Abstract
Background
Recently, lower estimates of influenza vaccine effectiveness (VE) against A(H3N2) virus illness among those vaccinated during the previous season or multiple seasons have been reported; however, it is unclear whether these effects are due to differences in immunogenicity.
Methods
We performed hemagglutination inhibition antibody (HI) assays on serum collected at preseason, ∼30 days post-vaccination, and postseason from a prospective cohort of healthcare personnel (HCP). Eligible participants had medical and vaccination records for at least four years (since July, 2006), including 578 HCP who received 2010–11 trivalent inactivated influenza vaccine [IIV3, containing A/Perth/16/2009-like A(H3N2)] and 209 HCP who declined vaccination. Estimates of the percentage with high titers (≥40 and > 100) and geometric mean fold change ratios (GMRs) to A/Perth/16/2009-like virus by number of prior vaccinations were adjusted for age, sex, race, education, household size, hospital care responsibilities, and study site.
Results
Post-vaccination GMRs were inversely associated with the number of prior vaccinations, increasing from 2.3 among those with 4 prior vaccinations to 6.2 among HCP with zero prior vaccinations (F[4,567] = 9.97, p < .0005). Thirty-two percent of HCP with 1 prior vaccination achieved titers >100 compared to only 11% of HCP with 4 prior vaccinations (adjusted odds ratio = 6.8, 95% CI = 3.1 – 15.3).
Conclusion
Our findings point to an exposure-response association between repeated IIV3 vaccination and HI for A(H3N2) and are consistent with recent VE observations. Ultimately, better vaccines and vaccine strategies may be needed in order to optimize immunogenicity and VE for HCP and other repeated vaccinees.
Keywords: influenza vaccination, hemagglutination inhibition antibody, healthcare personnel, immunogenicity
With the advent of universal recommendations for annual seasonal influenza vaccination in the USA, decades-old questions about the association between repeated vaccination and the effectiveness of influenza vaccines have new relevance [1–3]. Indeed, several recent studies have observed that influenza vaccine effectiveness (VE) against influenza illness is modified by prior vaccination history [4–8].
Of particular concern are lower point estimates of VE against influenza A(H3N2) virus illness among those vaccinated during the previous season [4–9] or repeatedly over the previous 5 seasons [9]. Although multiple studies have observed that previous vaccinees are less likely to seroconvert (or show a 4-fold increase in hemagglutination inhibition [HI]) following vaccination with inactivated trivalent influenza vaccines (IIV3s) [2,10–17], researchers have assumed this group is not at a disadvantage since they tend to have similar pre-season antibody titers due to higher baseline titers before vaccination [2,14].
From our prospective cohort study of healthcare personnel (HCP) in 2010–11, we have previously reported lower HI response to the A(H1N1)pdm09 component among those who received the monovalent inactivated A(H1N1)pdm09 vaccine a year earlier [4]. In a subsequent study focused on neuraminidase inhibition among vaccinees, we also noted lower response to N1 and N2 antigens following vaccination for those who received both the IIV3 or monovalent A(H1N1)pdm09 vaccine the prior year [18].
In this study, we expand our focus to examine vaccination history over four prior seasons. We examine the association between zero to four repeated annual IIV3 vaccinations and HI response to A/Perth/16/2009 (H3N2)-like virus. Specifically, we aimed to examine whether there is a dose-response (or exposure-response) association between the number of prior vaccinations and serologic immunogenicity, whether IIV3 vaccines with antigenically distinct A(H3N2) components contribute differently to any such association, and whether the association occurs regardless of baseline antibody levels and consistently across age groups among working age adults.
1. METHODS
1.1. Study Design and Setting
Samples for this evaluation were collected as part of a prospective cohort study of HCP aged 18–65 years old providing direct patient care full-time at both inpatient and ambulatory medical centers in Scott & White Healthcare (SWH) in Temple/Round Rock, Texas, and Kaiser Permanente Northwest (KPNW) in metropolitan Portland, Oregon (including parts of Washington). Details on cohort recruitment from September 18 to December 18, 2010, and the assessment of participant characteristics at enrollment have been described previously [19]. The study enrolled approximately 20% and 40% of the source HCP populations at KPNW and SWH, respectively; males and physicians were less likely to join the cohort [19].
This study focuses on a subset of participants with complete employee records and confirmation of health plan membership and/or receipt medical care from the study health systems for at least four years (since July, 2006). The choice of a four year prior vaccination period was made taking into account: record extraction limitations set by the Institutional Review Boards, the marked increased in employment stability starting at four years, and increased confidence in the thoroughness of employee records at both study sites starting in 2006.
Sera was collected at three times: pre-season (Time 1) for all participants (primarily in September and October), approximately 30 days post-vaccination for vaccinees (most of whom were vaccinated in October and provided blood again in November) (Time 2), and again for all participants in April and May, 2011, approximately 7 months after enrollment (Time 3). Participants who provided Time 2 sera < 14 days or >60 days from vaccination were excluded. Participants were monitored weekly for the development of acute respiratory illness, as described earlier [20–22], and influenza virus infection was confirmed via real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay. The 18 participants who experienced A(H3N2) illness have been described previously [20] and were excluded from analysis (Supplemental Figure A).
1.2. Vaccination Records and Composition of Vaccines
All participants received their personal medical care at the study medical centers. Vaccination history was documented from both their personal electronic medical records and employee health documentation in order to capture vaccinations received as a patient and those received though employee services. A few participants who received vaccination at a pharmacy or other non-traditional setting provided proof of vaccination to employee health, which was documented in their system. Since the predominant vaccine at both sites was inactivated and the immune response differs for live-attenuated influenza vaccine (LAIV) vs. IIV3 [23], we excluded participants who received LAIV during the study season or prior 4 seasons.
