Abstract
Solid organ transplant recipients have increased colorectal cancer (CRC) risk. We assessed CRC risk among transplant recipients and identified factors contributing to this association. The U.S. transplant registry was linked to 15 population-based cancer registries (1987–2010). We compared CRC risk in recipients to the general population using standardized incidence ratios (SIRs) and identified CRC risk factors using Poisson regression. Based on 790 CRCs among 224,098 transplants, recipients had elevated CRC risk (SIR=1.12, 95%CI:1.04–1.20). The increase was driven by an excess of proximal colon cancer (SIR=1.69, 95%CI:1.53–1.87), while distal colon cancer was not increased (SIR=0.93, 95%CI:0.80–1.07), and rectal cancer was reduced (SIR=0.64, 95%CI:0.54–0.76). In multivariate analyses, CRC was increased markedly in lung recipients with cystic fibrosis (incidence rate ratio [IRR]=12.3, 95%CI:6.94–21.9, vs. kidney recipients). Liver recipients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease also had elevated CRC risk (IRR=5.32, 95%CI:3.73–7.58). Maintenance therapy with cyclosporine and azathioprine was associated with proximal colon cancer (IRR=1.53; 95%CI:1.05–2.23). Incidence was not elevated in a subgroup of kidney recipients treated with tacrolimus and mycophenolate mofetil, pointing to the relevance of the identified risk factors. Transplant recipients have increased proximal colon cancer risk, likely related to underlying medical conditions (cystic fibrosis and PSC) and specific immunosuppressive regimens.
Keywords: colorectal cancer, anatomic sub-site, solid organ transplant, SIR
Introduction
Solid organ transplant recipients are at increased risk of cancer (1;2), especially virus-related cancers, suggesting that the increase is due to loss of immune control of oncogenic viruses (2). Most studies, although not all, have also reported an increased risk of colorectal cancer (CRC) among solid organ transplant recipients relative to the general population, with standardized incidence ratios (SIRs) ranging from no association to a 4.5-fold increase (1–10), and an overall SIR estimate of 1.69 reported from a meta-analysis (1). However, CRC is not known to be caused by a viral infection, and CRC risk is not elevated among human immunodeficiency virus (HIV)-infected people, who are also immunosuppressed (1). This raises the possibility of a different underlying biological mechanism.
Underlying medical conditions that often lead to organ transplantation may contribute to the increased CRC risk in transplant recipients. For example, a recent U.S. study (11) found evidence of increased CRC risk among cystic fibrosis (CF) patients, and risk was further elevated in those who received lung transplants. CF is the third leading indication for lung transplantation (11;12). Furthermore, up to 80% of people with primary sclerosing cholangitis (PSC, an indication for liver transplantation) have inflammatory bowel disease (IBD), with the majority being ulcerative colitis (UC), which is a risk factor for CRC (13–15).
Current U.S. recommendations for CRC screening in the general population include fecal occult blood testing, sigmoidoscopy, or colonoscopy from age 50 until 75 years (16). Screening reduces the incidence of CRC by detecting and removing precancerous polyps, and it improves cancer prognosis and outcomes by identifying some CRCs at localized stage. It is possible that CRC screening practices differ for transplant recipients because of their condition before transplant and close medical care after transplant.
While an overall increase in CRC after transplantation has been documented, previous studies have been small or have not identified the underlying factors that may contribute to risk (3;5–10;17–19). A few studies have looked at risk for colon and rectal cancers separately (3;17;20); however, due to small numbers, they have not been able to definitively assess possible differences in CRC risk by anatomical location (i.e., sub-site: proximal colon, distal colon, and rectum). To comprehensively characterize the relationship between solid organ transplantation and CRC, we used data from a large registry linkage study to evaluate transplant-related factors that may contribute to CRC risk.
Materials and Methods
Study Population
We used data from the U.S. Transplant Cancer Match (TCM) Study, which has been described in detail (2). Briefly, the Scientific Registry of Transplant Recipients (SRTR), which includes data from all U.S. solid organ transplant recipients since 1987, was linked with 15 U.S. population-based cancer registries. The TCM study currently includes 46% of the U.S. transplant population through 2010. The study was approved by the National Cancer Institute’s ethics committee and by the human subjects committees of participating cancer registries as required. It was exempted from human subjects approval by the Health Resources and Services Administration and the North Carolina Cancer Registry.
