Abstract
Background
The corpus callosum (CC) has been implicated in the pathogenesis of schizophrenia and bipolar disorder. Yet, it remains unclear whether CC alterations are related to the underlying familial diathesis for psychotic disorders. We examined CC, its sub-region volumes, and their relationship to cognition, psychotic symptoms and age in subjects with schizophrenia (SZ), psychotic bipolar disorder (PBD), schizoaffective disorder (SZA), their first-degree relatives and in healthy controls.
Methods
We present findings from morphometric and neurocognitive analyses of 1429 subjects [SZ (n=224), PBD (n=190), SZA (n=142), unaffected relatives n= 483) [SZ relatives (195), PBD relatives (175), SZA relatives (113)], and Controls (n=342)]. MPRAGE T1 scans across 5 sites were obtained using 3.T magnets. Image processing was done using FreeSurfer 5.1. Neurcognitive function was measured using the Brief Assessment of Cognition in Schizophrenia (BACS).
Results
Anterior and posterior splenia volumes were significantly reduced across the groups. SZ and PBD Probands showed robust and significant reductions while relatives showed significant reductions of intermediate severity. The splenial volumes were positively but differentially correlated with aspects of cognition in the probands and their relatives. Proband groups showed a significant age-related decrease in the volume of the anterior splenium in comparison with controls. Among the psychosis groups, the anterior splenium in PBD showed a stronger correlation with psychotic symptomatology as shown by the PANSS. All five sub regions showed significantly high familiality.
Conclusions
The splenial volumes were significantly reduced across the psychosis dimension. However, this volume reduction impacts cognition and clinical manifestation of the illnesses differentially.
Keywords: Psychosis, Schizophrenia, Schizoaffective, Bipolar, Corpus-Callosum, neuroimaging
1. INTRODUCTION
The corpus callosum (CC) is the largest white matter commissure connecting the two brain hemispheres, and consists of approximately 300–350 million fibers in humans (1,2). While these numbers are relatively fixed during development (3), fiber myelination continues throughout puberty, accounting for developmental morphological changes (3,4). The CC has an important role in the integration and communication of high-level motor, cognitive and sensory information (5). The CC can be considered as a set of multiple and overlapping transcallosal channels, defined by the number, type, and destination of the fibers in different sub-regions of the callosum (6).
The sub-regions of CC are topographically mapped to corresponding cortical regions. Thus, fibers running through the rostrum and genu interconnect the frontal association cortical areas. The midbody interconnects motor, somatosensory, and auditory cortices. Anterior splenial fibers connect the temporo-parietal association areas forming a single segment with the hippocampal commissure through which parahippocampal fibers cross. The posterior splenium interconnects the visual cortices. Fibers with large diameter (3–5 µm) are densest in the midbody. Small fibers (< 0.4 µm) are more numerous in the genu and anterior splenium interconnecting higher-order prefrontal and temporo-parietal association cortices. The largest fibers in the corpus callosum interconnect the primary auditory cortices (7,8). In healthy brains, the number of callosal fibers is dependent on developmental processes including axon outgrowth, synaptogenesis, and axon pruning (8), and are regulated by genetics (9).
CC has been well studied in schizophrenia (SZ) and bipolar disorder (PBD), but less so in schizoaffective disorder (SZA) (10,11); this structure has rarely been compared within the same study across these disorders. Abnormalities in transcallosal connectivity have been suggested to play a role in the pathogenesis of SZ (12–16) and PBD (17,18). The callosal fibers connecting the language centers of the prefrontal and temporoparietal cortices bilaterally (i.e. splenium and genu) have been specifically implicated (19,20). The splenium was positively correlated with verbal fluency and negatively with language lateralization in healthy women (21). These white matter abnormalities could possibly reflect alterations in myelin, axon membranes or axonal packing density (22–24). The loss of normal asymmetry resulting in reduced laterality is thought to underlie inter-hemispheric connectivity disturbances in SZ (25). An early meta-analysis of 11 studies (26) and a recent meta-analysis [Arnone et al., (27)] of 28 studies showed that the CC area was reduced in SZ in comparison to healthy controls, especially in first episode patients. An early study by Keshavan et al (2002) showed CC reductions in first episode patients (29); A meta-analysis in PBD examining 5 studies (28) showed that most CC areas were reduced in PBD. Thus, defects in interhemispheric communication may cut across psychotic disorders, irrespective of clinical diagnosis. Nevertheless, the CC literature in psychotic disorders remains somewhat inconsistent. A review of structural findings in SZ found that among 27 MRI studies of the CC, 17 (63%) reported positive findings while 10 (37%) did not (30).
The corpus callosum normally expands in size from childhood through adolescence (31,32). The age-related increase found in controls was absent in first episode schizophrenia (29). There is also evidence that white matter regions including the corpus callosum decrease with age in healthy middle aged and in elder subjects (33). However, it is not known whether such age-related CC changes are altered in SZ and related psychotic disorders.
The question of whether CC alterations might reflect the underlying familial susceptibility to psychotic disorders has not been well studied. Our group reported CC reductions in young at-risk relatives (34). One study found that volume and fractional anisotropy (FA) was reduced in patients and relatives in the whole CC, the inferior genu, the superior genu and the isthmus (35). Relatives had intermediate values in the volumetric and fiber integrity measurements between patients and controls. However, Johnson et al (2013) failed to observe CC alterations in healthy siblings of childhood onset SZ patients (36). Few studies have examined CC in relatives of SZA or PBP patients. Impaired inter-hemispheric communication has been thought to underlie the pathogenesis of psychotic symptoms (37). Lower CC volume and fiber integrity in SZ, and impaired frontotemporal connectivity have been associated with more severe auditory hallucinations (38). The CC also plays an important role in the integration of higher order cognitive functions such as verbal memory, working memory, motor speed, attention, executive functions and verbal fluency among others (reviewed in Schulte T, Müller-Oehring EM. (2010) (39). The relationships between psychotic symptoms, cognition and CC structural integrity, however have not been examined across the psychosis spectrum.