The study year’s 2010–11 IIV3 contained A/California/7/2009 pdm(H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 strains. At KPNW, a single lot of IIV3 manufactured by Novartis International was administered to approximately 90% of IIV3 vaccinees in the cohort; at SWH, 2 lots of IIV3 manufactured by GlaxoSmithKline Pharmaceuticals were administered to approximately 90% of IIV3 vaccinees.
The IIV3 composition during the previous 4 seasons is listed in Supplemental Table A. Of note, the 2010–11 A(H3N2) IIV3 component was antigenically distinct from the two components included in IIV3s during the previous four seasons: both the 2006–07 and 2007–08 IIV3s contained an A/Wisconsin/67/2005 (H3N2)-like strain, and both the 2008–09 and 2009–10 IIV3s contained an A/Brisbane/10/2007 (H3N2)-like strain.
1.3. Hemagglutination Inhibition Antibody Assay
Testing for HI titers to A/Perth/16/2009 (H3N2) was performed by Battelle Laboratory (Aberdeen, MD) using standard methods [24] as described previously [4]. An egg-grown wild type A/Perth/16/2009 virus was used. A standard 0.5% turkey erythrocyte was prepared, and serum samples were treated with receptor-destroying enzyme to remove nonspecific inhibitors. Nonspecific agglutinins were removed by serum adsorption with packed red blood cells. Serum was diluted 2-fold starting from 1:10. The HI titer was the reciprocal of the serum dilution in the last well with complete HI. The geometric mean titer (GMT) from duplicate results was reported; HI < 10 was considered to be 5 for GMT calculation.
1.4. Statistical Analysis
Since distributions of HI titer data were highly left-skewed, all statistical analyses were conducted using log base-2 transformed titer data; results were then back-transformed to the original scale for ease of interpretation [4,25]. Pre- and post-vaccine draws were assumed to be correlated within each person, thus repeated measures linear mixed models were fitted to estimate GMTs and geometric mean ratios (GMRs). Compound symmetric covariance error structures were assumed for repeated measures within individuals.
GMTs were calculated by back-transforming the least squares mean estimates of logged titer data. GMRs were calculated by back-transforming the difference of least squares means of post-vaccination and pre-vaccination logged titer estimates. GMRs are interpreted as the geometric mean fold ratio of post-vaccination titer to pre-vaccination titer. Multivariate estimates adjusted a priori for age, sex, race, and study site [19]. Linear, quadractic, and cubic terms for age were examined to consider possible nonlinear associations with age. Education, household size, and working in a hospital setting were added as covariates because they were associated with the number of prior vaccinations and either preseason GMT or post-vaccination GMRs among vaccinees (Supplemental Table B).
To test the hypothesis that the outcome (serologic vaccine response or GMR) varied depending on the exposure (the number of prior IIV3 vacciantions), we estimated an interaction term for time of sera draw (pre- and post-vaccination) by the number of prior vaccinations; after adjusting for main effects and covariates, a statistically significant interaction term (p < .025) indicated that vaccine response was significantly modified by prior IIV3 exposure.
In sensitivity analyses, all demographic and health variables listed in Table 1 were included in the adjusted models, but did not change the pattern of findings. Also in sensitivity analyses, days between Time 1 and 2 sera collection were not associated with Time 2 GMR; similarly, days between Time 1 and 3 (for unvaccinated HCP) and days between Time 2 and 3 (for vaccinees) were not associated with Time 3 GMR.
Table 1.
Characteristics of 816 Participants in a Healthcare Personnel Cohort with Vaccination Records through Four Prior Years and the 578 of these who Received 2010–11 Vaccination.
| Full Samplea | IIV3 Vaccineesb | |
|---|---|---|
| Categorical descriptors, N (%) | ||
| Study site (Oregon) | 361 (44) | 260 (45) |
| Sex (Female) | 700 (86) | 502 (87) |
| Race (White) | 650 (80) | 472 (82) |
| Ethnicity (Hispanic) | 8 (10) | 58 (10) |
| Child (age < 13) at home | 248 (30) | 159 (28) |
| Physician | 52 (6) | 42 (7) |
| Work in Hospital | 440 (54) | 319 (55) |
| Work in Emergency Dept. | 155 (19) | 100 (17) |
| Chronic medical conditionc | 151 (19) | 117 (20) |
| Continuous descriptors, Median (SD) | ||
| Age (years) | 47 (12) | 47 (12) |
| Household size (0–7) | 2 (2) | 2 (1) |
| Education (5-levels) | 16 (2) | 16 (2) |
| Self-rated health status (5-levels) | 4 (1) | 4 (1) |
| Body mass index (kg/m) | 28 (7) | 29 (7) |
| Direct patient care per week (hours) | 34 (11) | 34 (11) |
| Prior vaccinations, N (%) | ||
| 2006–07 IIV3 | 433 (53) | 379 (66) |
| 2007–08 IIV3 | 391 (48) | 342 (59) |
| 2008–09 IIV3 | 464 (57) | 399 (69) |
| 2009–10 IIV3 | 589 (72) | 503 (87) |
| 2009 MIV A(H1N1)pdm09 | 401 (49) | 358 (62) |
| 2010–11 IIV3 | 607 (74)b | 578 (100) |
| Sum of Prior IIV3, Median (SD) | 3 (1) | 3 (1) |
p < .05
p < .01
p < .001.
