Abstract
Objective
To test the null hypothesis that six factors representing potential fascial and muscular failure sites contribute equally to the presence and size of a cystocele: two vaginal attachment factors (apical support and paravaginal defects), two vaginal wall factors (vaginal length and width), and two levator ani factors (hiatus size and levator ani defects).
Methods
Thirty women with anterior-predominant prolapse (cases) and 30 controls underwent 3D stress magnetic resonance imaging. The location of the anterior vaginal wall at maximal Valsalva was identified with the modified Pelvic Inclination Coordinate System and the six factors measured. Analysis included repeated measure ANOVA, logistic regression, and stepwise linear regression.
Results
We identified a collinear triad consisting of apical location, paravaginal location, and hiatus size that were not only the strongest predictors of cystocele size, but were also highly correlated with one another (r = 0.84 – 0.89, p < 0.001) for the presence and size of the prolapse. Together they explain up to 83% of the variation in cystocele size. Amongst the less significant vaginal factors, vaginal length explained 19% of the variation in cystocele size, but no significant difference in vaginal width existed. Cases were more likely to have abnormalities in collinear triad factors (up to 80%) than vaginal wall factors (up to 23.3%). Combining the strongest collinear triad with the vaginal factors, the model explained 92.5% of the variation in cystocele size.
Conclusion
Apical location, paravaginal location, and hiatus size are highly correlated, and are strong predictors of cystocele presence and size.
Introduction
Cystocele, or anterior vaginal wall prolapse, isthe most common form of pelvic organ prolapse1,2 and the most frequent site of operative failure.3-7 Four connective tissue failure sites are often discussed as causal factors contributing to cystocele formation. Two failure sites concern the attachment of the vaginal wall to surrounding structures (that we will refer to as “attachment factors”) and include apical support8,9 and paravaginal defects10,11 (Figure 1). Two failure sites concern the fibromuscular wall of the vagina that surgeons refer to as fascia (“vaginal wall factors”) and include vaginal width12 and length.13,14 Levator ani muscle injuries, resulting in an enlarged hiatus, also play a major role in affecting pelvic organ support 15,16 .Surgical approaches have been proposed to address each hypothesized connective tissue failure site17; nonetheless, surgical success rates remain suboptimal.3-7
Figure 1.

Anatomic conceptual model indicating factors affecting anterior vaginal wall support, including the two attachment factors: apical attachments and paravaginal attachment; as well as the two vaginal wall factors: vaginal length and width. The figure represents the anterior vaginal wall, cervix, and surrounding structures after the bladder has been removed above the vesical neck (VN). ATFP, arcus tendineus fascia pelvis; ATLA, arcus tendineus levator ani. Modified from Reference 12. © 2016 John O.L. DeLancey. Used with permission.
The recent development of 3D Stress MRI now captures the geometry of the anterior vaginal wall during maximal Valsalva,18 allowing assessment of the status of the four connective tissue failure sites at maximal Valsalva.12 The pilot data reported in Larson, et al.12 did not include levator ani muscle injury or hiatus size in the analysis and did not examine the relative contributions of four connective tissue components. . In current study, we sought to quantify all four connective tissue support factors plus the two levator factors. We tested the null hypothesis that the vaginal factors, attachment factors, and levator factors all contribute equally and independently to predict the occurrence and size of a cystocele.