In summary, there is considerable evidence for CC abnormalities in SZ and PBD probands. Yet, important questions remain. Are the patterns of alterations similar or different across the three psychotic disorders? Are specific CC sub-regions more impaired than others? Do CC alterations in relatives exist and do the pattern of findings in relatives suggest familiality? Does their volume change with age at different rates in psychotic disorder probands than is seen in healthy controls? To answer these questions, we examined the CC in a large sample of 1429 subjects recruited via the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) consortium. We also performed a heritability analysis on the CC subregions to see if, as suggested by earlier studies [reviewed in Kaymaz and van Os, 2009 (40)], CC alterations might qualify as endophenotypes (41). Our hypotheses were that: a) Total CC and individual regional volumes of the CC would be reduced across the psychosis spectrum; b) In the probands, reduced total CC and individual volumes of the CC would be associated with higher psychosis symptom scores; c) Decreased total CC and individual volumes of the CC would be associated with impaired cognitive performance as measured by the Brief Assessment of Cognition in Schizophrenia (BACS); and d) CC and its subregional volumes would be familial.
2. METHODS
2.1 Subjects
Subjects (n=1501) were recruited as part of the BSNIP consortium, a multi-site collaboration (Wayne State University/Harvard University, Maryland Psychiatric Research Center, University of Chicago/University of Illinois at Chicago, University of Texas–Southwestern, and the Institute of Living/Yale University). Seventy-one relative subjects who had an axis I psychotic disorder and one subject with a missing diagnosis were excluded. 1429 participants [SZ (n=224), PBD (n=190), SZA (n=142), relatives with no psychotic symptoms (RNPS)(n= 483) [SZ relatives (195), PBD relatives (175), SZA relatives (113)], and healthy controls (n=342)] were included in the analyses. Among patients, all but 70 (SZ=11, SZA=14, PBD=45) were taking antipsychotics and 60 (SZ=12, SZA=8, PBD=40) were taking lithium (Supplemental Table S1). Dose calculations of antipsychotic medications were standardized across drugs using the method by Andreasen and colleagues (42).
Participants underwent a diagnostic interview using the Structured Clinical Interview for DSM-IV-TR (SCID-IV) (43). Symptom ratings were completed with probands using the Positive and Negative Symptom Scale (PANSS)(44), the Montgomery Asberg Depression Rating Scale (MADRS) (45), and the Young Mania Rating Scale (YMRS) (46). We assessed cognitive function using the BACS scale [Brief assessment of cognitive function in Schizophrenia (47)]. The cognitive functions measured by the BACS included verbal memory, working memory, motor speed, verbal fluency, attention and speed of information processing and executive functions.
MRI-Structural Imaging
High resolution isotropic T1-weighted MPRAGE scans (TR=6.7 msec, TE= 3.1 msec, 8° flip angle, 256×240 matrix size, total scan duration=10:52.6 minutes, 170 sagittal slices, 1mm slice thickness, 1×1×1.2 mm3 voxel resolution) were obtained in line with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol (http://www.loni.ucla.edu/ADNI). All raw images underwent rigorous data quality control. Images were converted to NIFTI format first and checked for scanner artifacts by trained raters. On passing this pre-check, the images were run through a first-level auto-reconstruction (auto-recon1) in FreeSurfer v5.1 (48). The extracted brains were checked for traces of dura or sinus that could interfere with accurate segmentation. When non-brain tissue was found, trained raters blind to subject identity edited images manually. All raters (AF, IM, NT) had inter-rater reliabilities (intra-class r) above 95%. When deemed sufficiently clean for segmentation by an independent rater, images were run through auto-recon 2 & 3, after which CC volume measures were extracted.
Outliers
Subjects with CC sub-regions exceeding three standard deviations from the mean (n=27; HC=11, SZ=3, SZA=6, BP=7) were excluded from the analysis.
