Abstract
There is a growing interest in understanding the role of inflammation in diet–depression relationship. The present study examined whether the dietary inflammatory index (DII, a measure of the inflammatory potential of individuals' diets) is associated with recurrent depressive symptoms (DepS) (CES-D score>16 or taking antidepressants both at baseline and follow-up) assessed over 5 years in middle-aged men (n=3178) and women (n=1068) from the Whitehall II Study. For each increment of 1 SD of DII score, odds of recurrent DepS increased by 66% (95 % CI:1.30–2.12) in women while no significant association between DII and recurrent DepS was observed in men (OR=1.12, 95 % CI: 0.92–1.36). This association was little attenuated after adjustment for confounders and after taking into account levels of interleukin-6 and C-reactive protein. In conclusion, there is an association between pro-inflammatory diet and recurrent DepS in women which seems not be driven by circulating inflammatory markers.
Introduction
With a prevalence of 350 million people worldwide, depression along with other mental health conditions is considered to be a major contributor to global disability (Whiteford et al., 2013). Despite significant developments, conventional treatment only address one-third of the disease burden linked to mood disorders (van Zoonen et al., 2014), Identifying new preventive strategies (as well as innovative approaches to delay their progression) thus seems crucial. In recent years, there has been an increasing interest in assessing the relationship between overall diet and mental health (Lai et al., 2014). A recent systematic review and meta-analysis provides a comprehensive evaluation of current evidence of the association between dietary patterns and depression indicating that a healthy dietary pattern is associated with a reduced odds of depression (Lai et al., 2014). An increased risk of depression associated with a high adherence to unhealthy dietary patterns, such as the western and processed food diets, has also been reported (Akbaraly et al., 2009; Jacka, Cherbuin, Anstey, & Butterworth, 2014; Jacka, Mykletun, Berk, Bjelland, & Tell, 2011; Jacka et al., 2010; Le Port et al., 2012). The epidemiologic evidence regarding the potential role of nutrition in depression indicates that nutritional medicine could have a place in mainstream psychiatric care (Sarris, Logan, Akbaraly, Amminger, et al., 2015; Sarris, Logan, Akbaraly, Paul Amminger, et al., 2015) by providing dietary recommendations to prevent depression (Opie et al., 2015). Amongst the biological mechanisms linking diet to depression, inflammatory processes have been strongly suggested, especially because of the common mechanism shared by depression and cardiometabolic disorders (Sanchez-Villegas & Martinez-Gonzalez, 2013).
A cumulative meta-analysis indicated higher levels of interleukin-6 (IL-6) and C-reactive protein (CRP) in patients with major depression compared to non–depressive individuals (Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimaki, 2015). Data from observational studies suggest predictive associations of these two inflammatory markers with the future development of mood disorders (Gimeno et al., 2009; Khandaker, Pearson, Zammit, Lewis, & Jones, 2014; Kivimaki et al., 2014; Virtanen et al., 2015; Wium-Andersen, Orsted, Nielsen, & Nordestgaard, 2013) in a dose response way (Kivimaki et al., 2014), and a role of IL-6 in depressive-like phenotypes (Sukoff Rizzo et al., 2012). However, few studies have examined the extent to which the diet–depression relationship may be directly driven by inflammation-modulating properties of the specific dietary pattern (and associated foods) assessed. To address this limitation, the present study aimed to examine whether the dietary inflammatory index which reflects the pro-inflammatory potential of diet - the dietary inflammatory index (DII) (Shivappa, Steck, Hurley, Hussey, & Hebert, 2014; Shivappa, Steck, Hurley, Hussey, Ma, et al., 2014) - is associated with the recurrence of depressive symptoms assessed over 5 years in a British cohort of men and women.
Methods
Study Population
Participants of the Whitehall II study were London based office staff, aged 35–55 years, who worked in 20 civil service departments at study inception (Marmot & Brunner, 2005). Baseline screening (phase 1: 1985–1988, n = 10,308) included a clinical examination and a self-administered questionnaire. Subsequent phases of data collection alternated between a clinical examination alongside a questionnaire survey (Phase 3: 1991/93, n = 8815; Phase 5: 1997/99, n=7263; Phase 7: 2002/04, n=6943; Phase 9 : 2007/09, n=6354) and a postal questionnaire alone (Phases 2, 4, 6, 8 and 10). Written informed consent was obtained after a thorough explanation of the study to each of the participants; the University College London ethics committee approved the study.