Sample (N = 816) includes health care personnel with medical and vaccination records since July, 2006 and excludes those who received live attenuated influenza vaccine (LAIV) in 2010–11 and/or in any season since 2006–07.
The post-vaccination study sample consists of 578 of 607 who received trivalent inactivated influenza vaccine (IIV3) and also had sera collected at both preseason and post-vaccination.
Presence of a chronic medical condition was identified by a medical visit during the prior year in the electronic medical record for a medical condition associated with increased risk of influenza complications (codes available from the authors).
Since baseline antibody titers are among the best predictors of serologic response [26], we repeated the GMR models stratifying participants by those with low baseline titers (GMT < 40) or high baseline titers (≥40) [4]. Given that only 27 participants had zero prior vaccinations, we excluded these participants from secondary stratified analyses.
As an additional outcome measure and to aid the interpretation of the association between prior vaccinations and GMR, we also report the percentage of participants with GMT of ≥40, a recognized immune marker associated with at least a 50% protection against influenza infection in populations [4], as well as a GMT of >100 which is associated with even higher clinical protection [25]. Given differences in baseline immunity, we also report the percentage who achieved titers ≥40 and >100 post-vaccination, excluding those with preseason GMT ≥40. These percentages were estimated using generalized linear models with the same covariates described for the mixed effects models and adjusted for baseline titers, as recommended [26], to minimize bias associated with prevaccination titers. We used logistic regression, with the same covariates, to illustrate the magnitude of the effect of prior vaccination on the dichotomous elevated titer outcomes.
In secondary analyses, we repeated the Time 2 post-vaccination GMR analyses and elevated GMT analyses (using the same covarites) considering separate counts for the number of 2006–07 and 2007–08 IIV3s received (containing A/Wisconsin/67/2005-like [H3N2]) and the number of 2008–09 and 2009–10 IIV3s received (containing A/Brisbane/10/2007-like [H3N2]). The vaccines’ match to circulating A(H3N2) strains was relatively low in 2006–07 and 2007–08 compared to subsequent years (Supplemental Table A). These results are presented for hypothesis generating purposes only, especially given the relatively small number of participants who received neither the 2008–09 nor 2009–10 IIV3s.
Vaccine exposure groups whose 95% confidence intervals (CI) (for GMT, GMR, or percentage with elevated titers) did not overlap were considered statistically different. Given the relatively small number of participants with zero prior vaccinations, we illustrated some differences between extreme groups comparing participants with 4 vs. 1 prior vaccination. For other analyses, a p-value of < .025 was considered statistically significant. Partial eta squared (η2) is reported for the mixed effect models to indicate the amount of variance in the outcome explained by the number of prior vaccinations after excluding variance explained by other covariates. All analyses were conducted using IBM SPSS Statistics 21 (Armonk, NY).
2. RESULTS
2.1. Participant Characteristics
As outlined in Supplemental Figure A, 1568 HCP were enrolled and gave preseason sera. Of these, 562 HCP (36%) were excluded because they lacked medical and vaccination records going back to July 2006, and an additional 37 (2%) and 153 (10%) were excluded because they received LAIV in 2010–11 or in any season since July 2006, respectively. Excluded participants were more likely to be under age 50 years old, to be male, have young children (aged < 9 years old), be physicians, and work at the Texas study site; excluded participants were less likely to have a chronic medical condition (data not shown). A similar percentage of excluded and included participants received IIV3 in the 2010–11 study year (71 vs. 74%).
The resulting study sample of 816 HCP with preseason sera, included 607 (74%) who subsequently received the 2010–11 IIV3 and 209 (26%) who were unvaccinated. Post-vaccination sera was available from 578/607 (95%) vaccinees. Post-season sera, excluding 18 HCP who had RT-PCR confirmed A(H3N2) illness during the season, were available from 194/209 (93%) unvaccinated and 564/578 (98%) vaccinated HCP.
The majority of study participants were female, white, and non-Hispanic (Table 1). The median number of IIV3 during the prior 4 seasons was 3, with 695/816 (85%) having received at least one IIV3 during this time. A greater number of prior vaccinations was associated with being older, having higher education and smaller households, as well as working outside of the hospital, having fewer hours of direct patient contact, and working at the KPNW study site (Supplemental Table B); these were all modest associations.
2.2. Repeated Vaccination History and Preseason Antibody Titers
In the Time 1 (preseason baseline) analysis, HCP with one or more IIV3 vaccinations in the previous 4 seasons had similar adjusted GMTs against the 2010–11 A(H3N2) vaccine component, and all were modestly higher than the GMT of HCP with no vaccinations (Supplemental Table C). A similar pattern of results was noted among HCP who were subsequently vaccinated with 2010–11 IIV3 or remained unvaccinated (Figure 1 and Table 2). Across prior vaccine exposure subgroups, the percentage with elevated pre-season titers of ≥40 or >100 was low (≤26% and ≤9%, respectively).
Figure 1.