2.2. Partitions of the Corpus Callosum using FreeSurfer 5.1
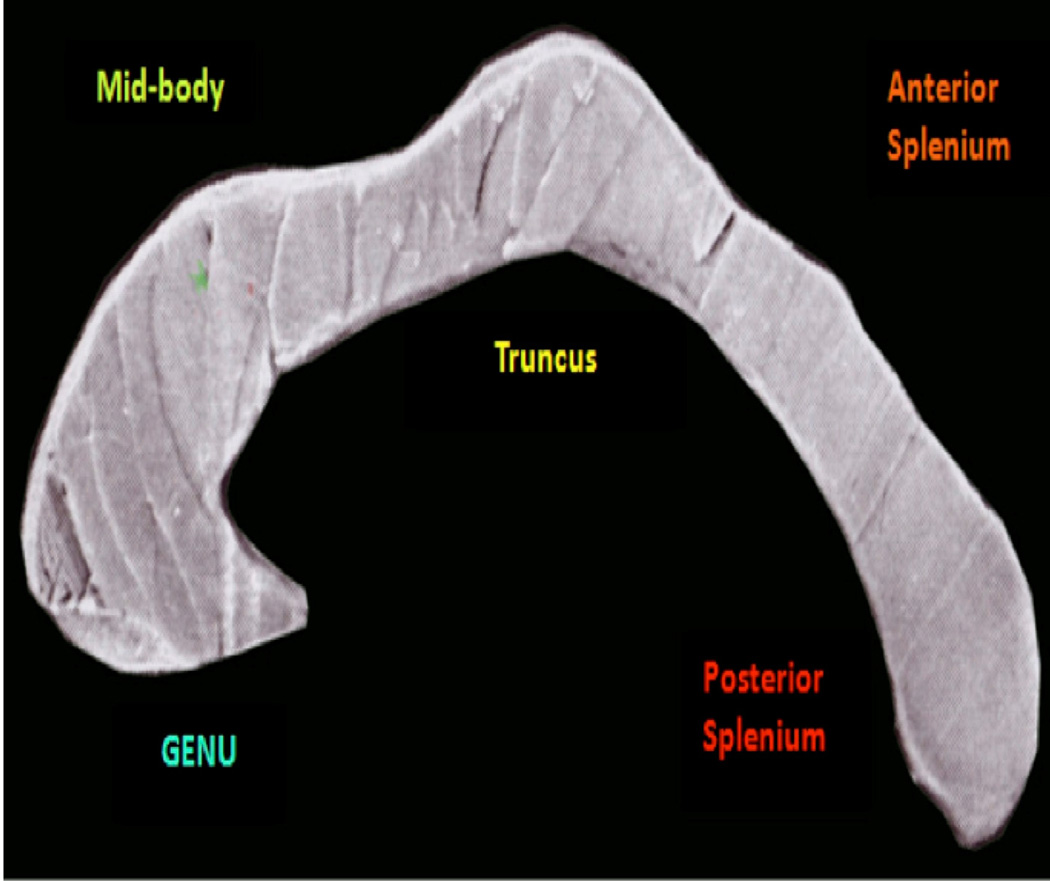
We used a classification based on Witelson at al, (1985) (49), to partition the CC. Using FreeSurfer 5.1, the CC was aligned to the ACPC plane and divided into five subregions: the anterior sixth (genu), the anterior half minus the anterior sixth (truncus), posterior half minus the posterior third (midbody), posterior third minus the posterior one-fourth (anterior splenium) and the posterior one-fourth (posterior splenium). The volume of the CC was taken as the sum total of the area of the CC in the mid-sagittal slice and two sagittal slices to the right and left side.
2.3. Statistical Analysis
We used Statistica 10 and R-Studio for statistical analysis. Our analysis strategy incorporated multivariate analyses to examine the effect of diagnosis and family membership on CC volume in comparison with a matched healthy control group. In addition to total CC volume, each callosal sub-regional volume was used as a dependent variable, with study group, site and gender as categorical predictors, and age, and intra cranial volume (ICV) as covariates. To correct for multiple comparisons, we used the Benjamini Hochberg (50) method. We performed planned pairwise univariate GLM analysis as a final method of analysis on CC sub-regions with a significant group effect. We ran Spearman rho correlations between sub-regions that showed significant differences between the groups and measures of symptom severity such as the PANSS, MADRS and YMRS since the data was not normally distributed. We also performed partial correlations controlling for age, and ICV, between significantly altered CC volumes and measures of cognition within the BACS inventory. Finally, the age range in our sample was 15 – 65. We examined the relationship of age and CC volumes in two ways: 1.age as a continuous variable and 2. as a categorical variable (into bins of 10 years: 15 – 25, – 26 – 35; 36–45; 46–55 and 56 –65). Age was used as a categorical variable to examine interactions with Diagnosis, sex and site. Effect sizes (Partial Eta squared) were calculated using pooled standard deviations and residualized means adjusted for covariates.
2.4. Familiality Analysis
To estimate familiality of the callosal volumes, we used Sequential Oligogenic Linkage Analysis Routines (SOLAR) software package. Heritability (h2) was estimated as the ratio of the variance of the individual measure explained by additive polygenic effects to the total variance for the individual measure. In the univariate heritability analyses, each callosal volume was used as a single dependent variable in the SOLAR models.
3. RESULTS
3.1 Effect of Diagnosis
Patients with psychotic disorders [SZ, SZA, PBD] had significantly smaller CC subregional volumes compared to controls [Wilks lambda=.95, F(15, 2269.6)=3.0573, p<0.001)] (see Table 1). Volumes of posterior and anterior splenia differed across groups after Hochberg correction for multiple comparisons. Site had a significant effect (p < 0.02) across all comparisons. However, there was no group × Site interaction.
Table 1. Effect of Diagnosis.
| CC region |
Controls (342) |
SZ (224) | SZA(142) | BD (190) | Effe ct Size ** |
F | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mea n |
SD | Mean | SD | ||||
| Total CC |
3164 .7 |
491 | 3094 .9 |
540 .7 |
303 2 |
549 .8 |
3005 .8 |
509 .6 |
0.01 | 5.4 1 |
<0.00 1 |
| GENU | 883 | 154 .6 |
880. 8 |
171 .7 |
861 | 163 .6 |
852. 3 |
156 .1 |
0.00 5 |
1.7 4 |
.157 |
| Truncu s |
460 | 94. 2 |
457. 9 |
108 | 446 | 99 | 434. 8 |
91. 4 |
0.00 8 |
2.4 7 |
0.061 |
| Midbo dy |
445. 9 |
95. 5 |
433. 7 |
90 | 427 .7 |
98 | 420. 2 |
94. 2 |
0.01 | 4.8 | 0.002 |
| Anteri or Spleni um |
431 | 96 | 405 | 98. 6 |
399 .4 |
96 | 403. 8 |
102 .3 |
0.02 | 8.0 3 |
<0.00 1* |
| Posteri or Spleni um |
945. 7 |
156 .4 |
916. 4 |
167 .5 |
897 .7 |
175 .1 |
894. 5 |
160 | 0.02 | 5.5 | <0.00 1* |
Corrected for multiple comparisons using Hochberg Method 1
- Partial Eta square
3.2 Effects in families with a schizophrenia proband
SZ, SZ relatives and controls were compared. A significant difference between the groups was obtained [Wilks lambda=.97, F(10, 1966)=3.3095, p<0.001)] (seen in Table 2). The total CC, anterior splenium and posterior splenium were significantly different across the groups and survived multiple comparisons.
