Summary
The re-emergence of Zika virus (ZIKV) and its suspected link with various disorders in newborn and adults led the World Health Organization to declare a global health emergency. In response, the stem cell field quickly established platforms for modeling ZIKV exposure using human pluripotent stem cell-derived neural progenitors and brain organoids, fetal tissues and animal models. These efforts provided significant insight into cellular targets, pathogenesis and underlying biological mechanisms of ZIKV infection as well as platforms for drug testing. Here we review the remarkable progress in stem cell-based ZIKV research and discuss current challenges and future opportunities.
Introduction
Stem cells are specialized cell types with hallmark properties of self-renewal and the ability to differentiate into other cell type(s) (Weissman, 2000). During development, somatic stem cells give rise to cellular building blocks for different tissues; in some organs, such as the brain, they continue to produce progeny to maintain tissue homeostasis and play important functions throughout life (Bond et al., 2015). Stem cells can also be derived in culture, for example, embryonic stem cells (ESCs) from inner cell mass of blastocysts and induced pluripotent stem cells (iPSCs) reprogrammed from somatic cells (Takahashi and Yamanaka, 2006). The last decade has witnessed tremendous progress in the stem cell field. It is now possible to derive iPSCs from patients with various disorders and differentiate them into various cell types in two-dimensional monolayer cultures (Tao and Zhang, 2016), or into three-dimensional organ-like tissues named organoids (Clevers, 2016). Stem cells have been used to investigate the basic biology of organ development, model human disorders, screen therapeutic compounds, and develop cell replacement strategies. Recent genome-editing technologies allow targeted activation or inactivation of specific genes or epigenetic modifications in stem cells to address their contributions to specific biological processes (Hsu et al., 2014). Technologies have also been developed to study somatic stem cells in vivo, in many cases at the single-cell level (Etzrodt et al., 2014). Cumulatively, key principles that have emerged from basic findings in the stem cell field have made major contributions to modern biology and medicine. Unexpectedly, the recent outbreak of Zika virus (ZIKV) in the Americas and its suspected link to microcephaly put stem cells at the forefront of an international research effort. Since the World Health Organization (WHO) declared a Public Health Emergency of International Concern on February 1 of 2016 (Heymann et al., 2016), the stem cell field has come together to develop versatile platforms for modeling ZIKV infection to understand its cellular targets, pathogenesis, and underlying mechanisms, and to test therapeutic interventions. Here we provide a general introduction of ZIKV and related viruses and summarize the remarkable progress made so far in this rapidly advancing area of research, with an emphasis on current challenges and future opportunities.
Zika Virus and Related Viral Pathogens
ZIKV is a member of the flavivirus genus in the Flaviviridae family of positive-strand RNA viruses (Lindenbach et al., 2007). Flavivirus is the largest genus of this family and contains many significant pathogens, such as dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus, and tick-borne encephalitis virus. Infection with flaviviruses causes a wide spectrum of diseases with clinical manifestations ranging from minor rashes to lethal hemorrhagic fever. ZIKV was first discovered in the blood of a rhesus macaque in the Ziika forest of Uganda in 1947 and re-isolated from Aedes mosquitoes from the same geographic area soon after (Dick et al., 1952). Even though ZIKV had subsequently spread to Asia Pacific, it only caused sporadic outbreaks and remained under the radar of clinicians, scientists, and the general public for over half a century until the recent outbreaks in South America. The re-emergence of ZIKV has become a global health concern because of its rapid spread and potentially severe pathogenic effects, especially during pregnancy (Heymann et al., 2016). Active local ZIKV transmission has been documented from the Americas to Asia. Like other flaviviruses, ZIKV is transmitted to humans and non-human primates via arthropod vectors, namely mosquitoes that bite vertebrate animals. Unlike any other known flavivirus, ZIKV can also be transmitted sexually in humans and passed from infected mothers to their fetuses though vertical transmission (D'Ortenzio et al., 2016), which can cause congenital defects, such as microcephaly, in a small percentage of infected babies (Mlakar et al., 2016; Rasmussen et al., 2016). While fetal microcephaly is perhaps the most dramatic and devastating consequence of ZIKV pathogenesis, it may only be the tip of the iceberg as the sequelae of ZIKV infection in babies born without overt microcephalic phenotypes are currently unknown. The Zika in Infants and Pregnancy (ZIP) study is underway to enroll as many as 10,000 pregnant women in their first trimester to determine if they become infected with ZIKV and, if so, to track the outcomes for both mother and child for at least one year after birth. ZIKV infection can cause Guillain-Barré Syndrome in a small percentage of infected adult humans and is linked to encephalitis, myelitis and conjunctivitis and uveitis associated with eye infection (Araujo et al., 2016; Cao-Lormeau et al., 2016; Parra et al., 2016).
ZIKV Life Cycle and Structures
Despite diversity in pathogenesis, members of the Flavivirus genus have highly similar genomic organization and share a common replication strategy. The ZIKV genome consists of a positive-sense, single-stranded RNA approximately 11,000 nucleotides in length, encoding a single open reading frame (ORF) flanked by 5′ and 3′ noncoding regions (NTRs; Figure 1A). The 5’ end of the genomic RNA is capped and both NTRs contain RNA secondary structures. In addition, the genomic RNA of ZIKV and several related positive-strand RNA viruses contain N6-adenosine methylation (m6A), which may regulate genome stability (Gokhale et al., 2016; Lichinchi et al., 2016).
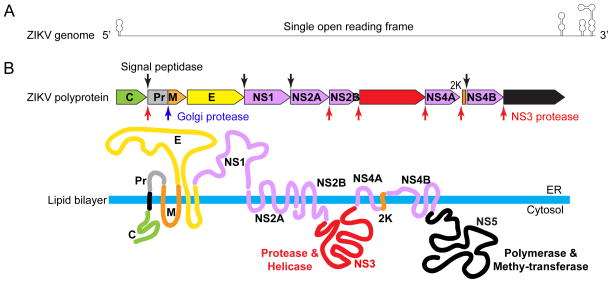
Figure 1. Zika virus genome and its encoded proteins.
(A) A diagram of a flavivirus genomic RNA, which is approximately 11kb in length and encodes a single open reading frame. Both 5’ and 3’ end of the genome contain non-translated, structured RNA sequences that are important for replication. (B) Processing strategy and protein products. The polyprotein is processed at various sites by host (red arrow heads) and viral (down black arrows) proteases. The protease responsible for cleaving the NS1/2A junction remains unknown at this time. The membrane topology and function of key ZIKV proteins are also illustrated. The topological arrangement of the structural proteins allows the lipid bilayer to wrap around the nucleocapsid structure and display the envelope proteins on the outer surface of the viral membrane. NS1 is a lumenal protein that functions during replication and can also be secreted to the outside of the cell to act as a pathogenesis factor.
The positive-sense nature of the flavivirus genome enables direct use of the RNA contained in the incoming virion as messenger RNA for protein translation, which produces viral proteins required to replicate more viral RNA. Translation of the long ORF produces a large polyprotein with over 3000 amino acid residues, which is then cleaved by both viral and host proteases to produce ten individual viral proteins (Figure 1B) (Lindenbach et al., 2007). The processing of the polyprotein occurs co- and post-translationally and the presence of signal peptides and transmembrane domains in individual proteins results in a distinctive topological arrangement of viral proteins on the endoplasmic reticulum (ER) membrane. There are three structural proteins (capsid, prM, and E), which make up the viral particles, and seven six nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), which participate in RNA replication (Figure 1B) (Lindenbach et al., 2007).
