Abstract
Aim
Accurate prediction of which individuals will go on to develop psychosis would assist early intervention and prevention paradigms. We sought to review investigations of prospective psychosis prediction based on markers and variables examined in longitudinal familial high-risk (FHR) studies.
Methods
We performed literature searches in MedLine, PubMed and PsycINFO for articles assessing performance characteristics of predictive clinical tests in FHR studies of psychosis. Studies were included if they reported one or more predictive variables in subjects at FHR for psychosis. We complemented this search strategy with references drawn from articles, reviews, book chapters and monographs.
Results
Across generations of familial high-risk projects, predictive studies have investigated behavioral, cognitive, psychometric, clinical, neuroimaging, and other markers. Recent analyses have incorporated multivariate and multi-domain approaches to risk ascertainment, although with still generally modest results.
Conclusions
While a broad range of risk factors has been identified, no individual marker or combination of markers can at this time enable accurate prospective prediction of emerging psychosis for individuals at FHR. We outline the complex and multi-level nature of psychotic illness, the myriad of factors influencing its development, and methodological hurdles to accurate and reliable prediction. Prospects and challenges for future generations of FHR studies are discussed in the context of early detection and intervention strategies.
Keywords: psychosis, prediction, high-risk, familial, early intervention
Introduction
Schizophrenia and related psychoses typically emerge in adolescence and young adulthood, although premorbid deficits are present in childhood. The chronic and debilitating nature of these illnesses makes their consequences profound across symptomatic, cognitive and functional domains. Accurate prospective identification of individuals who will go on to develop chronic psychosis would therefore be an important advance for early prevention and intervention paradigms.
Given the nonspecific symptomatology that precedes psychosis, prospective clinical assessment has unfortunately been a poor predictor of subsequent transition to psychosis,1,2 even among a more proximal, “ultra high-risk” group.3 Partly in response to this, researchers and clinicians have long hoped to identify and investigate markers and signs predictive of subsequent emergence of psychosis in a high-risk population.4,5 A compelling body of research has painstakingly described an array of predisposing epidemiologic factors, clinical and physical signs, cognitive measures, and genetic and neuroimaging biomarkers thought to be associated with the etiology and pathogenesis of these illness.
Yet despite these early hopes, studies have typically reported statistical significance or hazard ratios for risk factors and neurobiological markers in psychosis. These statistics are useful for determining important associations and markers, but cannot inform diagnosis and management of individual patients beyond the notion of accumulated risk. In contrast, only a minority of studies have documented measures of clinical validity and utility that can be brought to bear on diagnosis and patient care. In a recent comprehensive review, Lawrie et al6 point to the importance of sensitivity and specificity, or positive and negative predictive values (PPV and NPV), which reflect the utility of a test or prediction model for particular services or clinical settings (Table 1).
Table 1.
Calculating sensitivity, specificity, positive and negative predictive values for classification analyses.
| Statistic | Calculation | Comments | |
|---|---|---|---|
| Sensitivity |
|
|
|
| Specificity |
|
||
| Positive Predictive Value |
|
|
|
| Negative Predictive Value |
|
One factor likely contributing to sparse reporting of classification analyses is the vast heterogeneity across dimensions of clinical presentation, etiologic factors, and neurobiological characteristics seen in schizophrenia and related psychoses. Excessive heterogeneity in cross-sectional samples, overlapping diagnoses without clear boundaries, and variability in illness course can result in inconsistent findings and disappointing or insufficient predictive capacity. For these reasons, among others, the content validity of the schizophrenia construct itself is being increasingly called into question.7–9
In this selective review and synthesis, we discuss data from studies of individuals at familial high-risk (FHR) for schizophrenia which seek to assess predictive utility in a relatively etiologically homogenous population. Since familial causation does not necessarily mean genetic causation, we chose the term “familial” rather than “genetic” high risk strategy. Many recent reviews and meta-analyses have assessed “close-in” samples which are more proximal to the threshold of psychosis, or particular statistical approaches, but there is as of yet little summary available regarding the many approaches taken in prediction studies in FHR populations.10–12 We therefore begin by briefly describing the utility of familial high-risk studies alongside other high-risk studies of individuals at clinical or “ultra” high risk for psychosis (CHR). We go on to summarize reports of classification analyses (sensitivity, specificity, and positive/negative predictive values) based on clinical, cognitive, and other tests, markers or risk factors and their combinations in FHR populations. Finally, we synthesize this literature in the context of methodological considerations, discuss the implications of such approaches for diagnosis, treatment and early intervention strategies, and suggest lessons as well as potential future directions.
Methods
We identified and reviewed a range of reports from longitudinal familial high-risk studies that developed potential models for predicting psychosis development. Using keywords and MeSH headings, we conducted a comprehensive search on MedLine, PubMed, and PsycINFO databases which was subsequently restricted to articles in the English language, regarding human populations, and from age 6 to adulthood. MeSH headings for clinical prediction included “predictive value of tests”, “models, theoretical”, “algorithms”, “prognosis”, “early diagnosis”, “multivariate analysis” along with keywords “sensitivity”, “specificity”, and “predictive value”; headings for the disease entity included “schizophrenia” and “psychotic disorders”; headings to signify familial high risk studies included “risk”, “risk factors”, “family”, “child of impaired parents”, “genetic predisposition to disease”, and “brain”. Studies were to be included if they examined individuals at familial high risk, and reported more than one predictor variable for the onset of schizophrenia or related psychosis.
Using all of these headings and keywords in MedLine yielded over 4300 articles, the vast majority of which had no relation to the inclusion criteria. While our aim had been to conduct a systematic review,13 in delving into individual search hits we found that MeSH headings and keywords were inconsistent across studies, potentially because we were searching for predictive studies across methodologies, technologies and statistical approaches. For example, while early reports from “longitudinal studies” were consistently assigned such a MeSH heading, more recent follow-up reports from the same study populations were not. Similarly, most articles found using the heading “predictive value of tests” did not in fact document sensitivity, specificity and predictive value. Conversely, a slightly narrowed subset of the above MeSH headings yielded 189 articles, which did not include a majority of studies we previously knew to be relevant for the review.
We have therefore conceptualized this paper as a selective review that draws upon multiple search results from the above databases, from references from published articles and reviews, as well as monographs or book chapters known to the senior author. In order to ensure that the search source was not limiting our findings, we attempted a similar limited search using the PsycINFO database and found no additionally relevant studies. In total, 13 reports that investigated behavioural, cognitive, psychometric, clinical, imaging and other variables as predictors of psychosis onset in FHR populations met criteria for inclusion. These reports were examined in detail for study features and methods, along with parameters such as sensitivity, specificity and predictive values. While not a systematic review, we summarize these findings from disparate studies and synthesize their relevance for clinical utility at the present moment. We then draw on these observations to offer suggestions regarding future FHR studies that may improve their ability to understand etiology and pathogenesis, and for their linkage with early detection and intervention efforts.