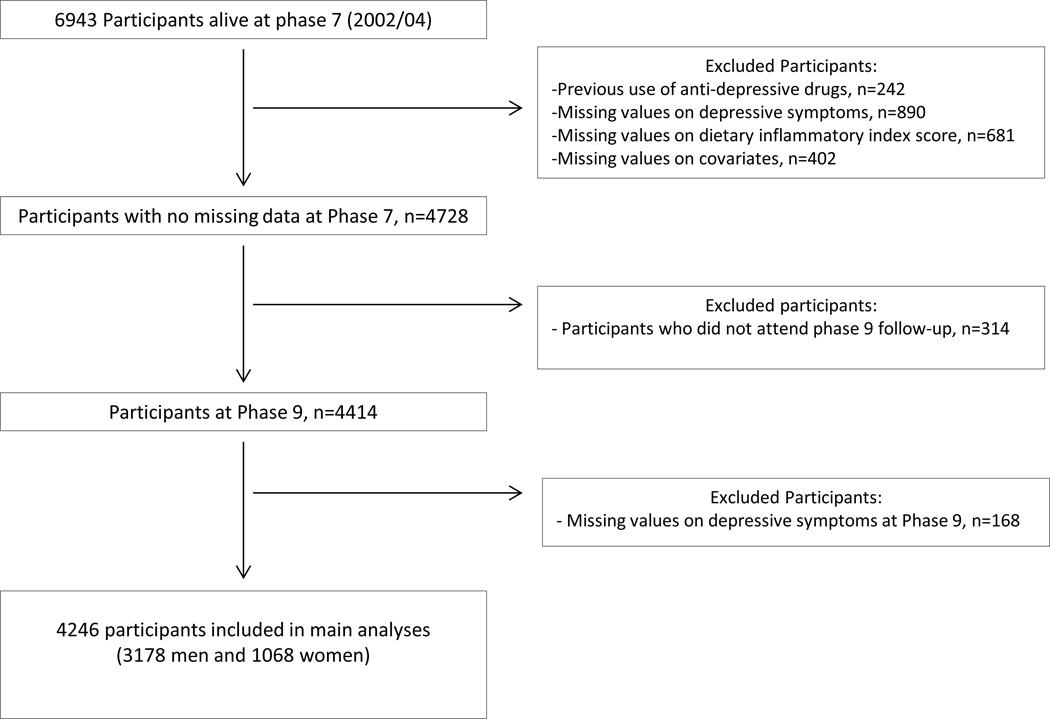
After excluding participants taking antidepressant medications at phases 3 or 5, analyses were restricted to participants with complete data on dietary assessment and covariates at phase 7 and depressive symptoms at phases 7 and 9 (n=4246) as detailed in the flow-chart diagram (Figure 1). Baseline and follow-up of this study were represented by phase 7 and 9 respectively.
Figure 1.
Flow chart diagram mapping the selection of participants
Data Collection
Dietary Assessment
Dietary intake of Whitehall II participants was assessed using a machine-readable Food Frequency Questionnaire (FFQ) (Brunner, Stallone, Juneja, Bingham, & Marmot, 2001) based on one used in the US Nurses’ Health study (Willett et al., 1985). The food list (127 items) in the FFQ was anglicised, and foods commonly eaten in the UK were added (Bingham et al., 1997). A common unit or portion size for each food was specified, and participants were asked how often, on average, they had consumed that amount of the item during the previous year. Response to all items was on a 9-point scale, ranging from ‘never or less than once per month’ to ‘six or more times per day’. The selected frequency category for each food item was converted to a daily intake. Nutrient intakes were computed by multiplying the consumption frequency for each food by its nutrient content (for specified portions) and then summing nutrient contributions from all foods. Frequency of consumption for multivitamin supplements was also collected. Nutrient values were calculated using the computerized system developed for the Whitehall II dietary data and based on the 4th and 5th editions of McCance and Widdowson’s The Composition of Foods and supplementary tables (Akbaraly et al., 2011). Nutrient supplement information was obtained from manufacturers of the supplements and added to the database. The validity and reliability of this FFQ in terms of nutrient and food consumption have been documented in Bingham et al. report (Bingham et al., 1997).
The dietary Inflammatory Index (DII)
The design and development of the DII has been described elsewhere (Shivappa, Steck, Hurley, Hussey, & Hebert, 2014; Shivappa, Steck, Hurley, Hussey, Ma, et al., 2014). Simply put, the DII is a scoring algorithm based on a review of the literature published from 1950 to 2010. A total of >6000 articles were scanned and 1943 linking one or more of these six inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, TNF-α and C-reactive protein) to diet were included in the final scoring algorithm. From these 1943 articles, 45 food parameters were identified as linked to one or more of the inflammatory biomarkers including various macronutrients, micronutrients, flavonoids and individual food items. The inflammatory potential for each food parameter was scored according to whether it increased (+1), decreased (−1) or had no effect (0) on six inflammatory biomarkers. A z-score (mean=0, standard deviation=1) for each food consumed was calculated by subtracting the “standard global mean” from the amount reported and dividing this value by its standard deviation. This value was then converted to a centered percentile score in order to reduce the risk of skewing. For each food parameter and for each participant this centered percentile score was then multiplied by the respective food parameter effect score derived from the literature review, in order to obtain a food parameter-specific DII score for a given participant. All of the food parameter-specific DII scores were then summed to create the overall DII score for each participant in the study. The greater the DII score, the more pro-inflammatory the diet when negative values are related to anti-inflammatory diets. Details regarding the construct validation of the DII have been enumerated elsewhere (Sanchez-Villegas, Ruiz-Canela, et al., 2015). In the present study, a total of 27 of 45 food parameters considered in the DII score were derived from the FFQ and could therefore be used to calculate the DII. These variables include energy, carbohydrate, protein, total fat, alcohol, fiber, cholesterol, saturated fat, MUFA, PUFA, omega-3, omega-6, trans-fat, niacin, thiamin, riboflavin, vitamin B12, B6, iron, magnesium, zinc, selenium, vitamin A, vitamin C, vitamin D, vitamin E, and folic acid.