For this study, we excluded recipients with a prior CRC (n=606) and with HIV infection (n=388). We evaluated CRC incidence from the date of transplantation or start of cancer registry coverage, whichever came later, until the first of the following events: diagnosis with incident CRC, failure of a transplanted organ, a subsequent transplant, death, loss to follow-up by the SRTR, or end of cancer registry coverage. Transplants occurring at different times in the same individual were considered separately. CRC was identified in transplant recipients using linked cancer registry data, based on the following International Classification of Disease for Oncology (3rd edition) topography codes: C18 for any colon cancer, and C19 and C20 for rectal cancer. We further investigated CRC risk specific to the sub-sites of proximal colon (cecum through transverse colon, C18.0–18.4); distal colon (splenic flexure through sigmoid colon, C18.5–18.7); and rectum (codes C19.9–20.9). Lymphomas, Kaposi sarcoma, and cancers with unspecified histologic subtype were excluded (2).
Statistical Analyses
We compared CRC risk in solid organ transplant recipients to the general population by calculating standardized incidence ratios (SIRs) as the number of cancers observed in the transplant cohort (determined from the linkage to cancer registries) divided by the number expected in the general population. Expected counts were estimated from general population cancer registry data using rates specific to age, sex, race/ethnicity, calendar year, and registry area (2). We present SIRs for overall CRC, by anatomic sub-site, and by cancer stage. We also evaluated SIRs stratified by transplanted organ (kidney, liver, heart, lung, or other/multiple), medical indication leading to the need for transplantation (such as CF and PSC), and medication used for transplant induction or maintenance. To explore a potential role of increased CRC screening in transplant recipients, we further calculated SIRs by sub-site separately for <50 and ≥50 year-olds.
Information about immunosuppressive medication use was available only at the time of transplant. For maintenance medications, we created a three-level variable to reflect medications that are commonly prescribed together: 1) cyclosporine and azathioprine but not tacrolimus or mycophenolate mofetil (MMF); 2) tacrolimus and MMF but not cyclosporine or azathioprine; 3) all other possibilities grouped as “other.”
To identify risk factors for CRC among transplant recipients, we used Poisson regression to estimate incidence rate ratios (IRRs) overall and separately for each sub-site. In univariate models, we examined recipient demographic characteristics (age, sex, race/ethnicity), transplant factors (organ type, medical indication, immunosuppressive medications, time since transplant, HLA mismatch), and other known CRC risk factors (overweight body mass index (BMI), diabetes mellitus (21)). Based on results from univariate models, multivariate models evaluated the independent roles of transplanted organ, indication for transplantation, induction and maintenance medications, time since transplant, and calendar year, with adjustment for age, sex, Hispanic ethnicity, BMI, and diabetes (see Table 3 footnote for details).
Table 3.
Multivariate associations between transplant factors and colorectal cancer incidence among transplant recipients, overall and by anatomic sub-site
| Characteristic | All CRC (n=790) IRR (95% CI) |
Proximal colon (n=408) IRR (95% CI) |
Distal colon (n=195) IRR (95% CI) |
Rectum (n=146) IRR (95% CI) |
|---|---|---|---|---|
| Organ and medical condition | ||||
| Kidney | Reference | Reference | Reference | Reference |
| Heart | 0.99 (0.79, 1.23) | 1.18 (0.87, 1.59) | 0.75 (0.48, 1.17) | 0.92 (0.55, 1.54) |
| Liver, no PSC | 1.03 (0.85, 1.25) | 1.32 (1.01, 1.73) | 0.64 (0.42, 0.96) | 1.01 (0.65, 1.58) |
| Liver, PSC without IBD | 2.72 (1.64, 4.52) | 3.78 (1.97, 7.25) | 2.29 (0.83, 6.31) | 1.67 (0.41, 6.91) |
| Liver, PSC and IBD | 5.32 (3.73, 7.58) | 7.91 (5.03, 12.5) | 3.43 (1.56, 7.52) | 2.08 (0.64, 6.72) |
| Lung, no CF | 1.88 (1.33, 2.64) | 2.07 (1.30, 3.32) | 1.72 (0.85, 3.47) | 1.65 (0.70, 3.90) |
| Lung, CF | 12.3 (6.94, 21.9) | 17.8 (7.91, 40.1) | 16.2 (5.95, 44.3) | 2.93 (0.39, 22.1) |
| Other or multiple | 1.08 (0.71, 1.63) | 1.52 (0.88, 2.65) | 0.63 (0.23, 1.74) | 0.83 (0.35, 2.00) |
| Time since transplant | ||||
| <2 years | Reference | Reference | Reference | Reference |
