit (BioWhittaker, Inc., Walkersville, Md.) according to the manufacturer's instructions.
Mouse cytokine response.
Concentrations of the cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were measured in triplicate by using the DuoSet kit (R&D Systems, Inc., Minneapolis, Minn.) as specified by the manufacturer.
Inoculation of mice and serum sample collection.
PAO1 cells were diluted in phosphate-buffered saline (PBS) plus 5% mucin, a procedure that reportedly leads to more-reproducible results (37), to yield either the minimal lethal dose (MLD) or three or five times the MLD per 100 μl. Four- to 5-week-old BALB/c mice were inoculated intraperitoneally (i.p.) with 100 μl of bacterial suspension. Phage dilutions in PBS (100 μl) were injected i.p. 45 min, 1 h, or 6 h after infection. Serum samples for measurement of TNF-α and IL-6 levels were obtained by bleeding from the tail vein before infection, shortly before treatment with either Pf3R or Pt1 at 1 h after infection, and 1, 3, and 5 h after treatment. Animals were purchased from Harlan-Winkelmann (Borchen, Germany) and held at InterCell's animal facilities in accordance with Austrian laws. The mice were allowed to eat and drink throughout the 7-day observation period. Statistical analysis of results was performed by using Fisher's exact test and forming two-by-two contingency tables, with results for mice receiving no treatment compared to those for the different treatments (26).
RESULTS
Rationale for construction of the Pf3R phage.
Pf3 is a filamentous single-stranded DNA phage of PAO1 that does not lyse its host during progeny release (32). In this study, phage Pf3 was equipped by means of recombinant DNA technology with the BglIIR gene, whose product does not cleave the replicative form of Pf3 DNA (17). The reasoning was that (i) Pf3R-mediated delivery of the BglIIR gene would result in nonrepairable breaks in double-stranded chromosomal DNA and thus in killing of PAO1 and that (ii) the recombinant phage can be stably propagated on a host strain expressing the BglII methylase gene. In addition, the coding region of an export protein gene (ORF430) was replaced with the BglIIR gene, whose expression in Pf3R is directed by an upstream phage promoter and by the translational initiation signals of ORF430. This rendered the recombinant Pf3R phage nonreplicative in wild-type PAO1. The Pf3R phage remained genetically stable, and its efficiency at killing PAO1 remained unaltered after 10 passages on strain PAO1(pUM430) (data not shown). High titers of Pf3R were obtained in the production strain harboring a plasmid-borne copy of the phage export protein gene and the BglII methylase gene (Fig. 1).
Pf3R-mediated killing of PAO1.
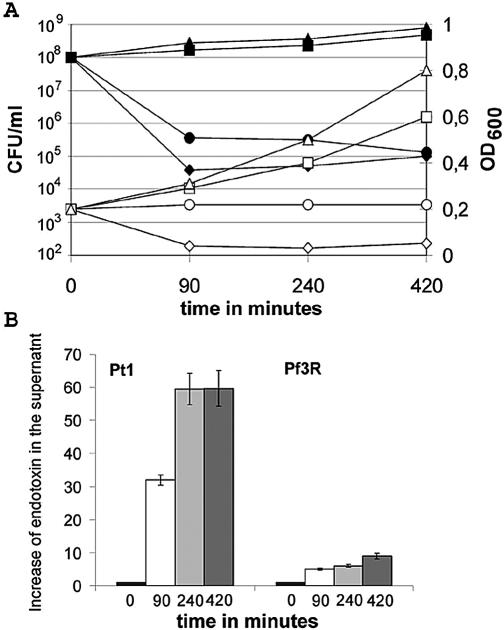
To test whether Pf3R efficiently killed PAO1, and whether the structural integrity of the cells was retained upon killing, we first determined the survival rate and the OD600 of PAO1 cultures upon infection with Pf3, Pf3R, or the lytic RNA phage Pt1. Cultures at an OD600 of 0.2 were infected with a MOI of 50. The growth of the strain infected with wild-type Pf3 was only slightly retarded relative to that of uninfected bacteria (Fig. 2A). After 90 min, PAO1 cultures infected with either Pf3R or Pt1 showed a >99% decline in CFU (Fig. 2A). As expected, the OD600 of the culture infected with the lytic Pt1 phage decreased to almost zero within 90 min. In contrast, the OD600 of the culture infected with Pf3R remained constant over 7 h, indicating that the nonviable cells remained structurally intact, whereas Pt1-infected bacteria were almost completely lysed (Fig. 2A).