Materials and Methods
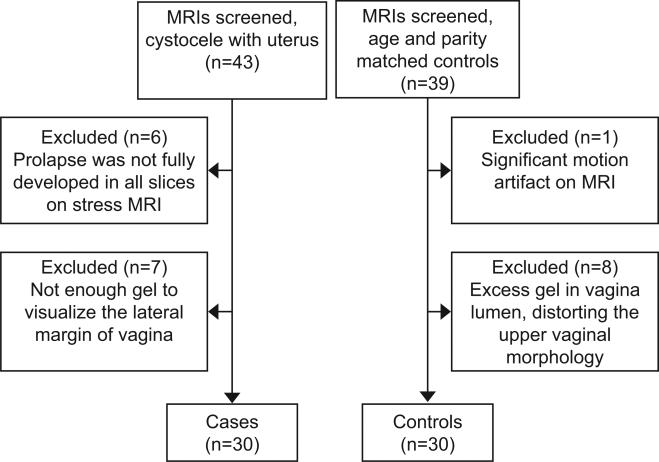
MRI scans from 30 women with cystocele-predominant prolapse (cases) and 30 asymptomatic women with normal support (controls), all with a uterus in situ, were selected from two IRB-approved case and control studies of pelvic organ prolapse (University of Michigan IRBMED HUM00043445 and HUM00031520). Cases had symptomatic cystocele-predominant prolapse, with supine Pelvic Organ Prolapse Quantification (POP-Q)12 location Ba at least 1 cm beyond the hymenal ring on clinical examination, and Point Ba greater than Point C. To find the 30 scans for cystocele evaluation, we screened 43 of the most recent MRI scans with a uterus and cystocele-predominant prolapse We also screened 39 age- and parity-matched controls to select 30 control participants who have adequate image quality. Inclusion and exclusion flow diagram is shown in Figure 2. All controls were asymptomatic based on Pelvic Floor Impact Questionnaires, had negative full bladder stress tests, and had support values on a POP-Q exam that lay within population-based norms (e.g. Ba and Bp points above the hymen).21 None of the participants had previously undergone hysterectomy or prior pelvic floor surgery. Among this group of participants, six cases and three controls had been included in an earlier technique development, description, and feasibility study,12 but all were standardized and re-measured in this study using the modified 3D Pelvic Inclination Coordinate System (PICS).22
Figure 2.
Participant screen inclusion and exclusion flow diagram.
We used the 3D Stress MRI technique to acquire images described previously.12 In brief, MRI sagittal images were acquired in the supine position during maximal Valsalva using a 3T Philips Achieva scanner (Philips Medical Systems, Best, the Netherlands) with a 6-channel, phased array coil. Ten to 20 ml of ultrasound gel were inserted into the vagina and distributed with a finger. Participants were then coached to perform a Valsalva and produce a prolapse similar in size to that previously identified during a supine clinical POP-Q examination. The MRI images were checked to make sure the prolapse seen in MRI was similar in size to the POP-Q data previously obtained. We used a maximal Valsalva effort to develop the prolapse as is done during clinical examination rather than using standardized Valsalva pressure to achieve the full size of the cystocele. The participants held the Valsalva for approximately 17 seconds with the prolapse protruding maximally in order to obtain 14 sagittal images from one side of the pelvis to the other in the sagittal plane (repetition time (TR) range 1249-1253 ms, echo time (TE) 80 ms, 6 mm slice thickness, 1 mm gap, SENSE factor 4, NSA 2, 320×178 voxels). If the prolapse did not reach its fullest extent, the MR study was repeated with additional coaching; if the patients could not produce a maximal prolapse, they were excluded from analysis.
Standard anatomical scans at rest were also acquired in sagittal, coronal, and axial planes using a turbo spin echo technique. Thirty images were obtained in each plane (TR range 2300-3000 ms, TE 30 ms, 4 mm slice thickness, 1 mm gap, number of signal average (NSA) 2, 256×256 voxels). These resting scans were aligned with one another using rigid registration in the 3D Slicer® software program (version 4.0. Brigham and Women's Hospital, Boston, MA), ensuring that structures co-localized in all three axes by simultaneous review of 3D scan planes in the viewer. The bony landmarks for the inferior point of the pubic symphysis (the arcuate pubic ligament), sacrococcygeal junction, and ischial spines were then identified. The sagittal maximal Valsalva images were aligned with the static sagittal images and bony landmarks using the inferior pubic point and the sacrococcygeal junction and a transformation matrix (both rotation and translation). This step ensure stress MRI and resting MRI were in the same local bony landmark-based coordinate system as an alternative to previous strategies that use the ischial spines because scan time requirements preclude inclusion of the ischial spines on the stress images.