Table 2. Effect of SZ Risk.
| CC region |
Controls (342) | SZ (224) | SZ-Rel (195) | Effect Size** |
F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Total CC | 3164.7 | 491 | 3094.1 | 540.7 | 3097.9 | 463.4 | 0.01 | 4.6 | 0.01 |
| GENU | 883 | 154.6 | 880.9 | 170.2 | 873 | 148.3 | 0.001 | 0.84 | .432 |
| Truncus | 460 | 94.2 | 457.9 | 107.6 | 446.4 | 86.7 | 0.003 | 1.22 | .29 |
| Midbody | 445.9 | 95.5 | 433.7 | 91 | 443.2 | 83.4 | 0.01 | 5.0 | 0.007 |
| Anterior Splenium |
431 | 96 | 405 | 98.3 | 418.8 | 95.2 | 0.02 | 10.9 | < 0.001* |
| Posterior Splenium |
945.7 | 156.4 | 916.4 | 167.5 | 923.2 | 149 | 0.01 | 4.01 | 0.02 |
Corrected for multiple comparisons using Hochberg Method
- Partial Eta squared
3.3. Effect of Bipolar Risk
We compared three groups - PBD probands, PBD relatives and healthy controls. We found a significant difference between the groups in the callosal sub-regions [Wilks lambda=.97, F(10, 1886)=2.5259, p=0.005)] (seen in Table 3). The total CC, midbody, truncus, anterior splenium and posterior splenium were significantly different across the groups. Only the anterior splenium and posterior splenium survived correction for multiple comparisons. Bipolar relatives showed volume reductions intermediate between controls and probands.
Table 3. Effect of Bipolar Risk.
| CC region | Controls (342) | BD (190) | BD-Rel (175) | Effect Size** |
F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Total CC | 3164.7 | 491 | 3005.8 | 509.6 | 3095.5 | 451.1 | 0.02 | 6.6 | < 0.001* |
| GENU | 883 | 154.6 | 852.3 | 156.1 | 878.5 | 139.1 | 0.007 | 2.23 | .109 |
| Truncus | 460 | 94.2 | 434.8 | 91.4 | 447.1 | 99 | 0.01 | 3.3 | 0.04 |
| Midbody | 445.9 | 95.5 | 420.2 | 94.2 | 438.7 | 88.2 | 0.02 | 5.4 | 0.004 |
| Anterior Splenium |
431 | 96 | 403.8 | 102.3 | 408.2 | 90.9 | 0.02 | 6.33 | < 0.002* |
| Posterior Splenium |
945.7 | 156.4 | 894.5 | 160 | 923 | 145.9 | 0.02 | 6.8 | < 0.001* |
Corrected for multiple comparisons using Hochberg Method
- Partial Eta squared
3.4. Effect of Schizoaffective Risk
We compared three groups – SZA probands, SZA relatives and healthy controls. The MANCOVA did not yield a significant difference between the groups.
3.5 Post-hoc single contrast analysis (Table 4)
Table 4. Planned pair-wise comparisons.
| CC -region | Comparison | F | p |
|---|---|---|---|
| Anterior Splenium |
Controls vs. Schizophrenia Probands | 18.44 | < 0.001 |
| Controls vs. Schizophrenia - Relatives | 10.00 | 0.002 | |
| Schizophrenia Prob vs. SZ Relatives | 2.98 | 0.08 | |
| Controls vs. Bipolar Probands | 10.45 | <0.001 | |
| Controls vs. Bipolar relatives | 10.00 | 0.002 | |
| Bipolar Probands vs. Bip Relatives | 2.72 | 0.09 | |
| Controls vs. Schizoaffective Probands | 9.65 | 0.002 | |
| Controls vs. Schizoaffective - Rels | 8.03 | 0.004 | |
| Schizoaffective Prob vs. SZA - Rel | 2.14 | 0.14 | |
| Schizophrenia Prob vs. Schizoaffective Prob | 0.014 | 0.91 | |
| Schizophrenia Prob vs. Bipolar Probands | 0.15 | 0.7 | |
| Schizoaffective Prob vs. Bipolar Probands | 0.04 | 0.83 | |
| Posterior Splenium |
Controls vs. Schizophrenia Probands | 5.51 | 0.019 |
| Controls vs Schizophrenia - Relatives | 2.85 | 0.09 | |
| Schizophrenia Prob vs. SZ Relatives | 4.74 | 0.03 | |
| Controls vs. Bipolar Probands | 11.2 | <0.001 | |
| Controls vs. Bipolar relatives | 2.84 | 0.09 | |
| Bipolar Probands vs. Bip Relatives | 2.72 | 0.09 | |
| Controls vs. Schizoaffective Probands | 3.30 | 0.06 | |
| Controls vs. Schizoaffective - Rels | 1.55 | 0.21 | |
| Schizoaffective Prob vs. SZA - Rel | 1.14 | 0.28 | |
| Schizophrenia Prob vs. Schizoaffective Prob | 0.28 | 0.6 | |
| Schizophrenia Prob vs. Bipolar Prob | 0.59 | 0.44 | |
| Schizoaffective Prob vs. Bipolar Prob | 0.92 | 0.33 |
3.5.1. Controls vs. Probands
We compared probands in all groups with controls separately. Across all comparisons, probands had significantly smaller anterior splenium. By contrast, while SZ and BP probands had significantly smaller posterior splenium, the SZA group showed a trend.