High-risk studies in psychosis
The so-called longitudinal familial high-risk (FHR) studies have drawn on the observations that genetic factors are among the best-established and strongest individual-level risk factors in schizophrenia.14,15 In examining first (and occasionally second) degree relatives of individuals with schizophrenia and schizoaffective disorder over time, these studies assess a sub-population with a particular vulnerability, have demonstrated elevated rates of conversion to psychosis, and provided higher-yield data on factors that might predict the later development of psychosis.16
At the same time, many individuals who develop psychosis have no family history of serious mental illness.17 FHR studies will thus not include the majority of individuals who are on a psychosis trajectory or who go on to manifest a psychotic illness. Such individuals might, however, instead come to the attention of studies documenting clinical high-risk (CHR) individuals seeking help if they manifest sub-threshold (‘prodromal’) symptoms and/or functional impairment. Significant family history is neither necessary nor sufficient for inclusion criteria in CHR studies, which typically incorporate attenuated or brief limited psychotic symptoms, or individuals with genetic risk (similar to FHR studies) who have experienced a recent and relatively rapid functional impairment.18,19 An additional criterion utilized for inclusion in some studies are measures of cognitive-perceptive “basic” symptoms in attentional, thought and speech, ideational, and abstract reasoning dimensions.20 Such studies tend to differ from FHR studies in that they focus on a diverse spectrum of individuals representing three different types of clinical symptomatology, all of which are putative markers of the acute “ultra high-risk” period proximal to the psychosis threshold.
FHR and CHR studies thus present somewhat distinct but overlapping data sets, approaches, and points along a continuum of illness.21 They have collected substantial data on risk antecedents and thus offer strategies to distil the heterogenous nature of the schizophrenia construct into component platforms for further assessment and analysis.
Findings from familial high-risk studies
Generations of observational, naturalistic studies of individuals at familial high-risk have been conducted. As described in earlier reviews,22,23 they vary in the range of information collected as well as the age range during which subjects (offspring or relatives of individuals with schizophrenia) are followed. Since data in FHR studies is recorded at baseline (study entry) and then at regular intervals, recall bias for certain information is reduced compared with “follow-back” studies (with exceptions for early life exposures24), allowing for examination of the influence of putative early predictors on subsequent illness development.
Early FHR studies25,26 hypothesized that anomalous patterns of early development represented inherited neurointegrative deficits (known as “pandysmaturation”) related to psychosis-spectrum outcomes, and collected socioenvironmental and neurobehavioral data across development. Later studies in Europe, Israel and North America examined the degree to which family and social environment conferred schizophrenia liability. A recent generation, exemplified by the Edinburgh and Pittsburgh FHR studies, have attempted to marry the developmental approach utilized in earlier projects with newer technologies such as neuroimaging, state-of-the-art neurophysiologic measures, and validated clinical assessments16,27.
Study windows range from birth onwards (New York Infant Study, Swedish High Risk Study, Jerusalem Infant Development Study),28–30 school-age onwards (New York High-Risk Project, Israeli Kibbutz Study),5,31 to adolescence onwards (Copenhagen High Risk Project, Edinburgh High Risk Study, Pittsburgh High Risk Study).16,27,32 Although the studies vary in their rates of conversion to psychoses, they consistently show elevated rates in offspring or relatives compared with relatives with no family history of psychosis.16,27
Neurobehavioral and cognitive meaures (Table 2)
Table 2.
FHR studies examining neurobehavioral and cognitive markers.
| Study (ref) |
Cohort | Population | Variables of interest | Outcome of interest | Results Reported | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||||||
| 32 | NYHRP | 63 offspring of psychotic parents, 43 offspring of affective disorder parents, 100 children of healthy control parents |
CPT, AST, DS | Behavioral Global Adjustment Scale | 0.36 | 0.91 | 0.38 | 0.91 | |
| 34 | NYHRP | 79 offspring of SZ parentsA, 57 offspring of AFF parentsB, 133 offspring of HC parents |
CPT, AST, DS, VADST, LOMDS |
Schizophrenia-or related psychoses versus affective disorders |
A | 0.500– 0.833 |
0.716– 0.896 |
0.333– 0.462 |
0.909– 0.960 |
| B | 0.000– 0.500 |
0.887– 1.00 |
0.000– 0.143 |
0.930– 0.962 |
|||||
| 35 | EHRS | 7 HC subjects, 67 HR without psychotic symptoms, 60 HR with psychotic symptoms, 20 HR subsequently ill |
RAVLT (total score, trials 1–5) |
HR subjects developing schizophrenia versus HR remaining well |
0.611 | 0.328 | 0.118 | 0.851 | |
Cohorts: NYHRP, New York High Risk Project; EHRS, Edinburgh High Risk Study.
Variables of Interest: CPT, Continuous Performance Test; AST, Attention Span Task; DS, Digit Span; VADST, Visual Aural Digit Span Test; LOMDS, Lincoln-Oseretsky Motor Development Scale; RAVLT, Rey Auditory Verbal Learning Test.
Model variables of interest include variables that were ultimately nonsignificant; readers are encouraged to refer to the text or the original article for additional information.
Cornblatt and Erlenmeyer-Kimling created a composite measure of “attentional deviance” in high-risk offspring composed of variables from three tasks reflecting different aspects of this processing capacity.33,34 Rather than a specific diagnostic outcome such as schizophrenia, cut-off values using this composite measure were assessed to measure their predictive power for severe behavioural impairments across family, peer and school functioning and interaction, with greater specificity and NPV than sensitivity or PPV. The authors point out that their 9–10% false-positive rates for the outcome of “behavioural deviance” might be either an underestimate of ultimate study results, since additional time would allow more subjects to shift into the true positive category of impaired behaviour and then to psychosis; or an overestimate, since many individuals flagged as behaviourally deviant might not go on to develop psychosis.
Subsequent research by the same group focused on prediction of childhood neurobehavioral and cognitive measures in high-risk offspring as predictors for subsequent schizophrenia-related psychosis,35 which were diagnosed using research diagnostic criteria. Attentional difficulties, verbal memory and gross motor skills respectively predicted 58%–85% of HR subjects subsequently developing psychosis-spectrum difficulties, although sensitivity was 50% and PPV 46% when combining all three tests. Sensitivity and PPV were significantly worse when assessing offspring of healthy parents and affectively ill parents. Nonetheless, the overall accuracy of such models remained notable given the low base-rate of conversion to psychosis in a familial high-risk population.
More recently, the Edinburgh High Risk Study examined a range of baseline variables (collected when high-risk subjects were clinically well) to assess their capacity for prediction of subsequent schizophrenia.36 A 5-trial version of the Rey Auditory Verbal Learning Test, which measures aspects of verbal learning and declarative memory, met thresholds for statistical significance while receiver operating characteristic analysis (to determine an optimal cut-off point) achieved moderate sensitivity and strong NPV, but low specificity and PPV.
Psychometric scales and clinical assessments (Table 3)
Table 3.
FHR studies examining psychometric and clinical markers.
| Study (ref) |
Cohort | Population | Model variables of interest | Outcome of interest | Results Reported | |||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |||||
| 36 | CHRP | 207 offspring of SZ parents | Standard/supplementary MMPI scales of frequency, phobias, depression, psychasthenia, schizophrenia, social introversion |
DSM-III-R criteria for schizophrenia versus no mental illness |
0.655 | 0.661 | 0.463 | 0.811 |
| 40 | NYHRP | 106 offspring of parents with schizophrenia 94 offspring of parents with affective illness 191 offspring of parents with no psychiatric diagnosis |
“Schizophrenia Proneness” derived from unique questions on MMPI Paranoid Schizophrenia scale |
Schizophrenia-related psychosis (schizophrenia, schizoaffective disorder) versus other outcomes |
0.500 | 0.939 | 0.258 | 0.978 |
| 41 | EHRS | 36 HC subjects, 113 non-symptomatic HR subjects, 38 symptomatic HR subjects, 25 FEP with schizophrenia |
Structured Interview for Schizotypy (SIS) total scores and subscores |
HR subjects developing schizophrenia versus those remaining well |
0.43–0.86 | 0.61–0.96 | 0.18– 0.67 |
0.94– 0.99 |
| 35 | EHRS | 7 controls subjects, 67 HR without psychotic symptoms, 60 HR with psychotic symptoms, 20 HR subsequently ill |
SIS total scores and subscores | HR subjects developing schizophrenia versus those remaining well |
0.444– 0.889 |
0.683– 0.902 |
0.289– 0.400 |
0.917– 0.977 |
| 35 | EHRS | 7 controls subjects, 67 HR without psychotic symptoms, 60 HR with psychotic symptoms, 20 HR subsequently ill |
Rust Inventory of Schizotypal Cognitions | HR subjects developing schizophrenia versus those remaining well |
0.611 | 0.913 | 0.500 | 0.943 |
| 42 | PHRS | 97 HR subjects followed longitudinally |
Cumulative ‘Psychosis Proneness’ index derived from two SOPS and two Chapman’s subscales |
HR subjects developing schizophrenia versus all others |
0.91 | 0.92 | 0.59 | 0.99 |
Cohorts: NYHRP, New York High Risk Project; CHRS, Copenhagen High Risk Project; EHRS, Edinburgh High Risk Study; PHRS, Pittsburgh High Risk Study. Model variables of interest include variables that were ultimately nonsignificant; readers are encouraged to refer to the text or the original article for additional information.