Blinded to participant clinical POPQ findings, the anterior vaginal wall shape and lateral margin locations were identified using an array of fiducial markers that established the x,y,z coordinates for a systematic series of locations (Figure 3). This fiducial technique was used rather than the previous technique of making 3D volume-rendered models in order to avoid (1) lofting and smoothing error introduced by the computer algorithms used for model construction, and (2) the need for a separate process for model creation and measurement. In addition, point placement was found to be more precise than the line drawing needed for model construction. The posterior fornix, external cervical os, anterior fornix, and distal end of the vagina, as well as four equally spaced sample points along the vagina were identified on the mid-sagittal MRI (Figure 3A). At each point, a line perpendicular to the vaginal axis in the sagittal plane was identified and projected onto all the parasagittal slices. At each parasagittal slice, the vaginal wall was marked at the level of the perpendicular projection where it could be identified (Figure 3B,C). These points were then displayed to represent the anterior vaginal wall at maximum Valsalva in 3D (Figure 3D). A second rater marks the points independently for a subset of 5 case and 5 controls to evaluate the inter-rater variability.
Figure 3.

Sampling the anterior vaginal wall at maximum Valsalva in 3D with point clouds. (A) Mid-sagittal MR image of participant with prolapse. Anatomical landmarks along the anterior vaginal wall, posterior fornix, external cervical os, anterior fornix, urethrovesical junction, and distal end of the vagina were marked with blue dots and other equally-spaced sampling locations were marked with yellow dots. Short blue lines were placed perpendicular to the vaginal axis at the level of those landmarks. (B) The anterior vaginal wall was marked at the level of perpendicular lines on a sample parasagittal plane. (C) Example of anterior vaginal wall points in multiple sagittal MRIs. (D) Illustration of the resulting point array for the anterior vaginal wall at maximum Valsalva in 3D. PS denotes pubic symphysis, SCJ – sacrum coccyx junction, L_IS – left ischial spine R_IS –right ischial spine. © 2016 John O.L. DeLancey. Used with permission.
Custom Python-based software was developed for data processing. All of the landmarks were transformed from scanner coordinates to the modified 3D Pelvic Inclination Coordinate System (PICS) (Figure 4). This process allows for all structural locations to be compared in the same coordinate system, thereby compensating for differences in the participant's location and pelvic inclination within the scanner. It is also aligned with the longitudinal body axis so that assessments can be made of “how high or low” (in a craniocaudal direction) a structure lies relative to the bony pelvis and the conceptual direction of gravity when standing.
Figure 4.

Example of the length, width, and location measurements in the PICS coordinate system: (A) 3D point array with complete pubic bone. (B) Normal support example showing width and lateral wall locations at 5 equidistant points from anterior fornix (1) to urethrovesical junction (5); the cervical os is marked with ٭. Vaginal length was defined from the cervical os to the external urethral meatus. The PICS coordinate system is shown with its origin at the inferior pubic point, x axis pointing to the left, y axis pointing dorsally, and z axis pointing caudally. (C) Similar markings in anterior compartment prolapse. (D) The vertical distance that lateral vaginal locations and apex lie from mean (x,y,z) location from the normal group (indicated in red) is shown with arrows extending from average normal location (red) to their location in prolapse in the y-z plane. © 2016 John O.L. DeLancey. Used with permission.
The anterior vaginal wall length was calculated from the cervico-vaginal junction to the distal end of the vagina at the external urethral meatus in the mid-sagittal plane by following the curve of the vaginal wall. The vaginal width was measured at five equally spaced sampling locations along the anterior vaginal wall from the anterior fornix (location 1) to the urethrovesical junction (location 5) (Figure 4B,C).
The vertical coordinates (z axis) of the external cervical os were used to assess apical support. The paravaginal locations were assessed as vertical coordinates (z axis) of the lateral vaginal edge points at the five sampling locations from the anterior fornix to the urethrovesical junction. The lowest vertical coordinates were used to define the prolapse size.
In addition, we defined a measure for the vertical “paravaginal descent” to estimate paravaginal defect size. To do that, we established a normal vertical location for the vaginal edge at each of the five sampling locations on both sides using the average z value in the control group. We then calculated the maximum difference from this normal location as the measure of “paravaginal descent” (Figure 4D).