FIG. 2.
Comparison of the killing efficiencies, effects on cell integrity, and endotoxin release mediated by phage Pf3R and Pt1. (A) PAO1 cultures at an OD600 of 0.2 were infected with Pf3, Pf3R, or Pt1 at an MOI of 50. The numbers of viable cells (CFU) upon infection with Pf3 (▪), Pf3R (•), or Pt1 (♦) and that in the uninfected culture (▴) were determined by plating serial dilutions on Luria-Bertani agar plates. OD600 values upon infection with wild-type Pf3 (□), Pf3R (○), or Pt1 (⋄) and in the uninfected culture (▵) are shown. Results are representative of three experiments. (B) Relative increases in endotoxin levels in the supernatant for samples taken before infection (solid bars) and 90 (open bars), 240 (light shaded bars), and 420 (dark shaded bars) min after infection with phage Pf3R or Pt1, normalized to the endotoxin level at time zero (∼8.3 × 106 endotoxin units/ml). Endotoxin levels were determined in triplicate with the QCL-1000 test kit (BioWhittaker). Error bars, standard deviations.
Endotoxin release upon infection of PAO1 with Pf3R or the lytic phage Pt1.
As expected, lysis by Pt1 was accompanied by a rapid increase in endotoxin levels in the supernatant. Compared to the amount present at the time of infection, endotoxin levels were 32- and 60-fold increased at 90 and 240 min, respectively, after infection with Pt1 (Fig. 2B). In contrast, when PAO1 was infected with Pf3R (Fig. 2B), endotoxin levels in the supernatant increased no more than five- and sevenfold at the respective times.
Pf3R and Pt1 therapy of experimental PAO1 infections of BALB/c mice.
To establish the MLD in an animal model of infection, BALB/c mice were injected i.p. with varying CFU of PAO1 suspended in PBS supplemented with 5% mucin. The MLD for the PAO1 strain used was found to correspond to 105 CFU. i.p. administration of the MLD of PAO1 resulted in symptoms of ruffled fur, lethargy, and a hunchback posture after 45 min and in severe illness, defined by those symptoms plus exudative accumulation around partially closed eyes, at 6 h postinfection. By 12 h the mice were moribund, and they died within 24 h.
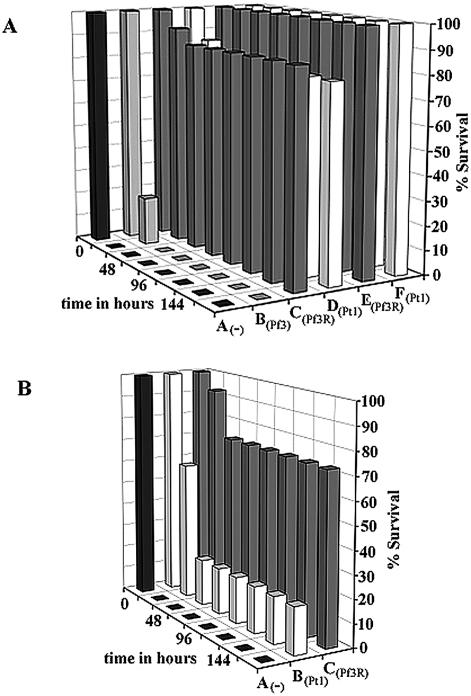
In the initial experiments, the mice were challenged with the MLD following treatment with 106 (MOI, 10), 107 (MOI, 100), 108 (MOI, 1,000), or 109 (MOI, 10,000) PFU of either Pf3R or Pt1 at 45 min postinfection. These studies revealed that an MOI of at least 1,000 of either phage was required to cure the mice of the lethal infection (data not shown). To obtain a more quantitative picture of the efficacy of phage treatment after infection, in the next experiments, 3 × 108 PFU of either Pf3, Pf3R, or Pt1 phage was administered at 45 min following infection with three times the MLD of PAO1 (MOI, 1,000). Under these conditions, both the lytic RNA phage Pt1 and the Pf3R phage (P < 0.00001), rescued ≥75% of the mice, whereas all untreated and Pf3-treated mice died within 24 and 48 h, respectively (Fig. 3A). In addition, the mice were challenged with three times the MLD and were then treated at 6 h postinfection with 3 × 109 PFU (MOI, 10,000) of either PF3R or Pt1. Both treatments resulted in rescue of all infected mice; survival was statistically significant, with a P value of 0.0003 (Fig. 3A).