Carter et al’s37 use of standard and supplementary psychometric scales derived from the Minnesota Multiphasic Personality Inventory (MMPI) battery built on cut-off and index score strategies that appeared to distinguish psychometrically “deviant” high-risk subjects from non-deviant offspring of parents with schizophrenia or other psychiatric conditions.38–40 Offspring were followed up at 10- and 25-year time-points and to compare MMPI scales with final diagnoses made using DSM-III-R criteria – allowing for ascertainment of ultimate illness outcome and not just “psychometric deviance”. MMPI-derived scores and scales successfully classified 65% of subsequent schizophrenia versus no mental illness, with sensitivity of 65.5%. A limited attempt to discriminate between paranoid and non-paranoid schizophrenia subtypes versus individuals with no mental illness was also moderately successful.
Further development of the MMPI as an indicator of schizophrenia liability was carried out by investigators using data from the New York High Risk Project. Bolinskey et al41 suggested a refined “Schizophrenia Proneness” scale based in part on questions that were unique to the MMPI’s Paranoid Schizophrenia scale in the hopes of increasing predictive power. They reported peak accuracy of 92.1% (although moderate sensitivity and PPV), and also tested other MMPI-derived scales with adjusted weightings. As in earlier analyses by this and other research teams, the authors commented on significant within-group heterogeneity, and their hope for improved predictive power when utilizing multiple predictive variables rather than a single measure.
In addition to neurobehavioral variables described in the previous section, Johnstone and colleagues assessed the predictive power of various Structured Interview of Schizotypy (SIS) clinical scales,36 which their group had previously reported to have statistically significant differences between converters and non-converters in the Edinburgh High-Risk Study.42 SIS total score was the most sensitive and least specific, whereas the social withdrawal subscale had high specificity and the oddness subscale intermediate between these two. Each measurement had relatively low PPV (ranging from 28.9%–40.0%) but high NPV (from 91.7%–97.7%). In the same study, baseline administration of the Rust Inventory of Schizotypal Cognitions (emphasizing cognitive content and bizarre/eccentric thoughts) was found to have modest sensitivity and PPV, but higher specificity and NPV.36
Tandon et al43 have attempted to operationalize recently proposed sub-threshold clinical criteria21 in first- or second-degree relatives of affected individuals. The top quartile of scores for each of the positive and disorganization subscales from the Scale of Prodromal Symptoms and the Chapman (perceptual aberration and magical ideation) Schizotypy subscales were identified; a resulting ‘psychosis proneness’ index resulted in sensitivity and specificity greater than 90%.
Neuroimaging biomarkers (Table 4)
Table 4.
FHR studies examining neuroimaging markers.
| Study (ref) |
Cohort | Population | Model variables of interest | Outcome of interest | Results Reported | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||||||
| 43 | EHRS | A –65 HR subjects B –18 HR subjects with transient or partial psychotic symptoms |
Grey matter changes in R cerebellum, L uncus, L inferior temporal gyrus |
HR subjects developing schizophrenia versus those remaining well |
A | 0.13– 0.38 |
0.88–0.96 | 0.20– 0.60 |
0.88– 0.92 |
| B | 0.38– 0.63 |
0.70–0.80 | 0.50– 0.71 |
0.58– 0.73 |
|||||
| 44 | EHRS | 21 control subjects, 41 HR without psychotic symptoms, 21 HR with psychotic symptoms (4 HR subsequently ill) |
fMRI parietal lobe activation, anterior cingulate deactivation, reduced right lingual gyrus and temporal activation |
HR subjects developing schizophrenia versus those remaining well |
ROIs | - | - | 0.17– 0.80 |
0.98– 1.00 |
| DFA | - | - | 0.50– 0.60 |
0.95– 0.98 |
|||||
Cohorts: EHRS, Edinburgh High Risk Study. ROIs, regions of interest. DFA, discriminant function analysis. Model variables of interest include variables that were ultimately nonsignificant; readers are encouraged to refer to the text or the original article for additional information.
Job et al44 studied voxel-based grey matter changes in a relatively small sample of high-risk individuals over two 1.0T structural MRI scans taken an average of 18 months apart. Individuals who developed schizophrenia had significantly greater reductions in grey matter over the study period in three areas: right cerebellum, left uncus and left inferior temporal gyrus, with strongest predictive power observed in the latter region using a receiver operating characteristic curve and optimized cut-off points.
Using a sentence completion task, fMRI approaches to psychosis prediction were subsequently tested as a potential biomarker by the same group.45 Overactivation at the primary region of interest, the parietal lobe, was used to discriminate between FHR subjects who later converted and those who remained well up to 18 months later, with PPV of 17% and NPV of 98%. Despite the low number of individuals (four) who developed psychosis, alternative classification analyses which combined parietal lobe and lingual gyrus regions of interest resulted in PPV of 80% and NPV of 100%.
Other single-variable studies (Table 5)
Table 5.
FHR studies examining other markers.
| Study (ref) |
Cohort | Population | Model variables of interest | Outcome of interest | Results Reported | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||||||
| 45 | CHRS | 207 HR subjects |
Diagnostic school report items: Females – lonely and rejected, nervous, uneasy about criticism, rare spontaneity, future psychotic or emotional problems, average intelligence, passivity. Males –lonely and rejected, repeated a school grade, passivity, future psychotic or emotional problems, persistent emotional reaction, high- strung |
Schizophrenia versus nonpsychotic diagnoses (reported here); (schizophrenia versus no mental illness, schizophrenia versus schizotypal and paranoid personality disorders, not reported here) |
F | 0.273 to 0.857 |
0.357 to 0.933 |
- | - |
| M | 0.250 to 0.438 |
0.812 to 0.944 |
- | - | |||||
Cohorts: CHRS, Copenhagen High Risk Project. Model variables of interest include variables that were ultimately nonsignificant; readers are encouraged to refer to the text or the original article for additional information.
Childhood teacher reports of FHR subjects in the CHRS were examined retrospectively to see if individuals who went on to develop psychosis were potentially identifiable earlier in life.46 Teacher ratings on a 25-item scale when subjects were between 9–20 years old were linked with final DSM-III diagnoses of schizophrenia, nonpsychotic diagnoses, Cluster A personality disorders, or no mental illness decades later. Compared with females, “pre-schizophrenic” males were incrementally easier to distinguish from those not developing psychosis or other mental illness using the 25-item scale, although the results of classification analyses remained modest in both cases.