The size of the urogenital hiatus in the levator ani muscle was measured as the distance between the inferior pubic point and the front of the perineal body (Figure 3A,D). The presence and magnitude of injury to the pubococcygeal portion of the levator ani muscle was assigned via the review of resting axial and coronal scans by two independent examiners.15,23 Each examiner scored both the left and right muscles from 0 (no injury) to 3 (complete loss of the pubococcygeus). The two scores were added and grouped as follows: scores of ‘0’ classified as normal; bilateral score 1-3 as minor; and bilateral score 4-6 or unilateral score 3 as major. Discrepancies in scoring were resolved by joint review. Standard statistical analyses were applied using t-tests to compare means of vaginal length and apex location. Repeated measure ANOVA and post-hoc comparisons of vaginal width and paravaginal location at levels 1-5 along the vaginal axis were carried out using SPSS (version 23, IBM, Armonk, NY). Spearman's correlation coefficients were determined to assess the bivariate relationships. Odds ratios and standardized odds ratios were also calculated using binary logistic regression for all of the supporting factors in order to determine the strength of their relationship with case–control status. The standardized odds ratio expresses the odds ratio based on a change of one standard deviation rather than a specific measurement (e.g. 1 cm change in vaginal length). This allows the standardized odds ratios among different metrics to be directly compared. Finally, we used step-wise linear regression to explore the combination of supporting factors to predict the size of the prolapse. To estimate the proportion of women in the cystocele group who fall outside the range of values seen in the normal group, we determined the expected direction of abnormalities (e.g. for vaginal width, wider than that seen in the normal women. or for the vaginal apex, lower than that seen in control group) and then assessed the proportion of cases that fell above (or below) that threshold.
Results
Participant characteristics and mean POP-Q values are shown in Table 1. No statistically-significant differences were found between the two groups, with the exception of the expected differences in POP-Q. The mean distance of points placement between two rater at various sampling segments ranged from 0.4mm to 2.1mm in the direction perpendicular to vaginal axis and 0.1mm to 0.5mm along the vagina axis.
Table 1.
Mean (standard deviation) Demographic Data
| Characteristics | Anterior Wall Prolapse (n=30) | Controls (n=30) | P-value |
|---|---|---|---|
| Age (years) | 59.0 ± 10.6 | 58.6 ± 5.3 | 0.85 |
| BMI (kg/m2) | 26.5 ± 4.4 | 27.4 ± 4.8 | 0.44 |
| Median parity (range) | 3 ± 1-5 | 2.5 ± 1-6 | 0.74 |
| POP-Q | |||
| Aa (cm) | 1.6 ± 1.1 | −2.0 ± 0.9 | < 0.001 |
| Ba (cm) | 2.7 ± 1.4 | −2.0 ± 0.9 | < 0.001 |
| C (cm) | −1.0 ± 2.9 | −6.9 ± 1.8 | < 0.001 |
| D (cm) | −5.8 ± 2.5 | −8.7 ± 1.7 | < 0.001 |
| Ap (cm) | −0.9 ± 1.3 | −2.1 ± 0.7 | < 0.001 |
| Bp (cm) | −1.0 ± 1.3 | −2.1 ± 0.7 | < 0.001 |
BMI, Body Mass Index; POP-Q, Pelvic Organ Prolapse Quantification
Group comparisons for all supporting factors are shown graphically in Figure 5 (the actual numbers for this graph are shown in the Table 2). Vaginal length was 23% longer in women with prolapse than controls (p < 0.001). The top of the vagina was 15% narrower in the anterior wall prolapse group than controls at both sampling segment 1 and 2 (p = 0.008, 0.03). Vaginal widths in sampling segments 3, 4, and 5 were not different. (Figure 5, top row).
Figure 5.

Comparison of vaginal wall factors (first row), attachment factors (second row), and levator ani factors (third row) by group. Vaginal wall and hiatus size error bars show the standard deviation. * denotes statistical significance, p < .05, ** denotes statistical significance p < 0.001. Attachment factors box plots show the median (center line) and interquartile range (box), as well as extreme values (the end of whiskers) and outliers. AW, anterior wall prolapse group
Table 2.
