Abstract
GABAergic interneurons of the parvalbumin-positive fast-spiking basket cells subtype (PV INs) are important regulators of cortical network excitability and gamma oscillations, involved in signal processing and cognition. Impaired development or function of PV INs has been associated with epilepsy in various animal models of epilepsy, as well as in some genetic forms of epilepsy in humans. In this review, we provide an overview of some of the experimental data linking PV INs dysfunction with epilepsy, focusing on disorders of the specification, migration, maturation, synaptic function or connectivity of PV INs. Furthermore, we reflect on the potential therapeutic use of cell-type specific stimulation of PV INs within active networks and on the transplantation of PV INs precursors in the treatment of epilepsy and its co-morbidities.
Keywords: epilepsy, GABA, interneurons, parvalbumin, basket cells, fast-spiking cells, genes
Introduction
Epilepsy is one of the most common neurological disorders, affecting 0.5–1% of the general population (Theodore et al., 2006). Unfortunately, up to one third of patients with epilepsy remain refractory to current therapies, which generally aim to control seizures but not the factors leading to the emergence of pathological circuits. Uncovering the mechanisms underlying the development of epilepsy is therefore necessary and urgent to improve the treatment and the prognosis of patients with refractory epilepsy.
Epilepsy is a diverse disorder with over 40 recognized epileptic syndromes (Berg et al., 2010). It is characterized by the repeated occurrence of highly synchronized and unprovoked bursts of neuronal activity, known as seizures (McCormick and Contreras, 2001). Focal seizures typically start in confined brain regions, and remain restricted to these areas (simple focal seizures) or spread to other brain regions (secondary generalization). By contrast, generalized seizures are characterized by the synchronous bi-hemispheric onset of epileptic activity on electroencephalograms (EEG). The mechanisms leading to these different types of seizures and epileptic syndromes still need to be clarified. However, recent genetic studies in patients with epilepsy, and the ongoing investigation of various animal models of epilepsy, have begun to shine light on some of the molecular and cellular determinants of epileptogenesis (the processes leading to epilepsy) and of ictogenesis (the process of seizure initiation).
Epileptogenesis is thought to involve progressive changes in gene expression, neuronal intrinsic properties, connectivity, network organization, astrocytic function and neuro-glial interactions that ultimately set up an imbalance between excitation and inhibition, leading to seizures (McNamara, 1994, Morimoto et al., 2004, Racine et al., 2002, Tasker et al., 1996). The initial trigger for this cascade of events can be an environmental insult (i.e. trauma, anoxia, infection, metabolic disturbances, febrile seizures, status epilepticus) or an intrinsic brain lesion (i.e. malformation, tumor, degeneration). However, in up to half of patients, no underlying cause can be found despite extensive investigations. In such situations, a genetic cause is often suspected and can sometimes be revealed by direct sequencing of candidate genes or by whole-exome/genome sequencing (as reviewed in (Sisodiya and Mefford, 2011)). The genetic etiologies of epilepsy are now recognized to be extremely diverse, including mutations in genes encoding ion channels, receptors, synaptic proteins, transcription factors, cell signaling factors, etc (as reviewed in (El Achkar et al., 2015, Sisodiya et al., 2007, Hani et al., 2015, Helbig, 2015)). The impact of such mutations on specific cell-types within epilepsy-prone networks must be investigated experimentally. Interestingly, recent advances in neurosciences methods, including genome editing to generate new mice models of epilepsy, and techniques such as paired and subcellular patch-clamp recordings, cell array recordings, optogenetics, in vivo electrophysiology and imaging technologies have begun to provide detailed descriptions of microcircuit function in both humans and animal models of epilepsy (see reviews: (Paz and Huguenard, 2015a, Rossignol et al., 2014)).
Appropriate brain function depends on highly interconnected and well-organized networks of inhibitory interneurons (INs) and excitatory projection pyramidal neurons (PNs). INs modulate the activity of PNs which transmit information between neuronal assemblies. Imbalances between these network components can give rise to disorders of brain function and neurological diseases, including epilepsy, schizophrenia and autism-spectrum disorder. Abnormalities of GABAergic inhibitory function have been observed in several genetic and experimental animal models of epilepsy and have been postulated to underlie epilepsy in some genetic forms of human epilepsy (see reviews (Powell, 2013, Rossignol, 2011)). Genetic mutations resulting in molecular and functional changes in GABA receptors (Faheem et al., 2014) or in the selective loss or functional impairment of GABAergic INs (Williams and Battaglia, 2013, Bender et al., 2012) may disrupt the regulation of local excitatory circuits, resulting in hyperexcitability of neuronal networks and contributing to epileptogenesis. Here, we review the progress in understanding the molecular factors that regulate cortical INs maturation, excitability, synaptic connectivity and integration within cortical networks, and how the perturbation of these processes leads to epilepsy, with a focus on parvalbumin (PV)-positive fast-spiking (FS) cortical INs of the basket cell (BC) sub-type (referred to as PV INs in this review).
Cortical interneuron diversity
Glutamatergic neurons constitute ~80 to 90% of the neuronal populations within cortical circuits, whereas GABAergic neurons account for the remaining 10 to 20% (Chu and Anderson, 2015, Druga, 2009). Although the GABAergic INs are a minority, they play vital functions within cortical networks: they provide feed-forward inhibition of incoming thalamocortical afferents and local inhibition within cortical microcircuits; they help generate or regulate specific rhythmic network oscillations important for proper signal processing; and they determine the onset and duration of cortical plasticity periods.
INs are substantially diverse: more than 20 distinct inhibitory cell types have been identified in the cerebral cortex and in the CA1 area of the hippocampus in rodents (Zeisel et al., 2015, Lovett-Barron and Losonczy, 2014). They can be divided into several subtypes sharing specific characteristics pertaining to their morphology, distribution, histochemical marker expression, intrinsic physiological properties, and connectivity ((Ascoli et al., 2008, Somogyi and Klausberger, 2005, Kawaguchi and Kubota, 1997, Butt et al., 2005) and see reviews (Rossignol, 2011, Sultan et al., 2013, Rudy et al., 2011)).
Neocortical GABAergic INs can be classified into several basic types according to their morphology: basket cells, chandelier cells, Martinotti cells, bouquet cells, bipolar cells, neurogliaform cells, etc. (Markram et al., 2004, Benarroch, 2013). They can also be classified based on their marker expression. About 40% of INs are PV-expressing cells, including basket cells and chandelier cells. About 30% of INs express somatostatin (SST), including the dendritic-targeting Martinotti cells, as well as other non-Martinotti cells, as described in the X98 and GINEGFP transgenic mice (Ma et al., 2006). The remaining ~30% of INs expresses the 5HT3A receptor, and includes bouquet cells and neurogliaform cells (Rudy et al., 2011, Lee et al., 2010). In addition, bipolar or bouquet cells frequently express vasointestinal peptide (VIP), and many of them also contain calretinin (CR). Most neurogliaform cells express reelin (see reviews: (Gelman and Marin, 2010, Rudy et al., 2011)).
Moreover, cortical INs differ in terms of their physiological properties, including their discharge patterns in response to depolarization (see reviews: (Fishell and Rudy, 2011, Druga, 2009, Rossignol, 2011)). PV-expressing basket cells and some chandelier cells are fast-spiking (PV-FS); they display high-frequency (>200 Hz) spike trains in response to depolarization, and have little spike frequency adaptation. The fast and high frequency discharges of PV-FS INs rely on the expression of the Nav1.1 voltage-gated sodium (Na+) channels, several potassium (K+) channels and calcium (Ca2+) permeable AMPA receptors (Geiger et al., 1995). SST-expressing Martinotti cells show a low-threshold burst firing pattern and a higher resting membrane potential than PV-FS INs; they are more readily activated than PV-FS INs (Fanselow et al., 2008). Bouquet cells show irregular and adapting spiking and high input resistance. Neurogliaform cells display a slow firing, late spiking firing pattern with slow adaptation (see reviews (Benarroch, 2013, Rossignol, 2011)).
In addition, different INs sub-types preferentially target distinct subareas of PNs, thus enabling a very efficient modulation of cortical activity. For instance, particularly interesting for this review, PV INs (BCs) make basket-like projections that target the soma and proximal dendrites of PNs, exerting a powerful inhibition of PNs excitability. By contrast, PV-positive chandelier cells project on the axonal initial segment (AIS) of PNs, thus modulating the output spike generation and spike timing of PNs. SST-positive Martinotti cells synapse on the dendrites of PNs, where they refine the glutamatergic inputs on cortical PNs and modulate the supralinear dendritic synaptic integration and plasticity of PNs (Lovett-Barron et al., 2012, Larkum, 2013). VIP-containing INs preferentially contact other subtypes of neocortical INs, in particular SST-, but also PV-positive INs, resulting in a net disinhibition of cortical PNs (Francavilla et al., 2015, Lee et al., 2013, Pi et al., 2013).
PV INs, which comprise 40–50% of GABAergic INs in the neocortex, constitute the dominant cortical inhibitory cell type in rodents (see review: (Rudy et al., 2011)). In addition, up to 30% of all INs in the hippocampus express PV (Bezaire and Soltesz, 2013, Katsumaru et al., 1988). Disorders of the specification or function of PV INs have been associated with epilepsy, which is why they will be the focus of this review. PV INs appear to be vulnerable to injury in chronic epileptogenic lesions of the neocortex (Drexel et al., 2011, Trotter et al., 2006, Zamecnik et al., 2006) and in the hippocampus (Andrioli et al., 2007, Scotti et al., 1997). Furthermore, as will be detailed in the following sections, genetic disorders altering the specification, migration, maturation, excitability and connectivity of PV INs have all been shown to cause seizures in rodents, and in some genetic human epilepsies (Figure 1).
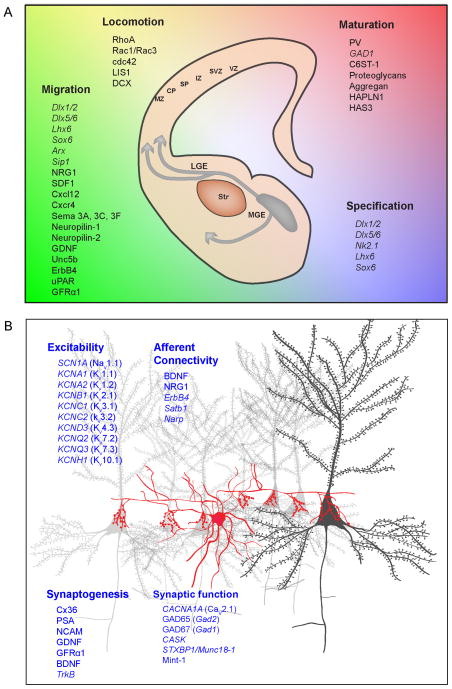
Figure 1. Molecular determinants of the development and function of cortical parvalbumin-positive fast-spiking basket cells (PV INs) linked with epilepsy.
A) Schematic representation of the migratory path of migrating INs from the medial ganglionic eminence (MGE) towards the cortical plate (CP). The MGE-derived INs, expressing the receptors neuropilin1 and 2, avoid the striatum that secretes repulsive semaphorins. Some of the molecular players involved in the control of the specification, migration (chemotaxis and locomotion) and maturation of PV INs that have been implicated in epilepsy are listed here (see text for details). B) Schematic representation of a PV IN basket cell (red) sending projections to the soma of multiple pyramidal cells (black and grey). Some of the sodium and potassium channels associated with epilepsy and governing neuronal excitability are listed here. Furthermore, genes and molecules involved in GABAergic synapse formation (synaptogenesis), synaptic function and afferent connectivity of PV INs are listed here (see text for details). MZ: marginal zone; CP: cortical plate; SP: sub-plate; IZ: intermediate zone; SVZ: sub-ventricular zone; VZ: ventricular zone; LGE: lateral ganglionic eminence; MGE: medial ganglionic eminence; Str: stiatum. See text for the abbreviations of genes and proteins listed here.
Cell type specification
Neocortical GABAergic INs originate in the ventral pallium (ventral forebrain), including in the medial (MGE) and the caudal (CGE) ganglionic eminences, as well as in the preoptic area ((Xu et al., 2004, Anderson et al., 2001, Fogarty et al., 2007, Gelman et al., 2009) and see review: (Batista-Brito and Fishell, 2009)). INs migrate tangentially to the cortical plate, and reach their final destination after radial migration across cortical layers (Anderson et al., 1997a, Anderson et al., 2001).
The MGE generates 70% of all cortical INs, including the PV-positive and the SST-positive INs (Butt et al., 2005, Miyoshi et al., 2007). PV INs derived mainly from the ventral MGE whereas SST INs are preferentially derived from the dorsal MGE, a phenomenon likely mediated by the combinational expression of particular transcription factors within different subdomains of the MGE (Fogarty et al., 2007, Wonders et al., 2008). By contrast, the CGE produces approximately 25% of cortical INs (Butt et al., 2005, Miyoshi et al., 2010, Nery et al., 2002). All CGE-derived INs express the 5HT3A serotonin receptor (Lee et al., 2010). These INs include, amongst others, the VIP-positive INs, the bipolar CR-positive INs and the multipolar neurogliaform cells (Lee et al., 2010, Miyoshi et al., 2010, Miyoshi et al., 2015, Nery et al., 2002). The lateral ganglionic eminence (LGE) is the source of GABAergic neurons to the striatum, amygdala and olfactory bulb, but does not produce cortical INs (Campbell et al., 1995, Batista-Brito et al., 2008, Wichterle et al., 2001, Wonders and Anderson, 2006, Anderson et al., 1997b, Waclaw et al., 2010).
The specification and the maturation of different sub-types of INs are tightly regulated, and some genes involved in these processes have been linked with epilepsy. The generation of MGE-derived INs relies on the serial expression of 5 important transcription factors: the Dlx homeobox genes Dlx1/2 and Dlx5/6, the NK2 homeobox 1 gene (Nkx2-1), the LIM homeobox protein 6 gene (Lhx6) and its downstream effector SRY-box 6 (Sox6) (see reviews: (Rossignol, 2011, Marín, 2012, Xu et al., 2004, Benarroch, 2013, Neves et al., 2013, Batista-Brito et al., 2009)).