Assessment of depressive symptoms
At baseline and follow-up, participants with the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) score ≥ 16 or treated by antidepressants were defined as “cases”, i.e., with depressive symptoms (DepS). Three categories of “5-y DepS status” were defined: “recurrent depressive symptoms” as DepS at both baseline and follow-up; “non-recurrent depressive symptoms” as DepS once out of the two phases; “no depressive symptoms” as DepS at none of both phases.
Covariates assessed at baseline
Socio-demographic factors included sex, age, ethnicity (White/ South Asian/ Black), marital status (married or cohabiting vs. single/divorced/widowed), and socio-economic status (SES) based on occupational position, categorized into three groups: high (administrative), intermediate (professional or executive) and low (clerical or support). This measure is a comprehensive marker of socioeconomic circumstances in the Whitehall II study being related to education, salary, social status and level of responsibility at work (Marmot & Brunner, 2005).
Health behaviors consisted of smoking, alcohol consumption, total energy intake (estimated from the FFQ) and physical activity. Smoking status was self-reported and classified as “current smoker” or “noncurrent smoker” (including former smokers). Alcohol consumption was assessed using questions on the number of alcoholic drinks consumed in the past week, and was classified as “no alcohol consumption in the previous week”, “moderate alcohol consumption” (1–14 units/week in women and 1–21 units/week in men), and “heavy drinkers” (15+ units in women and 21+ units in men). Physical activity was assessed by a questionnaire including 20 items on frequency and duration of participation in different physical activities (e.g., walking, cycling, sports) that were used to compute hours per week at each intensity level. Participants were classified as “active” (> 2.5hours per week of moderate physical activity or >1 hour per week of vigorous physical activity), “inactive” (< 1 hour per week of moderate physical activity and <1 hour per week of vigorous physical activity), or “moderately active” (if neither active nor inactive) (Sabia et al., 2012).
Health status factors included prevalent coronary heart disease (CHD) (denoted by clinically verified non-fatal myocardial infarction or definite angina); hypertension (defined by systolic/diastolic blood pressure ≥140 /90 mm Hg, respectively, or use of antihypertensive drugs); type 2 diabetes (diagnosed according to the WHO definition); serum high density lipoprotein (HDL) cholesterol measured in mmol/L, use of lipid-lowering drugs, central obesity defined by a waist circumference >102 cm in men and >88 cm in women and cognitive impairment defined by a score≤27 (Crum, Anthony, Bassett, & Folstein, 1993) in the Mini Mental State Examination (MMSE).
Circulating pro-inflammatory markers IL-6 and CRP have been measured. IL-6 was measured using a high-sensitivity ELISA assay (R&D Systems, Oxford, UK) (Gimeno et al., 2007; Gimeno et al., 2011). CRP was measured using a high-sensitivity immunonephelometric assay in a BN ProSpec nephelometer (Dade Behring, Milton Keynes, UK) (Gimeno et al., 2007; Gimeno et al., 2011). Blood samples were collected between 8 am and 1 pm, stored at −80°C and were not thawed or refrozen during storage. Stored serum samples were analyzed in the same laboratory. Values below the detection limit (0.154 mg/L for CRP and 0.08 pg/mL for IL-6) were assigned a value equal to half the detection limit as detailed elsewhere (Akbaraly et al., 2015).
Statistical Analysis
Characteristics of participants according to recurrent depressive symptoms status assessed over the 5-year follow-up were compared using the Chi-squared test for categorical variables and Student t-tests for continuous variables. Logistic regression models were used to assess the association of DII score at baseline with recurrent DepS. Non-recurrent DepS and “no DepS” were considered as non-cases. DII score was analyzed as a continuous standardized variable by using z-score (mean=0, standard deviation (SD) =1) allowing the odds of persistent and non-persistent DepS per 1 SD increment of the considered dietary score to be estimated. DII was also categorized into tertiles and the lowest tertile was used as the reference. Median and range were for Tertile 1 : −1.30 (−3.35; −0.68), (33.5% of men and 32.7% of women), Tertile 2 : −0.19 (−0.68; 0.43), (33.6% of men and 32.7% of women) and for Tertile 3 : 1.35 (0.43; 4.23), (32.9% of men and 34.6% of women). Models were first adjusted for age, sex, ethnicity and total energy intake (Model 1) then additionally for occupational grade, marital status, smoking behavior, physical activity, alcohol consumption (Model 2) and finally for type 2 diabetes, stroke, CHD, hypertension, use of lipid lowering drugs, HDL-cholesterol, central obesity and cognitive impairment (Model 3). Similar models were performed in which plasma inflammatory markers were additionally included as covariates. To examine effect modification, interactions between main covariates and DII score regarding DepS have been tested.