Group Comparisons for All Supporting Factors*
| Factors | AW (SD) | Control (SD) | Effect Size | p-value | % case outside normal range (CI) |
|---|---|---|---|---|---|
| Vaginal Factors (mm) | |||||
| Vaginal Length | 75.6 ± 16.3 | 61.2 ± 10.1 | 1.1 | < 0.001 | 23.3% (8.2%-38.5%) |
| Vaginal Width | |||||
| 1 (Anterior Fornix) | 38.0 ± 10.1 | 44.7 ± 9.0 | −0.7 | 0.008 | 0.0% (0.0% -0.0%) |
| 2.0 | 35.1 ± 11.9 | 41.1 ± 9.3 | −0.6 | 0.033 | 0.0% (0.0% -0.0%) |
| 3.0 | 35.0 ± 12.8 | 36.7 ± 10.6 | −0.1 | 0.574 | 0.0% (0.0% -0.0%) |
| 4.0 | 33.3 ± 14.3 | 32.5 ± 10.4 | 0.1 | 0.804 | 13.3% (1.2% - 25.5%) |
| 5 (UVJ) | 28.1 ± 12.6 | 28.7 ± 10.8 | −0.1 | 0.833 | 0.0% (0.0% -0.0%) |
| Attachment Factors (mm) | |||||
| Apex location | 0.8 ± 27.3 | 39.5 ± 14.8 | 1.8 | < 0.001 | 56.7% (38.9%-74.4%) |
| Paravaginal vertical location | |||||
| 1 (Anterior Fornix) | 13.7 ± 20.5 | −27.8 ± 15.6 | 2.3 | < 0.001 | 63.3% (46.% - 80.6%) |
| 2.0 | 24.8 ± 17.9 | −21.4 ± 16.4 | 2.7 | < 0.001 | 73.3% (57.5% - 89.1%) |
| 3.0 | 30.4 ± 14.8 | −14.6 ± 16.4 | 2.9 | < 0.001 | 80.0% (65.7%-94.3%) |
| 4.0 | 28.9 ± 10.7 | −7.8 ± 15.8 | 2.7 | < 0.001 | 50.0% (32.1% -67.9%) |
| 5 (UVJ) | 21.2 ± 8.0 | −1.6 ± 14.1 | 2.0 | < 0.001 | 63.3% (46.1% - 80.6%) |
| Levator Factors | |||||
| Hiatus size (mm) | 51.3 ± 11.8 | 33.0 ± 7.3 | 1.9 | < 0.001 | 56.7% (38.9%-74.4%) |
| Levator Score | |||||
| Major | 47 % | 20 % | 0.0 29 | ||
| Minor | 37% | 30% | 0.584 | ||
| Normal | 17% | 50% | 0.0062 |
AW, anterior wall prolapse group; UVJ, uretrovesical junction
Supporting factors are depicted graphically in Figure 5.
The mean apex location (cervical os) was 39 mm lower in women with cystocele than in controls (Case: −0.8 ± 27.4 mm; Control: −39.5 ± 14.8 mm, p < 0.0001). Repeated measure ANOVA showed no significant difference between the left and right sides in the vaginal edge location; therefore, data for the right and left sides were averaged to simplify the comparison. The lateral vaginal margin was lower at all sampling locations in women with cystocele than controls (Figure 5, middle row). The largest differences were found at location “2” where the margin was 46 mm lower (p<0.001), as well as “3” where it was 45 mm lower (p < 0.001).
The anterior-posterior diameter of the urogenital hiatus in the levator ani muscle at maximal Valsalva was 55% larger in women with prolapse (p < 0.001) than in controls (Figure 5, third row). In addition, women with prolapse were 2.5 times more likely to have major levator ani injury than controls (p = 0.015).
The frequency of which women with cystocele were abnormal is as follows: The lateral vaginal edge location was lower than the normal range in 63%, 73%, 80%, 50%, and 63% of sampling segments 1 to 5 respectively, with the highest percentage in the mid-portion of the vagina. Apical location was lower than the normal range in 57% of cases and vaginal length was longer than the normal range in 23% (Table 2). For vaginal width, only 13% were wider than the normal range at sampling segment 4, and all cases had normal widths at other sampling segments. Hiatal size was enlarged in 57% of cases (Table 2).