The Nkx2.1 transcription factor, which is expressed only in the MGE, is critical for the molecular specification of the MGE and for the generation of PV- and SST-positive INs (Sussel et al., 1999). Indeed, Nkx2.1 loss at E10.5 leads to a re-specification of MGE-derived INs into CGE INs sub-types, with gross deficits in PV- and SST-positive INs and a gain of VIP- and CR-positive INs (Butt et al., 2008). This loss of MGE-derived INs results in severe seizures and early mortality within the first 3 weeks of life in Nkx2.1 conditional mutants (Butt et al., 2008).
The Lhx6 transcription factor, an effector of Nkx2.1 (Du et al., 2008), also regulates the specification and the tangential migration of MGE-derived INs. Lhx6 is expressed by MGE-derived INs as they leave the ventricular zone, and its expression persists into adulthood (Grigoriou et al., 1998). In Lhx6−/− mutant mice, the loss of Lhx6 results in vastly decreased populations of PV- and SST-positive INs in the neocortex and hippocampus, with preserved total GABAergic INs numbers, suggesting a fate-switch phenotype (Liodis et al., 2007). Lhx6−/− mutant mice die before P21, most likely from seizures, although this was not formally studied. However, mice carrying an hypomorphic allele of Lhx6 (Lhx6LacZ) display a loss of PV INs in the dentate gyrus of the hippocampus and a widespread deficit of SST INs in the cortex and hippocampus, resulting in disinhibition and spontaneous seizures (Neves et al., 2013). Together, these data from the Nkx2.1 and Lhx6 mutant mice suggest that MGE-derived INs are particularly important in regulating cortical excitability and in preventing the development of seizures.
Migration
INs migrate to the cortical plate through the process of tangential migration, and invade the cortical plate using radial migration. The molecular cascades regulating the migration of INs have been extensively reviewed recently (Marin, 2013). Many genes involved in the regulation of INs migration have been linked to epilepsy in rodents and will be reviewed here.
Transcription factors regulating the migration of INs
As discussed above, Lhx6 regulates INs cell-fate and defines MGE-subtypes of INs (Liodis et al., 2007, Neves et al., 2013). Furthermore, its expression persists in migrating INs and in most MGE-derived mature INs, suggesting that it serves additional roles in INs development. Indeed, Lhx6 is a major regulator of MGE-INs migration, both tangential and radial, and it controls the final laminar position and maturation of MGE-derived INs (Alifragis et al., 2004, Liodis et al., 2007, Neves et al., 2013). In Lhx6−/− mice, MGE-derived INs have a markedly delayed migration and tend to remain in the lateral cortical plate, failing to reach the medial cortex (Liodis et al., 2007). Furthermore, they fail to populate the middle cortical layers, and tend to remain in the superficial and deep cortical layers (I–II and VI) (Liodis et al., 2007). As stated above, Lhx6−/− mice develop epilepsy and die in the first few weeks of age.
The Sry-related HMG-box containing transcription factor Sox6, a downstream effector of Lhx6, is also expressed in MGE-derived INs as they initiate their migration, and its expression persists into adulthood (Batista-Brito et al., 2009). Conditional Lhx6Cre; Sox6F/F mutant mice, carrying a targeted deletion of Sox6 in MGE-derived INs, display a malposition of cortical MGE-derived INs, similar to the one observed in Lhx6 mutant mice. Indeed, PV and SST INs fail to populate the middle cortical layers and tend to remain in superficial and deep layers. Furthermore, PV INs do not mature properly in Lhx6Cre; Sox6F/F mutant mice: FS basket cells display a reduction of PV expression and have immature electrophysiological properties with lower firing rates (Batista-Brito et al., 2009). Lhx6Cre; Sox6F/F mutant mice develop severe lethal seizures leading to death in the third or fourth post-natal week (Batista-Brito et al., 2009).
The Aristaless-related homeobox (Arx) transcription factor is one of the many genes up-regulated by Lhx6 and is thought to mediate some of the effects of Lhx6 on INs migration (Zhao et al., 2008). ARX mutations cause an X-linked disorder with epilepsy, intellectual disability and autism, with or without lissencephaly and agenesis of the corpus callosum in humans (Scheffer et al., 2002, Sherr, 2003). Arx plays multiple roles in brain development: it regulates neuronal proliferation and differentiation, as well as neuronal migration, and affects the development of both PNs and INs (Friocourt et al., 2006). In Arx−/− mice, CR-, neuropeptide Y- (NPY), and CB-positive INs are severely reduced while PV INs are relatively preserved (Friocourt and Parnavelas, 2010, Colombo et al., 2007, Kitamura et al., 2002). Conditional deletion of Arx in INs results in reduced cortical inhibition and epilepsy in hemizygous Arx−/y; Dlx5/6CIG male and in half of Arx−/+; Dlx5/6CIG female mice (Marsh et al., 2009).
The Dlx homeobox transcription factors are also major players in the regulation of INs development. Dlx genes are expressed in the sub-ventricular and mantle zones of the ganglionic eminences and in subsets of mature GABAergic INs. In particular, the Dlx1/2 genes are expressed in most immature INs, and become restricted to subsets of SST-, NPY- and CR-positive INs in the post-natal cortex (Cobos et al., 2005b, Cobos et al., 2006). The Dlx1/2 genes regulate the specification, migration and survival of forebrain INs (Cobos et al., 2007). Indeed, Dlx1/2 double knock-out mice have a striking reduction of cortical INs (>75%) and of hippocampal INs (>95%), with a near complete blockade of INs migration out of the ventral telencephalon (Anderson et al., 1997a). Arx is activated by the Dlx2 transcription factor (Colasante et al., 2008) and Dlx1/2−/− mutant mice show a severe reduction of Arx expression in ventral forebrain structures (Cobos et al., 2005a). Interestingly, the INs migration defect observed in Dlx1/2 mutant mice can be rescued in vitro by the overexpression of the Arx gene, suggesting that Arx is a major mediator of the effects of Dlx2 on INs migration (although Arx does not rescue the INs specification defect associated with the loss of the Dlx1/2 genes) (Colasante et al., 2008).
Dlx1/2 genes also induce the expression of the Dlx5/6 transcription factors in the LGE and MGE sub-ventricular zones (Anderson et al., 1997a). Contrary to the Dlx1/2 transcription factors, the Dlx5/6 genes are considered to preferentially control the migration and maturation of PV INs. Indeed, Dlx5/6−/− mutant mice display a significant delay in migration of MGE-derived Lhx6-positive INs in the deep and superficial migratory streams, partly due to a loss of the Cxcr4 receptor (Wang et al., 2010). This results in reduced numbers of cortical PV INs, and in an altered morphology of the surviving PV INs (increased dendritic branching) (Wang et al., 2010). Dlx5/6−/− mutant mice die prenatally and cannot be assessed for epilepsy. However, the heterozygous Dlx5/6+/− mice, which have normal numbers of cortical INs but altered cortical inhibition, develop spontaneous electrographic seizures and have a reduced power of cortical gamma oscillations (Wang et al., 2010), implying a dysfunction of PV INs.
Chemotaxis of INs towards the cortical plate
On their way to the cortical plate, INs are influenced by a variety of chemoattractive and chemorepulsive cues. For instance, as they exit the ganglionic eminences, migrating INs start expressing Sip1, which represses the expression of the Unc5b receptor that otherwise prevents INs from leaving the ventral telencephalon (van den Berghe et al., 2013). Conditional mutant mice carrying a targeted deletion of Sip1 in most of the ganglionic eminences (Gsh2Cre; Sip1fl/fl) have a striking reduction in cortical INs and develop spontaneous myoclonic seizures in the first few weeks of life (van den Berghe et al., 2013). Furthermore, neuregulin-1 (NRG1), a trophic factor, exerts short and long-range attraction on INs migrating towards the cortical plate by activating its receptor ErbB4 expressed by migrating INs (Flames et al., 2004). ErbB4 is one of the direct targets of Lhx6 and its deregulation could contribute to the INs migratory phenotype in Lhx6 mutants (Zhao et al., 2008). NRG1 signaling through its receptor ErbB4 also controls various aspects of PV INs function in mature cells, and has been implicated in epilepsy, as detailed in subsequent sections.
The urokinase plasminogen activator receptor (uPAR) (the mouse ortholog of the autism-susceptibility PLAUR gene) also appears to be a critical player for INs migration. Indeed, uPAR−/− mice show decreased numbers of INs in the parietal and anterior cingulate cortex, mainly affecting PV INs with relative preservation of CR+ and SST+ populations. uPAR−/− mice exhibit spontaneous seizures and increased susceptibility to pentylenetetrazol (PTZ)-induced seizures (Powell et al., 2003). The role of uPAR in INs migration had initially been attributed to its activation of the pleiotropic molecule hepatocyte growth factor/scatter factor (HGF/SF), a known INs mitogenic factor in vitro (Powell et al., 2001), and to the subsequent binding of HGF/SF to its receptor, MET (Powell et al., 2003). However, more recent evidence from the same authors suggests that MET is not expressed by migrating INs and that HGF/SF promotes the development of excitatory neurons, but not of INs (Eagleson et al., 2011). Therefore, the exact mechanisms by which uPAR facilitate INs migration in vivo remain to be resolved. uPAR also appears to promote PV INs maturation since PV INs in the adult uPAR−/− mice show decreased GABA expression (in the C57BL/6J background) (Eagleson et al., 2005).
Semaphorins are important repulsive guidance cues involved in growth cone guidance, axonal pathfinding, fasciculation and branching of growing axons (de Wit and Verhaagen, 2003). Semaphorin signaling has also been implicated in the regulation of INs migration. Indeed, the semaphorins 3A and 3F secreted by the striatum repulse INs expressing the neuropilin-1 and neuropilin-2 receptors away from the striatum and towards the cortical plate (Marin et al., 2001, Nobrega-Pereira et al., 2008). Furthermore, the expression of some semaphorins is maintained post-natally, as is the case for Sema 3C and 3F in the cortex and hippocampus (Barnes et al., 2003). Interestingly, these semaphorins are reduced in the hippocampus after kainic acid (KA)-induced status epilepticus (SE), and this may contribute to axonal sprouting post SE (Barnes et al., 2003). Sema3F−/− mice display spontaneous seizures (Sahay et al., 2003), which may in part be due to alterations in the migration of INs.
The neuropilins 1 and 2 are semaphorin receptors and are important for INs migration. Whereas neuropilin 1 binds Sema3A, 3C and 3F (Renzi et al., 1999), neuropilin 2 binds Sema 3C and 3F, but only weakly Sema3A (Chen et al., 1997). Neuropilin 1 null mice (Npn1−/−) are not viable. However, blocking neuropilin 1 expression (using dominant negative mutated proteins) (Marin et al., 2001) or function (using specific antibodies) (Tamamaki et al., 2003) results in altered INs migration, with most INs remaining in the striatum. Neuropilin 2 knock-out mice (Npn2−/−) have a normal lifespan, but they display a severe disorganization of major fiber tracts, including hippocampal mossy fibers (Chen et al., 2000, Giger et al., 2000). Furthermore, Npn2−/− mice have reduced numbers of cortical and hippocampal GABA-, PV- and NPY- positive INs, decreased GABAergic synapses, enhanced susceptibility to KA- or PTZ-induced seizures and increased vulnerability to seizure-related death (Gant et al., 2009, Marin et al., 2001).
The glial derived neurotrophic factor (GDNF) and its receptor GFR α 1, also participate in the guidance of migrating PV INs. Specifically, they are involved in the early differentiation and migration of MGE-INs (Pozas and Ibanez, 2005), and they regulate the final distribution of PV INs (Canty et al., 2009). Although GFR α 1 knock-out mice are not viable, the GFR α 1 “cis-only” mutants, that lack the GFR α 1 receptor in cells that do not express the RET signaling receptor subunit (including INs), are viable. These mutants display hole-like regions devoid of PV-expressing cells in rostral and caudo-lateral cortical regions, in a neuronal activity-dependent fashion (ie. the size of these regions increases after optic nerve transection) (Canty et al., 2009). GFR α 1 “cis-only” mutant mice are more prone to PTZ-induced seizures (Canty et al., 2009).
Neuronal locomotion, neurite extension, neurite branching and nukleokinesis
The migration of INs also involves multiple regulators of neuronal locomotion, the dynamic process of INs migration (see review (Marin, 2013)). INs locomotion starts with the extension and branching of a leading process that probes the environment for guidance cues, followed by the stabilization of a main branch that will guide the INs forward (Lysko et al., 2011). These steps involve an active remodeling of the actin cytosqueleton, regulated by various Rho-GTPAses, including RhoA, Rac1/Rac3 and cdc42 (Marin et al., 2010, Bellion et al., 2005, Tsai and Gleeson, 2005, Govek et al., 2011, Gallo and Letourneau, 2004, Valiente and Martini, 2009, Tivodar et al., 2015, Vidaki et al., 2012). The Rho-GTPAses are known to be deregulated following seizures in experimental models of epilepsy and in human surgical specimens from epilepsy patients (Zhang et al., 2015, Yuan et al., 2010, Dang et al., 2014). In addition, some of the guidance cues and trophic factors that promote the branching or directionality of the leading process of INs have been associated with epilepsy. For instance, neuregulin-1 (NRG1) is an attractant that promotes branch extension and guides INs on their migratory paths to the cortical plate (Flames et al., 2004).
The stromal-derived factor-1 (SDF1/CXCL12), a chemoattractant expressed in the meninges and in the subventricular/intermediate zones, is a key regulator of INs migration (Stumm et al., 2003, Borrell and Marin, 2006, Tiveron et al., 2006). SDF1/CXCL12 decreases the branching of the leading process of INs by stabilizing the microtubules within the leading process and the actin filaments at the leading process tip, resulting in increased migration speed (Lysko et al., 2014, Lysko et al., 2011). SDF1/CXCL12 acts on INs through its receptor Cxcr4 (Borrell and Marin, 2006). Conditional deletion of Cxcr4 in MGE-derived INs causes their premature entry in the cortical plate and results in altered regional distribution of INs (Li et al., 2008). A recent study showed that the CXCR4 antagonist AMD3100 reverses some of the pathological landmarks of temporal lobe epilepsy (TLE) (aberrant neurogenesis and distorted dendritic morphology of newborn DG neurons), and that it significantly reduces the duration and frequency of chronic seizures in the intracerebroventricular KA model of epilepsy (Song et al., 2016). Interestingly, although CXCR4 has not been formally associated with epilepsy in humans, we recently described a young child with severe early-onset epileptic encephalopathy carrying a de novo 2q22.2q21.3 chromosomal deletion encompassing the CXCR4 gene (Michaud et al., 2014). The identification of additional patients carrying deletion or deleterious mutations of the CXCR4 gene will be necessary to confirm this association with epilepsy.