Multi-domain multivariate studies (Table 6)
Table 6.
FHR studies examining markers from multiple domains.
| Study (ref) |
Cohort | Population | Model variables of interest | Outcome of interest | Results Reported | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||||||
| 46 | CHRP | 212 HR offspring, 99 matched controls |
Genetic risk, Rearing environment (disruptive behavior at school, unusual MMPI responses, unstable or hostile fathers, >1 schizophrenia-spectrum parent), Life functioning |
SZ versus: A – HR sample, no mental illness B – total sample, no mental illness C – total sample, all other outcomes D – HR subjects with 1 psychosis-spectrum parent, but no mental illness E –HR subjects with 2 psychosis-spectrum parents, no mental illness |
A | 0.66 | 0.73 | 0.53 | 0.82 |
| B | 0.67 | 0.80 | 0.46 | 0.90 | |||||
| C | 0.67 | 0.76 | 0.26 | 0.95 | |||||
| D | 0.50 | 0.77 | 0.43 | 0.82 | |||||
| E | 0.71 | 0.94 | 0.91 | 0.80 | |||||
| 47 | PHRS | 86 HR subjects (first- or second- degree relatives of patients with schizophrenia or schizoaffective disorder) |
Clinical Chapman’s scales for psychosis proneness, Cognitive impairment battery, Total brain volume |
Development of new or worsening psychopathology |
0.50 | 0.92 | 0.71 | 0.83 | |
| 48 | PHRS | 96 individuals with first- or second- degree relatives with schizophrenia or schizoaffective disorder |
Genetic risk, Early life biological and social/environmental influences (social services involvement, removal from parental home, cannabis misuse, obstetric complications, paternal age at conception, zip-code population density at childhood), Cognitive impairment battery, Total brain volume, Clinical Chapman’s scales for psychosis proneness, Development of new or worsening psychopathology |
Conversion to psychosis versus all other outcomes |
0.17 | 0.99 | 0.67 | 0.89 | |
Cohorts: CHRS, Copenhagen High Risk Project; PHRS, Pittsburgh High Risk Study. Model variables of interest includes variables that were ultimately nonsignificant; readers are encouraged to see the text or the original article for additional information.
The initial wave of prospective prediction studies in psychosis primarily investigated one or more variables from a single domain as correlates of risk status. As described above, however, their results (in the context of heterogeneity in signs, symptoms and course of onset) suggested that individual clinical and/or psychobiological markers were likely to be important but nonspecific (i.e. insufficient) for optimal prediction of psychosis conversion. Over time, attention thus shifted towards more complex models of interaction between multiple factors, including interaction between neurobiology and the social and physical environment (Table 6).
An initial multivariate study assessed a comprehensive range of neurobiological and socioenvironmental measures in FHR subjects through discriminant function analysis.47 Retrospective data were compiled on genetic risk, birth complications, rearing environment, early childhood experiences, parent characteristics, school behavior and socioeconomic status along with time-of-intake measures including autonomic response, cognitive functioning and personality traits (unusual thoughts or beliefs, oddness and peculiarity, and subclinical psychopathology). The important addition of this early childhood data produced an overall finding of genetic risk interacting with rearing environment to increase risk for schizophrenia, potentially in concert with disruptive school behavior. Results of classification analyses were generally of moderate strength and varied depending on the outcomes being compared.
More recently, Eack and colleagues48 integrated structural equation modelling to prospective estimate the contribution of a set of baseline clinical, neurobiological and cognitive factors to emerging psychopathology (rather than psychosis specifically) in a FHR population. Total brain volume, neurocognitive deficits and clinical schizotypy were found to predict subsequent general psychopathology development, albeit with little overlap among these domains.
In the same FHR population, Shah et al.49 brought together early risk factors (including perinatal complications, development of cannabis abuse, genetic/familial risk, and childhood adversity) along with Chapman rating scales and neurocognitive measures in a structural equation modelling approach, with the outcome of interest this time being psychosis conversion. Given the use of multiple distal (early) and nonspecific markers and the relatively low base-rate of conversion (12.5%), their findings of low sensitivity (17%) are not surprising. Moderate PPV (67%) and high specificity (99%) and NPV (89%) echoed the combination of neurobiologic and socioenvironmental dimensions employed by Carter et al.47
Summary and synthesis
What sorts of conclusions can be drawn from prediction studies in FHR populations? A number of broad observations stand out. First, the wide range of predictors utilized demonstrates that many etiologic variables or developmental pathways can contribute to a similar clinical end-point (multicausality). Yet not all indicators of schizophrenia liability are good predictors of illness development,22,50 and no single factor or combination of factors tested thus far is sufficient for accurate prediction of psychosis development. Since predictive studies are sparse, relatively few variables have been tested overall. Moreover, few FHR models reflect the dynamic interface between socioenvironmental and neurobiological factors (see Table 7).47,49 Multivariate and multi-domain approaches to prediction of outcomes may therefore be more productive than individual predictors examined alone, and are becoming increasingly common in the CHR literature.51,52 Novel pattern-recognition and machine-learning methodologies have had considerable within-domain success although primarily within a CHR population.53–55 Their application in FHR studies has not yet been attempted, but could be beneficial given the relative etiologic homogeneity of these subjects.
Table 7.
Study design, strengths and weaknesses across single-domain and multi-domain prospective prediction algorithms for emerging psychosis in FHR populations.
| Study (ref) |
Study design | Strength(s) | Weakness(es) |
|---|---|---|---|
| 32 | Composite index of attentional measures, with cutoff scores for each response variable of most poorly performing 5% of normal controls |
Multiple comparison groups; Multiple attentional measures used in composite index |
Individuals shifted between groups due to rediagnosis of parents; DSM-II diagnostic criteria used for parents; outcome of interests related to behavioral difficulties rather than diagnosis; Test scores weighted equally in composite index |
| 34 | Relationships between neurobehavioral deficits examined in path analysis using logistic regression |
Multiple comparison groups; Multiple attentional measures used in composite index; Modest sample size |
Varying mean age at time of final diagnosis; Limited follow-up period |
| 35 | Neuropsychological testing conducted at baseline ascertainment; serial psychopathological assessments conducted at 18 month intervals |
Multiple HR comparison groups; Moderately large sample size; ICD-10 diagnostic criteria used |
Mean age > 20; Limited follow-up period, particularly for women; |
| 36 | Retrospective analysis of MMPI subset scores on 25-year diagnostic follow-up using discriminant function analysis |
Attempted subgroup analysis (regarding paranoid subtypes) |
No validation of modified psychometric instrument utilized; High rate of non-response to test; Control group was unaffected; Post-hoc diagnostic re-evaluation years later using DSM-III-R criteria |