Results
Participant characteristics
Of the 6943 participants alive at phase 7, 4246 with complete data on DepS over the 5-year follow-up, dietary exposure and covariates at baseline were included in the present analysis. Compared to 2697 participants excluded, those participants included were more likely to be younger, men, white, with high SES and to have healthy behaviors (non smoker, high level of physical activity) and less likely to be living alone, having cardiometabolic diseases and disorders and to have cognitive impairment (Table A-Supplementary material).
DII score was computed for the 4246 participants included in the present analyses. The DII was built in a way that the greater the DII score, the more potentially pro-inflammatory the diet is. These preliminary analyses showed that the DII score was found to be positively associated with IL-6 and CRP levels categorized in tertiles. Participants in lowest tertiles of both inflammatory markers showed lower DII scores (Table B-Supplementary materials). Participants ’characteristics according to DII scores have also been compared (Table C-Supplementary materials). Higher mean DII scores was observed in men, in South Asian, in participants with low SES, and in participants living alone. Higher DII score was associated with unhealthy behavior and inversely associated with total energy intake. Regarding health status factors, high DII score was inversely associated with HDL cholesterol and lower scores were observed in participants taking lipid lowering drugs. No significant association was observed with other health status factors.
Over the 5-year of follow-up, 265 (166 men and 99 women) participants had recurrent DepS (having DepS at both baseline and follow-up, 6.2%), 580 (386 men and 194 women) had DepS in one measurement at baseline or follow-up (non-recurrent DepS, 13.7 %), while 3401 (2626 men and 775 women) of the sample did not have DepS at either phase (80.1 %). Table 1 presents the characteristics of participants according to recurrence of DepS in the whole sample and within each sex separately. Participants with recurrent DepS were more likely to be women, younger, South Asian with low SES and living alone. They were more likely to be current smoker, alcohol abstainers, physically inactive, with central obesity and to have coronary heart diseases and cognitive impairment. Similar characteristics associated with recurrent DepS were observed for each sex
Table 1.
Comparison of characteristics as a function of having recurrent depressive symptoms (DepS) in the 4246 Whitehall II participants
| Recurrence of DepS over the 5-year of follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics
of participants at baseline |
Whole Sample (n=4246) | Men (n=3178) | Women (n=1068) | |||||||
| No n=3981 |
Yes n=265 |
No n=3012 |
Yes n=166 |
No n=969 |
Yes n=99 |
|||||
| % or m±SD |
% or m±SD |
P | % or m±SD |
% or m±SD |
P | % or m±SD |
% or m±SD |
P | ||
| Sociodemographic factors | ||||||||||
| Sex | Men | 75.7 | 62.6 | <.001 | / | / | / | / | ||
| Women | 24.3 | 37.4 | / | / | / | / | ||||
| Age (y) | 61.0±5.9 | 60.1±5.9 | 0.02 | 61.0±5.9 | 59.7±5.7 | 0.01 | 61.0±5.9 | 60.8±6.2 | 0.75 | |
| Ethnicity | White | 95.6 | 86.8 | <.001 | 96.5 | 88.0 | <.001 | 93.1 | 84.9 | <.001 |
| South Asian | 3.1 | 12.6 | 2.9 | 11.5 | 3.8 | 14.1 | ||||
| Black | 1.2 | 0.8 | 0.6 | 0.6 | 3.1 | 1.0 | ||||
| SES | Low | 7.4 | 15.1 | <.001 | 2.3 | 6.6 | <.001 | 23.2 | 29.3 | 0.02 |
| Inverse ok | Medium | 40.9 | 50.2 | 38.2 | 46.4 | 49.4 | 56.6 | |||
| High | 51.7 | 34.7 | 59.5 | 47.0 | 27.4 | 14.1 | ||||
| Marital Status |
Married | 78.7 | 64.9 | <.001 | 84.6 | 74.7 | 0.001 | 60.4 | 48.5 | 0.02 |
| Living alone | 21.3 | 35.1 | 15.4 | 25.3 | 39.6 | 51.5 | ||||