The second step of INs locomotion is nukleokinesis, or the active movement of the nucleus. Nukleokinesis entails an initial movement of the centrosome in a swelling in the leading process, followed by the traction of the nucleus towards the centrosome. These processes rely on the reorganization of the microtubule network that surrounds the nucleus (the perinuclear cage) and extends between the nucleus and the centrosome. This step involves the action of various organizing complexes and motor proteins, some of which participate in the migration of both PNs and INs and have been linked with epilepsy. In particular, the microtubule-associated proteins (MAP) encoded by the LIS1 and DCX genes have both been associated, in humans, with epilepsy and lissencephaly type I, a disorganization of cortical layers attributed to impairments of PNs migration (see recent review (Kato, 2015)). Neuropathological evidence suggests that mutations in these genes also impair the tangential migration of cortical INs (Marcorelles et al., 2010). Both proteins are known to interact with dynein and to mediate the coupling between the nucleus and the centrosome (Tanaka et al., 2004). Doublecortin, encoded by the Dcx gene, binds and stabilizes microtubules (Horesh et al., 1999). Knock-down of Dcx in the ganglionic eminences of rat embryos results in delayed migration of INs to the cortical plate (Friocourt et al., 2007). However, in Dcx(−/Y) hemizygous mutant male mice, PV INs numbers are intact, suggesting a normal migration of INs. By contrast, PNs are disorganized in these animals, with abnormal lamination, altered dendritic morphology and enhanced excitability, suggesting that mechanisms other than disinhibition underlie the enhanced susceptibility to pharmacologically-induced epileptiform discharges in these animals (Bazelot et al., 2012).
Maturation and cell survival
The maturation of cortical PV INs involves the gradual expression of PV and glutamate acid decarboxylase 1 (GAD1), the acquisition of FS membrane dynamics, the progressive innervation of neuronal targets, the development of more complex dendritic morphology, and the development of perineuronal nets (PNNs). These processes are driven by the progressive expression of a variety of transcription factors, channels and membrane proteins (Doischer et al., 2008, Okaty et al., 2009), some of which have been linked with epilepsy, as reviewed here and in subsequent sections.
Maturation of PV INs: focus on the PNN
The PNNs are specialized extracellular matrix structures composed of hyaluronan (HA), link proteins, chondroitin sulfate proteoglycans (CSPGs), and tenascin-R (Tn-R), that surround the soma, dendrites, and AIS of neurons, and form stable structures around synapses, as reviewed in (Wang and Fawcett, 2012, Kwok et al., 2011). PNNs are found in almost all regions of the central nervous system (CNS), but they preferentially surround GABAergic PV INs (Soleman et al., 2013, Pollock et al., 2014). PNNs are critical regulators of synaptic plasticity and the development of PNNs has been linked with the timing of cortical plasticity periods and with epilepsy (see reviews: (Kwok et al., 2011, Dityatev and Fellin, 2008)). For instance, the progressive increase in the ratio of 4-sulfation/6-sulfation of chondroitin sulfate proteoglycans is required to enhance the expression of the transcription factor Otx2 and to induce the functional maturation towards FS properties of PV INs. Reduction of this 4-sulfation/6-sulfation ratio by overexpressing 6-sulfated chondroitin sulfate proteoglycans impairs the maturation of PV INs and delays the closure of the visual cortical plasticity period (Miyata et al., 2012). Interestingly, chondroitin 6-O-sulfotransferase-1 (C6ST-1) transgenic (TG) mice, which overexpress chondroitin 6-sulfated chains and retain a decreased 4-sulfation/6-sulfation ratio, have abnormal PNNs formation, aberrant PV cell maturation and are more susceptible to KA-induced seizures (Yutsudo and Kitagawa, 2015). Furthermore, status epilepticus induces a striking loss of the integrity of the PNNs surrounding PV INs, including alterations in aggregan, HAPLN1 and HAS3 (McRae et al., 2012). These changes are presumed to impair INs function and to contribute to the process of epileptogenesis.
Ambient activity can also regulate the maturation of PV INs. Indeed, the levels of GAD67 and PV in INs have been shown to be activity-regulated and to correlate with the closure of the cortical plasticity periods. For instance, dark-rearing reduces the level of PV expression in the visual cortex and prolongs the visual plasticity period (Tropea et al., 2006). Interestingly, PV knock-out mice are more susceptible to PTZ-induced seizures (Schwaller et al., 2004). The exact mechanisms underlying seizure susceptibility in Pv−/− mice are uncertain, but might reflect the critical role of PV as a calcium buffer that regulates calcium levels at the synapse and impacts on the dynamics of GABAergic neurotransmission.
Cell survival
A significant proportion of cortical PV INs undergoes programmed cell death during the early post-natal period, with a peak around post-natal day 7 in mice (Southwell et al., 2012). PV INs maturation and survival depends on the trophic support provided by astrocytes (as demonstrated in Fgfr1f/f; hGfapCre+ mice (Smith et al., 2014)) and on NMDA-signaling from ambient glutamate (Desfeux et al., 2010). However, the survival of PV INs appears to be mainly regulated by intrinsic cues, the nature of which remains to be determined (Southwell et al., 2012). Preliminary data suggests that PV INs survival is, at least in part, regulated by the Mef2c transcription factor in a cell-autonomous fashion (Jaglin et al., 2013). Interestingly, in humans, 5q14.3 chromosomal microdeletions and MEF2C point mutations cause early severe epileptic encephalopathy and intellectual deficiency with autistic features (Boutry-Kryza et al., 2015, Paciorkowski et al., 2013, Bienvenu et al., 2013).
In a similar fashion, mice lacking the Dlx1 gene show an age-dependent selective loss of SST-, NPY-, CR- and RELN-expressing INs (but no changes of PV INs, as these cells do not express the Dlx1 gene during post-natal ages) (Cobos et al., 2005b). The loss of INs is partly due to enhanced apoptosis of these cells between P21–P30. Furthermore, surviving bitufted INs have poorly differentiated dendrites (shorter total branch length and decreased branching), suggesting a role for Dlx1 in INs maturation (Cobos et al., 2005b). The loss and aberrant maturation of INs in the Dlx1−/− mice results in reduced GABAergic inhibitory transmission in the hippocampus and neocortex and induces epilepsy with generalized seizures (Cobos et al., 2005b). Interestingly, although PV INs appear to be spared in the Dlx1−/− mutant mice, recent data suggests that the selective ablation or silencing of SST INs before P10 reduces the density of excitatory thalamic afferents on PV INs, therefore reducing the effective feed-forward inhibition within cortical networks (Tuncdemir et al., 2016). This alteration of PV INs recruitment by thalamocortical afferents might thus contribute to the epileptic phenotype observed in the Dlx1−/− mutant mice.
Neuronal Excitability
Amongst the best-described epilepsy genes in human patients are various ion channels genes that encode primary determinants of membrane excitability (e.g., voltage-gated channels, inwardly rectifying ion channels, ligand-gated channels, ion transporters, and ion exchangers). Some of these genes are particularly important in the regulation of PV INs excitability and will be reviewed here.
Voltage-gated sodium channels
Mutations in the SCN1A gene, encoding the voltage-gated sodium channel Nav1.1, represent the most common genetic cause of sporadic Dravet’s syndrome (DS) and of various forms of familial generalized epilepsy (Generalized Epilepsy with Febrile Seizures (GEFS+), isolated febrile seizures, etc) (Escayg and Goldin, 2010, Bozzi et al., 2012, Claes et al., 2003, Claes et al., 2001, Escayg et al., 2000). Nav1.1 is expressed in PV INs, clustered at the AIS and sparsely distributed on the cell soma, and is absent from other INs sub-types (Ogiwara et al., 2007). Nav1.1 is also expressed at low levels on PNs, but Scn1a loss of function mutations predominantly affect the function of PV INs and do not significantly impair PNs function (Dutton et al., 2012, Yu et al., 2006). Indeed, Scn1a+/− mutant mice display a substantial reduction in sodium current density in hippocampal PV INs, associated with reduced threshold for febrile seizures, spontaneous seizures and premature death (Yu et al., 2006). In addition, mutant mice carrying a humanized Scn1a mutation (R1648H, Scn1arh mice) display a striking alteration of PV INs intrinsic properties, with slower recovery from inactivation and greater use-dependent inactivation of sodium current, as well as decreased action potential (AP) firing rate, resulting in spontaneous generalized seizures and a reduced threshold for febrile seizures and flurothyl-induced seizures (Martin et al., 2010). By contrast, PNs appear largely unaffected in these mutants. Furthermore, the conditional deletion of Scn1a restricted to forebrain INs (Dlx1/2Cre; Scn1afl/+) results in generalized seizures and premature death (Cheah et al., 2012). In addition, the conditional deletion of Scn1a in PV-positive populations results in reduced seizure threshold to flurothyl or febrile seizure, and induces spontaneous seizures in Ppp1r2Cre; Scn1afl/+ mutant mice. By contrast, conditional deletion of Scn1a in PNs (Emx1Cre; Scn1afl/+) does not cause epilepsy (Dutton et al., 2012). Interestingly, the cognitive deficits associated with SCN1A mutations have also been linked with impaired excitability of various populations of PV-positive neurons in the septum, hippocampus, amygdala and cortex, leading to disruption of critical network oscillations and altered network function (Bender et al., 2012, Bender et al., 2013, Han et al., 2012). Therefore, the pathogenesis of Dravet syndrome and other SCN1A-associated epilepsies is now largely attributed to PV INs dysfunction (Yamakawa, 2011), and therapies aiming at enhancing PV INs excitability are being actively sought for patients with these disorders (Baraban et al., 2013).
Voltage-gated potassium channels
Voltage-gated K+ channels (Kv) play vital roles in the regulation of neuronal excitability by promoting membrane repolarization after action potentials, thereby shaping spike firing windows. Mutations in various Kv channels have been linked with epilepsy in humans, including KCNA1 (Kv1.1), KCNA2 (Kv1.2), KCNB1 (Kv2.1), KCNC1 (Kv3.1), KCND3 (Kv4.3), KCNQ2 (Kv7.2), KCNQ3 (Kv7.3) and KCNH1 (Kv10.1) (Soh et al., 2014, Smets et al., 2015, Saitsu et al., 2015, Syrbe et al., 2015, Eunson et al., 2000).
KCNA1 point mutations cause episodic ataxia with myokimia and variable epilepsy phenotypes in humans (Liguori et al., 2001, Zuberi et al., 1999, Eunson et al., 2000). In addition, Kcna1−/− mice, carrying a deletion of Kv1.1 channels, develop severe epilepsy (Smart et al., 1998). This phenotype has been partly attributed to an increased excitability of the hippocampal CA3 recurrent axon collaterals (Smart et al., 1998). However, in the cortex, Kv1.1 channels are expressed selectively at the AIS of PV INs (Goldberg et al., 2008). Kv1.1 channels dynamically regulate the firing behavior and dampen the excitability of layers II/III cortical PV INs near AP threshold (Goldberg et al., 2008). It is therefore possible that impaired cortical PV INs excitability also contributes to the epilepsy phenotype in Kcna1−/− mice, for instance by inducing rapid depolarization block in hyperexcitable PV INs.
Kv3 channels express delayed-rectifier type currents that promote AP repolarization and are critical determinants of neuronal high-frequency spiking behaviors (as reviewed in (Gan and Kaczmarek, 1998, Rudy et al., 1999, Rudy and McBain, 2001)). Kv3 channels containing Kv3.1 subunits are prominently expressed in cortical and limbic PV-FS INs (Chow et al., 1999, Rudy et al., 1999). KCNC1 dominant-negative mutations have recently been associated with progressive myoclonic childhood epilepsy in humans (Muona et al., 2015). Kv3.1/3.3 knock-out mice have impaired thalamocortical oscillations and unstable slow-wave sleep (Espinosa et al., 2008). Although they do not display spontaneous seizures, they might have a decreased seizure threshold, but this was not formally tested. Kv3.2 subunits are also highly expressed in limbic and in deep-layer cortical PV INs, where they form heteromultimeric channels with Kv3.1 subunits (Chow et al., 1999, Tansey et al., 2002). Kv3.2-containing channels enable PV INs to sustain high-frequency firing rates (Lau et al., 2000). KCNC2 mutations have not yet been associated with epilepsy in humans, but ongoing genome studies may reveal such mutations in the near future. Nonetheless, Kv3.2 null mice display decreased cortical inhibition and enhanced seizure susceptibility (Lau et al., 2000). Given the fundamental role of Kv3 channels in promoting high-frequency behaviors in fast-spiking PV INs, modulators of Kv3 potassium channels are actively being sought as potential therapeutic tools for patients with interneuronopathies (i.e. schizophrenia and perhaps epilepsy) (Rosato-Siri et al., 2015).
