Abstract
Three types of multidrug-resistant Escherichia coli isolates, called GEN S, GEN R, and AMG S, according to their three different aminoglycoside resistance patterns, were responsible for urinary tract colonization or infection in 87, 12, and 13 new patients, respectively, in a French 650-bed geriatric hospital over a 13-month period. The three E. coli types belonged to the same clone and phylogenetic group (group B2) and had identical transferable plasmid contents (a 120-kb plasmid), β-lactam and fluoroquinolone resistance genotypes (blaTEM-1B, blaCTX-M-15, and double mutations in both the gyrA and the parC genes), and virulence factor genotypes (aer, fyuA, and irp2). They disseminated in the geriatric hospital, where the antibiotics prescribed most often were fluoroquinolones and ceftriaxone, but not in the affiliated acute-care hospital, where isolation precautions were applied to the transferred patients. Thus, E. coli isolates, both CTX-M-type β-lactamase producers and fluoroquinolone-resistant isolates, might present a new challenge for French health care settings.
For the last 10 years, sporadically but regularly, the microbiology literature has reported on multidrug-resistant (MDR) bacteria in geriatric hospitals, long-term-care facilities, and nursing homes (14, 26, 30, 44). The emergence of these bacteria in such health care facilities has become a public health concern because implementation of isolation precautions, which is the principal measure recommended in acute-care hospitals to avoid cross-transmission (12), is often considered deleterious for the quality of life of elderly people living in long-term-care facilities. Moreover, the elderly patients carrying MDR bacteria represent a reservoir of these bacteria in acute-care hospitals when they are transferred to them. The very least that can be done to address this double concern is to survey long-term-care facilities for the emergence and incidence of MDR bacteria and to inform clinicians in acute-care hospitals of the real or potential existence of these isolates in patients transferred from long-term-care facilities. Such a twofold approach, observation and an infection control program, is certainly more easily managed when the same microbiological department, as in Hôpital A. Paré, is in charge of the patients in both types of care settings.
This article reports on molecular analyses performed with three types of MDR Escherichia coli isolates which successively emerged and spread in the 650-bed geriatric hospital which is affiliated with a 450-bed acute-care hospital, Hôpital A. Paré. It also describes the particular hygiene measures which were successfully established in the acute-care hospital to avoid secondary cases of MDR E. coli colonization or infection from the patients transferred from the geriatric hospital.
MATERIALS AND METHODS
Population and collection of bacteria.
From October 2001 to October 2002, each first isolate of the family Enterobacteriaceae displaying an extended-spectrum β-lactamase (ESBL) production phenotype according to the disk synergy test (15) was collected from each patient in the geriatric hospital. The type and date of sampling and information on the locations in the hospital of the patients concerned (a particular unit, a rehabilitation subunit, or a long-term-care subunit) were gathered. Nine urinary tract E. coli isolates with an ESBL production phenotype and nine urinary tract E. coli isolates without an ESBL production phenotype, all isolated over the study period and responsible for urinary tract infections (leukocyte counts, >104/ml; bacterial counts, ≥105 CFU/ml), were selected for further analyses.
Antibiotic susceptibilities.
Antibiotic susceptibilities were tested by the agar disk diffusion method, and the results were interpreted according to the standards of the French Antibiogram Committee (1) for the following antibiotics: amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, piperacillin, piperacillin-tazobactam, cephalothin, cefuroxime, cefoxitin, cefotaxime, ceftazidime, cefepime, aztreonam, imipenem, gentamicin, kanamycin, tobramycin, netilmicin, amikacin, chloramphenicol, tetracycline, co-trimoxazole, nalidixic acid, pefloxacin, and ciprofloxacin.
Molecular typing, phylogenetic groups, and virulence factor genotypes of the uropathogenic E. coli isolates.
The 18 uropathogenic E. coli isolates were typed by the random amplified polymorphic DNA (RAPD) method, as described previously (24).
The phylogenetic groups of these isolates were determined by a previously described PCR-based method (10). The isolates were evaluated for the presence of nine genes encoding putative virulence factors (papC, papG [I, II, and III], sfa/foc, sfaS, aer, hly, cnf1, fyuA, and irp2) characteristic of extraintestinal pathogenic E. coli by PCR with the primers and under the conditions described previously (5).
Antibiotic resistance transfer and plasmid content analysis.
Transfer of antibiotic resistance from the MDR E. coli isolates to E. coli strain C600 (streptomycin resistant) was performed as described previously (27). Transconjugants were selected on agar plates containing streptomycin (100 μg/ml) and ampicillin (200 μg/ml) or cefotaxime (10 μg/ml). Plasmids were extracted from the MDR E. coli isolates and their transconjugants by the method of Kado and Liu (18), and their sizes were assessed by comparison with the sizes of four known plasmids (156, 66, 48, and 7 kb) contained in E. coli NCTC 50192 (19).