FIG. 3.
Phage rescue of mice after infection with PAO1. (A) Phage rescue after a challenge with three times the MLD. Mice were monitored for 7 days after i.p. injection with three times the MLD of PAO1 suspended in 100 μl of PBS-5% mucin. Group A (n = 10) did not receive treatment. Groups B, C, and D received phage treatment 45 min after bacterial challenge. Group B (n = 5) received an i.p. injection of 3 × 108 PFU of Pf3 suspended in 100 μl of PBS. Group C (n = 15) and group D (n = 15) were treated with 3 × 108 PFU of PF3R or Pt1, respectively. Groups E (n = 5) and F (n = 5) received 3 × 109 PFU of phage Pf3R or Pt1, respectively, at 6 h postinfection. (B) Phage rescue after challenge with five times the MLD. Group A (n = 10) was left untreated. One hour after bacterial challenge, groups B and C (n = 15) received i.p. injections of 5 × 108 PFU of phage Pt1 or Pf3R, respectively. Results in each panel are representative of two experiments.
The deleterious effects of endotoxin are expected to increase with higher CFU of PAO1. Therefore, the mice were next challenged with five times the MLD (5 × 105 CFU) of PAO1, and survival was monitored without phage treatment or after treatment with Pf3R or Pt1 (both at 5 × 108 PFU) at 60 min postinfection (Fig. 3B). All mice in the negative-control group (n = 15) died within 12 h. At 24 h, 95% of Pf3R-treated mice and 60% of Pt1-treated mice survived (n = 15 for each group). After 48 h, 73 and 20% of the Pf3R- and Pt1-treated mice, respectively, remained viable. No further changes were observed over the following 5 days of observation (Fig. 3B). The survival of Pf3R-treated mice was statistically significant (P = 0.009). These experiments showed for the first time that a nonreplicative phage can be as effective as, if not superior to, a replicating phage in treatment of an experimental infection.
Inflammatory response to treatment with Pf3R and Pt1.
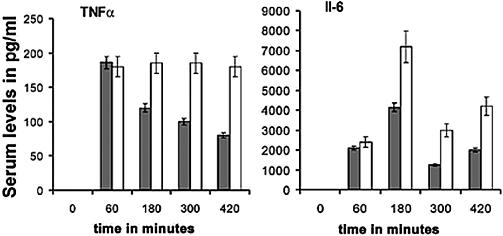
Next, we tested whether Pf3R and Pt1 treatments elicited different inflammatory responses. TNF-α is usually the first of the early cytokines found after the administration of endotoxin (11), whereas IL-6 levels increase later, as a direct response to elevated TNF-α levels (28). TNF-α and IL-6 levels in serum samples from two groups of mice inoculated with five times the MLD (5 × 108 CFU) were determined before bacterial challenge, 60 min after bacterial challenge, and 2, 4, and 6 h after treatment with phage Pt1 or Pf3R. TNF-α levels were below the detection threshold of 16 pg/ml in serum samples isolated before infection (time zero) but had increased significantly by 60 min postinfection (Fig. 4). Treatment with the two phage led to a significant difference in the inflammatory response. While the TNF-α levels in Pf3R-treated mice decreased after the initial rise, those in Pt1-treated mice remained constant during the observation period (Fig. 4). IL-6 levels were below the detection threshold of 8 pg/ml in serum samples withdrawn before infection (time zero), increased after infection, and peaked at 2 h after treatment with either phage. After treatment with Pf3R or Pt1, the IL-6 levels showed the same pattern, but the overall levels were almost twice as high in Pt1-treated mice as in Pf3R-treated mice (Fig. 4).
FIG. 4.