KCNQ2 mutations are known to cause a variety of epileptic disorders in humans, ranging from a benign form of neonatal seizures (benign familial neonatal epilepsy (BNFE)) due to KCNQ2 haploinsufficiency, to a severe epileptic encephalopathy due to dominant-negative KCNQ2 mutations (Bellini et al., 1993, Orhan et al., 2014, Weckhuysen et al., 2012, Weckhuysen et al., 2013, Kato et al., 2013, Saitsu et al., 2012a, Millichap and Cooper, 2012). KCNQ2 encodes the Kv7.2 potassium channels. KCNQ channels mediate the M-current, a non-inactivating potassium conductance triggered near the AP threshold (Wang et al., 1998). KCNQ channels assemble as tetramers and, in most neurons, Kv7.2 often combines with Kv7.3 to form KCNQ2/3 heteromers (Cooper et al., 2000, Saganich et al., 2001, Wang et al., 1998). Dominant-negative KCNQ2 mutations are often located in the voltage-sensor of pore domains of the channels, causing electromechanical uncoupling that prevents channel opening on depolarization (Millichap and Cooper, 2012). KCNQ2 mutations are thought to cause epilepsy by inducing a hyperexcitability of excitatory neurons. Indeed, dominant-negative Kcnq2 mutations in mice abolish M-currents and cause increased excitability, reduced spike-frequency adaptation and attenuated medium afterhyperpolarization in hippocampal CA1 PNs, resulting in epilepsy and cognitive impairment (Peters et al., 2005). Furthermore, conditional homozygote deletion of Kcnq2 in PNs is sufficient to cause epilepsy in Emx1Cre; Kcnq2fl/fl mice (Soh et al., 2014). However, Kcnq2 is also expressed in GABAergic INs, as was shown in different sub-population of hippocampal INs (both regular and PV-positive FS INs) (Grigorov et al., 2014, Cooper et al., 2001). In the hippocampus, Kv7 channels regulate interspike intervals of O-LM SST-positive INs (Lawrence et al., 2006). The roles of KCNQ channels in cortical INs remain uncertain. But since SST-INs mediate disinhibition by inhibiting PV INs in cortical layer IV (Xu et al., 2013, Cottam et al., 2013, Pfeffer et al., 2013), hyperexcitability or increased spike-frequency of SST-INs might be predicted to result in cortical disinhibition in the thalamocortical recipient layer, and could contribute to seizure generation.
Mutations in the KCNQ3 gene, encoding the associated subunit Kv7.3, have also been linked with epilepsy in humans, including with BNFE, benign familial infantile epilepsy (BFIE), and focal epilepsy with intellectual disability (Bellini et al., 1993, Miceli et al., 2015). The mechanisms underlying KCNQ3–associated epilepsy remain uncertain, but they do not involve hyperexcitability of pyramidal cells since Emx1Cre; Kcnq3fl/fl mutants do not develop epilepsy and have normal pyramidal cell properties (Soh et al., 2014). The impact of a Kcnq3 deletion on INs must therefore be investigated.
Neuromodulators of Kv channels directly participate in the regulation of neuronal excitability, including of PV INs excitability (see review:(Cooper and Jan, 2003)). For instance, M1 acetylcholine receptor agonists, such as pilocarpine, inhibit the KCNQ2/Q3 channels (M-channels) and increase the excitability of cortical and CA1 PV INs. This, in turn, induces depolarization block in a significant proportion of PV INs, resulting in disinhibition and seizures (Yi et al., 2014, Yi et al., 2015). The pilocarpine-induced enhancement of PV INs excitability is prevented in PVCre; M1fl/fl conditional knockout mice, which have reduced severity of pilocarpine-induced seizures compared to wild-type mice (Yi et al., 2015). Nonetheless, these mutant mice display cognitive impairment in tasks of novel object recognition and spatial memory (Yi et al., 2014). Of note, muscarinic agonists also robustly inhibit GABA release from cortical PV INs (Kruglikov and Rudy, 2008), compounding the effect of M1 activation on cortical disinhibition.
The brain-derived neurotrophic factor (BDNF) has been shown to decrease the excitability of PV INs in the rat dentate gyrus by activating an M-like current, presumably mediated by the KCNQ2/Q3 channels, thereby controlling PV INs resting membrane potential and excitability (Nieto-Gonzalez and Jensen, 2013, Holm et al., 2009). BDNF is significantly up-regulated following limbic seizures and may contribute to the pathogenesis of TLE (see review: (Scharfman, 2005)). BDNF also plays important roles in the regulation of the extent of PV INs innervation of PNs, as detailed in later sections of this review.
Synaptic connectivity
Synaptogenesis: GABAergic synapse formation and maturation
Loss or change of INs synaptic stability and organization can lead to disturbances in the excitation/inhibition balance and epilepsy. PV INs are critical to cortical local circuits. They are specialized to control cortical rhythms, and they regulate PNs spiking timely and precisely (Freund and Katona, 2007). PV INs respond to single-axon excitatory afferents much more strongly and rapidly than all other types of cortical neurons. They produce high-frequency trains of action potentials, and exert fast, stable and timed inhibitory output onto their target cells (Avermann et al., 2012, Sun et al., 2006). This ensures robust feed-forward inhibition and a resultant widespread cortical inhibition. PV INs are sufficient to generate network oscillations in the gamma frequency range that determine time windows for PNs activation (Cardin et al., 2009, Bartos et al., 2007, Sohal et al., 2009). These gamma oscillations are critical for adequate cortical processing (Cardin et al., 2009, Sohal et al., 2009), and participate in the processes of attention and memory (Howard et al., 2003). Therefore, selective impairment of PV INs and gamma oscillations may contribute to the cognitive impairments associated with epilepsy (see review: (Holmes, 2015)).
Apart from direct inhibitory control on PNs, PV INs are also mutually connected by GABAergic synapses (Jiang et al., 2013, Tamas et al., 2000) and electrical synapses (Traub et al., 2001, Buhl et al., 2003, Amitai et al., 2002, Szabadics et al., 2001, Tamas et al., 2000). Electrical coupling of PV INs is critical for the generation of gamma oscillations. Indeed, the deletion of connexin-36, impairing gap junction formation, results in decreased power of CA1 gamma oscillations and enhanced sensitivity to PTZ-induced seizures in Cx36 knock-out mice (Buhl et al., 2003, Jacobson et al., 2010). Moreover, PV INs can specifically form a large number of functional autapses (Jiang et al., 2015, Deleuze et al., 2014). The high connectivity amongst PV INs underlie their ability to synchronize their activity and to generate a coherent inhibitory output within cortical circuits (Bartos and Elgueta, 2012).
The pattern and extent of cortical PV INs innervation of PNs are tightly regulated by neural activity and experience (Baho and Di Cristo, 2012, Chattopadhyaya et al., 2004, Morales et al., 2002). Decreasing the activation of PV INs, either pharmacologically or by reducing afferent inputs with dark-rearing, results in a reduction of the pool of PNs innervated by each PV IN in the visual cortex (Baho and Di Cristo, 2012, Chattopadhyaya et al., 2004). Furthermore, the synaptic release of GABA regulates axonal pruning and GABAergic synapse elimination in the cortex (Wu et al., 2012). The maturation of cortical GABAergic innervation influences the onset and time course of cortical plasticity periods, as demonstrated for ocular dominance plasticity (Katagiri et al., 2007, Hensch and Fagiolini, 2005, Fagiolini and Hensch, 2000). In addition, some of the molecules involved in the activity-dependent maturation of GABAergic innervation have been associated with epilepsy and will be reviewed here.
Polysialic acid (PSA) is an elongated, linear polymer of α-2,8-linked sialic acid, preferentially attached to the neural cell adhesion molecule (NCAM) in vertebrates (Rothbard et al., 1982). This polyanionic and hydrated structure is central to the ability of NCAM and other adhesion molecules to induce intercellular attachment (Acheson et al., 1991, Fujimoto et al., 2001). PSA is involved in many processes entailing changes in cell shape or position, including neuronal migration, axonal fasciculation, axonal guidance, synaptogenesis and activity-dependent plasticity, as reviewed in (Di Cristo et al., 2007). PSA is central in the activity-mediated regulation of cortical GABAergic innervation. Indeed, the premature removal of PSA results in a precocious maturation of perisomatic innervation by PV INs, enhancing inhibitory transmission and causing an earlier onset of ocular dominance plasticity (Di Cristo et al., 2007). Interestingly, the selective ablation of NCAM during inhibitory synapse formation impairs axonal branching and synaptic bouton formation from PV INs (Chattopadhyaya et al., 2013). PSA-NCAM is upregulated in tissue from patients with medial temporal lobe epilepsy (MTLE) and in KA and kindling models of TLE (Le Gal La Salle et al., 1992, Mikkonen et al., 1998, Sato et al., 2002), possibly as a compensatory mechanism to favor stabilization of GABAergic innervation. Importantly, the PSA-NCAM assembly participates in neurotrophin signaling, as reviewed in (Ditlevsen et al., 2008). In particular, PSA-NCAM serves as a co-receptor for GDNF through its GFR α 1 receptor (Paratcha et al., 2003). GDNF-GFR α 1 signaling participates in axonal growth and synapse formation (Ledda et al., 2007). GDNF is a potent anticonvulsant (Kanter-Schlifke et al., 2007) and PSA-NCAM-dependent GDNF signaling was shown to limit neuronal loss and epileptogenesis in the KA model of epilepsy (Duveau and Fritschy, 2010). Furthermore, the ablation of PSA-NCAM increases acute seizure susceptibility (but not the progression to chronic epilepsy) in the amygdala-kindling model of epilepsy (Pekcec et al., 2007). The degree to which this neuroprotective effect of PSA-NCAM/GDNF signaling relates to its roles in sustaining GABAergic innervation remains to be established.
BDNF and its receptor TrkB are implicated in regulating activity-dependent synaptic plasticity and long-term potentiation (LTP), presumably by enhancing synaptic transmission from more active afferents (Kang et al., 1996, Kang and Schuman, 1995, Korte et al., 1995, Figurov et al., 1996). The activity-dependent expression of BDNF also regulates the expression of GABA in PV INs and controls the levels of cortical inhibition onto PNs, as shown in vitro (Rutherford et al., 1997, Rutherford et al., 1998). In vivo, the overexpression of BDNF shifts the visual ocular dominance plasticity period to earlier ages, possibly through its effect on the regulation of the maturation of GABAergic innervation (Hanover et al., 1999). Such precocious maturation of inhibitory innervation might have detrimental consequences. BDNF expression is enhanced in the hippocampi of patients with TLE (Martinez-Levy et al., 2016) and excessive activation of TrkB by status epilepticus was shown to promote the development of TLE (Liu et al., 2013). BDNF-TrkB signaling has therefore been identified as an important therapeutic target in the prevention of epileptogenesis. Interestingly, impairing BDNF-TrkB signaling, by uncoupling Trkb from phospholipase C γ 1, prevents the emergence of epilepsy after status epilepticus (Gu et al., 2015). Whether this neuroprotective effect depends on BDNF-TrkB signaling in INs or PNs remains to be investigated.
Disorganization of PV INs innervation may also be a prominent feature of symptomatic epilepsies due to cortical lesions or cortical malformations. For instance, in the undercut (UC) model of chronic post-traumatic epileptogenesis, the inhibitory transmission from FS cells to excitatory regular-spiking (RS) neurons (PNs or spiny stellate neurons) in layer IV of the barrel cortex is impaired. This disinhibition probably reflects presynaptic alterations in FS cells as indicated by a decreased GABA release probability, increased synaptic failures, and a reduced number of presynaptic boutons (Ma and Prince, 2012). In a similar fashion, the prenatal irradiation mouse model of cortical dysplasia displays a significant impairment of the synaptic release properties from PV INs on RS neurons, as illustrated by higher failure rates, decreased connection probability, decreased transmitter release probability, and decreased axonal terminal boutons in biocytin-filled PV INs in the dysplastic cortex. The electrical coupling between PV INs in the dysplastic cortex is also impaired, as shown by a reduction in the connection rates and coupling coefficients (Zhou and Roper, 2014). Of note, cortical INs are less abundant in the dysplastic cortex, and the excitatory drive on surviving FS INs (presumed PV INs) is reduced, further contributing to the disinhibition phenotype (Zhou et al., 2009).
Synaptic function
The functional specification of PV INs is defined by their physiological intrinsic properties, including short membrane time constants, ultra-fast AMPA receptor conductance, an abrupt, non-adapting high-frequency firing; and by their synaptic connectivity and synaptic properties, partly determined by their expression of the P/Q-type CaV2.1 calcium channels, as reviewed in (Wang et al., 2002, Rossignol, 2011, Rudy et al., 2011). Many molecular determinants of PV INs synaptic function have been associated with epilepsy, and some of these players will be reviewed here.
GABA synthesis occurs via two isoforms of the glutamic acid decarboxylase (GAD) enzyme, GAD65 (Gad2) and GAD67 (Gad1), in mammals. Although both enzymes are usually expressed by GABAergic cells in an activity-dependent fashion, they differ in their age-dependent expression profile, in their sub-cellular localization and in their kinetics (see review (Pinal and Tobin, 1998)). GAD67, responsible for approximately 90% of GABA synthesis, predominates during early development and after injury, and is expressed both at the cell body and axonal terminals. GAD65 tends to be expressed later and is particularly abundant at nerve terminals where it participates in GABA synthesis under conditions of heightened synaptic activity (Tian et al., 1999). Gad67 constitutive knockout mice (Gad1−/−) do not survive past the perinatal period, but the in vitro investigation of hippocampal slices from these animals reveals decreased miniature inhibitory postsynaptic currents (mIPSC) amplitude and reduced levels of GABA in the synaptic cleft of inhibitory synapses (Lau and Murthy, 2012). Mutant mice carrying a heterozygous Gad1 conditional deletion selectively in PV INs are viable and display substantial deficits in PV INs to PNs synaptic transmission, resulting in an enhancement of PNs excitability in the prefrontal cortex (Lazarus et al., 2015). Whether this deficit is sufficient to enhance seizure susceptibility remains to be investigated. However, constitutive GAD65 knockout mice (Gad2−/−), which do not display significant alterations in baseline mIPSC amplitudes or GABA level presumably due to compensation by GAD67, are more susceptible to PTZ and picrotoxin-induced seizures, suggesting an important role of GAD65 in contexts of high neuronal activity (Asada et al., 1996, Kash et al., 1997).