3.5.2. Controls vs. Relatives
Significantly reduced anterior splenium, intermediate between controls and probands, were seen in combined unaffected relative groups. By contrast, in the posterior splenium, SZ and BP relatives showed a trend while SZA relatives’ volume comparison was not significant.
3.5.3. Probands vs. Relatives
In the anterior splenium, trending values were observed in the SZ (F = 2.98; P = 0.08) and BP (F = 2.72; P = 0.09) groups but not the SZA group. By contrast, in the posterior splenium, SZ probands had significantly smaller volumes than relatives while a trend was observed in BP. SZA volumes were non significant.
3.5.4: Proband comparisons
Comparing the 3 proband groups, we found the anterior and posterior splenium volumes were not significantly different.
3.5. Familiality analysis
SOLAR analyses indicated that all the subregions of the CC showed significant heritability ratios across all three disorders. (See supplemental table S2).
3.6. Callosal Volume and Cognitive Function
We examined the association between regional volumes that had shown significant group effects (anterior and posterior splenia) and cognitive function measured by BACS in each of the diagnostic groups and relatives separately. After Hochberg correction, only SZ relatives’ BACS composite score and verbal memory survived and were positively correlated with the posterior splenium (p < 0.001). [supplemental tables S3 & S4]
3.7. Callosal Volume and symptomatology
We ran Spearman RHO correlations to examine the splenia and scores on the PANSS MADRS & YMRS in the proband groups. The anterior splenium was negatively correlated with the PANSS positive score in the SZ probands [r = −0.15 p < 0.03], SZA –BP [r = −0.27 p < 0.01] and with PANSS negative score in SZA [r = −0.55 p < 0.001]. The posterior splenium was negatively correlated with the PANSS total [r = −0.31 p < 0.04] in SZA and PANSS positive in SZA-BP [r = −0.27 p < 0.01] supplemental table S5. The correlations between the anterior and posterior splenium and the MADRS and YMRS were not significant.
3.8. Corpus Callosum & Age
We used group wise partial correlations controlling for ICV to examine the effect of age (as a continuous variable) on the volume of the posterior and anterior splenium. To control the experiment-wise error rate, partial correlations were limited to sub-regions showing volumetric deficits on the interrogative ANCOVAs. Benjamini Hochberg corrections were applied to the two-tailed p values of the correlations to achieve a corrected alpha error of 0.05. While the posterior splenium did not show a significant correlation with age, across the psychosis dimension, the anterior splenium showed a significantly greater decrement of volume with age (See supplemental table S6) than healthy controls.
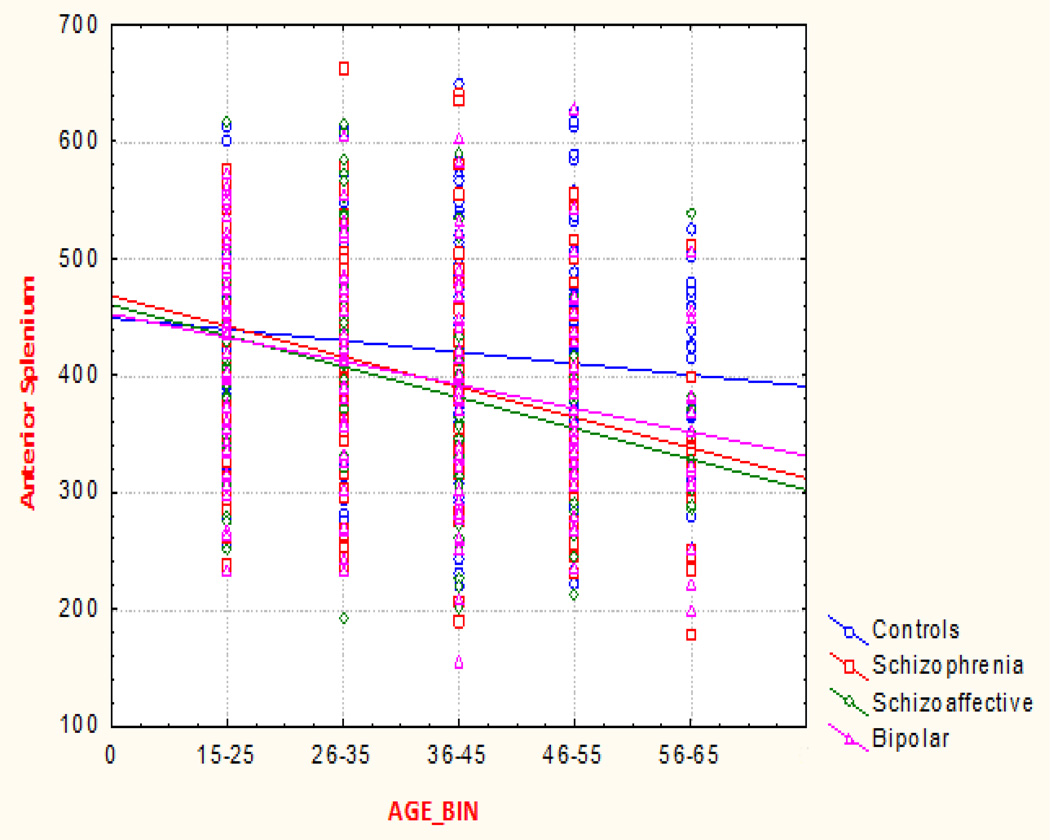
Examining the interaction of Age as a categorical variable with diagnosis in a GLM model, we found significant interactions between diagnosis and age in the anterior splenium (p < 0.023) See Figure 2 below. While controls showed a non-significant decrease over the age span, proband groups showed a significant age related decrease in the volume of the anterior splenium. The relatives did not show a significant difference from controls in the age continuum.
Figure 2.