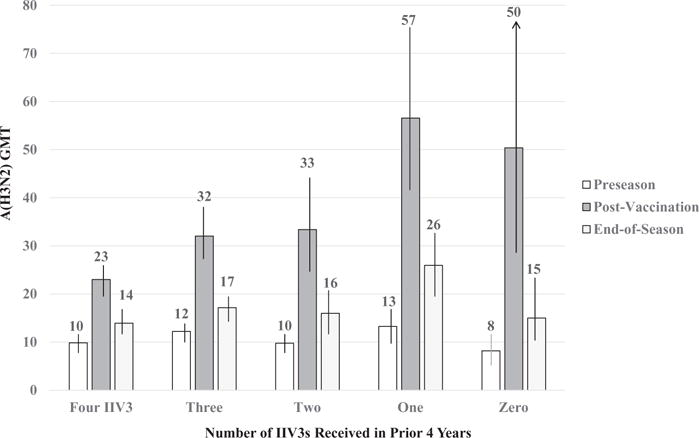
Estimated Geometric Mean Titer (GMT) for A/Perth/16/2009 (H3N2) and 95% Confidence Intervals at Preseason, Post 2010–11 Vaccination, and End-of-Season by the Number of Inactivated Influenza Vaccinations (IIV3s) during the Prior Four Seasons Received by Healthcare Personnel.
Table 2.
Serum Hemagglutination Inhibition Antibody (HI) Titers for A/Perth/16/2009 (H3N2) at Preseason, Post-Vaccination, and Post-Season by Number of Inactivated Influenza Vaccinations (IIV3s) during the Prior Four Seasons, including Estimated Geometric Mean Titer (GMT), Elevated Titers of ≥40 and >100, and Geometric Mean Fold Change Ratios (GMRs), among Healthcare Personnel Vaccinated and Unvaccinated with 2010–11IIV3.
| Preseason (Time 1)
|
Post-Vaccination (Time 2)
|
Post-Season (Time 3)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Sum of IIV3 2006–07 to 2009–10 |
N | GMT (95% CI) |
GMT >40% (95% CI) |
GMT >100% (95% CI) |
GMT (95% CI) |
GMR (95% CI) |
GMT>40% (95% CI) |
GMT >100% (95% CI) |
GMT (95% CI) |
GMR (95% CI) |
GMT >40%((95% CI) |
GMT >100% (95% CI) |
| 2010–11 Vaccinated | |||||||||||||
| 4 IIV3s | 227 | 10 (9–11) | 14 (9–18) | 3 (1–10) | 23 (20–27) | 2.3 (2.1–2.6) | 46 (40–51) | 11 (6–15) | 14 (12–16) | 0.6 (0.6–.7) | 23 (17–29) | 5 (2–8) | |
| 3 IIV3s | 153 | 12 (11–14) | 19 (13–25) | 2 (0–13) | 32 (28–38) | 2.6 (2.3–3.0) | 55 (48–61) | 8 (3–13) | 17 (15–20) | 0.5 (0.5–0.6) | 32 (25–39) | 2 (0–6) | |
| 2 IIV3s | 85 | 10 (8–12) | 17 (9–25) | 3 (0–7) | 33 (25–44) | 3.4 (2.8–4.3) | 56 (47–65) | 20 (13–26) | 16 (13–21) | 0.5 (0.4–0.6) | 34 (24–43) | 11 (5–16) | |
| 1 IIV3s | 86 | 13 (10–17) | 26 (18–33) | 9 (1–14) | 57 (42–76) | 4.3 (3.3–5.5) | 63 (54–72) | 32 (26–39) | 26 (20–32) | 0.5 (0.4–0.6) | 40 (31–50) | 19 (14–24) | |
| 0 IIV3s | 27 | 8 (6–11) | 12 (0–25) | 4 (0–6) | 50 (29–88) | 6.2 (3.4–11.3) | 69 (54–85) | 37 (26–48) | 15 (11–23) | 0.4 (0.2–0.5) | 25 (9–41) | 4 (0–14) | |
| 2010–11 Unvaccinated | |||||||||||||
| 4 IIV3s | 7 | 15 (8–28) | 26 (3–50) | 1 (0–15) | 16 (8–32) | 0.9 (0.9–0.9) | 15 (11–42) | 1 (0–15) | |||||
| 3 IIV3s | 15 | 12 (8–19) | 13 (0–29) | 7 (0–16) | 15 (10–25) | 0.8 (0.9–1.9) | 18 (1–36) | 1 (0–8) | |||||
| 2 IIV3s | 33 | 12 (9–16) | 18 (7–29) | 6(0–12) | 13 (10–19) | 0.9 (0.9–1.3) | 25 (13–37) | 3 (0–19) | |||||
| 1 IIV3s | 44 | 11 (8–14) | 18 (8–27) | 7 (0–12) | 10 (8–14) | 1.1 (0.9–1.4) | 14 (3–25) | 7 (1–12) | |||||
| 0 IIV3s | 110 | 7(6–7) | 7 (1–13) | 1 (0–6) | 8(7–9) | 1.2 (1.6–1.3) | 11 (5–18) | 3 (0–7) | |||||
Note: All estimates adjusted for sex, race (White), age (years) and age-squared, study site, work in a hospital setting or not, household size, and education (years). Among vaccinees, GMR at time 2 describes change in GMT post-vaccination (0r since Time 1); at Time 3, GMR describes change in GMT from post-vaccination to the end of season. Among unvaccinated HCP, GMR at Time 3 describes change in GMT since preseason (Time 1). GMRs were calculated using the log-transformed HI titers. GMTs were converted back to original GMT values. The GMR estimate was calculated by 2 to the power of mean difference estimate. The percentage with elevated tiers was estimated using generalized linear models with the same adjusted covariates. In addition, among vaccinees, estimates of post-vaccination elevated titers adjusted for preseason or baseline titers.