CaV2.1 P/Q-type calcium channels regulate neurotransmitter release at the majority of central synapses, but had been demonstrated to be particularly critical to sustain GABA release from PV INs (Zaitsev et al., 2007). Haploinsufficiency of the CACNA1A gene, encoding the α1 sub-unit of the CaV2.1 channels, causes epileptic encephalopathy with episodic ataxia in humans (Damaj et al., 2015, Allen et al., 2013, Jouvenceau et al., 2001). The loss of CaV2.1 channels can usually be compensated by a gain of function of CaV2.2 N-type calcium channels (Jun et al., 1999). However, we recently demonstrated that the targeted deletion of Cacna1a in MGE-derived cortical INs (Nkx2.1Cre; Cacna1ac/c mice) results in significant impairment of cortical PV INs synaptic function, with reduced connection probability to PNs and imprecise time-locking of IPSCs in PV INs to PNs pairs, despite compensation by N-type channels (Rossignol et al., 2013). By contrast, the synaptic function of SST INs was preserved in SSTCre; Cacna1ac/c mutants. We showed that this impairment of cortical and limbic PV INs synaptic function is sufficient to cause generalized epilepsy in Nkx2.1Cre; Cacna1ac/c mice (Rossignol et al., 2013). Interestingly, mutants carrying a targeted Cacna1a deletion in cortical PNs (Emx1Cre; Cacna1ac/c) did not develop epilepsy, but displayed decreased cortical excitability. Joint removal of Cacna1a in cortical INs and PNs reduced the seizure severity, leading to brief generalized spike-wave absence seizures in Nkx2.1Cre; Emx1Cre; Cacna1ac/c mice (Rossignol et al., 2013). Therefore, Cacna1a-associated epilepsy appears to be due, in part, to the specific cell-type requirement for CaV2.1 channels in PV INs and the resultant synaptic impairment of cortical PV INs in conditional Cacna1a mutants. Interestingly, mice carrying a later post-natal removal of Cacna1a restricted to PNs in cortical layer VI (Cacna1aNtsr(−/−)) were recently shown to have an enhancement of thalamocortical excitability and to display absence seizures, likely because the cortical excitability of PNs in other cortical layers was preserved (Bomben et al., 2016).
CASK mutations result in severe developmental delay, intellectual deficiency, microcephaly, ponto-cerebellar atrophy and epilepsy in humans (Moog et al., 2011, Najm et al., 2008, Saitsu et al., 2012b, Michaud et al., 2014). CASK is a membrane-associated guanylate kinase (MAGUK) with a conserved multidomain structure that includes a Ca2+/calmodulin-kinase domain, a PDZ and SH3 domain and a guanylate kinase domain (Hata et al., 1996). CASK plays a role in synaptic scaffolding and organization, by interacting with Mints, Veli/Mals and neurexins (Tabuchi et al., 2002, Butz et al., 1998, Hata et al., 1996). Cask knock-out mice are not viable due to severe respiratory failure. However, there is a net reduction of spontaneous inhibitory events (sIPSC) and a gain of excitatory events (sEPSC) in Cask−/− neuronal cultures, suggesting that the loss of Cask results in an interneuronopathy (Atasoy et al., 2007).
STXBP1 (syntaxin-binding protein 1) mutations have also been linked with severe epileptic encephalopathy and intellectual disability in humans (Deprez et al., 2010, Hamdan et al., 2009, Mastrangelo et al., 2013, Saitsu et al., 2008). Stxbp1b mutant zebrafish develop epilepsy and behavioral impairment (Grone et al., 2016). Stxbp1/Munc18-1 interacts with the synaptic machinery (including SNARE proteins) and is a key regulator of vesicular fusion and neurotransmission (Shen et al., 2015). The exact mechanisms underlying Stxbp1-associated epilepsy remain to be resolved. However, the Mint-1 protein, which is preferentially expressed in cortical INs (Ho et al., 2003), interacts both with CASK and Munc18-2/stxbp1 (Butz et al., 1998) and the loss of Mint-1 selectively impairs GABAergic neurotransmission (Ho et al., 2003). Therefore, a GABAergic deficit might underlie the epilepsy associated with perturbations of the CASK/Mint-1/Stxbp1 complex.
Excitatory and inhibitory innervation of PV INs
NRG1, through its receptor ErbB4, regulates PV IN function in multiple ways. First, it increases the intrinsic excitability of PV INs by phosphorylating and inhibiting Kv1.1 channels, thereby decreasing the voltage threshold for action potential in PV INs (Li et al., 2012). Second, it enhances the formation of excitatory synapses on PV INs (Fazzari et al., 2010). Third, it promotes the voltage-dependent release of GABA from PV INs onto PNs (Chen et al., 2010, Woo et al., 2007, Wen et al., 2010, Del Pino et al., 2013). Finally, NRG1–ErbB4 signaling in PV INs also regulates the expression of GABAA receptor subunits at GABAergic synapses on hippocampal PNs (Okada and Corfas, 2004) and suppresses LTP in PNs (Chen et al., 2010, Wen et al., 2010, Pitcher et al., 2011). NRG1 and ErbB4 are therefore critical regulators of PV INs excitability and function. In the cortex and hippocampus, ErbB4 is selectively expressed in PV INs (Vullhorst et al., 2009, Woo et al., 2007, Yau et al., 2003). Mice with targeted deletion of ErbB4 in PV INs (PvCre; Erbb4fl/fl) display a variety of cognitive behavioral impairments (hyperactivity, impaired working memory, deficits in pre-pulse inhibition and socialization) (Wen et al., 2010, Del Pino et al., 2013) and are more susceptible to PTZ- and pilocarpine-induced epilepsy (Li et al., 2012), as well as to kindling-induced seizures (Tan et al., 2012).
The density of thalamic afferents onto PV INs is also tightly regulated and is dependent on the integrity of SST INs before the second post-natal week. Indeed, SST INs are strongly activated by thalamocortical projections before P15, and they innervate PV INs and PNs broadly and equally at P6 (whereas they preferentially innervate PNs at P15) (Tuncdemir et al., 2016). Reducing the number of SST INs before the second post-natal week (in SSTCre; Rosa-DTA mutants or in SSTCre; Satb1c/c mutants) or impairing their ability to spontaneously release GABA in the extracellular space (SSTCre; Vgatc/+ mutants)results in a significant decrease of the thalamic innervation of PV INs (Tuncdemir et al., 2016). We previously demonstrated that Dlx6Cre; Satb1c/c mutants, with similar reduction of cortical SST INs, develop epilepsy in the second post-natal week (Close et al., 2012), which might be partly attributable to a reduced excitatory drive on PV INs.
The remodeling of cortical circuits promoting a progressive enhancement of feed-forward inhibition may be a central phenomenon to compensate enhanced excitation within cortical networks in chronic epilepsy. For instance, in the transcortical freeze lesion (FL) rodent model of epilepsy, the increased excitatory connectivity onto PN seems to be partially offset by an increase in excitatory connectivity and a decrease in inhibitory connectivity onto layer V PV FS cells, ultimately resulting in a relative balance between excitation and inhibition in the affected cortical area (Jin et al., 2014). Interestingly, a similar enhancement of excitatory synapse strength on PV INs was noted in cultured neurons from mutant mice carrying a deletion of the neuronal activity-regulated pentraxin (Narp) gene (Chang et al., 2010). Narp is an activity-regulated immediate-early gene that is preferentially enriched at excitatory synapses on PV INs and that is up-regulated by seizure activity. Notably, Narp−/− mice show an increased sensitivity to kindling-induced seizures (Chang et al., 2010). Together, this data suggests that Narp is a critical component of network remodeling following seizures and that it functions by raising excitatory transmission onto PV INs, which rebalances network excitation/inhibition dynamics following seizures (Chang et al., 2010).
Functional networks
The emergence of new techniques to selectively stimulate specific neuronal populations, including optogenetics and DREADD technologies, has raised significant hopes that selective manipulation of INs might represent new therapeutic avenues to correct defective INs function underlying some neuropsychiatric and neurological disorders, including epilepsy (see reviews: (Roth, 2016, Urban and Roth, 2015, Bui et al., 2015, Paz and Huguenard, 2015b)). In this section, we will summarize how modulating the activity of PV INs within neural networks may contribute to the development or prevention of epilepsy.
In recent years, several research groups have used optogenetic manipulations of INs to control seizure activity in various animal models of epilepsy in vivo and in vitro (Wykes et al., 2016, Krook-Magnuson et al., 2013, Shiri et al., 2015, Shiri et al., 2016, Ewell et al., 2015, Yekhlef et al., 2015, Ledri et al., 2014, Ellender et al., 2014). However, whether the stimulation of PV INs actually prevents or favors seizure generation appears to depend on the specific neuronal state at the onset of stimulation, on the site of stimulation and on the particular seizure models studied.
In a variety of experimental models of epilepsy in vitro, PV INs have been shown to fire intensely before seizure onset, which might suggest that they prevent seizure initiation. But because they are able to synchronize network activity (as reviewed in (Avoli and de Curtis, 2011, Jiruska et al., 2013)), it has been suggested that, in some circumstance, enhanced GABAergic signaling might actually trigger seizures. In two different cortical slice models of focal epilepsy (low Mg2+ or focal NMDA application), PV INs have been shown to fire actively before seizure onset and to provide an inhibitory barrage to excitation in PNs, preventing seizure propagation (Cammarota et al., 2013, Trevelyan et al., 2006). A similar area of desynchronized firing, the so-called ictal penumbra that surrounds the ictal focus, has also been observed in human patients undergoing multiunit recordings, suggesting that similar inhibitory barrages also occur in vivo (Schevon et al., 2012). However, after a period of excessive excitation, PV INs undergo depolarization block and the ensuing failure of PV INs coincides with the onset of seizures (Cammarota et al., 2013). Although this data suggests that PV INs prevent seizure onset, Sessolo et al. showed that the selective optogenetic or electric activation of PV INs at the epileptogenic focus failed to prevent seizures, but rather induced post-inhibitory rebound spiking in PNs, promoting ictal generation in the NMDA slice model of epilepsy (Sessolo et al., 2015). Similarly, the optogenetic activation of PV INs in the entorhinal cortex induces low-voltage fast-onset epileptic discharges in the 4-aminopyridine slice model of epilepsy (Shiri et al., 2015, Yekhlef et al., 2015), a GABAA receptor-mediated seizure pattern usually attributed to the synchronous activity of GABAergic INs (Gnatkovsky et al., 2008). These low-voltage fast-onset epileptic discharges differ from the glutamatergic-driven hypersynchronous periodic discharges occurring after the application of the GABAA receptor antagonist picrotoxin, together with GABAB receptor blockage, or by the optogenetic stimulation of PNs (Shiri et al., 2016, Shiri et al., 2015). This suggests that the stimulation of PV INs might actually trigger specific types of seizure activity. Furthermore, the selective optogenetic activation of PV INs can generate excitatory GABAergic responses onto CA3 PNs, due to a reversal of the chloride equilibrium potential, as shown in hippocampal organotypic slices, a post-traumatic model of epileptogenesis (Ellender et al., 2014). In this preparation, PV INs optic stimulation triggers afterdischarges that propagate across hippocampal networks and that resemble the epileptic activity recorded during the clonic phase of limbic seizures. Together, these studies suggest that, at least in situation of enhanced excitability (low Mg2+, local NMDA application and trauma), the coherent firing of PV INs may trigger seizures.
By contrast, PV INs appear critical in limiting the spread of seizures. Indeed, in cortical low Mg2+ cortical slices, PNs are recruited in spatially-restricted modules when the inhibitory barrage fails, leading to a slow progression of the epileptic activity in a step-wise fashion (Trevelyan et al., 2006). The speed of seizure progression inversely correlates with the inhibitory drive (Trevelyan et al., 2007). Furthermore, the stimulation of PV INs distant from the seizure focus prevents ictal propagation and shortens the duration of seizures at the primary focus in the NMDA slice model, presumably by preventing the propagation of afterdischarges back to the ictal focus (Sessolo et al., 2015).
Despite these controversies on the precise role of PV INs in seizure initiation in vitro, on-demand optogenetic activation of hippocampal PV INs or cerebellum PV-expressing neurons (Purkinje cells and INs) in vivo decreases the duration of spontaneous seizures in a mouse model of TLE (Krook-Magnuson et al., 2014, Krook-Magnuson et al., 2013), suggesting that the stimulation of PV INs may indeed be an appropriate therapeutic avenue in epilepsy. Furthermore, the transplantation of MGE INs precursors in the cortex of young and adult mice has been shown to reduce or abolish seizures in genetic mice models of epilepsy (Baraban et al., 2009, Hunt et al., 2013, Sebe and Baraban, 2010).
Furthermore, given the central role of PV INs in regulating network activities and in promoting gamma oscillation within different neuronal networks (Buhl et al., 2003, Cardin et al., 2009, Sohal and Huguenard, 2005, Sohal et al., 2009, Traub et al., 2003, Vida et al., 2006, Wulff et al., 2009), and given the suggested implication of gamma oscillations in sustaining various cognitive processes such as information processing, attention and memory (Colgin et al., 2009, Howard et al., 2003, Sohal et al., 2009, Cardin et al., 2009, Fries et al., 2001), it has been postulated that an impairment of PV INs disrupting gamma oscillations may underlie some of the cognitive and behavioral deficits associated with epilepsy. Indeed, several studies have reported that a disruption of the excitatory drive onto PV INs can compromise network oscillations and lead to cognitive and behavioral phenotypes (Yu et al., 2015, Huang et al., 2016, Billingslea et al., 2014, Saunders et al., 2013). Therefore, together with its suggested therapeutic benefit in seizure control in vivo, the manipulation of PV INs could potentially be beneficial for the treatment of the co-morbidities of epilepsy. As a proof of concept, the transplantation of MGE-derived INs in genetic models of epilepsy has been shown to correct the behavioral and cognitive phenotypes in adult mice with genetic epilepsy (Hunt et al., 2013).
Perspectives
In conclusion, multiple evidence point to a disruption of the development or function of PV INs as a pathophysiological substrate to the development of epilepsy in various genetic and lesional models of epilepsy, as well as in some genetic forms of epilepsy in humans. While the role of PV INs in the prevention or initiation of seizures in vitro remains controversial, the selective stimulation of PV INs or the transplantation of MGE-derived INs precursors have shown significant promises as therapeutic avenues in vivo. Therefore, advances in optogenetic and related technologies providing precise temporal and spatial control over particular populations of INs might pave the way for the future use of selective PV INs stimulation in the treatment of patients with epilepsy, particularly in patients where genomic studies reveal mutations in genes known to regulate the development or function of PV INs.