Replication of the viral genome (plus-strand RNA) occurs by RNA-directed RNA synthesis, which requires a negative-strand replicative intermediate (minus-strand RNA) (Garcia-Blanco et al., 2016). The negative-strand RNA is produced by NS5, a multi-functional enzyme that is, in part, an RNA-dependent RNA polymerase. The helicase activity encoded by NS3 likely also directly participates in RNA replication, while several other NS proteins contribute to the assembly and maintenance of replication complexes on intracellular membranes (Welsch et al., 2009). The formation of membrane-associated replication complexes, which may serve to increase local concentration of nonstructural proteins, is a common feature of positive-strand RNA replication.
The capsid protein (C) is a basic, dimeric protein that binds to the genome to form the nucleocapsid (Jones et al., 2003), which is further wrapped around by a lipid bilayer membrane derived from the host cell. The viral membrane, also called an envelope, is decorated by prM/M and E, two transmembrane viral glycoproteins that play major roles during the viral entry process (Rey et al., 1995). E is the receptor-binding protein as well as the fusion protein, while prM mainly functions as a chaperone of E protein to prevent premature fusion (Guirakhoo et al., 1992; Kuhn et al., 2002). Cleavage of prM by protease furin (Stadler et al., 1997), which generates M protein on the surface of more mature virions, occurs in the Golgi as the virion exits the cell via the secretory pathway (Kuhn et al., 2002).
Other Flaviviruses and Related Viral Pathogens
The similarities of flaviviruses at the molecular level belie the diverse range of diseases that they can cause in hosts. For example, although many flaviviruses cause encephalitis, ZIKV appears to be unique in its ability to cause fetal microcephaly. YFV bears the family name (flavus is Latin for “yellow” or “blonde”, for the jaundice that it causes) of the Flaviviridae and is the prototypic flavivirus. It was the first human pathogenic virus discovered and the first shown to be transmitted by an insect vector. A live attenuated YFV vaccine (YFV 17D) is available to protect humans and the experiences gained during YFV vaccine development may guide the current ZIKV vaccine effort. DENV infection causes dengue fever and more severe effects, dengue hemorrhagic fever and dengue shock syndrome. Serial infection by more than one of the four serotypes of DENV is associated with the more severe diseases. As such, travel-related re-emergence of DENV of a different serotype in historically endemic regions poses a potential health threat. A likely mechanism is antibody-dependent enhancement of infection when non-neutralizing antibodies bind dengue virions and facilitate entry into immune cells via the Fc receptors (Halstead and O'Rourke, 1977), although how broadly applicable is this mechanism applies to all flaviviruses is unclear. A potential DENV-ZIKV interaction is a concern because of the significant overlap between the major geographic areas affected by the two viruses. In vitro studies so far indicate that DENV differs from ZIKV in its inability to affect neural progenitor cells (Garcez et al., 2016; Zhang et al., 2016a) and there is no strong evidence of anti-dengue antibody enhancing ZIKV infection occurring in vivo. A recent study did show that anti-ZIKV antibodies may enhance DENV infection in mice, but the reverse did not occur in the same experimental setting (Stettler et al., 2016). Like ZIKV, WNV can invade the central nervous system (CNS) and lead to inflammation and neuronal degeneration. WNV, however, generally afflicts adults more often than children, as disease manifestation is more prevalent in adults, particularly the elderly. The recent finding of cognitive sequelae in mice after surviving acute WNV neuroinvasive infection (Vasek et al., 2016) raises concerns of similar consequences in apparently healthy babies born to ZIKV-infected mothers.
Teratogenic infectious agents that are vertically transmitted from mother to infant during pregnancy, childbirth, or breastfeeding have been traditionally classified as TORCH pathogens. TORCH stands for T: Toxoplasmosis; O: Other (syphilis, varicella-zoster, parvovirus B19, HIV, ect.); R: Rubella virus (German Measles); C: Cytomegalovirus; and H: Herpes simplex virus (Adams Waldorf and McAdams, 2013). These pathogens can cross the placenta and cause congenital defects, including microcephaly, intellectual disabilities, and other organ deficits. ZIKV now joins the list of viral TORCH pathogens (Coyne and Lazear, 2016) and our knowledge of these pathogens can inform current research concerning ZIKV fetal pathogenesis and strategies to fight the pandemic viral outbreak.
Modeling of ZIKV Exposure
The WHO declared a global health emergency for ZIKV, largely due to a suspected link between the ZIKV outbreak and the increase of incidence of microcephaly observed in Brazil (Heymann et al., 2016). ZIKV has been detected in almost all types of body fluids, including urine (Gourinat et al., 2015), saliva (Barzon et al., 2016), semen (Mansuy et al., 2016), and tears (Miner et al., 2016b) (Figure 2A). From the public health point of view, one of the most urgent questions at the time was whether ZIKV infection in fact causes microcephaly as over 80% of infections in humans are asymptomatic. ZIKV was found in microcephalic brains of fetuses from women infected with ZIKV during pregnancy (Driggers et al., 2016; Mlakar et al., 2016) (Figure 2A), yet clinical evidence alone does not prove causality and there were many plausible alternative hypotheses. For example, ZIKV may affect brain development indirectly via inflammation induced by infection of supporting cells or even cell types outside of the brain. To address this issue, multiple laboratories used stem cell-based cultures to identify tropism and to model pathogenesis of ZIKV.
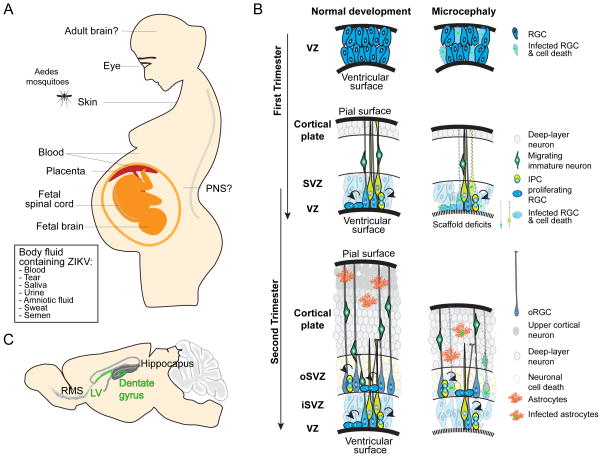
Figure 2. ZIKV infection of human body and pathological impact on cortical neurogenesis.
(A) An illustration of tissues and organs that are known to be infected by ZIKV in fetal and adult humans. The list of bodily fluids known to contain ZIKV is also shown. (B) Models illustrating normal human cortical development and impact of ZIKV infection. VZ: ventricular zone; SVZ: subventricular zone; iSVZ: inner ventricular zone; oSVZ: outer subventricular zone; RGC: radial glia cells; IPC: intermediate progenitor cell; oRGC: outer radial glia cell. (C) ZIKV also infects neural progenitors during adult neurogenesis in rodents at the lateral ventricles (LV) and dentate gyrus of the hippocampus. RMS: rostral migratory stream.