| 2 – <4 years | 1.10 (0.89, 1.37) | 1.19 (0.88, 1.60) | 1.05 (0.68, 1.62) | 1.03 (0.63, 1.68) |
| 4 – <6 years | 1.20 (0.95, 1.52) | 1.24 (0.89, 1.72) | 1.13 (0.70, 1.83) | 1.26 (0.74, 2.14) |
| 6 – <8 years | 1.23 (0.94, 1.60) | 1.10 (0.75, 1.61) | 1.65 (0.99, 2.73) | 0.99 (0.51, 1.94) |
| 8 – <12 years | 1.86 (1.43, 2.42) | 1.51 (1.04, 2.19) | 2.05 (1.21, 3.48) | 2.49 (1.39, 4.47) |
| >=12 years | 1.91 (1.30, 2.79) | 1.82 (1.09, 3.04) | 1.76 (0.78, 3.97) | 2.04 (0.80, 5.18) |
| Induction medication | ||||
| None | Reference | Reference | Reference | Reference |
| Any | 0.97 (0.82, 1.15) | 1.10 (0.87, 1.39) | 0.78 (0.55, 1.10) | 0.81 (0.55, 1.20) |
| Maintenance medications | ||||
| Tacrolimus/MMF | Reference | Reference | Reference | Reference |
| Cyclosporine/azathioprine | 1.14 (0.87, 1.48) | 1.53 (1.05, 2.23) | 0.76 (0.45, 1.29) | 0.87 (0.47, 1.59) |
| Other | 1.04 (0.85, 1.28) | 1.29 (0.96, 1.73) | 0.82 (0.54, 1.24) | 0.85 (0.54, 1.35) |
| Calendar year of transplant | ||||
| 1987–1994 | Reference | Reference | Reference | Reference |
| 1995–2002 | 1.06 (0.71, 1.59) | 1.12 (0.62, 2.02) | 1.17 (0.53, 2.56) | 0.98 (0.39, 2.46) |
| 2003–2006 | 0.88 (0.56, 1.39) | 1.26 (0.66, 2.40) | 0.73 (0.30, 1.78) | 0.57 (0.20, 1.62) |
| 2007–2010 | 0.69 (0.43, 1.13) | 0.79 (0.39, 1.58) | 0.58 (0.22, 1.51) | 0.61 (0.20, 1.84) |
Model are adjusted for age (modeled with one degree of freedom across categories of 0–17, 18–34, 35–49, 50–64, and 65+ years), sex, Hispanic ethnicity, body mass index (underweight, normal weight, overweight, obese, missing), and diabetes mellitus (yes, no, missing).
CRC=colorectal cancer; CF=cystic fibrosis; PSC=primary sclerosing cholangitis; IBD=inflammatory bowel disease; MMF=mycophenolate mofetil; IRR=incidence rate ratio; CI=confidence interval
Role of the funding source
This study was funded in part by the Intramural Research Program of the National Cancer Institute. Funding for the infrastructure of the TCM was provided by additional sources as detailed in the Funding section. H. Robbins was supported in part by the Cancer Epidemiology, Prevention, and Control Training Grant (NCI T32 CA009314). The funders had no role in the design, conduct, or reporting of the findings.
Results
A total of 224,098 solid organ transplants were included in our study (Table 1). The majority of transplants occurred in males (61.2%) and whites (61.5%), and in those over 35 years old (median age 48 years). Kidney was the most commonly transplanted organ (58.2%) followed by liver (21.8%), heart (9.9%), and lung (4.2%). Median follow-up was 3.7 years (interquartile range [IQR] 1.3–7.1 years). Eighteen percent of transplant recipients were known to be obese (BMI over 30 kg/m2), and 21.3% had diabetes.
Table 1.
Demographic characteristics of solid organ transplant recipients (at the time of transplant), U.S. Transplant Cancer Match Study
| Characteristic | Transplants (N=224,098) |
% |
|---|---|---|
| Sex | ||
| Male | 137,063 | 61.2 |
| Female | 87,035 | 38.8 |
| Age at transplant, years | ||
| 0–19 | 19,531 | 8.7 |
| 20–34 | 33,351 | 14.9 |
| 35–49 | 69,056 | 30.8 |
| 50–64 | 82,299 | 36.7 |
| 65+ | 19,861 | 8.9 |
| Race/ethnicity | ||
| White, non-Hispanic | 137,721 | 61.5 |
| Black, non-Hispanic | 38,815 | 17.3 |
| Hispanic | 35,208 | 15.7 |
| Asian/Pacific Islander | 12,354 | 5.5 |
| Transplanted organ | ||
| Kidney | 130,336 | 58.2 |
| Liver | 48,835 | 21.8 |
| Heart | 22,093 | 9.9 |
| Lung | 9,443 | 4.2 |
| Other or multiple | 13,391 | 6.0 |
| Body mass index | ||
| Underweight | 14,993 | 6.7 |
| Normal | 75,117 | 33.5 |
| Overweight | 58,861 | 26.3 |
| Obese | 39,558 | 17.7 |
| Missing | 35,569 | 15.9 |
| Diabetes mellitus | ||
| Yes | 47,714 | 21.3 |
| No | 130,738 | 58.2 |
| Missing | 45,646 | 20.4 |
Overall, there were 790 incident CRCs in this cohort, with a median age at cancer diagnosis of 63 years (IQR 55–68 years). The majority of CRCs (52%) were cancers of the proximal colon (n=408), while 25% were of the distal colon (n=195), and 18% (n=146) were of the rectum; the remaining 5% (n=41) were not-specified colon cancers. Compared to the general population, transplant recipients had an elevated risk of CRC overall (SIR=1.12, 95%CI 1.04–1.20; Figure 1). This excess was driven by increased risk of tumors in the proximal colon (SIR=1.69, 95%CI 1.53–1.87), as no elevated risk was observed for distal colon cancer (SIR=0.93, 95%CI 0.80–1.07), and a reduced risk was observed for rectal cancer (SIR=0.64, 95%CI 0.54–0.76).