Acknowledgments
ER is supported by a clinician-scientist career award form the Fond de recherche en Santé du Québec (FRQS). ER receives funding from the Canadian Health Research Institutes (CIHR), the CURE Foundation and the Réseau de Médecine Génétique Appliquée du Québec (RMGA).
Biographies
E.R. is a clinician scientist at the CHU Ste-Justine, and an assistant professor in the Departments of Neurosciences and Pediatrics at the Université de Montréal. Her work focuses on the genetic basis of pediatric epileptic encephalopathies and on the cellular and network mechanisms underlying these disorders, with a focus on GABAergic interneuron development and function.
X. J. is a post-doctoral fellow, trained electrophysiologist, conducting in vitro physiological investigations of various genetic mouse models of epilepsy generated in the Rossignol laboratory.
M. L. is a research assistant in Dr Rossignol’s laboratory.
Footnotes
Competing interests.
The authors have no competing interests.
Author contributions.
X. J. and E. R. wrote and edited the manuscript. M. L. designed the figure.
References
- ACHESON A, SUNSHINE JL, RUTISHAUSER U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol. 1991;114:143–53. doi: 10.1083/jcb.114.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALIFRAGIS P, LIAPI A, PARNAVELAS JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–8. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN AS, BERKOVIC SF, COSSETTE P, DELANTY N, DLUGOS D, EICHLER EE, EPSTEIN MP, GLAUSER T, GOLDSTEIN DB, HAN Y, HEINZEN EL, HITOMI Y, HOWELL KB, JOHNSON MR, KUZNIECKY R, LOWENSTEIN DH, LU YF, MADOU MR, MARSON AG, MEFFORD HC, ESMAEELI NIEH S, O’BRIEN TJ, OTTMAN R, PETROVSKI S, PODURI A, RUZZO EK, SCHEFFER IE, SHERR EH, YUSKAITIS CJ, ABOU-KHALIL B, ALLDREDGE BK, BAUTISTA JF, BORO A, CASCINO GD, CONSALVO D, CRUMRINE P, DEVINSKY O, FIOL M, FOUNTAIN NB, FRENCH J, FRIEDMAN D, GELLER EB, GLYNN S, HAUT SR, HAYWARD J, HELMERS SL, JOSHI S, KANNER A, KIRSCH HE, KNOWLTON RC, KOSSOFF EH, KUPERMAN R, MCGUIRE SM, MOTIKA PV, NOVOTNY EJ, PAOLICCHI JM, PARENT JM, PARK K, SHELLHAAS RA, SHIH JJ, SINGH R, SIRVEN J, SMITH MC, SULLIVAN J, LIN THIO L, VENKAT A, VINING EP, VON ALLMEN GK, WEISENBERG JL, WIDDESS-WALSH P, WINAWER MR. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMITAI Y, GIBSON JR, BEIERLEIN M, PATRICK SL, HO AM, CONNORS BW, GOLOMB D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–52. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON SA, EISENSTAT DD, SHI L, RUBENSTEIN JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–6. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- ANDERSON SA, MARIN O, HORN C, JENNINGS K, RUBENSTEIN JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- ANDERSON SA, QIU M, BULFONE A, EISENSTAT DD, MENESES J, PEDERSEN R, RUBENSTEIN JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- ANDRIOLI A, ALONSO-NANCLARES L, ARELLANO JI, DEFELIPE J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–43. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- ASADA H, KAWAMURA Y, MARUYAMA K, KUME H, DING R, JI FY, KANBARA N, KUZUME H, SANBO M, YAGI T, OBATA K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–5. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- ASCOLI GA, ALONSO-NANCLARES L, ANDERSON SA, BARRIONUEVO G, BENAVIDES-PICCIONE R, BURKHALTER A, BUZSAKI G, CAULI B, DEFELIPE J, FAIREN A, FELDMEYER D, FISHELL G, FREGNAC Y, FREUND TF, GARDNER D, GARDNER EP, GOLDBERG JH, HELMSTAEDTER M, HESTRIN S, KARUBE F, KISVARDAY ZF, LAMBOLEZ B, LEWIS DA, MARIN O, MARKRAM H, MUNOZ A, PACKER A, PETERSEN CC, ROCKLAND KS, ROSSIER J, RUDY B, SOMOGYI P, STAIGER JF, TAMAS G, THOMSON AM, TOLEDO-RODRIGUEZ M, WANG Y, WEST DC, YUSTE R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATASOY D, SCHOCH S, HO A, NADASY KA, LIU X, ZHANG W, MUKHERJEE K, NOSYREVA ED, FERNANDEZ-CHACON R, MISSLER M, KAVALALI ET, SUDHOF TC. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–30. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVERMANN M, TOMM C, MATEO C, GERSTNER W, PETERSEN CC. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol. 2012;107:3116–34. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- AVOLI M, DE CURTIS M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–32. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAHO E, DI CRISTO G. Neural activity and neurotransmission regulate the maturation of the innervation field of cortical GABAergic interneurons in an age-dependent manner. J Neurosci. 2012;32:911–8. doi: 10.1523/JNEUROSCI.4352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARABAN SC, DINDAY MT, HORTOPAN GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4:2410. doi: 10.1038/ncomms3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARABAN SC, SOUTHWELL DG, ESTRADA RC, JONES DL, SEBE JY, ALFARO-CERVELLO C, GARCIA-VERDUGO JM, RUBENSTEIN JL, ALVAREZ-BUYLLA A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–7. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES G, PURANAM RS, LUO Y, MCNAMARA JO. Temporal specific patterns of semaphorin gene expression in rat brain after kainic acid-induced status epilepticus. Hippocampus. 2003;13:1–20. doi: 10.1002/hipo.10041. [DOI] [PubMed] [Google Scholar]
- BARTOS M, ELGUETA C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–81. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTOS M, VIDA I, JONAS P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- BATISTA-BRITO R, CLOSE J, MACHOLD R, FISHELL G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–75. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATISTA-BRITO R, FISHELL G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATISTA-BRITO R, ROSSIGNOL E, HJERLING-LEFFLER J, DENAXA M, WEGNER M, LEFEBVRE V, PACHNIS V, FISHELL G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–81. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZELOT M, SIMONNET J, DINOCOURT C, BRUEL-JUNGERMAN E, MILES R, FRICKER D, FRANCIS F. Cellular anatomy, physiology and epileptiform activity in the CA3 region of Dcx knockout mice: a neuronal lamination defect and its consequences. Eur J Neurosci. 2012;35:244–56. doi: 10.1111/j.1460-9568.2011.07962.x. [DOI] [PubMed] [Google Scholar]
- BELLINI G, MICELI F, SOLDOVIERI MV, MIRAGLIA DEL GIUDICE E, COPPOLA G, TAGLIALATELA M. KCNQ2-Related Disorders. In: PAGON RA, ADAM MP, ARDINGER HH, WALLACE SE, AMEMIYA A, BEAN LJH, BIRD TD, FONG CT, MEFFORD HC, SMITH RJH, STEPHENS K, editors. GeneReviews(R) Seattle (WA): 1993. [PubMed] [Google Scholar]
- BELLION A, BAUDOIN JP, ALVAREZ C, BORNENS M, METIN C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25:5691–9. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENARROCH EE. Neocortical interneurons Functional diversity and clinical correlations. Neurology. 2013;81:273–280. doi: 10.1212/WNL.0b013e31829c002f. [DOI] [PubMed] [Google Scholar]
- BENDER AC, MORSE RP, SCOTT RC, HOLMES GL, LENCK-SANTINI PP. SCN1A mutations in Dravet syndrome: impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy Behav. 2012;23:177–86. doi: 10.1016/j.yebeh.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENDER AC, NATOLA H, NDONG C, HOLMES GL, SCOTT RC, LENCK-SANTINI PP. Focal Scn1a knockdown induces cognitive impairment without seizures. Neurobiol Dis. 2013;54:297–307. doi: 10.1016/j.nbd.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG AT, BERKOVIC SF, BRODIE MJ, BUCHHALTER J, CROSS JH, VAN EMDE BOAS W, ENGEL J, FRENCH J, GLAUSER TA, MATHERN GW, MOSHE SL, NORDLI D, PLOUIN P, SCHEFFER IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- BEZAIRE MJ, SOLTESZ I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23:751–85. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIENVENU T, DIEBOLD B, CHELLY J, ISIDOR B. Refining the phenotype associated with MEF2C point mutations. Neurogenetics. 2013;14:71–5. doi: 10.1007/s10048-012-0344-7. [DOI] [PubMed] [Google Scholar]
- BILLINGSLEA EN, TATARD-LEITMAN VM, ANGUIANO J, JUTZELER CR, SUH J, SAUNDERS JA, MORITA S, FEATHERSTONE RE, ORTINSKI PI, GANDAL MJ, LIN R, LIANG Y, GUR RE, CARLSON GC, HAHN CG, SIEGEL SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–13. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMBEN VC, AIBA I, QIAN J, MARK MD, HERLITZE S, NOEBELS JL. Isolated P/Q Calcium Channel Deletion in Layer VI Corticothalamic Neurons Generates Absence Epilepsy. J Neurosci. 2016;36:405–18. doi: 10.1523/JNEUROSCI.2555-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORRELL V, MARIN O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–93. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- BOUTRY-KRYZA N, LABALME A, VILLE D, DE BELLESCIZE J, TOURAINE R, PRIEUR F, DIMASSI S, POULAT AL, TILL M, ROSSI M, BOUREL-PONCHEL E, DELIGNIERES A, LE MOING AG, RIVIER C, DES PORTES V, EDERY P, CALENDER A, SANLAVILLE D, LESCA G. Molecular characterization of a cohort of 73 patients with infantile spasms syndrome. Eur J Med Genet. 2015;58:51–8. doi: 10.1016/j.ejmg.2014.11.007. [DOI] [PubMed] [Google Scholar]
- BOZZI Y, CASAROSA S, CALEO M. Epilepsy as a neurodevelopmental disorder. Frontiers in psychiatry. 2012:3. doi: 10.3389/fpsyt.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHL DL, HARRIS KD, HORMUZDI SG, MONYER H, BUZSAKI G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–8. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUI AD, ALEXANDER A, SOLTESZ I. Seizing Control: From Current Treatments to Optogenetic Interventions in Epilepsy. Neuroscientist. 2015 doi: 10.1177/1073858415619600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT SJ, FUCCILLO M, NERY S, NOCTOR S, KRIEGSTEIN A, CORBIN JG, FISHELL G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- BUTT SJ, SOUSA VH, FUCCILLO MV, HJERLING-LEFFLER J, MIYOSHI G, KIMURA S, FISHELL G. The requirement of Nkx2–1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–32. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTZ S, OKAMOTO M, SUDHOF TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–82. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- CAMMAROTA M, LOSI G, CHIAVEGATO A, ZONTA M, CARMIGNOTO G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. The Journal of physiology. 2013;591:807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL K, OLSSON M, BJORKLUND A. Regional incorporation and site-specific differentiation of striatal precursors transplanted to the embryonic forebrain ventricle. Neuron. 1995;15:1259–73. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- CANTY AJ, DIETZE J, HARVEY M, ENOMOTO H, MILBRANDT J, IBANEZ CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29:10695–705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARDIN JA, CARLEN M, MELETIS K, KNOBLICH U, ZHANG F, DEISSEROTH K, TSAI LH, MOORE CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG MC, PARK JM, PELKEY KA, GRABENSTATTER HL, XU D, LINDEN DJ, SUTULA TP, MCBAIN CJ, WORLEY PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nature neuroscience. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTOPADHYAYA B, BAHO E, HUANG ZJ, SCHACHNER M, DI CRISTO G. Neural cell adhesion molecule-mediated Fyn activation promotes GABAergic synapse maturation in postnatal mouse cortex. J Neurosci. 2013;33:5957–68. doi: 10.1523/JNEUROSCI.1306-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTOPADHYAYA B, DI CRISTO G, HIGASHIYAMA H, KNOTT GW, KUHLMAN SJ, WELKER E, HUANG ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEAH CS, YU FH, WESTENBROEK RE, KALUME FK, OAKLEY JC, POTTER GB, RUBENSTEIN JL, CATTERALL WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2012;109:14646–51. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H, BAGRI A, ZUPICICH JA, ZOU Y, STOECKLI E, PLEASURE SJ, LOWENSTEIN DH, SKARNES WC, CHEDOTAL A, TESSIER-LAVIGNE M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- CHEN H, CHEDOTAL A, HE Z, GOODMAN CS, TESSIER-LAVIGNE M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–59. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- CHEN YJ, ZHANG M, YIN DM, WEN L, TING A, WANG P, LU YS, ZHU XH, LI SJ, WU CY, WANG XM, LAI C, XIONG WC, MEI L, GAO TM. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–23. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOW A, ERISIR A, FARB C, NADAL MS, OZAITA A, LAU D, WELKER E, RUDY B. K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci. 1999;19:9332–45. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU J, ANDERSON SA. Development of cortical interneurons. Neuropsychopharmacology. 2015;40:16–23. doi: 10.1038/npp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAES L, CEULEMANS B, AUDENAERT D, SMETS K, LOFGREN A, DEL-FAVERO J, ALA-MELLO S, BASEL-VANAGAITE L, PLECKO B, RASKIN S, THIRY P, WOLF NI, VAN BROECKHOVEN C, DE JONGHE P. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat. 2003;21:615–21. doi: 10.1002/humu.10217. [DOI] [PubMed] [Google Scholar]
- CLAES L, DEL-FAVERO J, CEULEMANS B, LAGAE L, VAN BROECKHOVEN C, DE JONGHE P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE J, XU H, DE MARCO GARCIA N, BATISTA-BRITO R, ROSSIGNOL E, RUDY B, FISHELL G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32:17690–705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBOS I, BORELLO U, RUBENSTEIN JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–88. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBOS I, BROCCOLI V, RUBENSTEIN JL. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. J Comp Neurol. 2005a;483:292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- COBOS I, CALCAGNOTTO ME, VILAYTHONG AJ, THWIN MT, NOEBELS JL, BARABAN SC, RUBENSTEIN JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005b;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- COBOS I, LONG JE, THWIN MT, RUBENSTEIN JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–8. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- COLASANTE G, COLLOMBAT P, RAIMONDI V, BONANOMI D, FERRAI C, MAIRA M, YOSHIKAWA K, MANSOURI A, VALTORTA F, RUBENSTEIN JL, BROCCOLI V. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–86. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLGIN LL, DENNINGER T, FYHN M, HAFTING T, BONNEVIE T, JENSEN O, MOSER MB, MOSER EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–7. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- COLOMBO E, COLLOMBAT P, COLASANTE G, BIANCHI M, LONG J, MANSOURI A, RUBENSTEIN JL, BROCCOLI V. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007;27:4786–98. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER EC, ALDAPE KD, ABOSCH A, BARBARO NM, BERGER MS, PEACOCK WS, JAN YN, JAN LY. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc Natl Acad Sci U S A. 2000;97:4914–9. doi: 10.1073/pnas.090092797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER EC, HARRINGTON E, JAN YN, JAN LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–40. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER EC, JAN LY. M-channels: neurological diseases, neuromodulation, and drug development. Arch Neurol. 2003;60:496–500. doi: 10.1001/archneur.60.4.496. [DOI] [PubMed] [Google Scholar]