ZIKV Infection of the Central Nervous System
Some babies born from ZIKV-infected mothers exhibited thinner cortical layers, the hallmark of microcephaly (Mlakar et al., 2016; Rasmussen et al., 2016). In an attempt to make the connection between ZIKV exposure and microcephaly, stem cells were used to address the key question of whether ZIKV directly infects human cortical neural progenitors, cells that give rise to building blocks of human cortex (Tang et al., 2016). Indeed, ZIKV exposure readily infects forebrain-specific cortical neural progenitors derived from multiple lines of human iPSCs in monolayer cultures. At the cellular level, productive infection of neural progenitors by ZIKV affects cell cycle progression and increases cell death. At the molecular level, ZIKV infection leads to dysregulation of many signaling pathways, including downregulation of cell cycle genes and upregulation of apoptosis genes (Tang et al., 2016). The findings from this straightforward experiment provided the evidence of biological plausibility, which helped prompt the United States Center for Disease and Control (CDC) to declare that ZIKV causes microcephaly (Rasmussen et al., 2016). The decision by the CDC was unprecedented at such an early stage of the epidemic and in the absence of confirmative clinical evidence from large cohorts, which only came several months later (de Araujo et al., 2016). Many independent studies around the same time showed similar results of ZIKV infection and its pathological impact using both human iPSC- and fetal brain tissue-derived neural progenitors in monolayer and 3D neurosphere cultures (Brault et al., 2016; Cugola et al., 2016; Garcez et al., 2016; Liang et al., 2016; Simonin et al., 2016; Zhang et al., 2016a). Initial studies used the prototype ZIKV strain MR766 of African origin and the basic findings have since been confirmed with recent clinic isolates, including the Cambodian strain FSS13025 of Asian origin (Zhang et al., 2016a), and strains isolated from Brazil (Cugola et al., 2016), Mexico (ZIKV MEX_I_7) (Barrows et al., 2016), and Puerto Rico (PRVABC59) (Xu et al., 2016), all of which are originally of Asian origin. The capacity of different ZIKV strains to cause various human disorders is still not clear and remains an interesting question. In cellular models, some quantitative differences have been observed in terms of neural progenitor proliferation, cell death and gene expression (Cugola et al., 2016; Simonin et al., 2016; Zhang et al., 2016a). It should be pointed out that any strain-specific phenotypes in experimental models need to be considered in the context of passage histories of the strains. For example, the prototype MR766 strain has been passaged extensively in suckling mouse brain and may have gained adaptive properties accordingly, whereas the Cambodian strain and those from the recent outbreak in Americas have only been passaged a limited number of times in the laboratory.
During mammalian embryonic cortical development, radial glia cells are neural stem cells that generate neurons of different cortical layers either directly or via intermediate progenitors (Figure 2B) (Gotz and Huttner, 2005). The stereotypic organization of the developing cortex, including different progenitor layers and neuronal layers cannot be adequately modeled in monolayer or neurosphere cultures. In contrast, brain organoids from iPSCs can mimic endogenous human brain development to a remarkable degree, with similar structural organization, properties and molecular signatures (Kadoshima et al., 2013; Lancaster et al., 2013; Mariani et al., 2015; Pasca et al., 2015; Qian et al., 2016). This 3D model system allows direct investigation of effects of ZIKV infection on cortical layer thickness, to address additional questions related to cell type specificity in a complex tissue, and to investigate human-specific and developmental stage-specific features. Several groups have shown that ZIKV infection reduced growth of cerebral organoids generated using different methodologies (Cugola et al., 2016; Dang et al., 2016; Garcez et al., 2016; Qian et al., 2016). In a forebrain-specific human brain organoid model, transient exposure of ZIKV results in preferential infection of radial glia cells as compared to intermediate progenitors or immature neurons, leading to decreased proliferation and increased cell death (Figure 2B) (Qian et al., 2016). Interestingly, cell death was observed in infected neural progenitors and uninfected neurons, suggesting both cell autonomous and non-cell autonomous effects. Unlike rodents, the embryonic human cerebral cortex contains an abundant population of specialized outer radial glia cells (oRGCs) in the outer subventricular zone (oSVZ) (Figure 2B), the cell population considered pivotal in the evolutionary increase in the size and complexity of the human cortex (Lui et al., 2011). The oRGCs in forebrain organoids are also infected by ZIKV (Qian et al., 2016). Cerebral organoids with a mixture of cell types of different brain regions (Lancaster et al., 2013) have been used to compare the impact of different viruses or viral strains. Neither DENV (Garcez et al., 2016) nor YFV (Cugola et al., 2016), both of which have not been linked to microcephaly in humans, reduces the size of human brain organoids. Interestingly, the Brazilian ZIKV stain seems to have a larger effect on reducing neuronal layer thickness in human cerebral organoids compared to the African strain, and does not affect cerebral organoids derived from non-human primate (chimpanzee) iPSCs, suggesting both strain- and species-specific impact of ZIKV (Cugola et al., 2016).
These human organoid studies were accompanied by other important advances from the stem cell field using fetal tissues and animal models. First, direct infection of fetal human tissue in acute slices showed preferential infection of neuroepithelial stem cells and radial glia cells, as compared to immature neurons (Onorati et al., 2016). Notably, ZIKV infection caused scaffold disorganization of radial glia cells, which was also observed in a clinical sample of a ZIKV-infected microcephalic fetus (Mlakar et al., 2016) (Figure 2B). Second, several laboratories have independently performed in vivo animal modeling, including in both mouse and nonhuman primates, using multiple routes of ZIKV delivery, including direct injection into the embryonic lateral ventricles (Li et al., 2016a; Wu et al., 2016) or early postnatal brain (Shao et al., 2016), as well as subcutaneous (Adams Waldorf et al., 2016; Miner et al., 2016a), intraperitoneal (Wu et al., 2016), intravenous (Cugola et al., 2016), or vaginal tract injections (Yockey et al., 2016). Studies in mice revealed ZIKV infection of embryonic radial glia cells, increased cell death and thinner cortical layers resembling microcephaly (Cugola et al., 2016; Li et al., 2016a; Nguyen et al., 2016; Shao et al., 2016; Wu et al., 2016; Yockey et al., 2016). An important caveat with this approach, which we discuss in more detail below, is that most of these mouse studies relied on models with a deficient in interferon responses to avoid encountering an innate immune response that would prevent robust infection. ZIKV exposure also leads to a microcephaly-like phenotype in chick embryos, including decreased telencephalon and brain stem volume (Goodfellow et al., 2016). In addition, ZIKV delivered via retro-orbital injection to model the blood-borne route of arbovirus transmission infected neural progenitors in the lateral ventricles and the dentate gyrus of adult mice defective in interferon responses (Figure 2C) (Li et al., 2016b), leading to increased cell death and reduced proliferation, an effect resembling embryonic ZIKV infection.
Together, these studies employing multiple models, including 2D neural stem cell cultures, 3D neurospheres and human brain organoids, acute human fetal tissue cultures, animal models, and clinical fetal samples, all consistently point to one conclusion that neural progenitors in the mammalian brain are particularly vulnerable to ZIKV infection with significant pathological impact. These findings not only can explain, at least in part, how ZIKV infection during pregnancy leads to microcephaly, but also provide experimental models to address underlying cellular and molecular mechanisms and to test therapeutic interventions.