Figure 1. Standardized incidence ratios for colorectal cancer among transplant recipients.
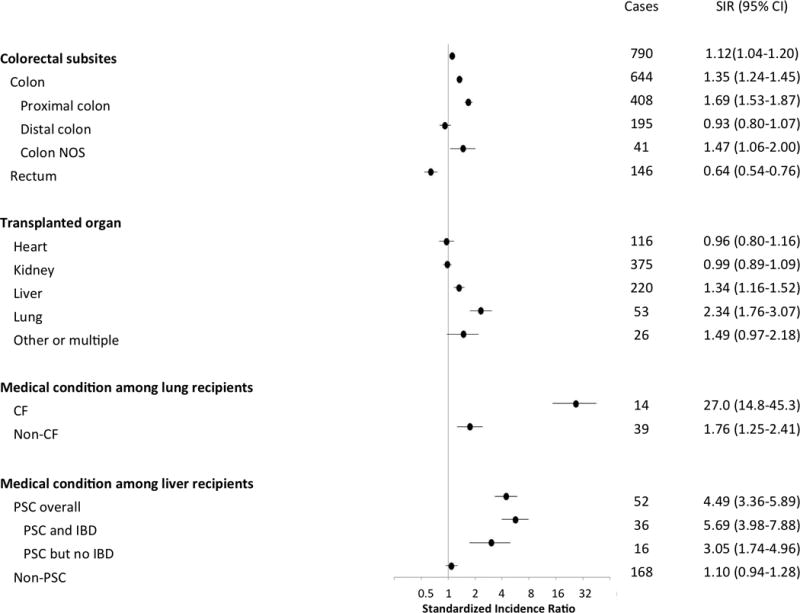
The figure presents standardized incidence ratios by anatomic sub-site, transplanted organ, and medical conditions leading to transplantation. The horizontal axis uses a logarithmic scale.
Abbreviations: CF=cystic fibrosis; PSC=primary sclerosing cholangitis; IBD=inflammatory bowel disease; NOS=not otherwise specified.
Examination of risk by transplanted organ revealed that the highest SIRs for CRC were among lung and liver transplant recipients (SIR=2.34, 95%CI 1.76–3.07 and SIR=1.34, 95%CI 1.16–1.52, respectively, Figure 1). Notably, recipients undergoing lung transplantation for CF had much higher risk than the general population (SIR=27.0, 95%CI 14.8–45.3), and risk was also substantially elevated among those with PSC as an indication for liver transplant (SIR=4.49, 95%CI 3.36–5.89). Although the SIR was higher among those PSC patients who had IBD (SIR=5.69, 95%CI 3.98–7.88), PSC with no report of IBD was also associated with elevated risk (SIR=3.05, 95% CI 1.74–4.96).
An evaluation of risk according to time since transplant showed that the risk of proximal colon cancer was elevated within two years of transplantation (SIR=1.36, 95%CI 1.09–1.67), increasing over time to an SIR of 2.68 (95%CI 1.91–3.65) for recipients living 12 or more years post-transplantation (Figure 2). In contrast, the reduced risk of rectal cancer was generally constant over time since transplant. Although there was a suggestive increase in distal colon cancer starting at six years post-transplantation, risk was not significantly different from the general population in any time interval. Evaluation of risk by cancer stage showed that SIRs for proximal colon cancer were consistently elevated for all cancer stages, while the decreased risk for rectal cancer was strongest for regional and distant stage tumors (Table 2).
Figure 2. Colorectal cancer risk among transplant recipients according to time since transplantation.