Isoelectric focusing.
Isoelectric focusing was performed as described previously (27). The pI values of the β-lactamases produced by the MDR E. coli isolates and their transconjugants were determined by comparison with the pI values of known β-lactamases: TEM-1, pI 5.4; TEM-2, pI 5.6; SHV-3, pI 7; SHV-1, pI 7.6; CTX-M-3, pI 8.4; and CMY-4, pI 9.2.
Molecular analysis of mechanisms of resistance.
On the basis of the β-lactamase pI values, the isolates were evaluated for the presence of defined bla genes (blaTEM, blaSHV, and blaCTX-M type) by using the specific primers published previously and indicated in Table 1. New primers (Table 1) were defined to amplify and sequence the 3′ end of the blaCTX-M-15 gene.
TABLE 1.
Primers used for amplification and sequencing of antibiotic resistance genes
| PCR target | Primer name (sequence) | Tm (°C)a | Fragment size (bp) | Reference or source |
|---|---|---|---|---|
| β-Lactam resistance genes | ||||
| blaTEM | TemA1 (5′-ATAAAATTCTTGAAGAC-3′) | 42 | 1,075 | 40 |
| TemB1 (5′-TTACCAATGCTTAATCA-3′) | ||||
| V167 (5′-ATCCTTGAGAGTTTTCGCCC-3′)b | ||||
| V267 (5′-GCTTTTCTGTGACTGGTGAG-3′)b | ||||
| blaSHV | SHV-F (5′-CACTCAAGGATGTATTGTG-3′) | 49 | 822 | 34 |
| SHV-R (5′-TTAGCGTTGCCAGTGCTCG-3′) | ||||
| blaCTX-M | CTX-C1 (5′-ATGTGCAGCACCAGTAAAGT-3′) | 54 | 545 | 2 |
| CTX-C2 (5′-ACCGCGATATCGTTGGTGG-3′) | ||||
| blaCTX-M gene group 1 | M13U (5′-GGTTAAAAAATCACTGCGTC-3′) | 52 | 863 | 38 |
| M13L (5′-TTGGTGACGATTTTAGCCGC-3′) | ||||
| blaCTX-M promoter region and gene 5′ end | ISEcplU2 (5′-AATACTACCTTGCTTTCTGA-3′) | 50 | Variable | 38 |
| MA3 (5′-ACYTTACTGGTRCTGCACAT-3′) | ||||
| blaCTX-M-15 gene 3′ end | CTX-M-F1 (5′-ATAAAACCGGCAGCGGTG-3′) | 50 | 483 | This study |
| CTX-M-F2 (5′-GAATTTTGACGATCGGGG-3′) | ||||
| Aminoglycoside resistance genes | ||||
| aac(6′)-1b | AAC(6)ID (5′-CATGACTGAGCATGACCTT-3′) | 49 | 528 | This study |
| AAC(6)IU (5′-GAAGGGTTAGGCATCACT-3′) | ||||
| aac(3)-II | ACC3F (5′-CAATAACGGAGGCAATTCG-3′) | 50 | 868 | This study |
| ACC3R (5′-GATTATCATTGTCGACGG-3′) | ||||
| Quinolone resistance genes | ||||
| gyrA | 5′-CGACCTTGCGAGAGAAAT-3′ | 58 | 625 | 9 |
| 5′-GTTCCATCAGCCCTTCAA-3′ | ||||
| parC | 5′-CGATTGCCGCCTGAGCCACTT-3′ | 58 | 605 | 9 |
| 5′-GCGAATAAGTTGAGGAATCAG-3′ | ||||
| Integron (classes I, II, and III) | hep35 (5′-TGCGGGTYAARGATBTKGATTT-3′) | 49 | Variable | 43 |
| hep36 (5′-CARCACATGCGTRTARAT-3′) |
Tm, melting temperature.
Primers used for sequencing.
The isolates were evaluated for fluoroquinolone resistance as a result of mutations in the genes encoding the antibiotic targets (GyrA and ParC) by amplifying and sequencing the corresponding genes with the specific primers indicated in Table 1.
The aminoglycoside resistance pattern transferred into E. coli strain C600 suggested the presence of different acc genes. These genes were looked for by PCR and then sequenced by using the specific primers designated in Table 1.
Detection of integrons of classes I, II, and III was carried out by using consensus primers (Table 1).
The various sequences obtained were compared with those of the corresponding genes available in GenBank.
Antibiotic consumption and ratio of urinary tract E. coli isolates resistant to the antibiotics concerned in the geriatric hospital.