In addition to neural stem cells, studies using human brain organoids and animal models have revealed ZIKV infection of other cell types in the CNS. A study over four decades ago found that injection of ZIKV into the adult mouse brain leads to infection of astrocytes and neurons (Bell et al., 1971). Recent studies also found that ZIKV readily infects human astrocytes in monolayer cultures and in forebrain organoids (Qian et al., 2016; Xu et al., 2016). Case reports from South America indicate that babies born to ZIKV-infected mothers display an array of eye malformations (Miranda et al., 2016). While modeling of ZIKV exposure in the developing eye using stem cell-derived human retinal organoids has not yet been reported, one study using adult lfnar1−/− or wildtype neonatal mice has shown ZIKV infection of multiple cell types in the retina, including ganglion cells and bipolar cells (Miner et al., 2016b). Defects in spinal cord have also been reported in a human fetus with microcephaly (Mlakar et al., 2016) and in ZIKV-infected mouse models (Lazear et al., 2016) (Figure 2A). In culture, ZIKV infects fetal human spinal cord-derived neural progenitors, leading to increased cell death (Onorati et al., 2016).
ZIKV Infection of the Peripheral Nervous System
In addition to microcephaly, other pronounced symptoms of ZIKV infection include retro-orbital and abdominal pain and diarrhea (Araujo et al., 2016), which are associated with the peripheral nervous system (PNS) and, more specifically, sensory and enteric neurons. It has now been established that ZIKV infection can cause Guillain-Barré syndrome, a PNS disorder in adults (Parra et al., 2016). It is not clear whether ZIKV infects PNS neurons or glia cells directly or whether it affects them indirectly via inflammation induced by ZIKV infection outside of the nervous system. In one recent study, ZIKV was shown to productively infect human ESC-derived cranial neural crest cells, which normally generate most cranial bones and exert paracrine effects on the developing brain (Bayless et al., 2016). ZIKV infection leads to secretion of cytokines, such as leukemia inhibitory factor (LIF) and vascular endothelial growth factor (VEGF), which in turn promote death and aberrant differentiation of CNS neural progenitors in co-culture. These results highlight the non-cell autonomous role of ZIKV infection in the nervous system. Protocols have been established to differentiate human ESCs/iPSCs into other neural crest stem cells, such as those that give rise to sensory neurons and Schwann cells (Lee et al., 2010; Lee et al., 2007), and future studies can explore whether ZIKV also infects these lineages and potential pathological consequences.
ZIKV Infection Beyond the Nervous System
As an arbovirus, ZIKV is known to infect cells of the skin and blood systems, such as dermal fibroblasts, keratinocytes and immature dendritic cells (Figure 2A) (Hamel et al., 2015). Whether ZIKV infects epidermal stem cells or haematopoietic stem cells remains to be determined. To address how ZIKV may bypass the placenta barrier to vertically transmit from mothers to fetuses, studies using mid- and late-gestation placentas and explants from first-trimester chorionic villi found ZIKV infection of many cell types, including placental macrophages, called Hofbauer cells, trophoblasts, umbilical cord mesenchymal stem cells and endothelial cells (Bayer et al., 2016; El Costa et al., 2016; Quicke et al., 2016; Tabata et al., 2016). Together, these results suggest multiple routes of vertical transmission. Infection of vascular endothelial cells by ZIKV also has implications for how ZIKV may cross the brain-blood-barrier (BBB) to enter the postnatal CNS (Liu et al., 2016). Similar to clinical findings that ZIKV can be detected in semen for over 6 months after the initial infection (Nicastri et al., 2016), a recent study has shown persistence of ZIKV, but not DENV, in the testis and epididymis of adult male mice (Nicastri et al., 2016). ZIKV preferentially infects spermatogonia, primary spermatocytes, and Sertoli cells in the testis, resulting in cell death and destruction of the seminiferous tubules. In vitro studies have further shown that many cells lines derived from humans, primates, rodents, pigs, rabbit hamsters, chickens and mosquitoes, support productive ZIKV replication in vitro (Chan et al., 2016). These studies paved the way to use genetically tractable human cell types derived from iPSCs to investigate the underlying pathophysiology and molecular mechanisms of ZIKV infection.
Mechanisms underlying ZIKV Infection and Pathogenesis
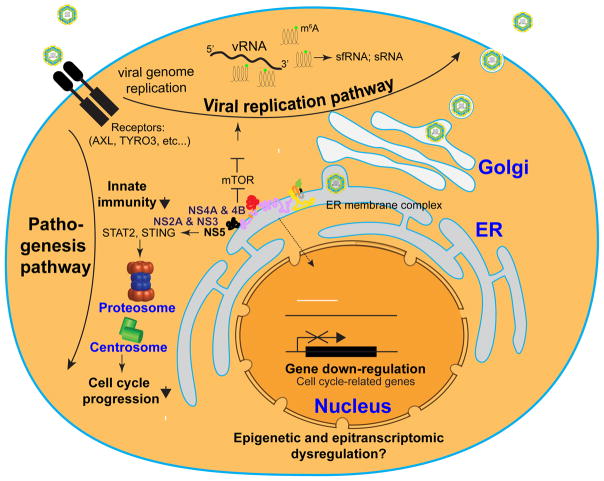
Although ZIKV has only begun to attract widespread attention this year since the WHO declared it to be a global health emergency, remarkable progress has already been made in understanding mechanisms underlying ZIKV infection and pathogenesis. At the molecular level, studies in human fibroblasts have implicated several cell surface adhesion factors, including TAM receptor proteins (AXL and Tyro3) and T-cell immunoglobulin and mucin domain 1 (TIM-1), as candidates to mediate ZIKV entrance to their targets (Hamel et al., 2015) (Figure 3). Notably, AXL is highly expressed in radial glia cells, outer radial glia cells, astrocytes, and microglia in the developing human brain (Nowakowski et al., 2016), neural progenitors in the adult mouse dentate gyrus (Shin et al., 2015), and trophoblast progenitors in placenta (Tabata et al., 2016), which correlates with the high efficiency of ZIKV infection in these cell types. However, deletion of AXL does not affect ZIKV infection in the mouse eye and brain (Miner et al., 2016b), nor human neural progenitors in monolayer or organoid cultures (Wells et al. this issue). Definitive identification of bona fide cellular receptor(s) for ZIKV or related flaviviruses remains a coveted prize for the field. Once ZIKV enters the cell, it hijacks cellular pathways for its replication and assembly, which interferes with cell proliferation and survival in neural progenitors (Figure 3). In some cell types, ZIKV infection also leads to upregulation and secretion of cytokines, which in turn exert a non-cell autonomous effect in neighboring cells.
Figure 3. A model of molecular mechanisms underlying ZIKV infection and pathogenesis.
Upon infection, ZIKV interacts with the host machinery to activate the viral replication pathway, which interferes with normal cell physiology. sfRNA: subgenomic flavivirus RNA; sRNA: viral small RNA.