Inflammatory responses upon phage treatment. Shown are levels of TNF-α (left) and IL-6 (right) in serum before infection with five times the MLD of PAO1 (time zero), 60 min after infection (immediately before phage treatment), and 2, 4, and 6 h after treatment with 5 × 108 PFU of phage Pf3R (shaded bars) or Pt1 (open bars). TNF-α and IL-6 cytokine concentrations were measured in triplicate by using the DuoSet kit (R&D Systems). Error bars, standard deviations. TNF-α and IL-6 levels were below the detection threshold (see Materials and Methods) before infection with PAO1 (time zero).
DISCUSSION
P. aeruginosa is one of the leading causes of hospital-acquired infections; it is able to cause a variety of diseases and is notorious among opportunistic pathogens for its high-level resistance to several antibiotics (6). This study was performed in order to develop genetically stable, efficient, and safe therapeutic phage for P. aeruginosa, the use of which should minimize endotoxin release. The Pf3R phage remained genetically stable, demonstrating the usefulness of the restriction endonuclease-methylase cassette as a means for propagation of the modified Pf3R phage on PAO1. In addition, the in vitro killing experiment (Fig. 2) and the in vivo phage therapy studies of experimental PAO1 infections of mice (Fig. 3) clearly demonstrated the efficacy of Pf3R as an antibacterial agent.
A statistically significant difference in survival after phage therapy with Pf3R versus Pt1 was observed upon administration of five times the MLD (Fig. 3B). More than 70% of the mice treated with Pf3R survived the 7-day observation period, whereas only 20% of the mice treated with Pt1 lived past the second day. The Pt1 phage was more effective than Pf3R at killing PAO1 in vitro (Fig. 2) and is able to replicate in PAO1, which is supposed to augment the therapeutic effect. In addition, the Pt1 phage was likewise effective in combating the P. aeruginosa infection when three times the MLD was administered (Fig. 3A). Therefore, we consider it rather unlikely that Pt1 is less efficient than Pf3R at reducing the load of PAO1 in vivo. The low release of endotoxin after Pf3R-induced killing of PAO1 in vitro (Fig. 2B) was mirrored by the reduced inflammatory response after Pf3R treatment relative to that after Pt1 treatment (Fig. 4).
The mouse inflammatory response to lethal doses of lipopolysaccharide (LPS) has been determined by several groups. Depending on the mouse strain, the source of endotoxin, and the cytokine assays used, considerable variations in the cytokine response were reported (1, 14, 22, 24). TNF-α levels peaked 1 h after LPS injection and ranged from 100 pg/ml (22) to 600 pg/ml (1) to 15,000 pg/ml (14). Reported serum IL-6 levels peaked between 2 and 4 h after LPS injection and ranged from 900 pg/ml (1) to 5,000 pg/ml (14) to 40,000 pg/ml (24). In a murine model of sepsis caused by P. aeruginosa pneumonia, serum IL-6 levels above 3,600 pg/ml were associated with 100% mortality, whereas levels below 1,200 pg/ml were associated with 100% survival (9).
Certainly, based on these data, more studies are necessary to assess whether the ΤNF-α and IL-6 levels upon phage Pt1 treatment determined in this study (Fig. 4) are indicative of endotoxemia. Nevertheless, this study suggests that the higher survival rate of Pf3R-treated mice (see Fig. 3B) could result from reduced endotoxin release. Based on a statistical analysis, it has been suggested that delivery of bactericidal agents by nonreplicating phage may represent a viable antimicrobial strategy (13). In fact, lethal agents delivered by a filamentous M13 phage variant have been shown to reduce the level of E. coli cells in the blood of mice (36). The present study indeed demonstrates that the use of nonreplicative phage in phage therapy of an experimental bacterial infection can be as effective as the use of replicating phage. It remains to be shown whether the same approach can be applied to phage of different bacterial pathogens or whether nonlytic phage are effective against infections where the causative agent is not easily accessible, e.g., in deep tissue infections. However, it seems feasible to use nonreplicative, nonlytic phage for topical treatment or for infections of the gut. Moreover, the use of nonreplicative phage, whether they are genetically modified or not, would allow application of a defined dose of the therapeutic phage rather than a dose that increases exponentially, as in the case of replicating phage. This, in turn, may facilitate the endeavor to obtain certification for phage as pharmaceuticals.