The levels of consumption of the most commonly prescribed antibiotics in the geriatric hospital (fluoroquinolones, ceftriaxone, and co-trimoxazole) in the 2 years preceding the study as well as the year during which the study took place were assessed by calculating the defined daily dose (DDD) for 1,000 days of hospitalization. This level of consumption was compared with the ratio of urinary tract E. coli isolates resistant to the antibiotics concerned over these two periods. Although the proportion of patients infected or colonized with several E. coli isolates with the same antibiotic susceptibility patterns was small during each of the years studied (13 of 297 patients in 2000, 21 of 267 patients in 2001, and 69 of 463 patients in 2002), only one E. coli isolate with a given antibiotic susceptibility pattern was retained per patient per year.
Implementation of specific hygiene measures in the acute-care hospital for patients transferred from the geriatric hospital.
The staff of each ward of the Hôpital A. Paré, an acute-care hospital, was informed of the presence of MDR E. coli isolates in the affiliated geriatric hospital. They were asked to (i) implement isolation barriers for each patient transferred from the geriatric hospital and (ii) to obtain a rectal swab and a urinary sample on the day of admission in order to detect any possible carriage of an MDR E. coli isolate. The isolation barriers were interrupted when the patient was found to be negative for these isolates but were maintained until the patient's discharge when the patient was found to be positive.
RESULTS
Emergence and spread of multiple-antibiotic-resistant E. coli isolates.
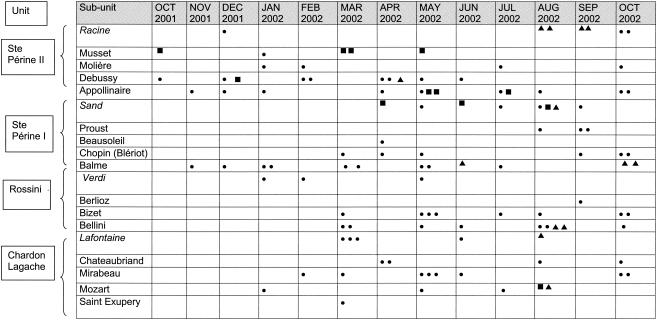
Between October 2001 and October 2002, the only enterobacterial isolates displaying an ESBL production phenotype were E. coli isolates. These isolates had identical β-lactam resistance patterns (resistance to amoxicillin, ticarcillin, piperacillin, cephalothin, cefuroxime, cefotaxime, ceftazidime, cefepime, and aztreonam; susceptibility to cefoxitin, imipenem, and piperacillin-tazobactam; and intermediate susceptibility to amoxicillin-clavulanate and ticarcillin-clavulanate), all isolates were resistant to fluoroquinolones, and all isolates were susceptible to co-trimoxazole and chloramphenicol. However, they differed from each other in their susceptibilities to the other antibiotics tested. One type of isolate, which emerged on 2 October 2001, was resistant to tetracycline and all aminoglycosides except gentamicin. This type and the first corresponding isolate were named GEN S and GEN Sa, respectively. A second type of isolate, which emerged 22 days after the first type had emerged in the same unit but in a different subunit, was still resistant to tetracycline but was also resistant to all aminoglycosides, including gentamicin. This type and its first isolate were named GEN R and GEN Ra, respectively. The third type of E. coli isolate, which emerged in April 2002 in the same unit as the first two, was susceptible to both tetracycline and all aminoglycosides and was therefore designated AMG S, and the first isolate of this type was designated AMG Sa. As indicated in Fig. 1, isolates of the GEN S and AMG S types spread into the different units and subunits of the geriatric hospital, whereas isolates of the GEN R type spread only in the unit where the first isolate of this type had emerged. The first isolate of type GEN S was thought to be imported into the geriatric hospital, as it was isolated from the urine of a patient on the day after his admission. No arguments to lead us to believe that this was also the case for the first isolates of the two other types were found.
FIG. 1.
Monthly distribution of new patients with urinary tract colonization or infection due to the three types (GEN S, GEN R, and AMG S) of E. coli with an ESBL production phenotype by unit and the long-term-care and rehabilitation subunits of the geriatric hospital over the study period (October 2001 to October 2002). Italic names indicate the rehabilitation subunits. Each symbol refers to one isolate of E. coli type GEN S (•), GEN R (▪), and AMG S (▴).
Overall, over the study period, 87 new patients had urinary tract colonization or infection with an E. coli isolate of type GEN S, 12 had urinary tract colonization or infection with an E. coli isolate of type GEN R, and 13 had urinary tract colonization or infection with an E. coli isolate of type AMG S. No cases of bacteremia due to these isolates were observed.
Molecular typing of E. coli isolates with and without an ESBL production phenotype.