Interferon Response, Autophagy and Cell Death Pathways
ZIKV infection is sensed by the innate immune receptor Toll-like-Receptor 3 (TLR3) in human fibroblasts, leading to Type I and Type II interferon (IFN) responses (Hamel et al., 2015). ZIKV also activates TLR3 in human brain organoids and TLR3 inhibition reduces ZIKV-induced dysregulation of neurogenesis and cell death (Dang et al., 2016). For effective replication in mammals, ZIKV and other flaviviruses must overcome innate immunity via host Type I IFN response. Indeed, ZIKV is capable of blocking Type I IFN receptor signaling in primate and human cells, but not in mouse cells (Grant et al., 2016). On the other hand, boosting IFN-stimulated genes, including small membrane-associated interferon-inducible transmembrane proteins (IFITMs) can inhibit ZIKV replication (Savidis et al., 2016b). The inability to block IFN responses in mice represents a barrier for modeling ZIKV exposure and most current studies have relied on IFN-deficient mouse models. Mechanistically, DENV and ZIKV infections lead to degradation of interferon-regulated transcriptional activator STAT2, thus inhibiting induction of interferon-stimulated genes in human but not mouse cells (Figure 3) (Grant et al., 2016). In addition, DENV infection also leads to degradation of Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173), in human, but not mouse cells (Aguirre et al., 2012). STING/TMEM173 is an adaptor protein that activates downstream transcriptional factors STAT6 and IRF3 via TANK-binding kinase 1 (TBK1) (Burdette and Vance, 2013). TBK1 is a multi-functional protein involved in innate immunity, cell proliferation, and apoptosis (Clement et al., 2008; Helgason et al., 2013; Pillai et al., 2015). Upon ZIKV infection, TBK1 relocates from centrosome to mitochondria, and pharmacological inhibition of TBK1 leads to supernumerary centrosomes and mitotic deficits of human neural progenitors, resembling ZIKV infection (Onorati et al., 2016). Therefore, TBK1 may serve as a major player in the cross-talk between innate immunity and neurogenesis pathways during ZIKV pathogenesis.
One of the pathways activated by ZIKV infection is autophagy, an intracellular degradation process that delivers cytoplasmic constituents to the lysosome (Ohsumi, 2014) (Figure 3). ZIKV infection of human skin fibroblasts leads to the formation of autophagosomes (Hamel et al., 2015). Multiple positive-strand RNA viruses, such as DENV, HCV, and ZIKV, modulate cellular autophagy to benefit their replication in host cells (Hamel et al., 2015; Heaton and Randall, 2010; Sir et al., 2012). Inhibition of autophagy with pharmacological or genetic approaches attenuates viral replication (Hamel et al., 2015; Liang et al., 2016). Transcriptional profiling of ZIKV-infected human neural progenitors has revealed dysregulation of autophagy related genes (ATGs), including upregulation of ATG2A, ATG4A, ATG16L1, STK38L, RAB7A, ULK1 and downregulation of LAMP2A and CASP2 (Tang et al., 2016). ZIKV infection of human fetal neural progenitors causes inhibition of the Akt-mTOR pathway, leading to defective neurogenesis and aberrant activation of autophagy (Liang et al., 2016).
Cell death, mainly through caspase-3 activation and apoptosis, has been frequently observed following ZIKV infection of neural progenitors in vitro, in animal models, and in clinical samples of ZIKV-infected fetuses (Cugola et al., 2016; Dang et al., 2016; Li et al., 2016a; Onorati et al., 2016; Qian et al., 2016; Shao et al., 2016; Tang et al., 2016; Wu et al., 2016; Yockey et al., 2016; Zhang et al., 2016a) (Figure 3). ZIKV infection increases total levels of tumor suppressor protein p53, its nuclear accumulation, and Ser15 phosphorylation (Ghouzzi et al., 2016) and p53 inhibitors block the apoptosis induced by ZIKV in human neural progenitors (Zhang et al., 2016a). Together these findings show that ZIKV interferes with key survival and homeostasis mechanisms, which helps explain the pleiotropic and destructive consequences of infection in cells and tissues.
Transcriptional, Epitranscriptomic, and Epigenetic Dysregulation
One of the most striking molecular phenotypes of ZIKV infection in neural progenitors is dysregulation of gene transcription (Figure 3). Transcriptome profiling of ZIKV infected human neural progenitors, brain organoids and mouse cortical tissues have revealed many dysregulated pathways related to cell cycle, transcription, metabolism, cell death, DNA replication and repair, and viral responses (Dang et al., 2016; Li et al., 2016a; Tang et al., 2016; Zhang et al., 2016a). The signature of transcriptional dysregulation is distinct from that induced by DENV infection in neural progenitors and varies depending on ZIKV strains (Zhang et al., 2016a). Over the past decade, molecular genetic studies have identified many autosomal recessive primary microcephaly genes (MCPHs), many of which encode proteins localized at the centrosome, an organelle that plays an important role in cell cycle progression (Gilmore and Walsh, 2013). Notably, upon ZIKV infection, human cortical neural progenitors in culture and embryonic mouse cortex in vivo downregulate many of the currently known microcephaly-associated genes, including ASPM/MCPH5, CASC5/MCPH4, CENPF, Microcephalin/MCPH1, RBBP8, STIL/MCPH5, and TBR2 (Li et al., 2016a; Tang et al., 2016). Centrosomal abnormalities have also been observed in human neural progenitors infected with ZIKV (Onorati et al., 2016).
ZIKV infection may also lead to epigenetic and epitranscriptomic dysregulation of the host cells (Figure 3), processes that play critical roles in regulating stem cells and neurogenesis (Yao et al., 2016). In human cortical neural progenitors, ZIKV infection upregulates all three DNA oxidases TET1-3 and downregulates DNA methyltransferase DNMT1 and DNMT3A (Tang et al., 2016), raising the possibility of genome-wide demethylation. How the DNA methylome, including both 5-methylcytosine methylation and 5-hydroxylmethylation, is altered by ZIKV infection in host cells remains to be characterized by genome-wide analysis (Shin et al., 2014). Among the many mRNA modifications identified so far, m6A is the most abundant and affects RNA structure and function, including mRNA decay, microRNA production and translational control (Yue et al., 2015). Two recent studies revealed that ZIKV RNA is modified at m6A and 2′-O-methylated nucleosides and depletion of m6A methyltransferases or m6A demethylases, respectively, increases or decreases infectious production of ZIKV and HCV (Gokhale et al., 2016; Lichinchi et al., 2016). Probably even more interesting is the finding that m6A mRNA profiles in host human HEK293 cells are also altered by ZIKV infection (Lichinchi et al., 2016). Future studies will address whether similar epitranscriptomic dysregulation occurs in neural progenitors and other relevant cell types and how such dysregulation may contribute to ZIKV-related pathophysiology.
ZIKV-encoded Viral Proteins and Noncoding RNAs
A central question to be addressed is how ZIKV interacts with the host machinery to replicate itself and affect host cellular behavior. While studies are underway to address how each of the individual ZIKV viral proteins may affect cellular properties (Figure 1), we can learn from previous studies of other flaviviruses. For example, DENV-NS4A upregulates autophagy in epithelial cells (McLean et al., 2011). Similarly, expression of ZIKV-NS4A and/or ZIKV-NS4B inhibits AKT phosphorylation, reduces mTOR signaling, and induces autophagy in human neural progenitors (Figure 3) (Liang et al., 2016). How ZIKV-NS4A/NS4B leads to inhibition of AKT phosphorylation remains to be determined. Given the ER localization of ZIKV-NS4A and NS4B, one possibility is that ER stress leads to inhibition of ATK phosphorylation. It will be also interesting to test whether inhibition of autophagy can rescue neural progenitor proliferation deficits induced by ZIKV infection and ZIKV-NS4A/4B expression. Recently, ZIKV-NS5 has been shown to interact with STAT2 and target it for proteasome degradation in human HEK293 cells, but not in mouse fibroblasts, highlighting a species-specific mechanism (Kumar et al., 2016). In addition, DENV-NS3B-NS3 protease complex cleaves STING in human, but not mouse, cells to inhibit innate immunity responses (Aguirre et al., 2012).