| 40 | Experimental scale derived from MMPI (“schizophrenia proneness”) and Moldin-Gottesman psychometric index. Cutoff scores defined using logistic regression, stepwise regression and discriminant function analysis |
Multiple comparison groups; Multiple scales and combinations tested; Extensive assessment of experimental scale and psychometric index; |
Diagnostic information and rating scales unclear; Highly derived measures and scales |
| 41 | Neuropsychological testing conducted at baseline ascertainment |
Multiple comparison groups, including symptomatic and nonsymptomatic HR subjects; ICD-10 diagnostic criteria used |
Mean age > 20; Surprisingly high numbers of converters; Limited follow-up period |
| 42 | Cutoff levels for positive and disorganized Scale of Prodromal Symptoms subscales, Chapman magical ideation and perceptual aberration scales confirmed by ROC analysis |
Multiple clinical measures used in composite index; Sensitivity analysis conducted with cognitive and social functioning data; Simple and clinically applicable |
No control group; Pre-set cutoff values; |
| 43 | Interval changing using region-specific longitudinal voxel- based morphometry |
Focus on dynamic versus static measures ICD-10 diagnostic criteria used |
Mean age > 20; Small sample size; No control group, subject group selected for minor symptoms in one test; Requires replication and validation; Short interval period between scans; Limited follow-up period |
| 44 | fMRI-based sentence completion task (verbal initiation section of the Hayling Sentence Completion Test) |
Multiple comparison groups; Multiple ROIs assessed; Pre-illness baseline measures; ICD-10 diagnostic criteria used |
Small sample size; Few converters; No sensitivity or specificity values provided; Extreme |
| 45 | Likelihood ratio and ROC analysis of 25-item childhood schoolteacher evaluations |
No inter-rater or test-retest reliability regarding school reports; Blind, independent reviews of diagnostic reliability; Multiple comparison groups; Attention to subgroups(e.g. gender- specific patterns in school reports) |
DSM-III-R diagnostic criteria used; No inclusion of low-risk subjects; Teacher reports compiled across a wide age range (9–20) |
| 46 | Discriminant function analysis across 7 domains and their interaction: genetic risk, birth factors, autonomic responsiveness, premorbid cognitive functioning, rearing environment, personality, school behavior |
Moderate sample size; Multiple comparison groups; Multivariate and multi-domain model, including early life data |
DSM-III-R diagnostic criteria used |
| 47 | Structural equation modeling across clinical, cognitive, and neurobiological domains |
Modest sample size; Mean entry age ~15.2; Multivariate and multi-domain model; DSM-IV diagnostic criteria used |
Crude neurobiological measure (total brain volume); No control group |
| 48 | Structural equation modeling across familial, neurobiologic, socioenvironmental, cognitive and clinical factors |
Modest sample size; Mean entry age ~15.9 Multivariate and multi-domain model, including early life data; DSM-IV diagnostic criteria used |
Low sensitivity; Crude neurobiological measure (total brain volume); No control group |
A second lesson is that an etiologic factor can also have multiple possible psychopathologic outcomes (multifinality). Despite the common familial risk that leads to enrolment in such studies, baseline and later symptomatology and clinical phenomenology in subjects can vary from none to significant, with a correspondingly wide spectrum of impairment. Indeed, emergence of general psychopathology appears to be a more common developmental end-point in this population than emergence of psychosis.48,56 The psychometric deviance measured in FHR studies may therefore reflect predisposition to nonspecific psychopathology shared amongst offspring of both parents with schizophrenia and other psychiatric disorders.40 Thus, given the infrequent development of psychosis and the nonspecific nature of early etiologic/risk markers, specificity might be a less important classification statistic than sensitivity or PPV.57
Third, at the level of methodology and design, the different study foci represented in different generations of FHR studies illustrates evolving understandings of psychosis over time, with a variety of strengths and weaknesses (Table 7). At times, however, this can present intractable challenges when no valid or consistent outcome measures exist. Early FHR studies, for example, were operating with very different diagnostic criteria than later ones,37 making this a ‘moving target’. Comparison across study eras is thus particularly difficult.
Finally, given the recent conceptualization of psychosis as a neurodevelopmental disorder and the long time-course over which such illnesses can emerge, FHR studies have collected data over differing periods of time (particularly for diagnostic and functional outcomes), in some cases completing data collection without following individuals through the acute risk period of adolescence and young adulthood (Table 7). This presents a problem of ‘shifting false negatives’ (individuals who were initially recorded as nonpsychotic but may have converted in later years), which may be a major influence on predictive power given the overall low base-rate of psychosis development. It is then compounded by another factor: the selection of outcomes of interest. As argued by Kapur58 and highlighted in the wide spectrum of psychopathology seen in FHR studies, the algorithmic distinction between individuals with severe mental illness (for example, psychosis) from those with overall mental health may have little practical relevance to real-world diagnostic conundrums. More complex – although more germane – are the clinically vexing and salient questions of whether such algorithms can accurately distinguish between affective versus nonaffective psychosis, or psychosis versus other major mental illness. Predictive investigations that explicitly aim to tackle the latter questions are as of yet in the minority.45,47
Looking forward: Implications for early course psychosis
“The appropriate level of analysis [is]… not in the discovery of singular environmental or constitutional factors, but in the interplay of both systems which have an inseparable role in producing all developmental outcomes, schizophrenic or otherwise.”
- Sameroff, Barocas, Seifer,59 p 513
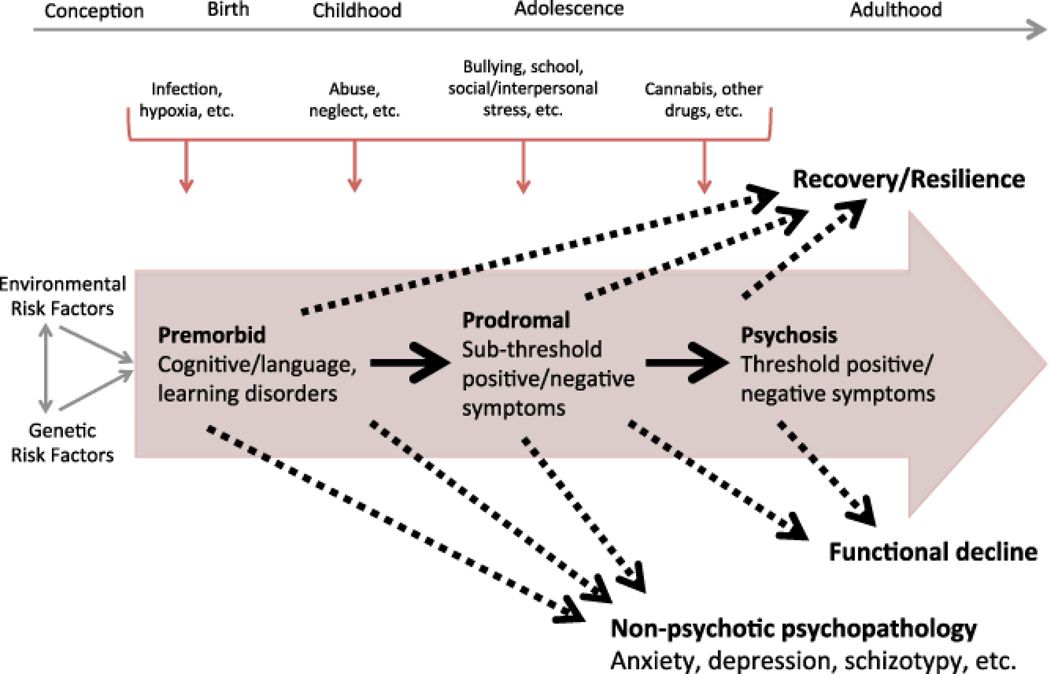
This review of FHR predictive studies in schizophrenia collects, summarizes and synthesizes data on the predictive power of various factors linked to psychosis onset. Across FHR analyses, the wide array of outcomes and markers shown to be relevant for risk ascertainment – although by no means individually or definitively predictive – are notable, given that family history is the strongest known risk factor for psychosis conversion. We now discuss the implications of this observation for familial high-risk studies, and make suggestions aimed at furthering the understanding of etiology and psychology/pathophysiology in the service of improved detection, prevention and early intervention. To do so, we develop a conceptual model that draws on these observations and integrates knowledge regarding etiologic and risk factors with the trajectory to and from psychosis, culminating in a range of diverse outcome domains (Figure 1).
Figure 1. Psychosis prediction and clinical utility in familial high-risk studies: selective review, synthesis, and implications for early detection and intervention.