| Health behavior factors | ||||||||||
| Smoking habits |
Never | 50.2 | 50.9 | 0.01 | 48.2 | 46.4 | 0.02 | 56.4 | 58.6 | 0.51 |
| Ex | 43.1 | 37.7 | 45.7 | 42.2 | 35.1 | 30.3 | ||||
| Current | 6.7 | 11.3 | 6.1 | 11.5 | 8.6 | 11.1 | ||||
| Alcohol Intake |
No | 13.4 | 21.9 | 0.001 | 9.8 | 13.3 | 0.20 | 24.6 | 36.4 | 0.03 |
| Moderate | 63.6 | 56.2 | 65.8 | 59.6 | 56.7 | 50.5 | ||||
| Heavy drinker | 23.0 | 21.9 | 24.3 | 27.1 | 18.8 | 13.1 | ||||
| Physical activity |
Inactive | 22.3 | 34.3 | <.001 | 20.8 | 31.9 | <.001 | 26.9 | 38.4 | 0.02 |
| Mod. Active | 16.0 | 23.4 | 14.6 | 24.1 | 20.3 | 22.2 | ||||
| Active | 61.7 | 42.3 | 64.6 | 44.0 | 52.7 | 39.4 | ||||
| Total energy intake (kcal/d) | 2191±636 | 2253±772 | 0.21 | 2255±636 | 2337±787 | 0.19 | 1994±596 | 2112±729 | 0.12 | |
| Health status factors | ||||||||||
| Type 2 diabetes | 8.4 | 10.6 | 0.23 | 8.2 | 11.5 | 0.14 | 9.3 | 9.1 | 0.95 | |
| Central obesity | 27.4 | 35.5 | 0.005 | 22.1 | 28.3 | 0.06 | 44.0 | 47.5 | 0.50 | |
| Cardiovascular Diseases | 8.0 | 12.5 | 0.01 | 8.7 | 11.5 | 0.23 | 5.8 | 14.1 | 0.001 | |
| Hypertension | 35.9 | 37.4 | 0.63 | 35.8 | 38.0 | 0.57 | 36.3 | 36.4 | 0.99 | |
| HDL cholesterol (mmol/L) | 1.6±0.4 | 1.6±0.4 | 0.37 | 1.5±0.4 | 1.5±0.4 | 0.31 | 1.9±0.5 | 1.7±0.5 | 0.01 | |
| Use of lipid-lowering drugs | 11.0 | 13.2 | 0.26 | 11.6 | 14.5 | 0.26 | 9.2 | 11.1 | 0.53 | |
| Cognitive impairment | 11.5 | 19.3 | <.001 | 11.1 | 18.7 | 0.003 | 12.7 | 20.2 | 0.04 | |
| Exposure | ||||||||||
| Dietary Inflammatory
Index (points) |
−0.03±1.3 | 0.12±1.5 | 0.12 | −0.03±1.3 | −0.02±1.5 | 0.92 | −0.04±1.4 | 0.33±1.5 | 0.01 | |
DII and Recurrent DepS over the 5-year of follow-up
Means (SD) and range of DII were −0.03 (1.3), range values: −3.35 to 4.23 in men and −0.002 (1.4) range values: −3.35 to 3.98) in women. Logistic regression models were performed to estimate the associations between DII score and recurrent DepS. Interaction between each covariates and DII score have been tested and a marginally significant interaction has been observed for sex (P for sex interaction 0.056) leading us to conduct analyses in men and women separately.
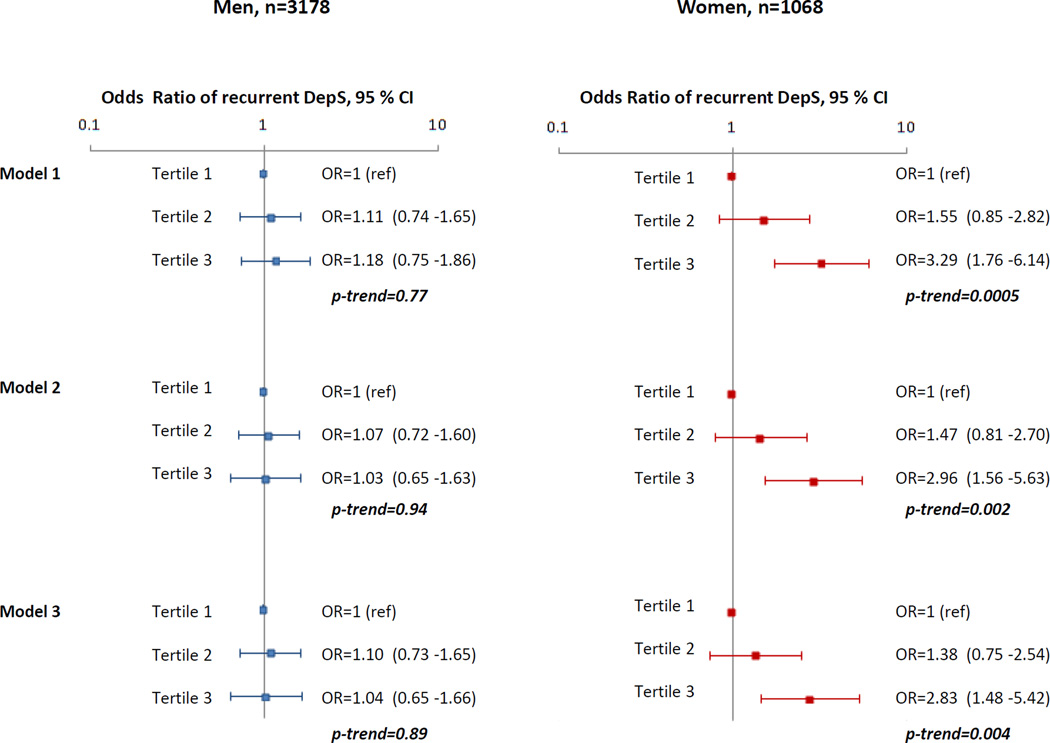
After adjusting for age, total energy intake and ethnicity, a significant association between high DII scores (reflecting pro-inflammatory potential of diet) and increased odds of recurrent DepS was observed in women but not in men. For each increment of 1 SD of DII score, odds of recurrent DepS increased by 66% (95 % CI: 1.30 to 2.12) in women while no significant association between DII and recurrent DepS was observed in men (odds ratio (OR) =1.12, 95 % CI : 0.92 to 1.36). Analyses in which the DII was categorized in tertile showed that women in the highest DII third were more likely to have recurrent Dep S over the 5-year of follow-up compared to women in the lowest third (OR= 3.29, 95 % CI: 1.76 to 6.14). Further adjustment for occupational grade, marital status, smoking behavior, physical activity, alcohol consumption (Model 2) and for type 2 diabetes, stroke, CHD, hypertension, use of lipid lowering drugs, HDL-cholesterol, central obesity and cognitive impairment (Model 3) did not substantially attenuate the results. After full adjustment, the odds of subsequent recurrent DepS over the 5-year of follow-up in women in the top tertile (Figure 2) compared to those in the bottom tertile was 2.83 (95 % CI: 1.48 to 5.42). No significant association between tertiles of DII and recurrent DepS was found in men. We repeated the analyses in which sex-specific tertiles of DII were computed. Similar results were obtained. Additionally instead of considering tertiles of DII, we assessed the DII-recurrent DepS associations by considering quintiles of DII, similar trends were observed.