A potential problem posed by using a single phage to treat bacterial pathogens is the emergence of phage-resistant bacterial mutants. There appear to be two ways to cope with phage resistance. Either different phage are used to treat a particular pathogen or, alternatively, phage that attach to bacterial surface structures, representing virulence factors, are used (16). For instance, the filamentous P. aeruginosa phage Pf1 adsorbs to type 4 pili (7), which are required for adhesion to epithelial cells. It is conceivable that resistance to phage may be accompanied by attenuated virulence of the phage-resistant mutants. Furthermore, additional genetic modifications of filamentous phage to broaden their host range are conceivable. It has been shown that filamentous phage containing chimeric Ike and Ff receptor binding domains attach to E. coli possessing either N or F pili (19). Broadening the host range in this way could be useful for therapeutic purposes. Endotoxin release from gram-negative bacteria is not the only side effect that can be addressed by avoidance of bacterial lysis. Release of superantigens as well as a variety of exotoxins from gram-positive bacteria is likely to increase patient morbidity and prolong recovery. The use of genetically modified lethal but nonlytic phage in therapy could help to avoid these adverse side effects during treatment of infections with gram-positive pathogens.
Acknowledgments
We are grateful to B. Anton, A. Kuhn, and H. P. Schweizer for providing materials.
This work was supported by grant 70.048-4 from the Austrian Ministry of Education, Science and Culture to U.B. and by InterCell AG.
REFERENCES
- 1.Agelaki, S., C. Tsatsanis, A. Gravanis, and A. N. Margioris. 2002. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect. Immun. 70:6068-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton, B. P., D. F. Heiter, J. S. Benner, E. J. Hess, L. Greenough, L. S. Moran, B. E. Slatko, and J. E. Brooks. 1997. Cloning and characterization of the BglII restriction-modification system reveals a possible evolutionary footprint. Gene 187:19-27. [DOI] [PubMed] [Google Scholar]
- 3.Barrow, P. A., and J. S. Soothill. 1997. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 5:268-271. [DOI] [PubMed] [Google Scholar]
- 4.Berchieri, A., Jr., M. A. Lovell, and P. A. Barrow. 1991. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 142:541-549. [DOI] [PubMed] [Google Scholar]
- 5.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodey, G. P., R. Bolivar, V. Fainstein, and L. Jadeja. 1983. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 5:279-313. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, D. E. 1973. The adsorption of Pseudomonas aeruginosa filamentous bacteriophage Pf to its host. Can. J. Microbiol. 19:623-631. [DOI] [PubMed] [Google Scholar]
- 8.Brosius, J. 1989. Superpolylinkers in cloning and expression vectors. DNA 8:759-777. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith, C. M., D. M. Amiot, P. E. Stromberg, W. M. Dunne, C. G. Davis, D. F. Osborne, K. D. Husain, I. R. Turnbull, R. S. Hothkiss, and T. G. Buchman. 2003. Antibiotics improve survival and alter the inflammatory profile in a murine model of sepsis from Pseudomonas aeruginosa pneumonia. Shock 19:408-418. [DOI] [PubMed] [Google Scholar]
- 10.Hagens, S., and U. Bläsi. 2003. Genetically modified filamentous phage as bactericidal agents: a pilot study. Lett. Appl. Microbiol. 37:318-323. [DOI] [PubMed] [Google Scholar]
- 11.Heumann, D., and T. Roger. 2002. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta 323:59-72. [DOI] [PubMed] [Google Scholar]
- 12.Jobling, M. G., and R. K. Holmes. 1990. Construction of vectors with the p15a replicon, kanamycin resistance, inducible lacZ alpha and pUC18 or pUC19 multiple cloning sites. Nucleic Acids Res. 18:5315-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasmann, L. A., A. Kasman, C. Westwater, J. Dolan, M. G. Schmidt, and J. S. Norris. 2002. Overcoming the phage replication threshold: a mathematical model with implications for phage therapy. J. Virol. 76:5557-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruzel, M. L., Y. Harari, D. Mailman, J. K. Actor, and M. Zimecki. 2002. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS induced inflammatory response in mice. Clin. Exp. Immunol. 130:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepper, P. M., T. K. Held, E. M. Schneider, E. Bolke, H. Gerlach, and M. Trautmann. 2002. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 28:824-833. [DOI] [PubMed] [Google Scholar]