Risk factors, illness trajectories and outcomes in familial high-risk studies for psychosis. Early high-risk studies commonly focused on a relatively narrow set of outcomes (as depicted inside the large arrow) whereas more recent studies have highlighted the need to consider a broader range of outcomes.
Figure 1 illustrates features drawn from knowledge regarding the evolution and trajectory of those at FHR for psychosis. Perinatal, childhood and adolescent and young adulthood periods are ripe for collection and measurement of early life risk variables; these could include genetic and environmental (or individual and ecological) factors such as advanced paternal age, obstetric and perinatal complications, childhood adversity and trauma, and cannabis or other substance exposure. As time goes on, a minority of individuals will develop premorbid disturbances; persistent premorbid symptoms may or may not evolve into prodromal features with sub-clinical severity (the typical outcomes assessed in FHR studies to date). In some cases, an Axis I psychotic disorder emerges. However, as with CHR subjects,60 a large fraction of those at FHR will experience either minimal psychopathology, general non-psychotic psychopathology, or development of a non-psychotic disorder Axis I which is also often influenced by similar early life variables. And – regardless of ultimate diagnostic outcome – hospitalization, educational and vocational attainment, and social role functioning are concrete recovery-oriented outcomes of concern to affected individuals.61 How can these observations shape or influence new generations of FHR studies?
Etiology and early detection
Concerns regarding previous FHR predictive studies have been noted: among others, they include short time windows for outcome assessment, surprisingly high conversion rates despite the use of validated clinical instruments (leading to potential for residual confounding), and diagnostic criteria that are (as with early versions of the DSM) unreliable or (as with more recent versions and the upcoming DSM-5) changing over time (see Table 7). Future studies could be designed with longer-term windows for outcome measurement in mind, and make use of clinical conferences that are blind to diagnoses at previous points. Creation of a set of ‘core’ and more stable diagnostic criteria will also better establish the clinical utility of putative measures as well as a ‘gold standard’ against which potential advances can be compared. Thus far, nosology has been based on symptoms but other approaches might be envisioned62: examining data that cuts across current diagnostic constructs may reveal unexpected relationships between phenotypic, biomarker, and etiological variables.
Recent reports by our group also suggest ways in which multiple predictive assessments can complement each other. Clinical assessments just prior to the point of conversion have strong sensitivity, and represent what might be late indicators or manifestations of a psychotic illness;43 in contrast, an algorithm taking more distal markers into account is less sensitive but appears to have stronger specificity.49 Combining these two findings, staged approaches involving initial screening tests (with high sensitivity) followed by confirmatory tests (with high specificity) may reduce the overall risk of false-positive and false-negative predictions.
At a conceptual level, FHR projects have been designed with a focus on baseline neurobiological measurement, self-reported socio-environmental and medical history, and regular follow-up periods for acquisition of further developmental information; reports have typically compared static early measurements with the later development of psychopathology. The lack of success in identifying specific etiologic factors or accurate predictive models under this approach illustrates the need to consider variables as potentially acting at multiple levels simultaneously (ranging from molecular neuroscience and genes at one extreme, to neighborhoods and cities on the other). Illustrative approaches may be found in the developmental psychopathology literature, where discussion regarding multi-level models of causation and propagation, dynamic changes (in risk or resiliency) and plasticity over time, multicausality (the notion that individuals with similar psychopathology may have many different starting points and pathways), and multifinality (similar starting points and pathways can lead to different outcomes across individuals) is of great relevance to the etiopathology of neurodevelopmental disorders.63
Key reasons for the limited predictive power revealed to date are the multifactorial etiology of psychosis, the multi-layered complexity of developmental trajectories, and the many potential confounding and mediating factors contained in longitudinal studies. Future FHR studies may begin to address this challenge by applying a ‘life-course perspective’ to risk markers, with biology, development and experience mutually influencing each other in a dynamic interplay over time that better appreciates latent effects acting at multiple levels and time periods.64,65 The life-course approach draws from initiatives in chronic disease epidemiology that have been applied to respiratory illness, breast cancer, type 2 diabetes and coronary heart disease.66 Clarity is also needed regarding the difference between risk factors (such as obstetric complications or maternal exposure to viral infections) and risk indicators (e.g. delayed milestones and early language difficulties), although this distinction is admittedly complex. Overall, such advances may more productively combine rich threads from both neurobiologic and social psychiatric approaches.
Intervention and prevention
In considering her notion of pandysmaturation, Fish26 suggested that if mechanisms contributing to early motor, perceptual, and cognitive deficits were better understood, early intervention to alleviate such difficulties could be evaluated in terms of their ability to prevent or reduce the chronic morbidity/mortality associated with psychoses. Alongside accurate prediction of psychosis conversion, then, the prospect of feasible and effective intervention early in the course of psychosis represents a holy grail.67
However, the focus on psychotic-spectrum diagnoses as the major outcome of interest may have resulted in inattention to other distressing psychopathology as well as outcomes beyond psychiatric diagnosis. Future FHR predictive analyses could thus consider a more diverse range of endpoints, incorporating sub-clinical symptomatology, nonpsychotic Axis I disorders, and measures of recovery or resilience. As depicted in Figure 1, examples of such outcomes might include development of any psychopathology over a study period, decrements in cognitive or other performance using validated instruments, or recovery-oriented goals such as vocational or educational attainment and social or role functioning. Through an appreciation of assorted outcomes, unknown or underappreciated protective factors, measures of resilience and recovery, or other sites for early intervention might also become apparent.
The low base-rate of conversion seen in FHR studies (and not just in population-based studies) has also stood in contrast to the enormous overall burden of illness. This dilemma has made consensus on intervention approaches difficult to achieve,68,69 in part due to the significant logistical, ethical and other challenges associated with use of a prophylactic treatment with substantial risk for a diagnosis that may never emerge.70,71
For practitioners and policy-makers, this debate suggests important implications for early psychosis prevention and intervention efforts across community and academic settings. Given the broad range of risk factors implicated in psychosis development – across cognitive, neurobiologic, familial, obstetric and prenatal, early childhood, substance-related, and clinical domains – future multivariate prediction analyses could emphasize “points of convergence”72 between primary and secondary approaches: multivariate prediction of emerging psychosis may not only identify subjects at especially high risk, but holds the potential to highlight individual and ecological risk and protective factors of importance, thereby informing both high-risk and population-based strategies.
At the individual level, this could manifest as categorical screening questionnaires drawing on model parameters to allow for calculation of an evidence-based “risk score”, permitting clinicians to stratify risk before deciding on referral or intervention (for those who are help-seeking) versus watchful waiting. Similar tools exist in other areas of medicine, in particular for chronic diseases.73 Identified high-risk individuals would benefit from closer monitoring, lower thresholds for management, and community programs aimed at de-coupling modifiable individual and ecological exposures, all forms of secondary intervention. CHR studies in Australia, Europe and North America have, for example, offered systems of care for help-seeking individuals that combine clinical services with associated resources for adolescents and young adults grappling with illness. Early evidence is also emerging that certain psychological and pharmacological interventions may be safe and efficacious for secondary prevention in the CHR period,74–77 although they are not without risk.
Since the FHR population is often non-help-seeking, less distressed, and with a lower rate of conversion, interventions for this group should have an even lower risk/benefit ratio before implementation. The multifinality of early life risk factors for a range of diagnoses and functional outcomes may also tilt the field towards less risky primary prevention and health promotion measures over secondary prevention. In that spirit, measures could be enacted to reduce risk exposures or to de-couple the link between FHR status and other factors.78 Clinically, this might manifest in robust prenatal nutrition and immunization programs and improved obstetric and perinatal care for at-risk individuals. And through public health and social policy measures, comprehensive early childhood education and care strategies aimed at mitigating the effect of childhood trauma and adversity, or programs aimed at preventing exposure to and use of noxious and precipitating substances. Such initiatives would, of course, likely benefit individuals at risk for psychosis as well as other chronic mental and physical illnesses.