The nine uropathogenic E. coli isolates with an ESBL production phenotype, which corresponded to four isolates of type GEN S, three of type AMG S, and two of type GEN R (Table 2), were typed by the RAPD method. Their RAPD profiles were compared with those of nine randomly selected uropathogenic E. coli isolates that displayed no ESBL production phenotype and that were either susceptible to all of the antibiotics tested or resistant to a few antibiotics, as indicated in Table 2. The nine E. coli isolates with an ESBL production phenotype displayed an identical and unique RAPD profile, whereas except for two isolates (strains 15 and 18), each E. coli isolate with no ESBL production phenotype had a unique profile. Strains 15 and 18 displayed a common unique profile (data not shown), had the same antibiotic resistance pattern, and had been isolated within an interval of 26 days from two patients hospitalized in the same rehabilitation subunit (Table 2).
TABLE 2.
Description of the 18 selected E. coli isolates
| Strain name or no. | Subunita | Isolation date (day-mo-yr) | Antibiotic susceptibility pattern |
|---|---|---|---|
| With ESBL | |||
| GEN Sa | Debussy | 02-10-01 | Multiresistant strain type GEN S |
| GEN Ra | Musset | 22-10-01 | Multiresistant strain type GEN R |
| 3 | Debussy | 07-12-01 | Multiresistant strain type GEN R |
| 4 | Balme | 12-03-02 | Multiresistant strain type GEN S |
| AMG Sa | Debussy | 25-04-02 | Multiresistant strain type AMG S |
| 6 | Mozart | 09-08-02 | Multiresistant strain type GEN R |
| 7 | La Fontaine | 12-08-02 | Multiresistant strain type AMG S |
| 8 | Bellini | 01-10-02 | Multiresistant strain type GEN S |
| 9 | Balme | 05-10-02 | Multiresistant strain type AMG S |
| Without ESBL | |||
| 10 | Racine | 31-05-02 | Susceptible to all antibiotics |
| 11 | St Exupéry | 11-06-02 | Resistant to AMX, TIC, SXT |
| 12 | Molière | 24-06-02 | Resistant to AMX, AMC, CEF, |
| 13 | Racine | 25-06-02 | Susceptible to all antibiotics |
| 14 | Berlioz | 28-06-02 | Resistant to AMX, AMC, CEF, CIP |
| 15 | La Fontaine | 23-08-02 | Resistant to AMX, TIC, SXT |
| 16 | Berlioz | 13-09-02 | Susceptible to all antibiotics |
| 17 | Chopin | 14-09-02 | Susceptible to all antibiotics |
| 18 | La Fontaine | 17-09-02 | Resistant to AMX, TIC, SXT |
Italic names indicate rehabilitation subunits, and nonitalic names indicate long-term-care subunits.
GEN, gentamicin; AMG, aminoglycosides; AMX, amoxicillin; AMC, amoxicillin-clavulanate; TIC, ticarcillin; SXT, co-trimoxazole; CEF, cephalothin; CIP, ciprofloxacin; S, susceptible; R, resistant.
Phylogenetic groups and virulence factor genotypes of E. coli strains GEN Sa, GEN Ra, and AMG Sa and the nine E. coli isolates with no ESBL production phenotype.
As indicated in Table 3, E. coli strains GEN Sa, GEN Ra, and AMG Sa, which correspond to the primary isolates of each of the three types, respectively, belonged to the same phylogenetic group (group B2), harbored the same three virulence factor genes (aer, fyuA, and irp2), and had no determinants for P or S fimbriae. The nine E. coli isolates without an ESBL production phenotype were classified into the four previously described phylogenetic groups (group D, n = 4; group B2, n = 2; group A, n = 2; and group B1, n = 1) and harbored from zero to eight of the nine genes tested (Table 3). Only four of these nine isolates harbored determinants for fimbriae. Strains 15 and 18, whose RAPD profiles and antibiotic resistance patterns were identical, belonged to phylogenetic group B2 and had the same virulence factor genotype (Table 3).
TABLE 3.
Phylogenetic group and virulence factor genes of E. coli strains GEN Ra, GEN Sa, and AMG Sa and the nine uropathogenic E. coli isolates with no ESBL production phenotypea
| Strain | Phylogenetic group | papC | papG allele | hly | cnf1 | sfa/foc | sfaS | aer | fuyA | irp2 |
|---|---|---|---|---|---|---|---|---|---|---|
| With ESBL | ||||||||||
| GEN Ra | B2 | − | − | − | − | − | − | + | + | + |
| GEN Sa | B2 | − | − | − | − | − | − | + | + | + |
| AMG Sa | B2 | − | − | − | − | − | − | + | + | + |
| Without ESBL | ||||||||||
| 10 | B2 | − | − | − | − | − | − | − | + | + |
| 11 | D | − | − | − | − | − | − | + | − | − |
| 12 | B1 | − | − | − | − | − | − | − | − | − |
| 13 | A | − | − | − | − | − | − | − | − | − |
| 14 | A | + | − | − | − | − | − | − | + | + |
| 15 | D | + | II | − | − | − | − | + | + | + |
| 16 | B2 | + | III | + | + | + | + | − | + | + |
| 17 | D | − | − | − | − | − | − | + | + | + |
| 18 | D | + | II | − | − | − | − | + | + | + |
+, presence; −, absence.