- COTTAM JC, SMITH SL, HAUSSER M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J Neurosci. 2013;33:19567–78. doi: 10.1523/JNEUROSCI.2624-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMAJ L, LUPIEN-MEILLEUR A, LORTIE A, RIOU E, OSPINA LH, GAGNON L, VANASSE C, ROSSIGNOL E. CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet. 2015;23:1505–12. doi: 10.1038/ejhg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANG J, TIAN F, LI F, HUANG W, SONG M, DING D, HUANG X. Roles of Rho guanine nucleotide triphosphatases in hippocampal mossy fiber sprouting in the pentylenetetrazole kindling model. Clin Lab. 2014;60:175–84. doi: 10.7754/clin.lab.2013.130320. [DOI] [PubMed] [Google Scholar]
- DE WIT J, VERHAAGEN J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71:249–67. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- DEL PINO I, GARCIA-FRIGOLA C, DEHORTER N, BROTONS-MAS JR, ALVAREZ-SALVADO E, MARTINEZ DE LAGRAN M, CICERI G, GABALDON MV, MORATAL D, DIERSSEN M, CANALS S, MARIN O, RICO B. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–68. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- DELEUZE C, PAZIENTI A, BACCI A. Autaptic self-inhibition of cortical GABAergic neurons: synaptic narcissism or useful introspection? Curr Opin Neurobiol. 2014;26:64–71. doi: 10.1016/j.conb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- DEPREZ L, WECKHUYSEN S, HOLMGREN P, SULS A, VAN DYCK T, GOOSSENS D, DEL-FAVERO J, JANSEN A, VERHAERT K, LAGAE L, JORDANOVA A, VAN COSTER R, YENDLE S, BERKOVIC SF, SCHEFFER I, CEULEMANS B, DE JONGHE P. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–65. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- DESFEUX A, EL GHAZI F, JEGOU S, LEGROS H, MARRET S, LAUDENBACH V, GONZALEZ BJ. Dual effect of glutamate on GABAergic interneuron survival during cerebral cortex development in mice neonates. Cereb Cortex. 2010;20:1092–108. doi: 10.1093/cercor/bhp181. [DOI] [PubMed] [Google Scholar]
- DI CRISTO G, CHATTOPADHYAYA B, KUHLMAN SJ, FU Y, BELANGER MC, WU CZ, RUTISHAUSER U, MAFFEI L, HUANG ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci. 2007;10:1569–77. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- DITLEVSEN DK, POVLSEN GK, BEREZIN V, BOCK E. NCAM-induced intracellular signaling revisited. J Neurosci Res. 2008;86:727–43. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- DITYATEV A, FELLIN T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol. 2008;4:235–47. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- DOISCHER D, HOSP JA, YANAGAWA Y, OBATA K, JONAS P, VIDA I, BARTOS M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–68. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREXEL M, PREIDT AP, KIRCHMAIR E, SPERK G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–29. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUGA R. Neocortical inhibitory system. Folia Biol (Praha) 2009;55:201–17. doi: 10.14712/fb2009055060201. [DOI] [PubMed] [Google Scholar]
- DU T, XU Q, OCBINA PJ, ANDERSON SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–67. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- DUTTON SB, MAKINSON CD, PAPALE LA, SHANKAR A, BALAKRISHNAN B, NAKAZAWA K, ESCAYG A. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol Dis. 2012;49C:211–220. doi: 10.1016/j.nbd.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUVEAU V, FRITSCHY JM. PSA-NCAM-dependent GDNF signaling limits neurodegeneration and epileptogenesis in temporal lobe epilepsy. Eur J Neurosci. 2010;32:89–98. doi: 10.1111/j.1460-9568.2010.07272.x. [DOI] [PubMed] [Google Scholar]
- EAGLESON KL, BONNIN A, LEVITT P. Region- and age-specific deficits in gamma-aminobutyric acidergic neuron development in the telencephalon of the uPAR(−/−) mouse. J Comp Neurol. 2005;489:449–66. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- EAGLESON KL, CAMPBELL DB, THOMPSON BL, BERGMAN MY, LEVITT P. The autism risk genes MET and PLAUR differentially impact cortical development. Autism Res. 2011;4:68–83. doi: 10.1002/aur.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL ACHKAR CM, OLSON HE, PODURI A, PEARL PL. The genetics of the epilepsies. Curr Neurol Neurosci Rep. 2015;15:39. doi: 10.1007/s11910-015-0559-8. [DOI] [PubMed] [Google Scholar]
- ELLENDER TJ, RAIMONDO JV, IRKLE A, LAMSA KP, AKERMAN CJ. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J Neurosci. 2014;34:15208–22. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCAYG A, GOLDIN AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCAYG A, MACDONALD BT, MEISLER MH, BAULAC S, HUBERFELD G, AN-GOURFINKEL I, BRICE A, LEGUERN E, MOULARD B, CHAIGNE D, BURESI C, MALAFOSSE A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–5. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- ESPINOSA F, TORRES-VEGA MA, MARKS GA, JOHO RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–81. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUNSON LH, REA R, ZUBERI SM, YOUROUKOS S, PANAYIOTOPOULOS CP, LIGUORI R, AVONI P, MCWILLIAM RC, STEPHENSON JB, HANNA MG, KULLMANN DM, SPAUSCHUS A. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–56. [PubMed] [Google Scholar]
- EWELL LA, LIANG L, ARMSTRONG C, SOLTESZ I, LEUTGEB S, LEUTGEB JK. Brain State Is a Major Factor in Preseizure Hippocampal Network Activity and Influences Success of Seizure Intervention. J Neurosci. 2015;35:15635–48. doi: 10.1523/JNEUROSCI.5112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAGIOLINI M, HENSCH TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–6. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- FAHEEM M, CHAUDHARY AG, KUMOSANI TA, AL-QAHTANI MH, YASIR M, BIBI F, KIM MO, RASOOL M, NASEER MI. Interaction of different proteins with GABAA receptor and their modulatory effect on inhibitory neural transmission leads to epilepsy. CNS Neurol Disord Drug Targets. 2014;13:1148–59. doi: 10.2174/1871527313666140917115121. [DOI] [PubMed] [Google Scholar]
- FANSELOW EE, RICHARDSON KA, CONNORS BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–52. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAZZARI P, PATERNAIN AV, VALIENTE M, PLA R, LUJAN R, LLOYD K, LERMA J, MARIN O, RICO B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–80. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- FIGUROV A, POZZO-MILLER LD, OLAFSSON P, WANG T, LU B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- FISHELL G, RUDY B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–67. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAMES N, LONG JE, GARRATT AN, FISCHER TM, GASSMANN M, BIRCHMEIER C, LAI C, RUBENSTEIN JL, MARIN O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–61. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- FOGARTY M, GRIST M, GELMAN D, MARIN O, PACHNIS V, KESSARIS N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCAVILLA R, LUO X, MAGNIN E, TYAN L, TOPOLNIK L. Coordination of dendritic inhibition through local disinhibitory circuits. Front Synaptic Neurosci. 2015;7:5. doi: 10.3389/fnsyn.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND TF, KATONA I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- FRIES P, REYNOLDS JH, RORIE AE, DESIMONE R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–3. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- FRIOCOURT G, LIU JS, ANTYPA M, RAKIC S, WALSH CA, PARNAVELAS JG. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J Neurosci. 2007;27:3875–83. doi: 10.1523/JNEUROSCI.4530-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIOCOURT G, PARNAVELAS JG. Mutations in ARX Result in Several Defects Involving GABAergic Neurons. Front Cell Neurosci. 2010;4:4. doi: 10.3389/fncel.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIOCOURT G, POIRIER K, RAKIC S, PARNAVELAS JG, CHELLY J. The role of ARX in cortical development. Eur J Neurosci. 2006;23:869–76. doi: 10.1111/j.1460-9568.2006.04629.x. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO I, BRUSES JL, RUTISHAUSER U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J Biol Chem. 2001;276:31745–51. doi: 10.1074/jbc.M104525200. [DOI] [PubMed] [Google Scholar]
- GALLO G, LETOURNEAU PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- GAN L, KACZMAREK LK. When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons. J Neurobiol. 1998;37:69–79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- GANT JC, THIBAULT O, BLALOCK EM, YANG J, BACHSTETTER A, KOTICK J, SCHAUWECKER PE, HAUSER KF, SMITH GM, MERVIS R, LI Y, BARNES GN. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–45. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIGER JR, MELCHER T, KOH DS, SAKMANN B, SEEBURG PH, JONAS P, MONYER H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- GELMAN DM, MARIN O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–41. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- GELMAN DM, MARTINI FJ, NOBREGA-PEREIRA S, PIERANI A, KESSARIS N, MARIN O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIGER RJ, CLOUTIER JF, SAHAY A, PRINJHA RK, LEVENGOOD DV, MOORE SE, PICKERING S, SIMMONS D, RASTAN S, WALSH FS, KOLODKIN AL, GINTY DD, GEPPERT M. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- GNATKOVSKY V, LIBRIZZI L, TROMBIN F, DE CURTIS M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–86. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- GOLDBERG EM, CLARK BD, ZAGHA E, NAHMANI M, ERISIR A, RUDY B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOVEK EE, HATTEN ME, VAN AELST L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71:528–53. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIGORIOU M, TUCKER AS, SHARPE PT, PACHNIS V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–74. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- GRIGOROV A, MOSKALYUK A, KRAVCHENKO M, VESELOVSKY N, VERKHRATSKY A, FEDULOVA S. Kv7 potassium channel subunits and M currents in cultured hippocampal interneurons. Pflugers Arch. 2014;466:1747–58. doi: 10.1007/s00424-013-1406-x. [DOI] [PubMed] [Google Scholar]
- GRONE BP, MARCHESE M, HAMLING KR, KUMAR MG, KRASNIAK CS, SICCA F, SANTORELLI FM, PATEL M, BARABAN SC. Epilepsy, Behavioral Abnormalities, and Physiological Comorbidities in Syntaxin-Binding Protein 1 (STXBP1) Mutant Zebrafish. PLoS One. 2016;11:e0151148. doi: 10.1371/journal.pone.0151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GU B, HUANG YZ, HE XP, JOSHI RB, JANG W, MCNAMARA JO. A Peptide Uncoupling BDNF Receptor TrkB from Phospholipase Cgamma1 Prevents Epilepsy Induced by Status Epilepticus. Neuron. 2015;88:484–91. doi: 10.1016/j.neuron.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMDAN FF, PITON A, GAUTHIER J, LORTIE A, DUBEAU F, DOBRZENIECKA S, SPIEGELMAN D, NOREAU A, PELLERIN S, COTE M, HENRION E, FOMBONNE E, MOTTRON L, MARINEAU C, DRAPEAU P, LAFRENIERE RG, LACAILLE JC, ROULEAU GA, MICHAUD JL. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Ann Neurol. 2009;65:748–53. doi: 10.1002/ana.21625. [DOI] [PubMed] [Google Scholar]
- HAN S, TAI C, WESTENBROEK RE, YU FH, CHEAH CS, POTTER GB, RUBENSTEIN JL, SCHEUER T, DE LA IGLESIA HO, CATTERALL WA. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–90. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANI AJ, MIKATI HM, MIKATI MA. Genetics of pediatric epilepsy. Pediatr Clin North Am. 2015;62:703–22. doi: 10.1016/j.pcl.2015.03.013. [DOI] [PubMed] [Google Scholar]
- HANOVER JL, HUANG ZJ, TONEGAWA S, STRYKER MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATA Y, BUTZ S, SUDHOF TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELBIG I. Genetic Causes of Generalized Epilepsies. Semin Neurol. 2015;35:288–92. doi: 10.1055/s-0035-1552922. [DOI] [PubMed] [Google Scholar]
- HENSCH TK, FAGIOLINI M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–24. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- HO A, MORISHITA W, HAMMER RE, MALENKA RC, SUDHOF TC. A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission. Proc Natl Acad Sci U S A. 2003;100:1409–14. doi: 10.1073/pnas.252774899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLM MM, NIETO-GONZALEZ JL, VARDYA I, VAEGTER CB, NYKJAER A, JENSEN K. Mature BDNF, but not proBDNF, reduces excitability of fast-spiking interneurons in mouse dentate gyrus. J Neurosci. 2009;29:12412–8. doi: 10.1523/JNEUROSCI.2978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17:101–16. doi: 10.1684/epd.2015.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORESH D, SAPIR T, FRANCIS F, WOLF SG, CASPI M, ELBAUM M, CHELLY J, REINER O. Doublecortin, a stabilizer of microtubules. Hum Mol Genet. 1999;8:1599–610. doi: 10.1093/hmg/8.9.1599. [DOI] [PubMed] [Google Scholar]