Acknowledgments
We thank Janice Glover MLS for assistance and advice with conducting the literature search. Support for this research was provided by a Dupont-Warren Fellowship, Harvard Medical School to JLS; National Institutes of Health (NIH) grants MH01180, MH64023, and MH78113 to MSK; and National Alliance on Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award to MSK.
References
- 1.Bell V, Halligan PW, Ellis HD. Diagnosing delusions: a review of inter-rater reliability. Schizophr Res. 2006;86(1–3):76–79. doi: 10.1016/j.schres.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Goldman D, Hien DA, Haas GL, Sweeney JA, Frances AJ. Bizarre delusions and DSM-III-R schizophrenia. American Journal of Psychiatry. 1992;149(4):494–499. doi: 10.1176/ajp.149.4.494. [DOI] [PubMed] [Google Scholar]
- 3.Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625–630. doi: 10.3109/00048671003620210. [DOI] [PubMed] [Google Scholar]
- 4.Mednick SA. A longitudinal study of children with a high risk for schizophrenia. Ment Hyg. 1966;50(4):522–535. [PubMed] [Google Scholar]
- 5.Erlenmeyer-Kimling L, Cornblatt B. The New York High-Risk Project: a followup report. Schizophr Bull. 1987;13(3):451–461. doi: 10.1093/schbul/13.3.451. [DOI] [PubMed] [Google Scholar]
- 6.Lawrie SM, Olabi B, Hall J, McIntosh AM. Do we have any solid evidence of clinical utility about the pathophysiology of schizophrenia? World Psychiatry. 2011;10(1):19–31. doi: 10.1002/j.2051-5545.2011.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuang MT, Faraone SV. The case for heterogeneity in the etiology of schizophrenia. Schizophr Res. 1995;17(2):161–175. doi: 10.1016/0920-9964(95)00057-s. [DOI] [PubMed] [Google Scholar]
- 8.Keshavan MS, Brady R. Biomarkers in schizophrenia: we need to rebuild the Titanic. World Psychiatry. 2011;10(1):35–36. doi: 10.1002/j.2051-5545.2011.tb00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keshavan MS, Morris DW, Sweeney JA, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133(1–3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobl EV, Eack SM, Swaminathan V, Visweswaran S. Predicting the risk of psychosis onset: advances and prospects. Early Intervention in Psychiatry. 2012;6(4):368–379. doi: 10.1111/j.1751-7893.2012.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 12.Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, Bechdolf A, Schimmelmann BG, Klosterkötter J. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012;18(4):351–357. doi: 10.2174/138161212799316064. [DOI] [PubMed] [Google Scholar]
- 13.Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–121. doi: 10.1258/jrsm.96.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson JS, Kley IB. On the application of genetic expectancies as age-specific base rates in the study of human behavior disorders. Psychol Bull. 1957;54(5):406–420. doi: 10.1037/h0045929. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan PF. The genetics of schizophrenia. Plos Med. 2005;2(7):e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3(3):163–168. [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman II, Erlenmeyer-Kimling L. Family and twin strategies as a head start in defining prodromes and endophenotypes for hypothetical early-interventions in schizophrenia. Schizophr Res. 2001;51(1):93–102. doi: 10.1016/s0920-9964(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 18.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 19.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 20.Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 21.Keshavan MS, DeLisi LE, Seidman LJ. Early and broadly defined psychosis risk mental states. Schizophr Res. 2011;126(1–3):1–10. doi: 10.1016/j.schres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97(1):65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Keshavan MS. High-risk studies, brain development and schizophrenia. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge: Cambridge University Press; 2010. pp. 455–472. [Google Scholar]
- 24.Cantor-Graae E, Cardenal S, Ismail B, McNeil TF. Recall of obstetric events by mothers of schizophrenic patients. Psychol Med. 1998;28(5):1239–1243. doi: 10.1017/s0033291798006953. [DOI] [PubMed] [Google Scholar]
- 25.Fish B. Neurobiologic antecedents of schizophrenia in children. Evidence for an inherited, congenital neurointegrative defect. Archives of General Psychiatry. 1977;34(11):1297–1313. doi: 10.1001/archpsyc.1977.01770230039002. [DOI] [PubMed] [Google Scholar]
- 26.Fish B. Characteristics and sequelae of the neurointegrative disorder in infants at risk for schizophrenia: 1952–1982. In: Watt NF, Anthony EJ, Wynne LC, Rolf JE, editors. Children at Risk for Schizophrenia: a Longitudinal Perspective. New York: Cambridge University Press; 1984. pp. 423–439. [Google Scholar]
- 27.Johnstone EC, Russell KD, Harrison LK, Lawrie SM. The Edinburgh High Risk Study: current status and future prospects. World Psychiatry. 2003;2(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 28.Fish B. Infant predictors of the longitudinal course of schizophrenic development. Schizophr Bull. 1987;13(3):395–409. doi: 10.1093/schbul/13.3.395. [DOI] [PubMed] [Google Scholar]
- 29.McNeil TF, Kaij L. Swedish high-risk study: sample characteristics at age 6. Schizophr Bull. 1987;13(3):373–381. doi: 10.1093/schbul/13.3.373. [DOI] [PubMed] [Google Scholar]
- 30.Marcus J, Auerbach J, Wilkinson L, Burack CM. Infants at risk for schizophrenia. The Jerusalem Infant Development Study. Archives of General Psychiatry. 1981;38(6):703–713. doi: 10.1001/archpsyc.1981.01780310103011. [DOI] [PubMed] [Google Scholar]
- 31.Mirsky AF, Silberman EK, Latz A, Nagler S. Adult outcomes of high-risk children: differential effects of town and kibbutz rearing. Schizophr Bull. 1985;11(1):150–154. doi: 10.1093/schbul/11.1.150. [DOI] [PubMed] [Google Scholar]
- 32.Mednick SA, Parnas J, Schulsinger F. The Copenhagen High-Risk Project, 1962–86. Schizophr Bull. 1987;13(3):485–495. doi: 10.1093/schbul/13.3.485. [DOI] [PubMed] [Google Scholar]
- 33.Cornblatt BA, Erlenmeyer-Kimling L. Global attentional deviance as a marker of risk for schizophrenia: specificity and predictive validity. J Abnorm Psychol. 1985;94(4):470–486. doi: 10.1037//0021-843x.94.4.470. [DOI] [PubMed] [Google Scholar]
- 34.Winters L, Cornblatt BA, Erlenmeyer-Kimling L. The Prediction of Psychiatric Disorders in Late Adolescence. In: Walker EF, editor. Schizophrenia: A Life-Course Developmental Perspective. Academic Press, Inc; 1991. pp. 124–137. [Google Scholar]
- 35.Erlenmeyer-Kimling L, Rock D, Roberts SA, et al. Attention, Memory, and Motor Skills as Childhood Predictors of Schizophrenia-Related Psychoses: The New York High-Risk Project. American Journal of Psychiatry. 2000;157(9):1416. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone EC, Ebmeier KP, Miller P, Owens DGC, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- 37.Carter JW, Parnas J, Cannon TD, Schulsinger F, Mednick SA. MMPI variables predictive of schizophrenia in the Copenhagen High-Risk Project: a 25-year follow-up. Acta Psychiatr Scand. 1999;99(6):432–440. doi: 10.1111/j.1600-0447.1999.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 38.Moldin SO, Gottesman II, Erlenmeyer-Kimling L. Psychometric validation of psychiatric diagnoses in the New York High-Risk Study. Psychiatry Res. 1987;22(2):159–177. doi: 10.1016/0165-1781(87)90103-x. [DOI] [PubMed] [Google Scholar]