Figure 2.
Association between dietary inflammatory Index score and recurrent depressive symptoms over 5 years in 3178 men and 1068 women from Whitehall II study DII was categorized in tertiles, median and range were for Tertile 1 : −1.30 (−3.35; −0.68), Tertile 2 : −0.19 (−0.68; 0.43) and for Tertile 3 : 1.35 (0.43; 4.23). Proportions of men and women in each tertile were : 33.5% of men and 32.7% of women in Tertile 1, 33.6% of men and 32.7% of women in Tertile 2, and 32.9% of men and 34.6% of women in Tertile 3.
Model 1 : adjusted for age, ethnicity, and total energy intake.
Model 2 : Model 1 + socio-economic status, marital status, smoking habits, physical activity, and alcohol intake.
Model 3 : Model 2 + coronary heart diseases, type 2 diabetes, hypertension, HDL-cholesterol, use of lipid-lowering drugs, central obesity, and cognitive impairment.
The role of circulating inflammatory markers
To assess the extent through which association between DII and recurrent DepS is explained by circulatory inflammatory markers - IL-6 and CRP- measured at baseline, the main analyses were repeated after entering these two inflammatory markers in the models. Analyses were performed on data from participants for whom measures of these inflammatory markers were available. After exclusion of cases with possible acute inflammation and immune activation due to current illness (defined as having CRP values>10 pg/mL) assessable data was available from a total of 3749 participants (2848 men and 901 women). The additional adjustment for IL-6 and CRP had little effect on results as we obtained similar associations between DII and recurrent DepS as those reported in main analyses. This was the case in analyses stratified by sex and irrespective of the level of adjustments. For example, in the total cohort the multivariate adjusted odds ratio for the DII z-score and recurrent DepS was 1.22 before and 1.21 after the additional adjustment for IL-6 and CRP. These results suggest that markers of inflammation are not shaping the association between DII and recurrent depressive symptoms.
Discussion
In this longitudinal study, we assessed the association between the DII - a dietary index designed to assess the pro-inflammatory potential of overall diet- and recurrence of DepS in a large cohort of British men and women. In this analysis from Whitehall II study, we showed a sex-specific association between DII and recurrent depressive symptoms. While no significant association was found in men, women with the highest DII score (representing diet with high pro-inflammatory potential) were more likely to develop recurrent depressive symptoms over the 5-year follow-up. We showed that these associations were little attenuated after adjustment for a large range of socio-economic variables, lifestyle habits, cardiometabolic risk factors and cognitive impairment. Our findings add a supplementary argument to the presupposed role of inflammatory processes in the diet-depression relationship.
The inverse association between DII and recurrence of DepS observed concurs with results found in other analyses assessing the association between overall diet, DepS and depressive symptoms and depression. In several reports including our previous work, an increased risk of depression/DepS was observed in participants showing a high adherence to western or processed food dietary patterns (Akbaraly et al., 2009; Jacka et al., 2014; Le Port et al., 2012) and in those with low adherence to prudent or traditional (Lai et al., 2014) dietary patterns whereas a reduced risk of depression was associated with high diet quality scores including Mediterranean diet (Sanchez-Villegas, Henriquez-Sanchez, et al., 2015) and Alternative Healthy Eating Index (Akbaraly, Sabia, Shipley, Batty, & Kivimaki, 2013). The anti- and pro-inflammatory properties of specific foods or nutrients included in healthy and unhealthy diet respectively have been proposed as potential explanations linking overall diet to depression.
However, little evidence is available on the extent to which the pro- or anti-inflammatory potential of diet shapes the association between diet and depression. To date, two cohort studies have assessed the association between dietary patterns linked to inflammation and depression. One derived dietary patterns related to inflammatory markers in American women (Lucas et al., 2014) and the other used the DII in a Spanish population (Sanchez-Villegas, Ruiz-Canela, et al., 2015). Both showed an association with incident depression. In the present study, which also used the DII, we found a similar positive association with recurrent DepS.