Transfer of antibiotic resistance and analysis of the plasmid contents of E. coli strains GEN Sa, GEN Ra, and AMG Sa and their transconjugants.
The transconjugant of E. coli strain GEN Ra displayed the same antibiotic resistance pattern, except for that to quinolones and fluoroquinolones, to which it was susceptible, and the same plasmid content, meaning a plasmid of approximately 120 kb, as E. coli strain GEN Ra (data not shown). The same feature was observed for the antibiotic resistance patterns of the respective transconjugants of E. coli strains GEN Sa and AMG Sa but not for the plasmid contents. In fact, these transconjugants harbored the plasmid of 120 kb but not the plasmid of 7 kb, which was also present in the two donor E. coli strains (data not shown). The identities between the antibiotic resistance patterns of strains GEN Sa and AMG Sa and their respective transconjugants suggested that no resistance-encoding genes were harbored by the plasmid of 7 kb.
Mechanisms of antibiotic resistance displayed by E. coli strains GEN Sa, GEN Ra, and AMG Sa.
E. coli strains GEN Sa, GEN Ra, and AMG Sa produced two β-lactamases, one with a pI of 5.4 and one with a pI of >8.4 and <9.2. No blaSHV gene was found, whereas the blaTEM-1B and the blaCTX-M-15 genes were found in the three strains. The region upstream of the blaCTX-M-15 gene comprised the ISEcp1 insertion sequence separated from the coding region by the same 48-bp nucleotide sequence in the three strains.
E. coli strains GEN Sa and GEN Ra, as well as their respective transconjugants, harbored the aac(6′)-1b gene, whereas the acc(3)-II gene was found only in E. coli strain GEN Ra. None of these genes was detected in E. coli strain AMG Sa.
E. coli strains GEN Ra, GEN Sa, and AMG Sa displayed the same double nucleotide substitution in the quinolone resistance-determining regions of their gyrA and parC genes, which led to the following amino acid substitutions: Ser83Leu and Asp87Asn in GyrA and Ser80Ile and Glu84Val in ParC.
The class I, class II, and class III integrons were not detected in E. coli strain GEN Sa, GEN Ra, or AMG Sa.
Antibiotic consumption and ratio of E. coli isolates resistant to the antibiotics concerned.
As indicated in Fig. 2, the most prescribed antibiotic family, independently of the year, was the fluoroquinolone family. This prescription decreased from 17 to 12 DDDs for 1,000 hospitalization days between 2000 and 2002, whereas the co-trimoxazole prescription increased from 2 to 5 DDDs for 1,000 hospitalization days in the same period. This shift, which was not statistically significant, was, however, correlated to the gradual increase in the ratio of urinary E. coli isolates resistant to fluoroquinolones (12% in 2000, 17% in 2001, and 50% in 2002) and the decrease in the ratio of urinary tract E. coli isolates resistant to co-trimoxazole, notably, between 2001 (35%) and 2002 (18%). On the contrary, no modification in the rates of prescription of ceftriaxone was observed over the 3 years (approximately 7 DDDs for 1,000 hospitalization days), although a significant increase in the ratio of urinary tract E. coli isolates resistant to ceftriaxone was observed in 2002 (43%) in comparison with the ratios observed in 2001 (4%) and 2000 (less of 1%).
FIG. 2.
Consumption of fluoroquinolones (♦), ceftriaxone (•), and co-trimoxazole (×), expressed as the DDDs for 1,000 hospitalization days in the geriatric hospital and the ratio of urinary E. coli isolates resistant to these antibiotics in 2000, 2001, and 2002. The bars represent data for E. coli isolates not susceptible to fluoroquinolones (solid bars), ceftriaxone (stippled bars), and co-trimoxazole (gray bars).
Survey of patients transferred from the geriatric hospital into the acute-care hospital.
The impact of geriatric patient transfer on the introduction and the dissemination of MDR isolates in an acute-care hospital, Hôpital A. Paré, was taken into consideration only when a geriatric patient was found to be positive for ESBL-producing E. coli on the day of admission (in May 2002) to the intensive care medical unit (ICMU), where for a long time systematic samplings had been applied to any transferred patient. A retrospective study showed that approximately seven patients per month had been transferred from the geriatric hospital into 11 wards of the acute-care hospital between October 2001 and May 2002 and that only the two patients (4.6%) transferred into the ICMU had been screened for MDR isolates. However, according to the microbiological database, four patients other than transferred geriatric patients had had a clinical sample positive for E. coli with both the ESBL production phenotype and fluoroquinolone resistance during this period. Therefore, isolation precautions and systematic sampling were recommended from June 2002 in each ward where geriatric patients had been transferred. Thus, 24 of the 35 geriatric patients (68%) transferred into 13 different wards between June and October 2002 were sampled, and 5 (21% of sampled patients and 14% of transferred patients) had a sample positive for ESBL-producing E. coli isolates. This type of isolate was never found in clinical samples from nongeriatric patients.