2.3. Repeated Vaccination History and GMR Serologic Response
Among HPC who received 2010–11 IIV3, there was a statistically significant interaction between number of prior vaccinations and GMR vaccine response, as indicated by the linear mixed effect model: F(4,567) = 9.97, p < .0005, partial η2 = .07. The adjusted GMRs were inversely associated with the number of prior vaccinations, increasing from 2.3 (95% CI = 2.1 – 2.6) among those with 4 prior vaccinations to 4.3 (95% CI = 3.3 – 5.5) among HCP with only 1 prior vaccination and to 6.2 (95% CI = 3.4 – 11.3) among those with no vaccinations (Table 2 and Supplemental Figure B). The same pattern of findings, including a significant interaction between number of prior vaccinations and GMR, applied to HCP with preseason GMT of < 40 (F[3,458] = 7.343, p < .0005, partial η2 = .05) and those with high baseline titers (≥40) (F[3,86] = 4.45, p = .006, partial η2 = .13) (Supplemental Table E).
We observed a curvilinear association between age and GMR (as indicated by statistically significant linear and quadratic terms for age), reflecting lower GMR among adults age 35–49 years old compared to younger or older HCP (Supplemental Table D). Nonetheless, we observed the same inverse association between GMR and the number of prior vaccinations (including significant interaction terms) in all three age groups (Supplemental Table D).
2.4. Repeated Vaccination History and Elevated Post-Vaccination Titers
The percentage of HCP with elevated titers post-vaccination increased as the number of prior vaccinations decreased (Figure 2). This was a significant trend for titers of ≥40 (Wald Chi-Square [4]=15.25, p = .004) and >100 (Wald Chi-Square [4]=52.77, p < .0005), with statistically significant differences in percentages between the extreme groups (Table 2). Less than half (46%) of HCP with 4 prior vaccinations had titers ≥40 compared to about two-thirds of those with 0 or 1 prior vaccinations (69% and 63%, respectively). About one-third of HCP with zero or one prior vaccination (37% and 32%, respectively) had titers of >100 compared to about one-in-ten of HCP with 3 or 4 prior vaccinations (8% and 11%, respectively). To illustrate, compared to HCP who had been consistently vaccinated for 4 prior years, the odds of having post-vaccination titers of ≥40 were 3-fold higher (adjusted odds ratio [AOR] = 3.2, 95% CI = 1.7 – 6.2) and the odds of having titers of >100 were over 6-fold higher (AOR = 6.8, 95% CI = 3.1 – 15.3) among HCP who had only been vaccinated once in recent years.
Figure 2.
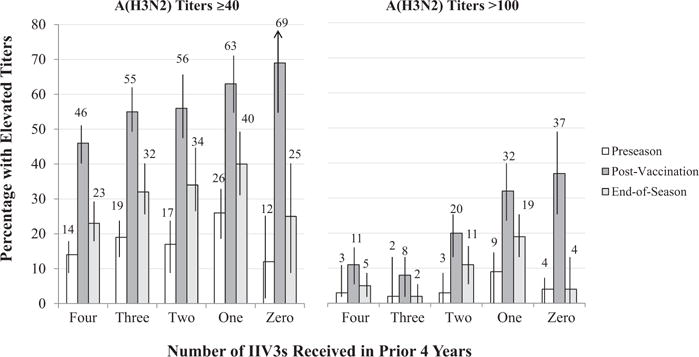
Percentage of Healthcare Personnel with Geometric Mean Titer (GMT) ≥40 and >100 for A/Perth/16/2009 (H3N2) (and 95% Confidence Intervals) at Preseason, Post 2010–11 Vaccination, and Post-Season by the Number of Inactivated Influenza Vaccinations (IIV3s) during the Prior Four Years.
In secondary analyses that excluded HCP with preseason GMT ≥40, we observed the same inverse association, with the percentage who achieved elevated titers declining as the number of prior vaccinations increased (Supplemental Table E). For example, among HCP with preseason GMT < 40, 57% (95% CI = 47–68%) of those with only 1 prior vaccination achieved post-vaccination GMT ≥40 compared to 37% (95% CI = 30–43%) of HCP with 4 prior vaccinations.
2.5. Associations with Antigenically Distinct IIV3s
We repeated the post-vaccination analyses looking at responses among individuals that received the two IIV3s containing the A/Wisconsin/67/2005-like H3N2 component (in 2006–07 and 2007–08) and the two IIV3s containing the A/Brisbane/10/2007-like H3N2 component (in 2008–09 and 2009–10) (Supplemental Table F). In linear mixed effects models with the number of prior vaccinations represented in these separate counts, an inverse association with GMR to the 2010–11 A/Perth/16/2009 (H3N2)-like vaccine component was clearest for the number of 2006–07 and 2007–08 vaccinations (F[2,567] = 14.57, p < .0005, partial η2 = .05), but was marginally significant for the number of 2008–09 and 2009–10 vaccinations (F[2,567] = 2.95, p = .056, partial η2 = .01). Similarly, a significant inverse association with the percentage with GMT ≥40 was noted for the number of 2006–07 and 2008–09 IIV3s (Wald Chi-Square [2] = 18.61, p < .0005), but not for the number of 2008–09 and 2009–10 IIV3s. In fact, we observed similar percentages of participants with GMT ≥40 (52–54%) for those who received neither, one, or both of the 2008–09 and 2009–10 IIV3s. However, a significant inverse association between GMT >100 and the number of prior IIV3s was noted for both the 2006–07 and 2008–09 IIV3s and the 2008–09 and 2009–10 IIV3s counts (Supplemental Table F).