We also examined the bivariate correlations between supporting factors (Figure 6). Two attachment factors (apex location and “paravaginal descent”) and hiatus size were highly correlated with each other, with the correlation coefficient r ranging from 0.84 to 0.89 (all statistically significant, with p < 0.001). In what follows we shall refer to these three factors as a “collinear triad.” There were moderate correlations (r = 0.32 – 0.51, p < 0.05) between vaginal length and the collinear triad, but no significant association with vaginal width (r = 0.05 – 0.23, p = 0.08 – 0.77). Cystocele size was strongly associated with the collinear triad (r = 0.87 – 0.92, p < 0.001) and moderately associated with vaginal length (r = 0.54, p < 0.001), but not significantly associated with vaginal width (r = 0.05, p = 0.721) (Figure 6).
Figure 6.

Bivariate scatter plot for each support factor when considering prolapse size. Note the highly correlated “collinear triad”: paravaginal descent, apex location, and hiatal diameter. Cases shown as filled circles and controls as open circles.
The relative importance of these support factors in predicting the presence of cystocele was evaluated using binary logistic regression analysis. For factors measured at multiple levels, we analyzed both maximum vaginal width and the maximum difference in vaginal width as compared to the mean width calculated from women with normal support. We chose the better predictor—maximum vaginal width—as the measure of vaginal width. Similarly, we use the “maximum vertical paravaginal descent” that represents the largest vertical distance that the lateral vaginal edge is below the mean value in the normal participants (Figure 4D) as the measure of paravaginal defect. The odds ratio and the standardized odds ratio of all supporting factor predictors are listed in Table 3. Maximum paravaginal descent, apical location, and hiatus diameter had the largest standardized OR; these were much larger than the standardized OR for the vaginal factors. That is, with one standard deviation change in vaginal location as well as hiatus size, there is a much greater risk for women to have cystocele than with changes in the vaginal wall itself.
Table 3.
The Odds Ratio of Predictors to Cystocele Presence (Yes or No)
| Factors | Parameter | Odds Ratio (95% CI for OR) | Standardized OR (95% CI) | P value |
|---|---|---|---|---|
| Vaginal Factors | Vaginal Length | 1.08 (1.02 – 1.13) | 3.53 (1.43 – 8.72) | < 0.001 |
| Attachment Factors | Maximum l Vertical Paravaginal Gap | 1.19 (1.02 – 1.29) | 11.26 (3.39 – 37.44) | < 0.001 |
| Apex Vertical Location | 1.05 (1.06 – 1.09) | 2.97 (1.38 – 6.37) | 0.002 | |
| Perineal Body Vertical Location | 1.19 (1.08 – 1.31) | 35.71 (4.92 – 250.0) | < 0.001 | |
| Levator Ani Factors | Hiatus Diameter | 1.23 (1.11 – 1.37) | 30.15 (5.13 – 177.3) | < 0.001 |
| Major LA Defect | 4 (1.27 – 12.58) | NA | 0.018 |
LA, Levator Ani; NA, standardized odds ratio cannot be calculated for dichotomous variables
To evaluate how strongly each parameter contributed to the size of the cystocele among cases, we used step-wise linear regression models (Table 4). With the strongest collinear triad, maximum paravaginal descent explained 82.6% of the variation in cystocele size. With the strongest vaginal factor, vaginal length explains 18.6% of the variation in cystocele size. The combination of the collinear triad and vaginal factors explained 92.5% of the variation in cystocele size. The difference between model 2 (R2= 82.6%) and model 3 (R2=92.5%) has a p value of 0.10.
Table 4.