DISCUSSION
Although the first description of CTX-M-type β-lactamases was from Western Europe in 1992 (4), the geographic areas widely concerned with these enzymes up to the beginning of the 21st century were South America (37), Eastern Europe (13), and Japan (25). In the last 2 years, different studies have highlighted the emergence and the increasing prevalence of these β-lactamases within the ESBL-producing members of the family Enterobacteriaceae in new areas, comprising the United States (39), countries in Western Europe where they were not previously detected (8, 31, 32), and other parts of the world (Australia and South Africa) (31). These class A β-lactamases, called “CTX-M” because of their potent hydrolytic activities for cefotaxime, may be classified into five groups, represented by CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25, respectively (7). Moreover, numerous variants have already been described within each group, including variants displaying increased hydrolytic activities against ceftazidime. Among these ceftazidime-hydrolyzing CTX-M-type enzymes, the CTX-M-15 enzyme seems to be the most common, as isolates producing it have already been described in India (19), Japan (GenBank accession no. AY013478), Poland (3), France (29), the United Kingdom (28), Turkey (22), and Russia (11). Poirel et al. (36) characterized the CTX-M-15 enzyme genetically and biochemically and suggested that the clinical use of ceftazidime should be evaluated for its role in the selection of ceftazidime-hydrolyzing CTX-M-type enzymes. For our part, we observed the emergence and the dissemination of three types of CTX-M-15-producing E. coli isolates in a geriatric hospital where ceftazidime has been extremely rarely prescribed. Subsequently, other factors, including bacterial, host, and environmental factors, were required to explain the persistence of such CTX-M-15-producing strains in the geriatric hospital.
We demonstrated that the three types of CTX-M-15-producing strains (i) belonged to the same clone, despite variable levels of resistance to aminoglycosides and tetracycline, and (ii) harbored a transferable plasmid of the same size on which the blaCTX-M-15 gene was located at a distance of 48 bp downstream from the ISEcp1element, as described previously (19). All these findings were quite different from previous descriptions of the local emergence of CTX-M-type enzymes, which generally showed the concomitant emergence of different E. coli clones that produced the same CTX-M-type enzyme and whose corresponding gene was often harbored by different plasmids and often separated from ISEcp1 by sequences of various sizes (8, 19, 28, 31).
One of our goals was to determine whether our three clonally related strains were more virulent than apparently nonepidemic E. coli isolates without an ESBL production phenotype. We demonstrated that the three strains belonged to the same phylogenetic group (group B2) corresponding to one of the two predominant groups observed among extraintestinal pathogenic E. coli strains (33) and harbored the same three virulence factor determinants which have classically been shown to be associated with urosepsis E. coli strains (6, 17). According to this analysis, the three epidemic CTX-M-15-producing strains had an arsenal of virulence factor determinants more or less robust than that displayed by urinary E. coli strains with no ESBL production phenotype. Among the latter strains, two were demonstrated to be clonally related, suggesting that strains other than those producing ESBL could spread in the geriatric hospital.
Three other remarkable points characterized our three types of CTX-M-15-producing strains. They lacked determinants for P and S fimbriae, they were never responsible for bacteremia, and they were resistant to fluoroquinolones, meaning that they were resistant to the antibiotic family most prescribed in the geriatric hospital. We determined that fluoroquinolone resistance was related to a double amino acid substitution in each of the gyrA and the parC quinolone resistance-determining regions, which has rarely been described. Moreover, we found an amino acid, Val, rarely involved in the Glu84 substitutions in ParC (9, 21, 42).
According to previous publications, the uropathogenic E. coli strains displaying multiple-antibiotic resistance and particularly those resistant to fluoroquinolones had a decreased invasive capacity (41) and harbored fewer virulence factor genes than susceptible strains (16, 17). In 1988, Johnson et al. (17) found that the urosepsis E. coli strains which lacked determinants for P fimbriae and hemolysin were significantly more prevalent among the multiple-antibiotic-resistant strains and were isolated exclusively from compromised patients. Lautenbach et al. (23), who in 2001 investigated the risk factors for fluoroquinolone resistance in ESBL-producing E. coli and Klebsiella pneumoniae isolates, found three independent risk factors: fluoroquinolone use, aminoglycoside use, and residence in a long-term-care facility. Interestingly, all the features determined in those different studies except aminoglycoside use were present in the microbiological and clinical situation described in the present study. Is the presence of all of these factors sufficient to explain the amplitude of the outbreak observed in the geriatric hospital? As we demonstrated that these strains did not disseminate in the acute-care hospital, in which isolation precautions were established for each patient transferred from the geriatric hospital, it seems reasonable to suggest that cross-transmission was one of the main environmental factors involved in this outbreak.