Anterior splenium volume across the AGE continuum Psychotic Groups only (p < 0.02).
3.9 Duration of Illness and anterior splenium
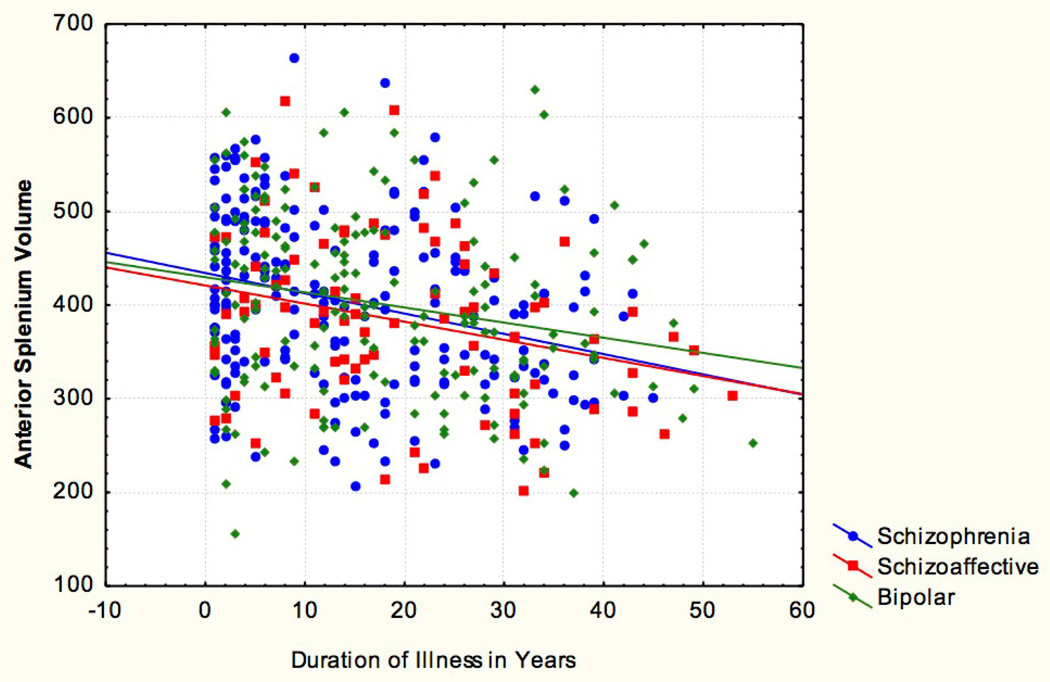
We examined illness duration in the three proband groups (see figure 2). Duration of illness was defined as the difference in time (measured in years) between the year at which a subject was first diagnosed with a psychotic illness and the date of his/ her scan. The proband groups showed a significant negative correlation in volume of the anterior splenium[r = −0.27 p < 0.001] [See supplemental table S7]. This was not observed in the posterior splenium.
3.10 Sex and the splenia
We did not find a significant group × sex interaction across all comparisons.
4. DISCUSSION
The present study examined the morphology of the CC in a large sample of 1429 subjects comprising three psychosis groups, their non-psychotic first-degree relatives and control subjects. While total CC and anterior splenium volume decreases were seen across all proband groups, volume reductions of the posterior splenium were significant in the SZ and PBD proband groups and at a trend-level in SZA. Similarly, relatives of SZ and BP showed a trend in the anterior and posterior splenium while SZA relatives’ volume difference was not significant. Overall, while psychosis probands robustly differed from healthy controls, few differences were seen in CC or its subregions between DSM diagnostic categories. This is consistent with a number of reports from the BSNIP consortium showing relatively few differences across psychotic disorders in most structural, functional and cognitive endophenotypes (Tamminga et al 2014). These findings also confirm previous studies by our group examining the morphology of the CC in early course SZ and their young relatives (29, 34), and extend these findings to other disorders. The subregions of the CC showed a significant degree of heritability as measured by SOLAR in all 3 disorders studied. Thus, CC alterations may reflect a dimensional, familial biomarker related to the risk for psychotic disorders.
Among CC sub-regions, the anterior splenium appears to have the most consistent reduction in the SZ and PBD groups across the psychosis spectrum and in those at familial risk for psychosis. The posterior forceps (splenial fibers) interconnect the parietal and occipital cortices, while the anterior splenial fibers interconnect the inferior and medial temporal and posterior cingulate cortices in a bandage- like manner (51) The splenia can be considered a constituent of the hippocampal commissure which carries fibers connecting the hippocampi together with those linking the posterior parietal, medial temporal and medial occipital cortices of the hemispheres (7,52,53). Our observations of reductions in the anterior splenial volume are consistent with previous results from the BSNIP group showing hippocampal and sub-region volume decrements psychosis probands and their relatives across diagnoses (54, 55). Such alterations could result in decreased or aberrant information flow between the medial temporal structures, parietal cortices, hippocampi and the subfields (35); this interhemispheric hypoconnectivity may be related to abnormal information processing, a core abnormality of these illnesses (14, 56).
In relation to abnormal information processing, we found that the posterior splenium correlated with the composite score and verbal memory in the SZ relatives but not with the other groups. Cognitive impairment may in part result from impaired myelin integrity of the splenium (57, 58). Innocenti (59) proposed that myelination facilitates interhemispheric interaction by enhancing more efficient recruitment of the target neural population to common activity. This facilitation and co-ordination is reduced when splenia are reduced and this may be the reason for impaired cognitive function across the psychosis spectrum (56). Some evidence also points to abnormalities of callosal metabolism and neurotransmission as contributory factors to the altered inter-hemispheric transfer of information across the CC (59). The cognitive manifestations of splenial deficits have been thought to contribute to psychotic symptomatology (61, 62). In line with this, we found modest negative correlations (ranging from r = −0.15 to 0.55) between splenia and positive and negative symptom scores of the PANSS indicating that the greater the psychotic features the smaller the splenia. This is consistent with DTI studies that have shown FA abnormalities of the splenium, which were positively correlated with severity of psychotic symptoms (60–62). Other studies show a correlation between splenial volume and severity of auditory verbal hallucinations (62–64), supporting theories positing the role of the anterior splenium in two core illness domains: psychosis, cognitive/negative symptoms.