Size of Cystocele in Women with Cystocele: Linear Regression Analysis
| Model | Predictors | Parameter Estimation | P value | Model R2 |
|---|---|---|---|---|
| Model 1 Strongest Colinear Triad Predictor | (Constant) Maximum Vertical Paravaginal Descent | 2.32 0.794 |
0.525 < 0.001 |
0.826 |
| Model 2 Strongest Vaginal Predictor | (Constant) Vaginal Length | 8.938 0.347 |
0.501 0.017 |
0.186 |
| Model 3 Colinear Triad + Vaginal Factor | (Constant) Apex Vertical location Vaginal Length | 14.519 0.451 0.292 |
0.01 <0.001 <0.001 |
0.925 |
Discussion
This study indicates that vaginal attachment factors (apical and paravaginal) and measures related to the levator ani muscle—not the vaginal wall itself—are the factors most strongly associated with cystocele occurrence and size. Three factors formed a collinear triad: paravaginal descent, apical descent, and anteroposterior hiatal diameter. These 3 factors are highly correlated and were the best predictors for the occurrence of cystocele, accounting for 83% of variations in cystocele size. Vaginal length, not vaginal width, is the more predominant vaginal wall factor and explains only 19% of the variation in cystocele size, less than a quarter of that accounted for by the collinear triad. We conclude that without evidence to support a role for vaginal width, and only a small contribution of vaginal wall length, that the vaginal wall and its fascia are not the primary cause of cystocele.
Our findings provided comprehensive subject-specific data comparing measurement for each factor in women with cystocele to those with normal support and determine the magnitude of these differences. These data address the causal factors outlined in recent reviews24,25 that include apical support, paravaginal attachment, vaginal wall fascial tissues, or pelvic musculature. At present, the “ideal” cystocele correction plan remains undefined.26 Having objective measures to identify failure sites in each women whose measurements fall outside a normal range could, in the future, help identifying the best surgical plan for the individual patient.
Our findings corroborate earlier studies showing that women with prolapse have increased apical descent and increased vaginal length when compared to women with normal support.8,9,13 They also confirm and expand upon our pilot findings presented in our description of the 3D Stress MRI technique,12 reinforce the importance of paravaginal support as well as the high correlation between apical descent and paravaginal gap. This study also elucidates the inter-relationships between other factors while adding parameters concerning the levator ani.
Mechanistically, perhaps the most important finding is discovery of the collinear triad. This observation demonstrates that these individual factors—previously postulated as separate failure sites—are actually facets of a singular and more complex support failure phenomenon. This imaging study finding confirms our earlier theoretical computer simulations suggesting such a relationship19 The results support a conclusion that there is a strong functional relationship between both muscular and connective tissue supports and interplay between different facial defect sites. Further research will be needed to assess the details of how and why these factors are so highly related.
The finding that the mean vaginal width was not greater in women with cystocele came as a surprise. Our data do not eliminate the possibility that certain women with cystocele have a wider than normal vagina, but it does show that factor as not being as important in determining anterior vaginal prolapse as others. The clinical impression that the vagina is wider in women with cystocele probably arises when the normally wider upper vagina becomes visible through the introitus , giving the impression that the vagina has increased in width.
The limitations of this study deserve consideration. The study was not powered to detect subtle changes that may contribute to cystocele formation. This sample is not population-based and excludes other types of prolapse., Inferences must be limited to those appropriate to our design and inclusion criteria. It is challenging to acquire these images. we screened 43 qualifying cases to find 30 adequate images in women with prolapse, so selection bias must be considered. Most of the exclusions were due to technical problems with gel distribution and the woman's inability to hold the Valsalva that could bias our results towards having fewer frail women. This would probably not have affected the basic pattern of our results. Vaginal gel was used to illuminate the lateral extent of the vagina on MR and does result in slight distortion of the vaginal shape.. We think the effect was minimal since those with too much gel were eliminated.
In conclusion, there are changes in all the supporting domains of vaginal support— attachment factors, vaginal wall factors, and levator factors—but the highly correlated changes in attachment and levator factors are much larger than the changes in vaginal wall factors. A need to routinely reinforce the vaginal wall with mesh or biological tissues is not supported by our data.
Acknowledgments
Supported by NIH ORWH grant P50 HD044406 and the National Institute of Child Health and Human Development R01 HD038665. Investigator support for Luyun Chen was provided by NIH ORWH BIRCWH Career Development Award K12 HD004438.