Conclusion.
CTX-M-type enzymes, whose presence is now described all over the world, appear to be a new challenge for the medical community, as they occur in a wide range of species and in clones of given species and plasmids, probably in relation to the fact that the corresponding genes are located downstream of an insertion sequence element (an ISEcp1-like element) which seems to act as a key factor in the dissemination of blaCTX-M type genes and as a strong positive factor for the expression of these genes (35). However, to our knowledge, outbreaks due to CTX-M-type enzyme-producing isolates have essentially been described so far in countries where CTX-M-type-producing isolates are endemic, notably, Japan (20, 26) and South America (37). The present article, which reports on the dissemination of clonally related CTX-M-15-producing E. coli strains in a French 650-bed geriatric hospital, might prefigure the near future for Western European countries, which now seem to be concerned with CTX-M-type enzymes.
Acknowledgments
We thank P. Nordmann for providing us with E. coli strain NCTC 50192 and E. Collatz for help with aminoglycoside resistance analysis.
This work was supported by grants from the Direction de la Recherche des Etudes Doctorales, Ministère de l'Education Nationale, Paris, France.
REFERENCES
- 1.Anonymous. 2003. Comité de l'Antibiogramme de la Société Française de Microbiologie report 2003. Int. J. Antimicrob. Agents 21:364-391. [DOI] [PubMed] [Google Scholar]
- 2.Armand-Lefèvre, L., V. Leflon-Guibout, J. Bredin, F. Barguellil, A. Amor, J. M. Pagès, and M. H. Nicolas-Chanoine. 2003. Imipenem resistance in Salmonella enterica serovar Wien related to porin loss and CMY-4 β-lactamase production. Antimicrob. Agents Chemother. 47:1165-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraniak, A., J. Fiett, W. Hryniewicz, P. Nordmann, and M. Gniadkowski. 2002. Ceftazidime-hydrolyzing CTX-M-15 extended spectrum β-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 50:393-396. [DOI] [PubMed] [Google Scholar]
- 4.Barthelemy, M., J. Peduzzi, H. Bernard, C. Tancrede, and R. Labia. 1992. Close amino acid sequence relationship between the new plasmid-mediated extended spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim. Biophys. Acta 13:15-22. [DOI] [PubMed] [Google Scholar]
- 5.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardin, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of E. coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 6.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Eschecrichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou, G., M. Cartelle, M. Tomas, D. Canle, F. Molina, R. Moure, J. M. Eiros, and A. Guerrero. 2002. Identification and broad dissemination of the CTX-M-14 β-lactamase in different Escherichia coli strains in the northwest area of Spain. J. Clin. Microbiol. 40:4030-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J. Y., L. K. Siu, Y. H. Chen, P. L. Lu, M. Ho, and C. F. Peng. 2001. Molecular epidemiology and mutations at gyrA and parC genes of ciprofloxacin-resistant Escherichia coli isolates from a Taiwan medical center. Microb. Drug Resist. 7:47-53. [DOI] [PubMed] [Google Scholar]
- 10.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein, M., M. Pimkin, I. Palagin, I. Edelstein, and L. Stratchounski. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother. 47:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner, J. S., and The Hospital Infection Control Practices Advisory Committee. 1996. Guideline for isolation precautions in hospitals. Infect. Control Hosp. Epidemiol. 17:53-80. [DOI] [PubMed] [Google Scholar]
- 13.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouby, A., C. Neuwirth, G. Bourg, N. Bouziges, M. J. Carles-Nurit, E. Despaux, and M. Ramuz. 1994. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J. Clin. Microbiol. 32:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. R., M. A. Kuskowski, K. Owens, A. Gajewski, and P. L. Winokur. 2003. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 188:759-768. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. R., S. L. Moseley, P. L. Roberts, and W. E. Stamm. 1988. Aerobactin and other virulence factor genes among strains of Escherichia coli causing urosepsis: association with patient characteristics. Infect. Immun. 56:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu, M., N. Ikeda, M. Aihara, Y. Nakamachi, S. Kinoshita, K. Yamasaki, and K. Shimakawa. 2001. Hospital outbreak of MEN-1-derived extended spectrum β-lactamase-producing Klebsiella pneumoniae. J. Infect. Chemother. 7:94-101. [DOI] [PubMed] [Google Scholar]