Flaviviruses, including ZIKV, contain 3’- and 5’-NTRs with secondary structures (Figure 1A) that are important for viral translation, transcription and replication regulation (Bavia et al., 2016). Flaviviruses also produce subgenomic flavivirus RNAs (sfRNAs) (Akiyama et al., 2016) and viral small RNAs (sRNAs), including miRNAs (Figure 3). How these noncoding RNAs are produced, regulated and are involved in viral infection and host cell responses is not well understood. Previous studies of WNV and DENV have shown that 3’-NTR interacts with two cellular RNA-binding proteins, TIA-1 and TIAR (Emara and Brinton, 2007). TIA-1 and TIAR are essential for the formation of stress granules and are concentrated at the site of viral RNA replication. Functionally, deletion of these genes attenuates viral replication. Additional stress granule-related proteins that interact with the sfRNA of DENV include G3BP1, G3BP2, and CAPRIN1 (Bidet et al., 2014). Finally, the sfRNA from a DENV type 2 strain has been shown to interact with TRIM25 in a sequence-specific manner to suppress innate immunity and potentially facilitate virus transmission (Manokaran et al., 2015). Given similarities of secondary structures of 3’-NTRs among flaviviruses, many of these proteins may play a similar role in ZIKV replication. In addition to interacting with essential host factors, these sfRNAs and sRNA may hijack the cellular machinery for small RNA processing and therefore affect the endogenous noncoding RNAs, such as miRNAs, which in turn may affect cellular host properties (Schnettler et al., 2012). Identification of ZIKV-derived small RNAs in physiologically relevant cell types and investigation of their functions in both viral infection and host responses will be an interesting topic for future studies.
Opportunities and Challenges for ZIKV Research using Stem Cells
Remarkable progress has been made in a short period of time for stem cell-based Zika research. While we are only scratching the surface, these initial studies have built a foundation for many lines of research and many questions to be answered in the near future.
Pathophysiology
In addition to congenital defects and adult neurological pathology, we are learning more about other abnormalities upon ZIKV infection. Are other organs and cell types affected by ZIKV (Figure 2A)? In addition, a more complete picture of potential pathology and developmental abnormalities caused by ZIKV infection during pregnancy is emerging from clinical observations; it is clear that microcephaly is just one feature of this congenital malformation disorder (Melo et al., 2016; Moura da Silva et al., 2016). ZIKV infection during embryonic development can have a long-lasting impact beyond neurogenesis, due to direct infection by ZIKV and associated inflammation. Therefore, it is important to look at effects on later stages of neuronal development, including neuronal migration, axon guidance, synapse formation and circuitry formation. Such modeling will become more important as we begin to perform longitudinal studies of infants born from women infected with ZIKV during pregnancy, with or without microcephaly, and new clinical information of neurological outcomes becomes available.
Aided by clinical observations, stem cell-based modeling can address many basic questions to help establish causality, as in the case of ZIKV-induced microcephaly, and furthermore, provides a platform to investigate underlying mechanisms and intervention strategies. For example, using iPSC lines derived from different human populations can test the degree to which population-specific features and environmental factors determine vulnerability. The genetically tractable human stem cell system also allows quantitative comparison of the impact of different ZIKV strains on different cell types. So far, studies in the CNS have been mostly focused on the cerebral cortex. It is not clear how other areas could be affected. Protocols have been established to differentiate human pluripotent stem cells into neural lineages corresponding to different CNS regions in both monolayer cultures (Tao and Zhang, 2016) and as brain organoids, including forebrain, midbrain, hypothalamus, retina, and heterogeneous organoids of multiple brain regions (Kelava and Lancaster, 2016). On the other hand, we need to be aware of limitations of current stem cell-based models, such as a lack of any immune cells or blood vessels for natural viral delivery. Protocols have been developed to derive hematopoietic lineages (Rowe et al., 2016), endothelial cells (Lippmann et al., 2012), and microglia (Muffat et al., 2016), from human pluripotent stem cells. Future models with co-cultures of multiple cell populations in monolayer cultures and organoids may serve as better models for ZIKV research. All of these in vitro stem cell-based models should be complemented with animal studies of different species, including nonhuman primates (Adams Waldorf et al., 2016; Osuna et al., 2016). In addition, the physiological relevance and validity of findings in these models needs to be confirmed with clinical samples of ZIKV-infected fetal or adult human tissues (Onorati et al., 2016).
Cellular and Molecular Mechanisms
There is a wealth of information about viral replication and interaction with the host for other flaviviruses and viral TORCH pathogens, which will facilitate our current effort to understand mechanisms of ZIKV infection. In addition, new technologies now allow for a much faster way to interrogate the whole genome in an unbiased fashion to elucidate underlying molecular mechanisms. For example, a number of genome-wide screens using RNAi and CRISPR/Cas9 have recently identified host factors and pathways that are essential for replication of multiple flaviviruses, including ZIKV (Marceau et al., 2016; Savidis et al., 2016a; Zhang et al., 2016b). These unbiased approaches have recovered AXL, host factors involved in endocytosis (RAB5C and RABGEF), and the ER membrane complex (Figure 3). Not surprisingly, these studies also identified virus-specific host factors. Such unbiased approaches can also be used to identify host factors that mediate pathological effects of ZIKV infection, such as neural progenitor proliferation deficits. Similar to previous studies of HIV, HCV and influenza viruses (de Chassey et al., 2008; Heaton et al., 2016; Jager et al., 2011; Luo et al., 2016), generation of comprehensive ZIKV-host protein interactome maps between each of the ZIKV proteins and the host proteome will provide important clues into how ZIKV rewires the host's cellular machinery during the course of infection and pathogenesis. It is also now feasible to generate a comprehensive interactome between viral RNAs, including 3’- and 5’-NTRs, sfRNAs and sRNAs, with or without modifications (e.g. m6A methylation) and the host proteome (Hu et al., 2013).
How ZIKV infection, such as in neural progenitors, leads to large scale transcriptional dysregulation is not clear. ZIKV infection may also lead to large-scale changes of epigenomes (e.g. DNA methylation due to expression changes in TETs and DNMTs) (Tang et al., 2016) and epitranscriptomes (e.g. m6A) (Lichinchi et al., 2016) in host cells. In addition, computational analyses suggest that the ZIKV genomic RNA possesses conserved G-Quadruplexes (Fleming et al., 2016) as well as the capacity to generate a large number of sfRNAs and sRNAs (Akiyama et al., 2016). Whether any of these mechanisms contribute to ZIKV replication and host cell pathogenesis remains to be functionally determined.
Therapeutics
Traditionally, screening of antiviral compounds has used tumor cell lines (e.g. astrocytoma cells and hepatoma cells) or animal cell lines (e.g. Vero cells). The establishment of human stem cell-based model systems allows direct screening using relevant human physiological targets of ZIKV for discovery or to validate primary hits. In one recent repurposing screen of 774 drugs approved by the US Food and Drug Administration (FDA), several inhibitors of ZIKV infections were identified first using a human hepatoma cell line and then confirmed in human placental and neural stem cell lines (Barrows et al., 2016). In another repurposing screen of over 6000 FDA approved drugs, clinical trial drug candidates, and pharmacologically active compounds, a pan-caspase inhibitor was found to inhibit ZIKV-induced increases in caspase-3 activity and protected human neural progenitors from cell death in both monolayer and forebrain organoid cultures (Xu et al., 2016). Interestingly, ten structurally unrelated inhibitors of cyclin-dependent kinases (CDKs) inhibit ZIKV replication. Given that none of the ZIKV encoded proteins are CDKs, these results suggest an important role of host CDKs in ZIKV replication. These compounds can be used as tools to help delineate mechanisms underlying ZIKV-induced pathogenesis and as leading compounds for medicinal chemistry for further drug development. Future studies are necessary to test these FDA approved drugs or clinical trial drug candidates in animal models for their efficacy as well as toxicity before moving to clinical trials. In addition to nonbiased large scale screens, studies based on mechanistic insights have also identified individual drugs and compounds that inhibit ZIKV replication and/or protect neural progenitors from cell death (Deng et al., 2016; Mounce et al., 2016; Onorati et al., 2016; Zmurko et al., 2016). Some of these compounds have been validated in animal models, but remain to be tested in the microcephaly model in vivo (Deng et al., 2016; Zmurko et al., 2016).