- 16.Levin, B. R., and J. J. Bull. 1996. Phage therapy revisited: the poulation biology of a bacterial infection and its treatment with bacteriophage and antibiotics. Am. Nat. 147:881-898. [Google Scholar]
- 17.Luiten, R. G., D. G. Putterman, J. G. Schoenmakers, R. N. Konings, and L. A. Day. 1985. Nucleotide sequence of the genome of Pf3, an IncP-1 plasmid-specific filamentous bacteriophage of Pseudomonas aeruginosa. J. Virol. 56:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinus, M. G., M. Carraway, A. Z. Frey, L. Brown, and J. A. Arraj. 1983. Insertion mutations in the dam gene of Escherichia coli K-12. Mol. Gen. Genet. 192:288-289. [DOI] [PubMed] [Google Scholar]
- 19.Marzari, R., D. Sblattero, M. Righi, and A. Bradbury. 1997. Extending filamentous phage host range by the grafting of a heterologous receptor binding domain. Gene 185:27-33. [DOI] [PubMed] [Google Scholar]
- 20.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merril, C. R., D. Scholl, and S. L. Adhya. 2003. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2:489-497. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, A., Y. Mori, K. Hagiwara, T. Suzuki, T. Sakakibara, T. Kikuchi, T. Igarashi, M. Ebina, T. Abe, J. Miyazaki, T. Takai, and T. Nukiwa. 2003. Increased susceptibility to LPS-induced toxic shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J. Exp. Med. 5:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien, A. D., L. R. Marques, C. F. Kerry, J. W. Newland, and R. K. Holmes. 1989. Shiga-like toxin converting phage of enterohemorrhagic Escherichia coli strain 933. Microb. Pathog. 6:381-390. [DOI] [PubMed] [Google Scholar]
- 24.Purswani, M. U., S. J. Eckert, H. K. Arora, and G. J. Noel. 2002. Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. J. Antimicrob. Chemother. 50:51-58. [DOI] [PubMed] [Google Scholar]
- 25.Rietsch, A., and U. Bläsi. 1993. Non-specific hole formation in the Escherichia coli inner membrane by λ S proteins is independent of cellular secY and secA functions and of the proportion of membrane acidic phospholipids. FEMS Microbiol. Lett. 107:101-106. [DOI] [PubMed] [Google Scholar]
- 26.Riffenburgh, R. H. 1999. Statistics in medicine. Academic Press, Inc., San Diego, Calif.
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Shalaby, M. R., A. Waage, L. Aarden, and T. Espevik. 1989. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin. Immunol. Immunopathol. 53:488-498. [DOI] [PubMed] [Google Scholar]
- 29.Slopek, S., I. Durlakowa, B. Weber-Dabrowska, A. Kucharewicz-Krukowska, M. Dabrowski, and R. Bisikiewicz. 1983. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch. Immunol. Ther. Exp. (Warsaw) 31:267-291. [PubMed] [Google Scholar]
- 30.Smith, H. W., and M. B. Huggins. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307-318. [DOI] [PubMed] [Google Scholar]
- 31.Soothill, J. S. 1992. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 37:258-261. [DOI] [PubMed] [Google Scholar]
- 32.Stanisich, V. A. 1974. The properties and host range of male-specific bacteriophages of Pseudomonas aeruginosa. J. Gen. Microbiol. 84:332-342. [DOI] [PubMed] [Google Scholar]
- 33.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 35.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 36.Westwater, C., L. M. Kasman, D. A. Schofield, P. A. Werner, J. W. Dolan, M. G. Schmidt, and J. S. Norris. 2003. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob. Agents Chemother. 47:1301-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagel, S., J. F. Barrett, D. J. Amaratunga, and M. B. Frosco. 1996. In vivo oral efficiacy of levofloxacin for treatment of Pseudomonas aeruginosa infections in a murine model of septicemia. Antimicrob. Agents Chemother. 40:2894-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, R., and U. Bläsi. 1995. Holins: form and function in bacteriophage lysis. FEMS Microbiol. Rev. 17:191-205. [DOI] [PubMed] [Google Scholar]