- 39.Moldin SO, Gottesman II, Erlenmeyer-Kimling L, Cornblatt BA. Psychometric deviance in offspring at risk for schizophrenia: I. Initial delineation of a distinct subgroup. Psychiatry Res. 1990;32(3):297–310. doi: 10.1016/0165-1781(90)90035-4. [DOI] [PubMed] [Google Scholar]
- 40.Moldin SO, Rice JP, Gottesman II, Erlenmeyer-Kimling L. Psychometric deviance in offspring at risk for schizophrenia: II. Resolving heterogeneity through admixture analysis. Psychiatry Res. 1990;32(3):311–322. doi: 10.1016/0165-1781(90)90036-5. [DOI] [PubMed] [Google Scholar]
- 41.Bolinskey PK, Gottesman II, Nichols DS, et al. A New MMPI-Derived Indicator of Liability to Develop Schizophrenia: Evidence from the New York High-Risk Project. Assessment. 2001;8(2):127–143. doi: 10.1177/107319110100800202. [DOI] [PubMed] [Google Scholar]
- 42.Miller P, Byrne M, Hodges A, Lawrie SM, Owens DGC, Johnstone EC. Schizotypal components in people at high risk of developing schizophrenia: early findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2002;180:179–184. doi: 10.1192/bjp.180.2.179. [DOI] [PubMed] [Google Scholar]
- 43.Tandon N, Montrose D, Shah J, Rajarethinam RP, Diwadkar VA, Keshavan MS. Early prodromal symptoms can predict future psychosis in familial high-risk youth. J Psychiatr Res. 2012;46(1):105–110. doi: 10.1016/j.jpsychires.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Job DE, Whalley HC, McIntosh AM, Owens DGC, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whalley HC, Simonotto E, Moorhead W, et al. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60(5):454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Olin SS, John RS, Mednick SA. Assessing the predictive value of teacher reports in a high risk sample for schizophrenia: a ROC analysis. Schizophr Res. 1995;16(1):53–66. doi: 10.1016/0920-9964(94)00063-e. [DOI] [PubMed] [Google Scholar]
- 47.Carter JW, Schulsinger F, Parnas J, Cannon T, Mednick SA. A multivariate prediction model of schizophrenia. Schizophr Bull. 2002;28(4):649–682. doi: 10.1093/oxfordjournals.schbul.a006971. [DOI] [PubMed] [Google Scholar]
- 48.Eack SM, Prasad KMR, Montrose DM, et al. An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1873–1878. doi: 10.1016/j.pnpbp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah J, Eack SM, Montrose DM, et al. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophr Res. 2012;141(2–3):189–196. doi: 10.1016/j.schres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erlenmeyer-Kimling L, Cornblatt B. High-risk research in schizophrenia: a summary of what has been learned. J Psychiatr Res. 1987;21(4):401–411. doi: 10.1016/0022-3956(87)90087-2. [DOI] [PubMed] [Google Scholar]
- 51.Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the Prodrome to Psychosis in the NAPLS Consortium: Relationship to Family History and Conversion to Psychosis. Archives of General Psychiatry. 2010;67(6):578. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riecher-Rössler A, Pflueger MO, Aston J, et al. Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol Psychiatry. 2009;66(11):1023–1030. doi: 10.1016/j.biopsych.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Gothelf D, Hoeft F, Ueno T, et al. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psychiatr Res. 2011;45(3):322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koutsouleris N, Davatzikos C, Bottlender R, et al. Early Recognition and Disease Prediction in the At-Risk Mental States for Psychosis Using Neurocognitive Pattern Classification. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66(7):700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1–3):114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones PB, Tarrant CJ. Specificity of developmental precursors to schizophrenia and affective disorders. Schizophr Res. 1999;39(2):121–125. doi: 10.1016/s0920-9964(99)00110-3. discussion 161. [DOI] [PubMed] [Google Scholar]
- 58.Kapur S. Looking for a "biological test" to diagnose “schizophrenia”: are we chasing red herrings? World Psychiatry. 2011;10(1):32. doi: 10.1002/j.2051-5545.2011.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sameroff AJ, Barocas R, Seifer R. The Early Development of Children Born to Mentally Ill Women. In: Watt NF, Anthony EJ, Wynne LC, Role JE, editors. Children at Risk for Schizophrenia: A Longitudinal Perspective. Cambridge University Press; 1984. pp. 482–513. [Google Scholar]
- 60.Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Windell D, Norman R, Malla AK. The personal meaning of recovery among individuals treated for a first episode of psychosis. Psychiatr Serv. 2012;63(6):548–553. doi: 10.1176/appi.ps.201100424. [DOI] [PubMed] [Google Scholar]
- 62.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36(6):1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cicchetti D. Development and Psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Hoboken: Wiley; 2006. pp. 1–23. [Google Scholar]
- 64.Dutta R, Greene T, Addington J, McKenzie K, Phillips M, Murray RM. Biological, Life Course, and Cross-Cultural Studies All point Toward the Value of Dimensional and Developmental Ratings in the Classification of Psychosis. Schizophr Bull. 2007;33(4):868–876. doi: 10.1093/schbul/sbm059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 66.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 67.Tandon N, Shah J, Keshavan MS, Tandon R. Attenuated psychosis and the schizophrenia prodrome: current status of risk identification and psychosis prevention. Neuropsychiatry. 2012;2(4):345–353. doi: 10.2217/NPY.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yung AR, Woods SW, Ruhrmann S, et al. Whither the attenuated psychosis syndrome? Schizophr Bull. 2012;38(6):1130–1134. doi: 10.1093/schbul/sbs108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carpenter WT, van Os J. Should Attenuated Psychosis Syndrome Be a DSM-5 Diagnosis? Am J Psychiatry. 2011;168(5):460–463. doi: 10.1176/appi.ajp.2011.10121816. [DOI] [PubMed] [Google Scholar]
- 70.McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206–1212. doi: 10.4088/JCP.08r04472. [DOI] [PubMed] [Google Scholar]
- 71.Francey SM, Nelson B, Thompson A, et al. Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurobiology and ethics in the era of early intervention. Schizophr Res. 2010;119(1–3):1–10. doi: 10.1016/j.schres.2010.02.1071. [DOI] [PubMed] [Google Scholar]
- 72.Mojtabai R, Malaspina D, Susser E. The concept of population prevention: application to schizophrenia. Schizophr Bull. 2003;29(4):791–801. doi: 10.1093/oxfordjournals.schbul.a007047. [DOI] [PubMed] [Google Scholar]
- 73.D'Agostino RBS, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 74.Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 75.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125(1):54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 76.Yung AR, Phillips LJ, Nelson B, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry. 2011;72(4):430–440. doi: 10.4088/JCP.08m04979ora. [DOI] [PubMed] [Google Scholar]
- 77.Woods SW, Walsh BC, Hawkins KA, et al. Glycine treatment of the risk syndrome for psychosis: Report of two pilot studies. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah J, Mizrahi R, McKenzie K. The four dimensions: a model for the social aetiology of psychosis. Br J Psychiatry. 2011;199:11–14. doi: 10.1192/bjp.bp.110.090449. [DOI] [PubMed] [Google Scholar]