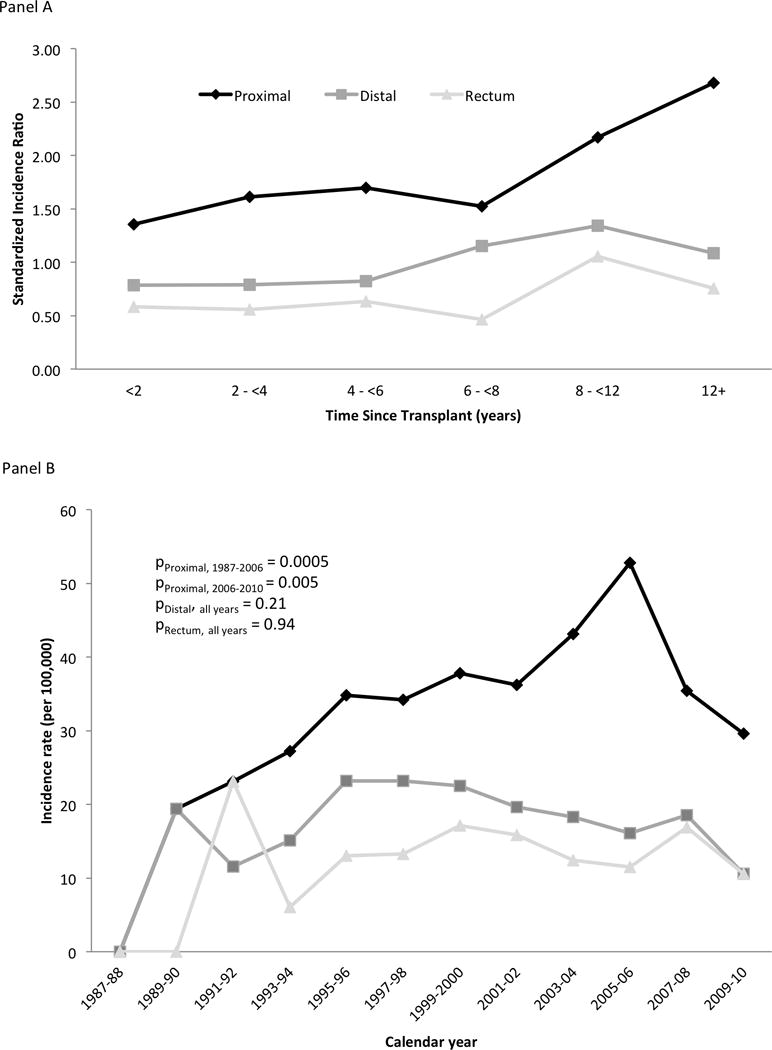
Panel A shows standardized incidence ratios for CRC (y-axis) according to time since transplantation (x-axis). Diamond markers correspond to proximal colon cancer, square markers to distal colon cancer, and triangle markers to rectal cancer. In panel A, the dashed line at a standardized incidence ratio of 1.0 indicates risk similar to the general population.
Table 2.
Standardized incidence ratios for colorectal cancer among transplant recipients by cancer sub-site and stage
| CRC Sub-site | Stage | CRC Cases (N) | SIR (95% CI) |
|---|---|---|---|
| Overall CRC | Local | 326 | 1.21 (1.09, 1.36) |
| Regional | 247 | 0.97 (0.85, 1.09) | |
| Distant | 178 | 1.31 (1.26, 1.52) | |
| Unknown | 39 | 0.84 (0.60, 1.14) | |
| Proximal Colon | Local | 152 | 1.80 (1.53, 2.11) |
| Regional | 144 | 1.45 (1.23, 1.71) | |
| Distant | 94 | 1.98 (1.60, 2.42) | |
| Unknown | 18 | 1.82 (1.08, 2.88) | |
| Distal Colon | Local | 86 | 1.02 (0.81, 1.26) |
| Regional | 64 | 0.85 (0.66, 1.09) | |
| Distant | 41 | 1.03 (0.74, 1.39) | |
| Unknown | 4 | 0.38 (0.10, 0.98) | |
| Colon NOS | Local | 11 | 2.26 (1.13, 4.04) |
| Regional | 3 | 0.56 (0.12, 1.65) | |
| Distant | 19 | 1.83 (1.10, 2.87) | |
| Unknown | 8 | 1.10 (0.47, 2.16) | |
| Rectum | Local | 77 | 0.82 (0.65, 1.02) |
| Regional | 36 | 0.47 (0.33, 0.66) | |
| Distant | 24 | 0.63 (0.41, 0.94) | |
| Unknown | 9 | 0.47 (0.22, 0.90) |
CRC=colorectal cancer; NOS=not otherwise specified; SIR= standardized incidence ratio; CI=confidence interval. 1987–2010 used for calculating SIRs.