2.6. Maintenance of Antibody Titers Post-Season
In the time 3 (post-season) analysis, HCP with only 1 prior vaccination in the previous 4 seasons maintained higher adjusted GMT for A(H3N2) and had a larger percentage with high titers (>40 and ≥100) post-season than HCP with 4 prior vaccinations (Table 2). This higher maintained response was not observed for participants with zero prior doses; indeed, similar to those with 3 or 4 prior vaccinations, significantly fewer participants with zero prior doses had GMT > 100 (4%) compared to those with 1 prior dose (19%) at time 3.
3. DISCUSSION
Serum HI titers for A(H3N2) following 2010–11 IIV3 vaccination varied significantly among our HCP participants depending on the number of previous IIV3 vaccinations. The magnitude of vaccine response (as indicated by GMR or GMT) declined with additional IIV3 received during the prior 4 years. This was noted for all age groups in our cohort of HCP aged 18 to 65 years old. With the exception of modestly lower baseline titers among HCP with no prior vaccinations, HCP with one or more prior vaccinations had similar preseason antibody titers for A/Perth/16/2009 (H3N2)-like virus. However, after vaccination, HCP with zero or one previous vaccinations had significantly higher GMR resulting in greater proportions with elevated titers (≥40 and >100) than frequent vaccinees.
Our findings point to an inverse exposure-response association between repeated vaccination and serologic response to an A(H3N2) component of IIV3. This is consistent with results from McLean et al.’s recent multi-season analysis that observed the highest protection against A(H3N2) illness among vaccinees who had been unvaccinated in the previous 5 years (VE = 65%, 95% CI = 36–80) and the lowest VE among those repeatedly vaccinated in 4 or 5 recent seasons (VE = 25%, 95% CI = 3–41%) [9]. Our findings are also consistent with reports from Ohmit et al.’s household cohort study which noted lower VE against A(H3N2) illness and lower preseason A(H3N2) antibody titers among those vaccinated in both the current and prior year compared to those vaccinated in the current season only [27].
It is unclear whether our serologic findings correspond with clinical protection. Indeed, high HI titers do not guarantee protection and most individuals with low titers will not become infected [28]. Nonetheless, HCP who received the 2010–11 IIV3 but had only one or no vaccinations in the prior 4 years were substantially more likely to enter influenza season with elevated titers (≥40 and >100). For example, one in three vaccinees (32%) with only one prior vaccination had A(H3N2) titers of >100, which may confer greater protection against influenza infection [25], compared to 1 in 10 consistent vaccinees (11%); this represents a 6-fold difference in odds of having very high post-vaccination titers.
One possible explanation for our HI findings and recently observed differences in VE is that repeated vaccination negatively interferes with immunogenicity [1,4,17,29,30]. In a modeling study, Smith et al. [1] predicted that revaccination is most likely to interfere with serologic response when vaccine components are unchanged or the antigenic distance between strains in consecutive vaccines is small. The magnitude of antigenic and genetic differences between vaccine strains (and more importantly, between the egg-adapted high growth reassortant viruses used in IIV3 manufacturing) sufficient to interfere with influenza vaccine response is unclear. We noted an inverse exposure-response association even though the IIV3 in 2010–11 was antigenically distinct from previous IIV3s and the A(H3N2) component had changed twice in the prior 4 years.
Although our study was unable to disentangle the potential influence of different combinations of prior vaccinations, our findings suggest that the prior IIV3 formulations may have differed in their relevance to subsequent serologic vaccine response. We observed a clearer and more consistent inverse association for GMR with the number of 2006–07 and 2007–08 IIV3s (which contained A/Wisconsin/67/2005-like H3N2) than with the two more recent IIV3s (which contained A/Brisbane/10/2007-like H3N2). Receipt of the 2006–07 and 2007–08 IIV3 may have offered little clinical protection since the vaccine’s match to circulating strains was low in both seasons. However, if the vaccines with A/Wisconsin/67/2005-like H3N2 had higher immunogenicity than those with A/Brisbane/10/2007-like H3N2 due to genetic changes in the egg-adapted high growth reassortant viruses used in IIV3 manufacturing [31], this might explain why the number of earlier (2006–07 and 2007–08) IIV3s may have played a greater role in interfering with GMR than the more recent IIV3s.1 Therefore, looking at the association between immunogenicity (0r VE) and the receipt of IIV3 during the previous season or summed across multiple seasons is likely a crude and incomplete way to study this effect, and more sophisticated approaches are needed that consider the period of time between vaccine exposures and take into account antigenic and genetic relatedness between vaccines.
One of the limitations of our study is that we could not quantify participants’ antibody titers against previous vaccine components and especially A/Wisconsin/67/2005-like H3N2, which was the IIV3 component in the 2006–08 and 2007–08 vaccines; these two IIV3s had the clearest association with vaccine response in our study. Future research is needed to map vaccination history to HI responses for a panel of A(H3N2) viruses included in previous IIV3s in order to examine how baseline antibody titers may mediate the impact of prior vaccination. Nonetheless, the fact that we observed an inverse association between repeated annual vaccination and GMR among HCP with elevated baseline antibody titers against the vaccine strain as well as those with low baseline titers suggests differences in humoral immunity cannot fully explain the effect; future studies will need to examine virus neutralization assays and consider the role of other mechanisms including differences in cell mediated immunity.