Additionally, we found that DII was positively associated with IL6 and CRP levels at baseline making our results compatible with the hypothesis of the involvement of inflammatory process in the diet-depression relationship. However it is interesting to note that adjustment for IL-6 and CRP did not yield a material change in the estimate of the DII-DepS recurrence association. There are several possible explanations for this finding. One explanation is that DII might not sufficiently capture the inflammatory properties of diet in Whitehall II participants. It is also possible that the direct effect of diet-related inflammation is not entirely mediated by CRP or IL-6. Also, they are both subject to fluctuations in measurements and levels may be affected by short-term disturbances in the metabolic milieu. Further investigations assessing the extent by which DII is a proxy measure of inflammatory activity in the present cohort is needed to fully interpret our findings whose conclusion is compatible with the multifactorial nature of mechanisms (and not only inflammatory pathways) linking overall diet to depression processes.
The association between DII and DepS yielded significance for women but not for men. A similar sex-difference was also observed in previous analyses assessing the association between adherence to the Alternative Healthy Eating Index and recurrent depressive symptoms in the Whitehall II study (Akbaraly et al., 2013). This contrasts with findings from the SUN cohort in which DII-depression association was observed in both sexes. Further research is needed to determine whether sex-differences in the diet-depression relationship are real or attributable to methodological limitations in assessing male depression. Indeed, some CES-D items (the measure used in this study) have been shown to produce biased responses when comparing male and female respondents (Stommel et al., 1993).
Our study has limitations. First, even if the CES-D scale has been shown to be a reliable and valid measurement tool indicating the presence of DepS (St John, Blandford, & Strain, 2006), it does not capture the severity of DepS. We sought to take into account this limitation by focusing recurrent DepS episodes defined as participants who met the CES-D criteria at two consecutive measurements. Nevertheless, our results cannot be extended to major depression. Secondly, the methodological design did not allow us to clearly separate recurrent from chronic depression. However, the current epidemiological data about depression can infer that chronic states were restricted to a minority of the participants (Blanco et al., 2010). Thirdly, our study was limited by the assessment of dietary intake. We used a semi-quantitative FFQ that covers only specific foods, recognized to be less precise than dietary assessments by diary records. FFQ has been showed to be associated with a variety of measurement biases in some studies (Hebert, Clemow, Pbert, Ockene, & Ockene, 1995; Hebert et al., 2002; Hebert et al., 1997; Hebert et al., 2001). Moreover, the extent to which our results are generalizable is an important consideration. Whitehall II study participants are mainly white, office-based civil servants and not fully representative of British general population (Marmot & Brunner, 2005). Furthermore the present analysis carried out on participants without any missing value on depression outcomes, dietary exposures and other covariates. Finally, even with the prospective design and after excluding participants under antidepressant treatment before measuring the exposure, the possibility remains that high DII score could be the consequence, rather than the cause, of DepS.
Despite these limitations, our results confirm an association between high score of DII and onset of recurrent DepS at least in women of a large British population. We also found this association to be largely independent of plasma levels of inflammatory markers, suggesting that further research is needed to identify the exact biological mechanisms underlying the DII-recurrent DepS association.
Supplementary Material
Acknowledgments
We thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Sources of support
The Whitehall II study has been supported by grants from the British Medical Research Council (MR/K013351); the British Heart Foundation (PG/11/63/29011 and RG/13/2/30098); the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (R01HL036310); the National Institute on Aging, National Institute of Health (R01AG013196, R01 AG034454); the Economic and Social Research Council (ES/J023299). Mika Kivimaki is supported by the Medical Research Council (K013351), UK; NordForsk, the Nordic Research Programme on Health and Welfare and by a professorial fellowship from the Economic and Social Research Council. Tasnime Akbaraly is supported by the Economic and Social Research Council and a regional grant ARPE from Languedoc Roussillon district in South of France (“Aide à la recherche en partenariat avec les Entreprises”). Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
The funding organization or sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosures : Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. The subject matter of this paper has not had any direct bearing on that work, nor has that activity exerted any direct influence on this project.
Footnotes
Conflicts of interest: None to report.
Authors’ contribution
TNA and MK developed the research question; TNA and MK conducted research; NS and JH designed the dietary Index; CK analyzed the data; TNA drafted the manuscript; and TNA, CK,MW, NC, LN, NS, JH and MK made critical revisions of the manuscript for important intellectual content. TNA has primary responsibility for final content.