We also examined risk separately for recipients below or above age 50 years, the recommended age to begin CRC screening. Incidence of proximal colon cancer was elevated for both age <50 years (SIR=2.52, 95%CI 1.78–3.46) and ≥50 years (SIR=1.64, 95%CI 1.48–1.82). Incidence of distal colon cancer was significantly elevated in recipients aged <50 years (SIR=1.77; 95% CI 1.18–2.54), but was decreased in those aged ≥50 years (SIR=0.86; 95% CI: 0.73–1.00). For rectal cancers, incidence was similar to the general population among recipients aged <50 years (SIR=1.15, 95%CI: 0.74–1.69), but significantly reduced among those aged ≥50 years (SIR=0.59, 95%CI: 0.49–0.70). To confirm that that the increases in those aged <50 years were not due to recipients with CF (who were mostly <50 years old), we excluded recipients with CF, which did not alter these observations (not shown).
Univariate associations of selected demographic and transplant-related factors with CRC incidence are shown in eTables 1 and 2, respectively. In multivariate models (Table 3), CRC incidence increased significantly with increasing time since transplant (p-trend=0.0002 for overall CRC), with the greatest increase 8 years after transplant (IRR=1.86, 95%CI 1.43–2.42; and IRR=1.91, 95%CI 1.30–2.79, respectively for 8–12 years and greater than 12 years after transplant, compared with recipients <2 years after transplant). CRC risk was increased in lung recipients with CF compared with kidney recipients (IRR=12.3, 95% CI 6.94–21.9); this was strongest for proximal colon cancer (IRR=17.8), followed by distal colon cancer (IRR=16.2). While the risk associated with CF appeared elevated for rectal cancer, the association was not statistically significant (IRR=2.93, 95% CI: 0.39–22.1). Liver recipients with concurrent PSC and IBD also had elevated risk of CRC compared to kidney recipients (IRR=5.32, 95% CI: 3.73–7.58); again, the strongest association was for proximal colon cancer (IRR=7.91). Liver recipients with PSC (but no IBD) also had increased CRC risk; in contrast, CRC risk for liver recipients without PSC was similar to kidney recipients.
As shown in Table 3, maintenance immunosuppression with cyclosporine/azathioprine was associated with increased risk of proximal colon cancer (IRR=1.53, 95%CI: 1.05–2.23, compared to use of tacrolimus/MMF). As azathioprine may be used to treat PSC, we conducted a sensitivity analysis that excluded recipients with PSC, and the association persisted (not shown). Induction immunosuppressive medication use was not associated with CRC after adjustment, overall or by sub-site (Table 3)
In an effort to investigate whether the elevation in CRC risk is mainly confined to recipients with the risk factors we identified (i.e. those with CF, PSC, and treatment with cyclosporine/azathioprine), we examined CRC risk in a subgroup that had none of these risk factors. Specifically, we estimated the SIR among kidney transplant recipients who were treated with tacrolimus/MMF. The SIR for overall CRC was 0.79 (95% CI: 0.64–0.98). Furthermore, the incidence of proximal colon cancer was not elevated (SIR=0.92, 95% CI: 0.65–1.27).
Discussion
In this large study of over 224,000 solid organ transplants in the U.S., we found an increased risk of CRC among transplant recipients compared to the general population. To our knowledge this is the first report to provide estimates for CRC risk among transplant recipients by anatomic sub-site. Of note, risk of proximal colon cancer was significantly higher than in the general population, risk of distal colon cancers was similar to the general population, and risk was actually reduced for rectal cancer. Regardless of anatomic sub-site, risk was highest among recipients living 8 or more years after transplant, which suggests a potential etiologic role for long-term immunosuppression or other chronic effects of transplantation medications.
Further, our analysis demonstrates strongly elevated risk of proximal and distal colon cancers associated with underlying medical conditions that are indications for transplantation. In our study, lung recipients with CF had a greatly increased risk of proximal and distal colon cancers. Cystic fibrosis is a genetic disorder caused by mutations in the cystic fibrosis transmembrane regulator (CFTR) gene, resulting in epithelial changes in the respiratory tract and similar abnormalities of the gastrointestinal system, which may predispose to the development of colon cancer (12).
Liver recipients with both PSC and IBD also had a substantially elevated risk of proximal and distal colon cancers. Recipients with PSC but no documented IBD had increased proximal colon cancer risk, which might reflect an independent effect of PSC, occult IBD at the time of transplant, incomplete reporting of IBD by transplant programs, or development of IBD after transplantation (22;23). Our results are consistent with previous studies that reported very high risk of CRC among liver recipients with PSC (24–26). PSC is a chronic, progressive liver disease that involves the intra-hepatic and extra-hepatic bile ducts and can lead to end-stage liver disease requiring transplantation. Up to 80% of people with PSC have IBD, primarily ulcerative colitis. PSC patients are known to have increased risk of CRC, and PSC patients with UC have higher CRC risk than those with UC only (13–15), as such there are specific CRC screening recommendations for patients with PSC that include surveillance with colonoscopy (27).