Another possible explanation for effect modification by vaccination history is confounding by indication, if individuals with poorer immune response are more likely to be repeated vaccinees [17]. Possible selection bias in this convenience sample is also a concern. However, these possible biases are unlikely to explain fully our findings, since we adjusted for age, study site, and other potential confounders in our models, and conducted additional sensitivity analyses to rule out the possible influence of demographic and health characteristics associated with repeated vaccination [19], cohort participation, exclusion from our multi-year analysis, and even baseline antibody titers. Nonetheless, residual and unmeasured confounding may have biased our estimates in unknown ways.
There are at least three other noteworthy study limitations. First, our study only examined HI response and could not consider other indicators of humoral or cell mediated immune response. For example, results from a microneutralization assay may have been more sensitive to A(H3N2) antibodies. Further studies examining B-cell responses are also needed, especially since one possible mechanism of the exposure-response effect could be that injected hemagglutinin protein in IIV3 could form antigen–antibody complexes with preexisting HI antibodies, which could reduce the amount of HI antigen available for stimulating B cells [29].
Second, our findings are limited to A(H3N2) viruses during the study year and prior 4 years. Although we previously reported lower HI response to the 2010–11 A(H1N1)pdm09 vaccine component among participants who received the monovalent A(H1N1)pdm09 vaccine in 2009 [4], we have not examined HI response to the B virus vaccine component. Nonetheless, concern about lower VE among repeated vaccinees has most consistently been noted for A(H3N2) viruses and less so for B viruses [6,27].
Third, we lacked information on past infections. Associations between vaccination history and immunogenicity are likely interconnected with differences in the history of natural infections with influenza viruses. An individual that foregoes vaccination would have more “opportunities” to be infected with wild influenza viruses that may provide a broader spectrum of subsequent immune protection. However, if such an effect existed in our sample, it was not reflected in differences in baseline antibodies, since HCP with different numbers of prior IIV3 vaccinations had largely similar baseline antibodies against A/Perth/16/2009 (H3N2)-like virus. Nonetheless, differences in influenza exposure history may have contributed to the age-related differences in GMR that we observed; indeed, some have proposed that antigenic seniority of A(H3N2) strains in circulation since 1968 may explain differences in immunogenicity among middle-aged adults [32,33].
Our findings should not be interpreted as indicating that HCP can forego annual vaccination or occasionally skip seasons. Annual vaccination for HCP continues to be a safe and effective preventive strategy that must be combined with other infection control practices to protect HCP and their patients [20,34]. Nonetheless, given the high morbidity and mortality associated with A(H3N2) virus illness [35] and the sub-optimal VE observed for IIV3 against A(H3N2) illness in recent years [5–7,36], research is needed to differentiate host, virus, and vaccine technology factors that may contribute to the imperfect immunogenicity and clinical protection afforded by this component of IIV3.
The exposure-response association we observed for repeated IIV3 vaccination with HI response supports the biological plausibility of a similar stepwise difference in VE against A(H3N2) illness reported by McLean et al. [9]. Taken together, this suggests that annual VE studies, which traditionally ignored vaccine history or considered the prior year only [5,6,37,38], need to expand their investigations to consider multiple years of vaccination history. Certainly, further observational studies are needed with other populations, including children and the elderly, and ideally a randomized clinical trial in a setting without universal vaccination which can vary multi-year vaccination regimens. Ultimately, better vaccines and vaccine strategies may be needed in order to optimize immunogenicity and VE for HCP and other populations that may receive repeated annual vaccinations for decades.
Supplementary Material
Acknowledgments
The authors thank the following persons at the Influenza Division, National Center for Immunization and Respiratory Diseases, CDC, for critical review of this manuscript: Eduardo Azziz-Baumgartner, MD; David Shay, MD, MPH; Joe Breese, MD; and Jerome Tokars, MD. We appreciate the research teams at Scott & White Healthcare, Kaiser Permanente Center for Health Research, and Abt Associates. We also thank the healthcare personnel in direct patient care at the study sites who volunteered to participate in this study.
Financial support: This work was supported by the CDC (contract 200-2010-F-33396 to Abt Associates Inc). This research was supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.10.119.
Footnotes
The authors would like to thank an anonymous peer reviewer for this observation.
4. Conflicts of Interest
M. G. has received research funding from MedImmune and Novartis, and A. N. has received research funding from GlaxoSmithKline. All other authors report no potential conflicts. Supported by the Centers for Disease Control and Prevention (contract 200-2010-F-33132 to Abt Associates Inc.).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC, Abt Associates, Inc, Kaiser Permanente Center for Health Research, or Scott & White Healthcare.