References
- Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195(5):408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, Kivimaki M. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr. 2011;94(1):247–253. doi: 10.3945/ajcn.111.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly TN, Sabia S, Shipley MJ, Batty GD, Kivimaki M. Adherence to healthy dietary guidelines and future depressive symptoms: evidence for sex differentials in the Whitehall II study. Am J Clin Nutr. 2013;97(2):419–427. doi: 10.3945/ajcn.112.041582. doi: ajcn.112.041582 [pii]10.3945/ajcn.112.041582 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly TN, Shipley MJ, Ferrie JE, Virtanen M, Lowe G, Hamer M, Kivimaki M. Long-term adherence to healthy dietary guidelines and chronic inflammation in the prospective Whitehall II study. Am J Med. 2015;128(2):152 e154–160 e154. doi: 10.1016/j.amjmed.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Day NE. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–S151. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- Blanco C, Okuda M, Markowitz JC, Liu SM, Grant BF, Hasin DS. The epidemiology of chronic major depressive disorder and dysthymic disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2010;71(12):1645–1656. doi: 10.4088/JCP.09m05663gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86(3):405–414. doi: 10.1079/bjn2001414. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Gimeno D, Brunner EJ, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. Eur J Epidemiol. 2007;22(10):675–683. doi: 10.1007/s10654-007-9171-9. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Delclos GL, Ferrie JE, De Vogli R, Elovainio M, Marmot MG, Kivimaki M. Association of CRP and IL-6 with lung function in a middle-aged population initially free from self-reported respiratory problems: the Whitehall II study. Eur J Epidemiol. 2011;26(2):135–144. doi: 10.1007/s10654-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24(2):389–398. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged women's estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol. 2002;12(8):577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Ma Y, Clemow L, Ockene IS, Saperia G, Stanek EJ, 3rd, Ockene JK. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol. 1997;146(12):1046–1055. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Peterson KE, Hurley TG, Stoddard AM, Cohen N, Field AE, Sorensen G. The effect of social desirability trait on self-reported dietary measures among multi-ethnic female health center employees. Ann Epidemiol. 2001;11(6):417–427. doi: 10.1016/s1047-2797(01)00212-5. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Cherbuin N, Anstey KJ, Butterworth P. Dietary patterns and depressive symptoms over time: examining the relationships with socioeconomic position, health behaviours and cardiovascular risk. PLoS One. 2014;9(1):e87657. doi: 10.1371/journal.pone.0087657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med. 2011;73(6):483–490. doi: 10.1097/PSY.0b013e318222831a. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Shipley MJ, Batty GD, Hamer M, Akbaraly TN, Kumari M, Singh-Manoux A. Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psychiatry. 2014;19(2):149–150. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99(1):181–197. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- Le Port A, Gueguen A, Kesse-Guyot E, Melchior M, Lemogne C, Nabi H, Czernichow S. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7(12):e51593. doi: 10.1371/journal.pone.0051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Chocano-Bedoya P, Schulze MB, Mirzaei F, O'Reilly EJ, Okereke OI, Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014;36:46–53. doi: 10.1016/j.bbi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34(2):251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, Jacka FN. Dietary recommendations for the prevention of depression. Nutr Neurosci. 2015 doi: 10.1179/1476830515Y.0000000043. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measures. 1977;1:385–401. [Google Scholar]
- Sabia S, Dugravot A, Kivimaki M, Brunner E, Shipley MJ, Singh-Manoux A. Effect of intensity and type of physical activity on mortality: results from the Whitehall II cohort study. Am J Public Health. 2012;102(4):698–704. doi: 10.2105/AJPH.2011.300257. doi: AJPH.2011.300257 [pii]10.2105/AJPH.2011.300257 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Henriquez-Sanchez P, Ruiz-Canela M, Lahortiga F, Molero P, Toledo E, Martinez-Gonzalez MA. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015;13(1):197. doi: 10.1186/s12916-015-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Martinez-Gonzalez MA. Diet, a new target to prevent depression? BMC Med. 2013;11:3. doi: 10.1186/1741-7015-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Ruiz-Canela M, de la Fuente-Arrillaga C, Gea A, Shivappa N, Hebert JR, Martinez-Gonzalez MA. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr. 2015:1–9. doi: 10.1017/S0007114515003074. [DOI] [PubMed] [Google Scholar]
- Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanza-Martinez V, Freeman MP, Jacka FN. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- Sarris J, Logan AC, Akbaraly TN, Paul Amminger G, Balanza-Martinez V, Freeman MP, Jacka FN. International Society for Nutritional Psychiatry Research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. 2015;14(3):370–371. doi: 10.1002/wps.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John PD, Blandford AA, Strain LA. Depressive symptoms among older adults in urban and rural areas. Int J Geriatr Psychiatry. 2006;21(12):1175–1180. doi: 10.1002/gps.1637. [DOI] [PubMed] [Google Scholar]
- Stommel M, Given BA, Given CW, Kalaian HA, Schulz R, McCorkle R. Gender bias in the measurement properties of the Center for Epidemiologic Studies Depression Scale (CES-D) Psychiatry Res. 1993;49(3):239–250. doi: 10.1016/0165-1781(93)90064-n. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, Brandon NJ. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF, 3rd, Beekman AT, Cuijpers P. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43(2):318–329. doi: 10.1093/ije/dyt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen M, Shipley MJ, Batty GD, Hamer M, Allan CL, Lowe GD, Kivimaki M. Interleukin-6 as a predictor of symptom resolution in psychological distress: a cohort study. Psychol Med. 2015:1–8. doi: 10.1017/S0033291715000070. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70(2):176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.