Cyclosporine and azathioprine, used as maintenance immunosuppressive medications to prevent graft rejection, were associated in our study with increased risk of proximal colon cancer compared with tacrolimus and MMF. Both cyclosporine and azathioprine are classified as Group I carcinogens by IARC (28) and are linked to non-Hodgkin lymphoma (28). Increased cancer risk with cyclosporine and/or azathioprine has been observed in this cohort previously, specifically in relation to melanoma (29), Merkel cell carcinoma (30), post-transplant hepatobiliary carcinomas (31), and some anogenital cancers (32). The adverse association with cyclosporine/azathioprine maintenance therapy was present after we excluded recipients with PSC, suggesting that the increased risk was not due to confounding related to other indications for azathioprine.
Interestingly, in an exploratory subgroup analysis, we found that CRC risk was not elevated among kidney transplant recipients treated with tacrolimus/MMF, whom we selected as a subgroup lacking the transplant-related CRC risk factors that we identified. Along with the observation that HIV-infected people do not have an elevated risk of CRC (1), these findings suggest that the elevated risk in the overall transplant population may largely be explained by the underlying medical conditions and use of azathioprine/cyclosporine, rather than by the presence of immunosuppression.
An important limitation of our study is that we did not have individual level information on colorectal screening practices before or after transplantation. Nonetheless, some of our findings might be tentatively interpreted with regard to likely patterns of screening. In the age-stratified analysis, we observed significant deficits in risk for distal colon and rectal cancers among recipients ≥50 years old (the recommended age for CRC screening in the U.S.). In contrast, the risks for these cancers were similar to or higher than the general population among recipients <50 years old. Risk for proximal colon cancer was increased in recipients both under and over age 50.
One possible way to explain these results might be that transplant recipients in our study were screened more frequently than the general population, and given the reduced risk of distal colon and rectal cancers but not proximal colon cancer, that this screening may have been primarily sigmoidoscopy which does not reach the proximal colon. There has been a gradual shift over time from use of sigmoidoscopy to colonoscopy for CRC screening in the U.S. (33). The excess cases of proximal colon cancer that we observed may therefore represent failures in screening and might be preventable through enhanced screening efforts. However, it is possible that risks associated with colonoscopy (such as complications of anesthesia, bleeding, or infection following a biopsy) could be greater in transplant recipients than the general population, so clinicians must weigh these risks against the potential benefit. We emphasize that given the absence of data on screening in our population, these interpretations must be considered speculative.
Although the reasons for the reduced risk of rectal cancer compared to the general population are unknown, our results are consistent with other studies that showed lower rectal cancer risk in kidney and heart recipients (17;20). As mentioned above, it is possible that this reduced risk is due to increased screening compared to the general population. This possibility is further supported by the observation that the reduced risk was most pronounced for regional and distant stage rectal cancers, as would be seen if sigmoidoscopy screening had a down-staging effect (34).
The large sample size, well-characterized transplant population representative of the overall US transplant population (2), and case ascertainment through population-based cancer registries are substantial strengths of this study. Given the large number of CRC cases, we were able to conduct in-depth analyses of factors associated with CRC arising post-transplant both overall and by sub-site. Out-migration and the associated loss to follow-up are generally low in this cohort (2), but if this occurred, it would have led to slight underestimation of the SIRs. Although we lacked information about screening, the duration of use and dose of specific immunosuppressive medications, and use of non-steroidal anti-inflammatory medications, our study had information on other important factors such as the underlying medical condition leading to transplantation. We did not have information on other potential risk factors for CRC such as smoking and diet. It is unclear whether these risk factors differ substantially between transplant recipients and people in the general population, or if the associations with these risk factors are strong enough to explain our findings.
In summary, our registry linkage study of solid organ transplant recipients revealed an increased risk of CRC, particularly proximal colon cancer. Multiple factors may contribute to this increased risk, including underlying medical conditions that are indications for transplant, use of specific maintenance medications, and possibly screening practices and/or long-term immunosuppression. Since there are no generally accepted guidelines for CRC screening among transplant recipients, clinicians have presumably based decisions on guidelines for the general population (35). However, our study suggests that additional or more frequent screening may be warranted for some patients undergoing transplantation. The benefits of screening must be weighed against the risks, but especially for subgroups at highest risk such as CF and PSC patients, it is likely that screening would be of benefit. Further prospective studies should determine the degree to which CRC morbidity and mortality among transplant recipients could be reduced through better screening, and characterize the safety of different screening approaches.