Beyond the ZIKV Epidemics
It is the hope that rapid development of potent ZIKV therapeutics and vaccines, along with effective vector control, will stop the spread and eliminate large ZIKV epidemics in the near future (Thomas et al., 2016). The recent report of potent neutralizing human antibodies and the mapping of a relevant epitopes is encouraging in light of both treatment and vaccination efforts (Sapparapu et al., 2016). Nevertheless, ZIKV research will continue to benefit the scientific community for years to come. For example, understanding how ZIKV interacts with the host machinery and replicates itself will generate insights into mechanisms of other flaviviruses, which also cause serious diseases, whereas understanding how ZIKV passes through the placenta and BBB barriers to affect brain development may help fight other viral TORCH pathogens. The established stem cell-based platform will also facilitate research and drug discovery efforts for other viruses.
Analogous to how the study of v-Src encoded by Rous sarcoma virus greatly transformed current knowledge of cancer and related basic cell biology, the study of ZIKV-induced microcephaly provides an entry point to understand normal human brain development, about which we know little. The advent of lentiviral vectors based on HIV (Naldini et al., 1996) has had widespread applications to allow efficient and stable transduction of non-dividing cells both in culture and in vivo. The tropism of ZIKV towards neural progenitor cells in the developing and adult brain also offers unique opportunities to engineer safe ZIKV-based vectors to target this population for lineage-tracing and genetic manipulation. ZIKV readily infects and kill glioblastoma cells (Xu et al., 2016), which raises the possibility of new strategies to fight glioblastoma. The availability of molecular clones of ZIKV can facilitate the construction of ZIKV-based vectors (Gadea et al., 2016; Schwarz et al., 2016; Shan et al., 2016; Tsetsarkin et al., 2016). Future studies will determine whether ZIKV-based viral tools could have broad applications in basic research and gene therapy.
Conclusion
Zika epidemics represent a major challenge, but also an opportunity for the scientific community to collaborate across disciplines to learn fundamental principles of virology, stem cell biology and human development. Stem cell-based modeling of ZIKV exposure is one of the clearest examples of how the promise of human stem cell research may be realized and how decades of advances in basic stem cell biology allowed researchers to immediately address a global health emergency. Future modeling of the ZIKV syndrome will address the extent of pathophysiology associated with ZIKV infection and the underlying biological mechanisms, as well as furthering the development of platforms to facilitate drug screen and development. It is certain that by the time this review is in print, additional progress will have been made and there will be much more to follow.
The re-emergence of Zika virus (ZIKV) and its suspected link with various disorders in newborn and adults led the WHO to declare a global health emergency. Ming et al review the progress in stem cell-based ZIKV research and insights into cellular targets, pathogenesis, development of therapeutics and underlying mechanisms of infection.
Acknowledgments
We apologize for not citing many original papers due to the space limit. We thank Kim Christian for comments. The research in the authors’ laboratories were supported by grants from NIH (R01MH105128, R21MH110160 and R35NS097370 to G-l.M., R21AI119530 to H.T., R37NS047344, U19MH106434, P01NS097206, R21HD086820, RM1HG008935 to H.S.), Maryland Stem Cell Research Fund (G-l.M. and H.S.), Foundation for Prader-Willi Research (G.L.M.) and ZIKV seed funding from Florida State University (H.T.)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146:R151–162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016 doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama BM, Laurence HM, Massey AR, Costantino DA, Xie X, Yang Y, Shi P-Y, Nix JC, Beckham JD, Kieft JS. Zika virus produces noncoding RNAs using a multi-pseudoknot structure that confounds a cellular exonuclease. Science. 2016 doi: 10.1126/science.aah3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo LM, Ferreira ML, Nascimento OJ. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. 2016;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Munoz G, McGrath EL, Urrabaz-Garza R, Gao J, et al. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin E, Lavezzo E, Brugnaro P, Palu G. Isolation of infectious Zika virus from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy, January 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.10.30159. [DOI] [PubMed] [Google Scholar]
- Bavia L, Mosimann AL, Aoki MN, Duarte Dos Santos CN. A glance at subgenomic flavivirus RNAs and microRNAs in flavivirus infections. Virol J. 2016;13:84. doi: 10.1186/s12985-016-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless NL, Greenberg RS, Swigut T, Wysocka J, Blish CA. Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host Microbe. 2016;20:423–428. doi: 10.1016/j.chom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35:183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- Bidet K, Dadlani D, Garcia-Blanco MA. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog. 2014;10:e1004242. doi: 10.1371/journal.ppat.1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault JB, Khou C, Basset J, Coquand L, Fraisier V, Frenkiel MP, Goud B, Manuguerra JC, Pardigon N, Baffet AD. Comparative Analysis Between Flaviviruses Reveals Specific Neural Stem Cell Tropism for Zika Virus in the Mouse Developing Neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF, Yip CC, Tsang JO, Tee KM, Cai JP, Chik KK, Zhu Z, Chan CC, Choi GK, Sridhar S, et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93. doi: 10.1038/emi.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell research. 2008;18:889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol. 2016;14:707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo TV, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo AP, Valongueiro S, de Albuquerque MF, Souza WV, Braga C, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugue S, Meiffren G, Pradezynski F, Faria BF, Chantier T, et al. Hepatitis C virus infection protein network. Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YQ, Zhang NN, Li CF, Tian M, Hao JN, Xie XP, Shi PY, Qin CF. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect Dis. 2016;3:ofw175. doi: 10.1093/ofid/ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016 doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Scientific reports. 2016;6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzrodt M, Endele M, Schroeder T. Quantitative single-cell approaches to stem cell research. Cell Stem Cell. 2014;15:546–558. doi: 10.1016/j.stem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Fleming AM, Ding Y, Alenko A, Burrows CJ. Zika Virus Genomic RNA Possesses Conserved G-Quadruplexes Characteristic of the Flaviviridae Family. ACS Infect Dis. 2016;2:674–681. doi: 10.1021/acsinfecdis.6b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea G, Bos S, Krejbich-Trotot P, Clain E, Viranaicken W, El-Kalamouni C, Mavingui P, Despres P. A robust method for the rapid generation of recombinant Zika virus expressing the GFP reporter gene. Virology. 2016;497:157–162. doi: 10.1016/j.virol.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- Garcia-Blanco MA, Vasudevan SG, Bradrick SS, Nicchitta C. Flavivirus RNA transactions from viral entry to genome replication. Antiviral Res. 2016;134:244–249. doi: 10.1016/j.antiviral.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Ghouzzi VE, Bianchi FT, Molineris I, Mounce BC, Berto GE, Rak M, Lebon S, Aubry L, Tocco C, Gai M, et al. ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly and p53. Cell Death Dis. 2016;7:e2440. doi: 10.1038/cddis.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013;2:461–478. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale NS, McIntyre AB, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, et al. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow FT, Tesla B, Simchick G, Zhao Q, Hodge T, Brindley MA, Stice SL. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 2016 doi: 10.1089/scd.2016.