Footnotes
Presented at the 35th American Urogynecology Society (AUGS) Annual Scientific Meeting, Washington, D.C. in 2014.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: A prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175(6):1418–1421. doi: 10.1016/s0002-9378(96)70084-4. discussion 1421-2. [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen JK. Current concepts in the diagnosis and surgical repair of anterior vaginal prolapse due to paravaginal defects. Obstet Gynecol Surv. 2001;56(4):239–246. doi: 10.1097/00006254-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: A randomized controlled trial. Obstet Gynecol. 2008;111(4):891–898. doi: 10.1097/AOG.0b013e31816a2489. [DOI] [PubMed] [Google Scholar]
- 5.Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98(1):40–44. doi: 10.1016/s0029-7844(01)01378-3. [DOI] [PubMed] [Google Scholar]
- 6.Maher C, Baessler K. Surgical management of anterior vaginal wall prolapse: An evidencebased literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):195–201. doi: 10.1007/s00192-005-1296-3. [DOI] [PubMed] [Google Scholar]
- 7.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–1373. doi: 10.1067/mob.2000.110910. Discussion 1373-4. [DOI] [PubMed] [Google Scholar]
- 8.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195(6):1837–1840. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 9.Summers A, Winkel LA, Hussain HK, DeLancey JOL. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187(1):93–8. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 12.Larson KA, Luo J, Guire K, Chen L, Ashton-Miller JA, DeLancey JOL. 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal and apical defects. Int Urogynecol J. 2012;23(3):285–293. doi: 10.1007/s00192-011-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JOL. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swenson CW1, Simmen AM, Berger MB, Morgan DM, DeLancey JO. The long and short of it: anterior vaginal wall length before and after anterior repair. Int Urogynecol J. 2015;26(7):1035–9. doi: 10.1007/s00192-015-2636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLancey JOL, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 16.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. Br J Obstet Gynaecol. 2008;115:979–84. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 17.Maher C. Anterior vaginal compartment surgery. Int Urogynecol J. 2013;24:1791–1802. doi: 10.1007/s00192-013-2170-3. [DOI] [PubMed] [Google Scholar]
- 18.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JOL. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21(9):1103–9. doi: 10.1007/s00192-010-1161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Ashton-Miller JA, DeLancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42(10):1371–7. doi: 10.1016/j.jbiomech.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 21.DeLancey JOL, Fenner DE, Guire K, Patel D, Howard D, Miller JM. Differences in continence system between community-dwelling black and white women with and without urinary incontinence in the EPI study. Am J Obstet Gynecol. 2010;202(6):584. doi: 10.1016/j.ajog.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betschart C, Chen L, Ashton-Miller JA, DeLancey JOL. On pelvic reference lines and the MR evaluation of genital prolapse: a proposal for standardization using the Pelvic Inclination Correction System. Int Urogynecol J. 2013;24(9):1421–8. doi: 10.1007/s00192-013-2100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger MB, Morgan DM, DeLancey JOL. Levator ani defect scores and pelvic organ prolapse: is there a threshold effect? Int Urogynecol J. 2014;25(10):1375–9. doi: 10.1007/s00192-014-2388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamblin G, Delorme E, Cosson M, Rubod C. Cystocele and functional anatomy of the pelvic floor: review and update of the various theories. Int Urogynecol J. 2015 Sep 4; doi: 10.1007/s00192-015-2832-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Kerkhof MH, Hendriks L, Brölmann HAM. Changes in connective tissue in patients with pelvic organ prolapse – a review of the current literature. Int Urogynecol J. 2009;20:461–74. doi: 10.1007/s00192-008-0737-1. [DOI] [PubMed] [Google Scholar]
- 26.Lensen EJM, Withagen MIJ, Kluivers KB, Milani AL, Vierhout ME. Surgical treatment of pelvic organ prolapse: a historical review with emphasis on the anterior compartment. Int Urogynecol J. 2013;24:1593–1602. doi: 10.1007/s00192-013-2074-2. [DOI] [PubMed] [Google Scholar]