Reference List
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011 Nov 2;306(17):1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003 Oct 6;89(7):1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009 Oct 15;125(8):1747–54. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 5.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007 Apr;7(4):941–8. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 6.Kyllonen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13(Suppl 1):S394–S398. doi: 10.1007/s001470050369. [DOI] [PubMed] [Google Scholar]
- 7.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010 Aug;10(8):1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Villeneuve PJ, Wielgosz A, Schaubel DE, Fenton SS, Mao Y. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant. 2010 Mar;10(3):637–45. doi: 10.1111/j.1600-6143.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008 Nov;14(11):1588–97. doi: 10.1002/lt.21554. [DOI] [PubMed] [Google Scholar]
- 10.Aberg F, Pukkala E, Hockerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008 Oct;14(10):1428–36. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 11.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013 Jan 16;105(2):122–9. doi: 10.1093/jnci/djs481. [DOI] [PubMed] [Google Scholar]
- 12.Meyer KC, Francois ML, Thomas HK, Radford KL, Hawes DS, Mack TL, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros. 2011 Sep;10(5):366–9. doi: 10.1016/j.jcf.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009 Jan;50(1):158–64. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002 Jul;56(1):48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 15.Tsaitas C, Semertzidou A, Sinakos E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J Hepatol. 2014 Apr 27;6(4):178–87. doi: 10.4254/wjh.v6.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 17.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006 Dec 20;296(23):2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 18.Roithmaier S, Haydon AM, Loi S, Esmore D, Griffiths A, Bergin P, et al. Incidence of malignancies in heart and/or lung transplant recipients: a single-institution experience. J Heart Lung Transplant. 2007 Aug;26(8):845–9. doi: 10.1016/j.healun.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JH, Koh SJ, Kim JY, Kim JW, Lee KL, Kim BG, et al. Prevalence of advanced colorectal neoplasm after kidney transplantation: surveillance based on the results of screening colonoscopy. Dig Dis Sci. 2015 Jun;60(6):1761–9. doi: 10.1007/s10620-015-3525-z. [DOI] [PubMed] [Google Scholar]
- 20.Stewart T, Henderson R, Grayson H, Opelz G. Reduced incidence of rectal cancer, compared to gastric and colonic cancer, in a population of 73,076 men and women chronically immunosuppressed. Clin Cancer Res. 1997 Jan;3(1):51–5. [PubMed] [Google Scholar]
- 21.Giovannucci E, Wu K. Cancers of the Colon and Rectum. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. pp. 809–29. [Google Scholar]
- 22.Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, et al. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006 Jun;6(6):1422–9. doi: 10.1111/j.1600-6143.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 23.Hampton DD, Poleski MH, Onken JE. Inflammatory bowel disease following solid organ transplantation. Clin Immunol. 2008 Sep;128(3):287–93. doi: 10.1016/j.clim.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen KK, Lindstrom L, Cvancarova M, Castedal M, Friman S, Schrumpf E, et al. Colorectal neoplasia in patients with primary sclerosing cholangitis undergoing liver transplantation: a Nordic multicenter study. Scand J Gastroenterol. 2012 Sep;47(8–9):1021–9. doi: 10.3109/00365521.2012.685754. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen KK, Lindstrom L, Cvancarova M, Karlsen TH, Castedal M, Friman S, et al. Immunosuppression After Liver Transplantation for Primary Sclerosing Cholangitis Influences Activity of Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2013 Jan 16; doi: 10.1016/j.cgh.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Vera A, Gunson BK, Ussatoff V, Nightingale P, Candinas D, Radley S, et al. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003 Jun 27;75(12):1983–8. doi: 10.1097/01.TP.0000058744.34965.38. [DOI] [PubMed] [Google Scholar]
- 27.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010 Feb;51(2):660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 28.IARC. Monographs on the evaluation of carcinogenic risks to humans, volume 100-A. A review of human carcinogens. 2008 [Google Scholar]
- 29.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, et al. Melanoma Risk and Survival Among Organ Transplant Recipients. J Invest Dermatol. 2015 Aug 13; doi: 10.1038/jid.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015 Feb;107(2) doi: 10.1093/jnci/dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koshiol J, Pawlish K, Goodman MT, McGlynn KA, Engels EA. Risk of Hepatobiliary Cancer After Solid Organ Transplant in the United States. Clin Gastroenterol Hepatol. 2013 Dec 19; doi: 10.1016/j.cgh.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013 Dec;13(12):3202–9. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012 Jun;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012 Jun 21;366(25):2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009 Nov;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]