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nature reviews Molecular cell biology. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirakhoo F, Bolin RA, Roehrig JT. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, et al. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Moshkina N, Fenouil R, Gardner TJ, Aguirre S, Shah PS, Zhao N, Manganaro L, Hultquist JF, Noel J, et al. Targeting Viral Proteostasis Limits Influenza Virus, HIV, and Dengue Virus Infection. Immunity. 2016;44:46–58. doi: 10.1016/j.immuni.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PF, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016 doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Wan J, Su Y, Song Q, Zeng Y, Nguyen HN, Shin J, Cox E, Rho HS, Woodard C, et al. DNA methylation presents distinct binding sites for human transcription factors. eLife. 2013;2:e00726. doi: 10.7554/eLife.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. Global landscape of HIV-human protein complexes. Nature. 2011;481:365–370. doi: 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Ma L, Burgner JW, Groesch TD, Post CB, Kuhn RJ. Flavivirus capsid is a dimeric alpha-helical protein. J Virol. 2003;77:7143–7149. doi: 10.1128/JVI.77.12.7143-7149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA. Stem Cell Models of Human Brain Development. Cell Stem Cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016 doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016a;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016b doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, Rana TM. Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2007. pp. 1101–1152. [Google Scholar]
- Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, DeLalio LJ, Isakson BE, Wang TT. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.309866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Jacobs EY, Greco TM, Mohammed KD, Tong T, Keegan S, Binley JM, Cristea IM, Fenyo D, Rout MP, et al. HIV-host interactome revealed directly from infected cells. Nat Microbiol. 2016;1:16068. doi: 10.1038/nmicrobiol.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science. 2015;350:217–221. doi: 10.1126/science.aab3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, Delobel P, Gonzalez-Dunia D, Malnou CE, Izopet J, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis. 2016;16:1106–1107. doi: 10.1016/S1473-3099(16)30336-X. [DOI] [PubMed] [Google Scholar]
- Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, Swaminathan K, Mata MA, Elias JE, Sarnow P, et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature. 2016;535:159–163. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J Biol Chem. 2011;286:22147–22159. doi: 10.1074/jbc.M110.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, Batista AG, Ferreira T, Dos Santos MP, Sampaio VV, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016a;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016b;16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda HA, 2nd, Costa MC, Frazao MA, Simao N, Franchischini S, Moshfeghi DM. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology. 2016;123:1788–1794. doi: 10.1016/j.ophtha.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Mounce BC, Cesaro T, Moratorio G, Hooikaas PJ, Yakovleva A, Werneke SW, Smith EC, Poirier EZ, Simon-Loriere E, Prot M, et al. Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J Virol. 2016;90:9683–9692. doi: 10.1128/JVI.01347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura da Silva AA, Ganz JS, Sousa PD, Doriqui MJ, Ribeiro MR, Branco MD, Queiroz RC, Pacheco MJ, Vieira da Costa FR, Silva FS, et al. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg Infect Dis. 2016;22:1953–1956. doi: 10.3201/eid2211.160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, Bakiasi G, Tsai LH, Aubourg P, Ransohoff RM, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016 doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Qian X, Song H, Ming GL. Neural stem cells attacked by Zika virus. Cell research. 2016;26:753–754. doi: 10.1038/cr.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.32.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y. Historical landmarks of autophagy research. Cell research. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorati M, Li Z, Liu F, Sousa AM, Nakagawa N, Li M, Dell'Anno MT, Gulden FO, Pochareddy S, Tebbenkamp AT, et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016 doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B, Lizarazo J, Jimenez-Arango JA, Zea-Vera AF, Gonzalez-Manrique G, Vargas J, Angarita JA, Zuniga G, Lopez-Gonzalez R, Beltran CL, et al. Guillain-Barre Syndrome Associated with Zika Virus Infection in Colombia. N Engl J Med. 2016 doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Nguyen J, Johnson J, Haura E, Coppola D, Chellappan S. Tank binding kinase 1 is a centrosome-associated kinase necessary for microtubule dynamics and mitosis. Nature communications. 2015;6:10072. doi: 10.1038/ncomms10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- Rowe RG, Mandelbaum J, Zon LI, Daley GQ. Engineering Hematopoietic Stem Cells: Lessons from Development. Cell Stem Cell. 2016;18:707–720. doi: 10.1016/j.stem.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016 doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, John SP, Aker AM, Renzette N, Robbins DR, et al. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Rep. 2016a;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Savidis G, Perreira JM, Portmann JM, Meraner P, Guo Z, Green S, Brass AL. The IFITMs Inhibit Zika Virus Replication. Cell Rep. 2016b;15:2323–2330. doi: 10.1016/j.celrep.2016.05.074. [DOI] [PubMed] [Google Scholar]
- Schnettler E, Sterken MG, Leung JY, Metz SW, Geertsema C, Goldbach RW, Vlak JM, Kohl A, Khromykh AA, Pijlman GP. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and Mammalian cells. J Virol. 2012;86:13486–13500. doi: 10.1128/JVI.01104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MC, Sourisseau M, Espino MM, Gray ES, Chambers MT, Tortorella D, Evans MJ. Rescue of the 1947 Zika Virus Prototype Strain with a Cytomegalovirus Promoter-Driven cDNA Clone. mSphere. 2016;1 doi: 10.1128/mSphere.00246-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, Yang Y, Tesh RB, Bourne N, Barrett AD, et al. An Infectious cDNA Clone of Zika Virus to Study Viral Virulence, Mosquito Transmission, and Antiviral Inhibitors. Cell Host Microbe. 2016;19:891–900. doi: 10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, Chen JF. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016 doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Ming GL, Song H. Decoding neural transcriptomes and epigenomes via high-throughput sequencing. Nat Neurosci. 2014;17:1463–1475. doi: 10.1038/nn.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin Y, Loustalot F, Desmetz C, Foulongne V, Constant O, Fournier-Wirth C, Leon F, Moles JP, Goubaud A, Lemaitre JM, et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Kuo CF, Tian Y, Liu HM, Huang EJ, Jung JU, Machida K, Ou JH. Replication of hepatitis C virus RNA on autophagosomal membranes. J Biol Chem. 2012;287:18036–18043. doi: 10.1074/jbc.M111.320085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Zhang SC. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell. 2016;19:573–586. doi: 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, L'Azou M, Barrett AD, Jackson NA. Fast-Track Zika Vaccine Development - Is It Possible? N Engl J Med. 2016;375:1212–1216. doi: 10.1056/NEJMp1609300. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. MBio. 2016;7 doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell research. 2016;26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Christian KM, He C, Jin P, Ming GL, Song H. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256. e1244. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes & development. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Hammack C, Ogden SC, Cheng Y, Lee EM, Wen Z, Qian X, Nguyen HN, Li Y, Yao B, et al. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016a doi: 10.1093/nar/gkw765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature. 2016b;535:164–168. doi: 10.1038/nature18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J. The Viral Polymerase Inhibitor 7-Deaza-2'-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl Trop Dis. 2016;10:e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]