Finally, anterior splenium was inversely correlated with age across the psychosis spectrum. The significant interaction of age category and diagnosis shows that illnesses across the psychosis spectrum have different trajectories. In the psychosis groups, an age related decrement in volume was observed. This may be due to an abnormal loss of myelination and/or reduction is axonal numbers and size over time brought about by the neurotoxic effects of psychotic episodes as shown by the relationship between illness duration and anterior splenium volume. This derailment in myelination and/or axonal loss could potentially be the cause of abnormal information transmission and cognitive processing.
Morphometric analyses do not address the question whether the observed CC alterations primarily reflect myelination or axonal integrity, or both. To explore the relationship of splenium and myelination, Fornari et al, (65) used Magnetization Transfer Imaging (MTI) and showed a robust correlation between MTI indices and a child’s age for the splenial area. Fields (66) showed that myelination is a plasticity dependent process. It is possible that the reduced splenial volumes in the present study may reflect altered myelination leading to decreased plasticity, which in turn may be related to impaired cognition and psychotic symptomatology. In sum, the present study has shown that the illnesses across the psychosis dimension and risk for psychoticism share a common neurobiological substrate within the CC: anterior splenium. The anterior splenium partially fulfills the criteria for classification as an intermediate phenotype.
In conclusion, since the CC is purported to be the latest developing higher order neural network in the brain (67), longitudinal studies on CC morphology are crucial to elucidating the relationship of this commissure and the etiopathogenesis of psychotic disorders.
Limitations
The proband groups of our study were under diverse medications with many probands treated with multiple medications. Subjects with longstanding antipsychotic treatment may not accurately reflect original microstructural CC abnormalities representative of these disorders. The effects of neuroleptic/ psychotropic medication on the CC was not significant as shown by a post hoc analysis of chlorpromazine equivalents (CPZ-EQ) data.
Supplementary Material
Figure 1.
Corpus Callosum Anatomical Divisions
Figure 3.
Anterior splenium against Duration of Psychotic Illness r = −0.27 p < 0.001
Acknowledgments
Funding Source: This research was funded in part by NIMH grants MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888.
We thank Dr. Gunvant Thaker for his input and collaboration on this project.
Carol Tamminga has the following disclosures to make: Intracellular Therapies (ITI, Inc.)—Advisory Board, drug development; PureTech Ventures—Ad Hoc Consultant; Eli Lilly Pharmaceuticals—Ad Hoc Consultant; Sunovion—Ad Hoc Consultant Astellas—Ad Hoc Consultant; Cypress Bioscience—Ad Hoc Consultant; Merck—Ad Hoc Consultant; International Congress on Schizophrenia Research—Organizer, unpaid volunteer; National Alliance on Mental Illness—Council Member, unpaid volunteer; American Psychiatric Association— Deputy Editor. Dr. Pearlson has served on an advisory panel for Bristol-Myers Squibb. Dr. Keshavan has received research support from Sunovion and GlaxoSmithKline. Dr. Sweeney has been on advisory boards for Bristol-Myers Squibb, Eli Lilly, Roche, and Takeda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest Statement: All other authors report no biomedical financial interests or potential conflicts of interest.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992a;598(1–2):143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Res. 1992b;598(1–2):154–161. doi: 10.1016/0006-8993(92)90179-d. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010 Aug 18;30(33):10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain. 1997 Jul;120(Pt 7):1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- Yazgan M, Kinsbourne M. Functional consequences of changes in callosal area in Tourette’s syndrome and attention deficit/hyperactivity disorder. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. MIT Press; 2003. pp. 423–432. [Google Scholar]
- Zaidel E, Clarke J, Suyenbu B. Hemispheric independence: A paradigm case for cognitive neuroscience. In: Scheibel AB, Wechsler AF, editors. Neurobiology of higher cognitive function. New York: Guilford Press; 1990. pp. 297–355. [Google Scholar]
- Fabri M, Pierpaoli C, Barbaresi P, Polonara G. Functional topography of the corpus callosum investigated by DTI and fMRI. World J Radiol. 2014 Dec 28;6(12):895–906. doi: 10.4329/wjr.v6.i12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Topography of occipital lobe commissural connections in the rhesus monkey. Brain Res. 1986 Feb 12;365(1):174–178. doi: 10.1016/0006-8993(86)90736-5. [DOI] [PubMed] [Google Scholar]
- Newbury AJ, Rosen GD. Genetic, morphometric, and behavioral factors linked to the midsagittal area of the corpus callosum. Front Genet. 2012 May 31;3:91. doi: 10.3389/fgene.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Rogers J, Barrett EA, Glahn DC, Kochunov P. Genetic contributions to the midsagittal area of the corpus callosum. Twin Res Hum Genet. 2012 Jun;15(3):315–323. doi: 10.1017/thg.2012.10. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58(1):19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, et al. Corpus callosum size and shape in first-episode affective and schizophrenia-spectrum psychosis. Psychiatry Res. 2009 Jul 15;173(1):77–82. doi: 10.1016/j.pscychresns.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, et al. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989 Dec;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Temporolimbic or transcallosal connections: where is the primary lesion in schizophrenia and what is its nature? Schizophr Bull. 1997;23(3):521–523. doi: 10.1093/schbul/23.3.521. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as a transcallosal misconnection syndrome. Schizophr Res. 1998;30(2):111–114. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999 Jan;122(Pt. 1):99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first episode patients with bipolar disorder. Psychol Med. 2007;37:699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007 Aug;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Rev. 2000 Mar;31(2–3):118–129. doi: 10.1016/s0165-0173(99)00029-6. Review. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull. 2001;27(3):481–496. doi: 10.1093/oxfordjournals.schbul.a006889. Review. [DOI] [PubMed] [Google Scholar]