References
- 1.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America. 1999 Nov 23;96(24):14001–6. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Archives of internal medicine. 1999 Jan 25;159(2):182–8. doi: 10.1001/archinte.159.2.182. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013 Aug;57(3):474–6. doi: 10.1093/cid/cit255. [DOI] [PubMed] [Google Scholar]
- 4.Gaglani M, Spencer S, Ball S, Song J, Naleway A, Henkle E, et al. Antibody Response to Influenza A(H1N1)pdm09 Among Healthcare Personnel Receiving Trivalent Inactivated Vaccine: Effect of Prior Monovalent Inactivated Vaccine. The Journal of infectious diseases. 2014 Jun;209(11):1705–14. doi: 10.1093/infdis/jit825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014 Feb;58(3):319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson MG, Li DK, Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014 Feb;58(4):449–57. doi: 10.1093/cid/cit750. [DOI] [PubMed] [Google Scholar]
- 7.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. Influenza vaccine effectiveness in the community and the household. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013 May;56(10):1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PloS one. 2014;9(3):e92153. doi: 10.1371/journal.pone.0092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of Repeated Vaccination on Vaccine Effectiveness AgainstInfluenza A(H3N2) and B During 8 Seasons. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014 Sep 29; doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp GG, Knook DL. Quality and quantity of the humoral immune respose in healthy elderly and young subjects after annually repeated influenza vaccination. Journal of Infectious Diseases. 1999;179:31–6. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- 11.Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001 Sep 14;19(32):4610–7. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 12.Pyhala R, Kumpulainen V, Alanko S, Forsten T. HI antibody kinetics in adult volunteers immunized repeatedly with inactivated trivalent influenza vaccine in 1990–1992. Vaccine. 1994 Aug;12(10):947–52. doi: 10.1016/0264-410x(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 13.Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, et al. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine. 1996 Oct;14(14):1331–9. doi: 10.1016/s0264-410x(96)00058-8. [DOI] [PubMed] [Google Scholar]
- 14.Kunzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 1996 Aug;14(12):1108–10. doi: 10.1016/0264-410x(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 15.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997 Jul;15(10):1114–22. doi: 10.1016/s0264-410x(97)00003-0. [DOI] [PubMed] [Google Scholar]
- 16.Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010 May 21;28(23):3929–35. doi: 10.1016/j.vaccine.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 17.Huijskens E, Rossen J, Mulder P, van Beek R, van Vugt H, Verbakel J, et al. Immunogenicity, boostability, and sustainability of the immune response after vaccination against Influenza A virus (H1N1) 2009 in a healthy population. Clinical and vaccine immunology: CVI. 2011 Sep;18(9):1401–5. doi: 10.1128/CVI.05046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laguio-Vila MTM, Reynolds S, Spencer SS, et al. Comparison of Serum Hemagglutinin and Neuraminidase Inhibition Antibody Production Following 2010–2011. Trivalent Inactivated Influenza Vaccination among Healthcare Personnel. doi: 10.1093/ofid/ofu115. CID IN REVIEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MG, Gaglani MJ, Naleway A, Ball S, Henkle EM, Sokolow LZ, et al. The expected emotional benefits of influenza vaccination strongly affect pre-season intentions and subsequent vaccination among healthcare personnel. Vaccine. 2012 May 21;30(24):3557–65. doi: 10.1016/j.vaccine.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkle E, Irving SA, Naleway AL, Gaglani MJ, Ball S, Spencer S, et al. Comparison of laboratory-confirmed influenza and noninfluenza acute respiratory illness in healthcare personnel during the 2010–2011 influenza season. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2014 May;35(5):538–46. doi: 10.1086/675832. [DOI] [PubMed] [Google Scholar]
- 21.Thompson MG, Gaglani MJ, Naleway A, Thaker S, Ball S. Changes in Self-Rated Health and Subjective Social Status over Time in a Cohort of Healthcare Personnel. Journal of health psychology. 2013 May 16; doi: 10.1177/1359105313485486. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MG, Naleway A, Ball S, Henkle EM, Sokolow LZ, Williams J, et al. Subjective social status predicts wintertime febrile acute respiratory illness among women healthcare personnel. Health psychology: official journal of the Division of Health Psychology. American Psychological Association. 2014 Mar;33(3):282–91. doi: 10.1037/a0032764. [DOI] [PubMed] [Google Scholar]
- 23.Haaheim LR, Katz JM. Immune correlates of protection against influenza: challenges for licensure of seasonal and pandemic influenza vaccines, Miami, FL, USA, March 1–3, 2010. Influenza Other Respir Viruses. 2011 Jul;5(4):288–95. doi: 10.1111/j.1750-2659.2011.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Network WHOGIS. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva, Switzerland: WHO; 2011. Serological diagnosis of influenza by haemagglutination inhibition testing. [Google Scholar]
- 25.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC medical research methodology. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus research. 2004 Jul;103(1–2):125–32. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza Vaccine Effectiveness in Households with Children during the 2012–2013 Season: Assessments of Prior Vaccination and Serologic Susceptibility. The Journal of infectious diseases. 2015 Nov 21;211:1519–28. doi: 10.1093/infdis/jiu650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. The Journal of infectious diseases. 2011 Dec 15;204(12):1879–85. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PloS one. 2008;3(8):e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhr JW, Moller G. Regulatory effect of antibody on the immune response. Advances in immunology. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Zhou H, Jin H. The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine. 2010 May 28;28(24):4079–85. doi: 10.1016/j.vaccine.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 32.Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJ, et al. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS pathogens. 2012;8(7):e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucharski AJ, Gog JR. The role of social contacts and original antigenic sin in shaping the age pattern of immunity to seasonal influenza. PLoS computational biology. 2012;8(10):e1002741. doi: 10.1371/journal.pcbi.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014 Jan;58(1):50–7. doi: 10.1093/cid/cit580. [DOI] [PubMed] [Google Scholar]
- 35.Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morbidity and mortality weekly report. 2010 Aug 27;59(33):1057–62. [PubMed] [Google Scholar]
- 36.Interim adjusted estimates of seasonal influenza vaccine effectiveness–United States, February 2013. MMWR Morbidity and mortality weekly report. 2013 Feb 22;62(7):119–23. [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine. 2011 Aug 5;29(34):5785–92. doi: 10.1016/j.vaccine.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 38.McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, et al. Influenza Vaccine Effectiveness in the United States During 2012–2013: Variable Protection by Age and Virus Type. The Journal of infectious diseases. 2014 Nov 18; doi: 10.1093/infdis/jiu647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