- 21.Komp Lindgren, P., A. Karlsson, and D. Hughes. 2003. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 47:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lartigue, M. F., L. Poirel, C. Héritier, V. Tolün, and P. Nordmann. 2003. First description of CTX-M-15-producing Klebsiella pneumoniae in Turkey. J. Antimicrob. Chemother. 52:315-316. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbach, E., B. L. Strom, W. B. Bilker, J. B. Patel, P. H. Edelstein, and N. O. Fishman. 2001. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin. Infect. Dis. 33:1288-1294. [DOI] [PubMed] [Google Scholar]
- 24.Leflon-Guibout, V., G. Ternat, B. Heym, and M. H. Nicolas-Chanoine. 2002. Exposure to co-amoxiclav as a risk factor for co-amoxiclav-resistant Escherichia coli urinary tract infection. J. Antimicrob. Chemother. 49:367-371. [DOI] [PubMed] [Google Scholar]
- 25.Ma, L., Y. Ishii, M. Ishiguro, H. Matsuzawa, and K. Yamaguchi. 1998. Cloning and sequencing of the gene encoding Toho-1, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob. Agents Chemother. 42:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, L., H. Matsuo, Y. Ishii, and K. Yamaguchi. 2002. Characterization of cefotaxime-resistant Escherichia coli isolates from a nosocomial outbreak at three geriatric hospitals. J. Infect. Chemother. 8:155-162. [DOI] [PubMed] [Google Scholar]
- 27.Mariotte, S., P. Nordmann, and M. H. Nicolas. 1994. Extended-spectrum β-lactamase in Proteus mirabilis. J. Antimicrob. Chemother. 33:925-935. [DOI] [PubMed] [Google Scholar]
- 28.Mushtaq, S., N. Woodford, N. Potz, and D. M. Livermore. 2003. Detection of CTX-M-15 extended-spectrum β-lactamase in the United Kingdom. J. Antimicrob. Chemother. 52:528-529. [DOI] [PubMed] [Google Scholar]
- 29.Neuwirth, C., E. Siebor, M. Pruneaux, M. Zarnayova, C. Simonin, J. P. Kisterman, and R. Labia. 2003. First isolation of CTX-M-15-producing Escherichia coli from two French patients. J. Antimicrob. Chemother. 51:471-473. [DOI] [PubMed] [Google Scholar]
- 30.Nicolle, L. E., L. J. Strausbaugh, and R. A. Garibaldi. 1996. Infections and antibiotic resistance in nursing homes. Clin. Microbiol. Rev. 9:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagani, L., E. Dell'Amico, R. Migliavacca, M. M. D'Andrea, E. Giacobone, G. Amicosante, E. Romero, and G. M. Rossolini. 2003. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 41:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson, D. L., K. M. Hujer, A. M. Hujer, B. Yeiser, M. D. Bonomo, L. B. Rice, R. A. Bonomo, and the International Klebsiella Study Group. 2003. Extended-spectrum β-lactamase in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob. Agents Chemother. 47:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Eliion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitout, J. D. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, E. S. Moland, and C. C. Sanders. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poirel, L., J. W. Decousser, and P. Nordmann. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of blaCTX β-lactamase gene. Antimicrob. Agents Chemother. 47:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolyzing extended-spectrum β-lactamase CTX-M-15 and of its structurally-related β-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 37.Radice, M., P. Power, J. Di Conza, and G. Gutkind. 2002. Early dissemination of CTX-M-derived enzymes in South America. Antimicrob. Agents Chemother. 46:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saladin, M., C. Van Thi Bao, T. Lambert, J. L. Donay, J. L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M β-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 39.Smith Moland, E., J. A. Black, A. Hossain, N. D. Hanson, K. S. Thomson, and S. Pottumarthy. 2003. Discovery of CTX-M-like extended-spectrum β-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob. Agents Chemother. 47:2382-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speldooren, V., B. Heym, R. Labia, and M.-H. Nicolas-Chanoine. 1998. Discriminatory detection of inhibitor-resistant β-lactamases in Escherichia coli by single strand conformational polymorphism-PCR. Antimicrob. Agents Chemother. 42:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velasco, M., J. P. Horcajada, J. Mensa, A. Moreno-Martinez, J. Vila, J. A. Martinez, J. Ruiz, M. Barranco, G. Roig, and E. Soriano. 2001. Decreased invasive capacity of quinolone-resistant Escherichia coli in patients with urinary tract infections. Clin. Infect. Dis. 133:1682-1686. [DOI] [PubMed] [Google Scholar]
- 42.Vila, J., J. Ruiz, P. Goni, and M. Teresa Jimenez De Anta. 1996. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob. Agents Chemother. 40:491-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, P. A., J. C. McIver, and W. D. Rawlison. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiener, J., J. P. Quinn, P. A. Bradford, R. V. Goering, C. Nathan, K. Bush, and R. A. Weinstein. 1999. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281:517-523. [DOI] [PubMed] [Google Scholar]