- Hines M, Chiu L, McAdams LA, Bentler PM, Lipcamon J. Cognition and the corpus callosum: verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav Neurosci. 1992 Feb;106(1):3–14. doi: 10.1037//0735-7044.106.1.3. [DOI] [PubMed] [Google Scholar]
- Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B, et al. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr Res. 2009 Mar;108(1–3):41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007 Jan-Feb;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, et al. The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N Y Acad Sci. 2005;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzelier JH. Functional neuropsychophysiological asymmetry in schizophrenia: a review and reorientation. Schizophr Bull. 1999;25(1):91–120. doi: 10.1093/oxfordjournals.schbul.a033370. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, et al. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58(4):457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2008;101(1–3):124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008 Nov;118(5):357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72(6):757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010 Jun;51(2):501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis AN, Bhojraj TS, Prasad KM, Kulkarni S, Montrose DM, Eack SM, et al. Abnormalities of the corpus callosum in non-psychotic high-risk offspring of schizophrenia patients. Psychiatry Res. 2011 Jan 30;191(1):9–15. doi: 10.1016/j.pscychresns.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel C, O'Dwyer L, Alves G, Reinke B, Magerkurth J, Rotarska-Jagiela A, et al. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr Res Sep. 2012;140(1–3):129–135. doi: 10.1016/j.schres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Greenstein D, Clasen L, Miller R, Lalonde F, Rapoport J, et al. Absence of anatomic corpus callosal abnormalities in childhood-onset schizophrenia patients and healthy siblings. Psychiatry Res. 2013 Jan 30;211(1):11–16. doi: 10.1016/j.pscychresns.2012.09.013. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985 May-Jun;(3):273–282. doi: 10.1016/0010-440x(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Whitford TJ, Alvarado J, Pelavin P, McCarley RW, Kubicki M, Salisbury DF, Shenton ME. Hearing voices: a role of interhemispheric auditory connectivity? World J Biol Psychiatry. 2012 Feb;13(2):153–158. doi: 10.3109/15622975.2011.570789. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring E. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol Rev June. 2010;20(2):174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymaz N, van Os J. Heritability of structural brain traits an endophenotype approach to deconstruct schizophrenia. Int Rev Neurobiol. 2009;89:85–130. doi: 10.1016/S0074-7742(09)89005-3. 2009. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003 Apr;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. Review. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. In: Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I. Michael B, First MD, Spitzer Robert L, Gibbon Miriam, Williams Janet BW, editors. Google Books; 2012. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004 Jun 1;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection. The corpus callosum is larger in left-handers. Science. 1985 Aug 16;229(4714):665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289–300. 1995. [Google Scholar]
- Knyazeva MG. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast. 2013:639430. doi: 10.1155/2013/639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res. 2003 Apr;36(4):409–420. doi: 10.1590/s0100-879x2003000400002. 2003. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006 Sep;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Matthew I, Gardin TM, Tandon N, Eack S, Francis AN, Clementz BA, et al. Medial Temporal structures and Hippocampal subfields in Psychotic disorders: Findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. JAMA Psychiatry. 2014 Jul 1;71(7):769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- Arnold SJM, Ivleva EI, Gopal TA, Reddy AP, Sacco CB, Francis AN, et al. Hippocampal Volume as a Putative Psychosis Biomarker: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Schizophr Bull. 2015 Jan;41(1):233–249. [Google Scholar]
- Li J, Kale Edmiston E, Chen K, Tang Y, Ouyang X, Jiang Y, et al. A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Res. 2014 Jan 30;221(1):58–62. doi: 10.1016/j.pscychresns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009 Jan 1;65(1):84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C, Donati A, Antille V, Viceic D, Ghika J, von Gunten A. Demyelination in mild cognitive impairment suggests progression path to Alzheimer's disease. PLoS One. 2013 Aug 30;8(8) doi: 10.1371/journal.pone.0072759. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8(3):261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010 Jul 1;68(1):70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprihan A, Jones T, Chen H, Lemke N, Abbott C, Qualls C, et al. The Paradoxical Relationship between White Matter, Psychopathology and Cognition in Schizophrenia: A Diffusion Tensor and Proton Spectroscopic Imaging Study. Neuropsychopharmacology. 2015 Aug;40(9):2248–2257. doi: 10.1038/npp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel C, Oertel-Knöchel V, Schönmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, et al. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012 Jan 16;59(2):926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Sanders RD, Goldberg TE, Bigelow LB, Christison G, Torrey EF, et al. Morphometry of the corpus callosum in monozygotic twins discordant for schizophrenia: a magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 1990;53(5):416–421. doi: 10.1136/jnnp.53.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Bigelow LB. Quantitative brain measurements in chronic schizophrenia. Br J Psychiatry. 1972;121(562):259–264. doi: 10.1192/bjp.121.3.259. [DOI] [PubMed] [Google Scholar]
- Fornari E, Knyazeva MG, Meuli R, Maeder P. Myelination shapes functional activity in the developing brain. Neuroimage. 2007 Nov 15;38(3):511–518. doi: 10.1016/j.neuroimage.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005 Dec;11(6):528–531. doi: 10.1177/1073858405282304. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junqué C, Martí-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34(1):71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.