- HOWARD MW, RIZZUTO DS, CAPLAN JB, MADSEN JR, LISMAN J, ASCHENBRENNER-SCHEIBE R, SCHULZE-BONHAGE A, KAHANA MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- HUANG Y, YOON K, KO H, JIAO S, ITO W, WU JY, YUNG WH, LU B, MOROZOV A. 5-HT3a Receptors Modulate Hippocampal Gamma Oscillations by Regulating Synchrony of Parvalbumin-Positive Interneurons. Cereb Cortex. 2016;26:576–85. doi: 10.1093/cercor/bhu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT RF, GIRSKIS KM, RUBENSTEIN JL, ALVAREZ-BUYLLA A, BARABAN SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16:692–7. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON GM, VOSS LJ, MELIN SM, MASON JP, CURSONS RT, STEYN-ROSS DA, STEYN-ROSS ML, SLEIGH JW. Connexin36 knockout mice display increased sensitivity to pentylenetetrazol-induced seizure-like behaviors. Brain Res. 2010;1360:198–204. doi: 10.1016/j.brainres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- JAGLIN X, BATISTA-BRITO R, BENEDETTI BL, GUIGUE PRC, FISHELL G. Neuroscience 2013 Abstracts. San Diego, CA: Society for Neurosciences; 2013. The Myocyte Enhancer Factor 2C (Mef2c) is essential for the maturation of Parvalbumin-expressing cortical interneurons. Program No. 318.17/D7. [Google Scholar]
- JIANG M, YANG M, YIN L, ZHANG X, SHU Y. Developmental reduction of asynchronous GABA release from neocortical fast-spiking neurons. Cereb Cortex. 2015;25:258–70. doi: 10.1093/cercor/bht236. [DOI] [PubMed] [Google Scholar]
- JIANG X, WANG G, LEE AJ, STORNETTA RL, ZHU JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–8. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN X, JIANG K, PRINCE DA. Excitatory and inhibitory synaptic connectivity to layer V fast-spiking interneurons in the freeze lesion model of cortical microgyria. J Neurophysiol. 2014;112:1703–13. doi: 10.1152/jn.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIRUSKA P, DE CURTIS M, JEFFERYS JG, SCHEVON CA, SCHIFF SJ, SCHINDLER K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol. 2013;591:787–97. doi: 10.1113/jphysiol.2012.239590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOUVENCEAU A, EUNSON LH, SPAUSCHUS A, RAMESH V, ZUBERI SM, KULLMANN DM, HANNA MG. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358:801–7. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- JUN K, PIEDRAS-RENTERIA ES, SMITH SM, WHEELER DB, LEE SB, LEE TG, CHIN H, ADAMS ME, SCHELLER RH, TSIEN RW, SHIN HS. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A. 1999;96:15245–50. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG H, JIA LZ, SUH KY, TANG L, SCHUMAN EM. Determinants of BDNF-induced hippocampal synaptic plasticity: role of the Trk B receptor and the kinetics of neurotrophin delivery. Learn Mem. 1996;3:188–96. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- KANG H, SCHUMAN EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–62. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- KANTER-SCHLIFKE I, GEORGIEVSKA B, KIRIK D, KOKAIA M. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther. 2007;15:1106–13. doi: 10.1038/sj.mt.6300148. [DOI] [PubMed] [Google Scholar]
- KASH SF, JOHNSON RS, TECOTT LH, NOEBELS JL, MAYFIELD RD, HANAHAN D, BAEKKESKOV S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–5. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAGIRI H, FAGIOLINI M, HENSCH TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–12. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- KATO M. Genotype-phenotype correlation in neuronal migration disorders and cortical dysplasias. Front Neurosci. 2015;9:181. doi: 10.3389/fnins.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO M, YAMAGATA T, KUBOTA M, ARAI H, YAMASHITA S, NAKAGAWA T, FUJII T, SUGAI K, IMAI K, USTER T, CHITAYAT D, WEISS S, KASHII H, KUSANO R, MATSUMOTO A, NAKAMURA K, OYAZATO Y, MAENO M, NISHIYAMA K, KODERA H, NAKASHIMA M, TSURUSAKI Y, MIYAKE N, SAITO K, HAYASAKA K, MATSUMOTO N, SAITSU H. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–7. doi: 10.1111/epi.12200. [DOI] [PubMed] [Google Scholar]
- KATSUMARU H, KOSAKA T, HEIZMANN CW, HAMA K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72:347–62. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- KAWAGUCHI Y, KUBOTA Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- KITAMURA K, YANAZAWA M, SUGIYAMA N, MIURA H, II, ZUKA-KOGO A, KUSAKA M, OMICHI K, SUZUKI R, KATO-FUKUI Y, KAMIIRISA K, MATSUO M, KAMIJO S, KASAHARA M, YOSHIOKA H, OGATA T, FUKUDA T, KONDO I, KATO M, DOBYNS WB, YOKOYAMA M, MOROHASHI K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- KORTE M, CARROLL P, WOLF E, BREM G, THOENEN H, BONHOEFFER T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–60. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROOK-MAGNUSON E, ARMSTRONG C, OIJALA M, SOLTESZ I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROOK-MAGNUSON E, SZABO GG, ARMSTRONG C, OIJALA M, SOLTESZ I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014:1. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUGLIKOV I, RUDY B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–24. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWOK JC, DICK G, WANG D, FAWCETT JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–89. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- LARKUM M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36:141–51. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- LAU CG, MURTHY VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 2012;32:8521–31. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU D, VEGA-SAENZ DE MIERA EC, CONTRERAS D, OZAITA A, HARVEY M, CHOW A, NOEBELS JL, PAYLOR R, MORGAN JI, LEONARD CS, RUDY B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–85. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE JJ, SARAGA F, CHURCHILL JF, STATLAND JM, TRAVIS KE, SKINNER FK, MCBAIN CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26:12325–38. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZARUS MS, KRISHNAN K, HUANG ZJ. GAD67 Deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cerebral Cortex. 2015;25:1290–1296. doi: 10.1093/cercor/bht322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE GAL LA SALLE G, ROUGON G, VALIN A. The embryonic form of neural cell surface molecule (E-NCAM) in the rat hippocampus and its reexpression on glial cells following kainic acid-induced status epilepticus. J Neurosci. 1992;12:872–82. doi: 10.1523/JNEUROSCI.12-03-00872.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDDA F, PARATCHA G, SANDOVAL-GUZMAN T, IBANEZ CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- LEDRI M, MADSEN MG, NIKITIDOU L, KIRIK D, KOKAIA M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J Neurosci. 2014;34:3364–77. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, HJERLING-LEFFLER J, ZAGHA E, FISHELL G, RUDY B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, KRUGLIKOV I, HUANG ZJ, FISHELL G, RUDY B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–70. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G, ADESNIK H, LI J, LONG J, NICOLL RA, RUBENSTEIN JL, PLEASURE SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–98. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI KX, LU YM, XU ZH, ZHANG J, ZHU JM, ZHANG JM, CAO SX, CHEN XJ, CHEN Z, LUO JH, DUAN S, LI XM. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2012;15:267–73. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- LIGUORI R, AVONI P, BARUZZI A, DI STASI V, MONTAGNA P. Familial continuous motor unit activity and epilepsy. Muscle Nerve. 2001;24:630–3. doi: 10.1002/mus.1048. [DOI] [PubMed] [Google Scholar]
- LIODIS P, DENAXA M, GRIGORIOU M, AKUFO-ADDO C, YANAGAWA Y, PACHNIS V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–89. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU G, GU B, HE XP, JOSHI RB, WACKERLE HD, RODRIGUIZ RM, WETSEL WC, MCNAMARA JO. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79:31–8. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVETT-BARRON M, LOSONCZY A. Behavioral consequences of GABAergic neuronal diversity. Curr Opin Neurobiol. 2014;26:27–33. doi: 10.1016/j.conb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- LOVETT-BARRON M, TURI GF, KAIFOSH P, LEE PH, BOLZE F, SUN XH, NICOUD JF, ZEMELMAN BV, STERNSON SM, LOSONCZY A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. 2012;15:423–30. S1–3. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- LYSKO DE, PUTT M, GOLDEN JA. SDF1 regulates leading process branching and speed of migrating interneurons. J Neurosci. 2011;31:1739–45. doi: 10.1523/JNEUROSCI.3118-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYSKO DE, PUTT M, GOLDEN JA. SDF1 reduces interneuron leading process branching through dual regulation of actin and microtubules. J Neurosci. 2014;34:4941–62. doi: 10.1523/JNEUROSCI.4351-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA Y, HU H, BERREBI AS, MATHERS PH, AGMON A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–82. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA Y, PRINCE DA. Functional alterations in GABAergic fast-spiking interneurons in chronically injured epileptogenic neocortex. Neurobiol Dis. 2012;47:102–13. doi: 10.1016/j.nbd.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCORELLES P, LAQUERRIERE A, ADDE-MICHEL C, MARRET S, SAUGIER-VEBER P, BELDJORD C, FRIOCOURT G. Evidence for tangential migration disturbances in human lissencephaly resulting from a defect in LIS1, DCX and ARX genes. Acta Neuropathol. 2010;120:503–15. doi: 10.1007/s00401-010-0692-z. [DOI] [PubMed] [Google Scholar]
- MARIN O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci. 2013;38:2019–29. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- MARÍN O. Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- MARIN O, VALIENTE M, GE X, TSAI LH. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIN O, YARON A, BAGRI A, TESSIER-LAVIGNE M, RUBENSTEIN JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–5. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- MARKRAM H, TOLEDO-RODRIGUEZ M, WANG Y, GUPTA A, SILBERBERG G, WU C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- MARSH E, FULP C, GOMEZ E, NASRALLAH I, MINARCIK J, SUDI J, CHRISTIAN SL, MANCINI G, LABOSKY P, DOBYNS W, BROOKS-KAYAL A, GOLDEN JA. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–76. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN MS, DUTT K, PAPALE LA, DUBE CM, DUTTON SB, DE HAAN G, SHANKAR A, TUFIK S, MEISLER MH, BARAM TZ, GOLDIN AL, ESCAYG A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–34. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ-LEVY GA, ROCHA L, LUBIN FD, ALONSO-VANEGAS MA, NANI A, BUENTELLO-GARCIA RM, PEREZ-MOLINA R, BRIONES-VELASCO M, RECILLAS-TARGA F, PEREZ-MOLINA A, SAN-JUAN D, CIENFUEGOS J, CRUZ-FUENTES CS. Increased expression of BDNF transcript with exon VI in hippocampi of patients with pharmaco-resistant temporal lobe epilepsy. Neuroscience. 2016;314:12–21. doi: 10.1016/j.neuroscience.2015.11.046. [DOI] [PubMed] [Google Scholar]
- MASTRANGELO M, PERON A, SPACCINI L, NOVARA F, SCELSA B, INTROVINI P, RAVIGLIONE F, FAIOLA S, ZUFFARDI O. Neonatal suppression-burst without epileptic seizures: expanding the electroclinical phenotype of STXBP1-related, early-onset encephalopathy. Epileptic Disord. 2013;15:55–61. doi: 10.1684/epd.2013.0558. [DOI] [PubMed] [Google Scholar]
- MCCORMICK DA, CONTRERAS D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–46. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- MCNAMARA JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–25. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCRAE PA, BARANOV E, ROGERS SL, PORTER BE. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur J Neurosci. 2012;36:3471–82. doi: 10.1111/j.1460-9568.2012.08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICELI F, STRIANO P, SOLDOVIERI MV, FONTANA A, NARDELLO R, ROBBIANO A, BELLINI G, ELIA M, ZARA F, TAGLIALATELA M, MANGANO S. A novel KCNQ3 mutation in familial epilepsy with focal seizures and intellectual disability. Epilepsia. 2015;56:e15–20. doi: 10.1111/epi.12887. [DOI] [PubMed] [Google Scholar]
- MICHAUD JL, LACHANCE M, HAMDAN FF, CARMANT L, LORTIE A, DIADORI P, MAJOR P, MEIJER IA, LEMYRE E, COSSETTE P, MEFFORD HC, ROULEAU GA, ROSSIGNOL E. The genetic landscape of infantile spasms. Hum Mol Genet. 2014;23:4846–58. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- MIKKONEN M, SOININEN H, KALVIANEN R, TAPIOLA T, YLINEN A, VAPALAHTI M, PALJARVI L, PITKANEN A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–34. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- MILLICHAP JJ, COOPER EC. KCNQ2 Potassium Channel Epileptic Encephalopathy Syndrome: Divorce of an Electro-Mechanical Couple? Epilepsy Curr. 2012;12:150–2. doi: 10.5698/1535-7511-12.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYATA S, KOMATSU Y, YOSHIMURA Y, TAYA C, KITAGAWA H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–22. S1–2. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- MIYOSHI G, BUTT SJ, TAKEBAYASHI H, FISHELL G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–98. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYOSHI G, HJERLING-LEFFLER J, KARAYANNIS T, SOUSA VH, BUTT SJ, BATTISTE J, JOHNSON JE, MACHOLD RP, FISHELL G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–94. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYOSHI G, YOUNG A, PETROS T, KARAYANNIS T, MCKENZIE CHANG M, LAVADO A, IWANO T, NAKAJIMA M, TANIGUCHI H, HUANG ZJ, HEINTZ N, OLIVER G, MATSUZAKI F, MACHOLD RP, FISHELL G. Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J Neurosci. 2015;35:12869–89. doi: 10.1523/JNEUROSCI.1164-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOG U, KUTSCHE K, KORTUM F, CHILIAN B, BIERHALS T, APESHIOTIS N, BALG S, CHASSAING N, COUBES C, DAS S, ENGELS H, VAN ESCH H, GRASSHOFF U, HEISE M, ISIDOR B, JARVIS J, KOEHLER U, MARTIN T, OEHL-JASCHKOWITZ B, ORTIBUS E, PILZ DT, PRABHAKAR P, RAPPOLD G, RAU I, RETTENBERGER G, SCHLUTER G, SCOTT RH, SHOUKIER M, WOHLLEBER E, ZIRN B, DOBYNS WB, UYANIK G. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48:741–51. doi: 10.1136/jmedgenet-2011-100218. [DOI] [PubMed] [Google Scholar]
- MORALES B, CHOI SY, KIRKWOOD A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–90. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIMOTO K, FAHNESTOCK M, RACINE RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- MUONA M, BERKOVIC SF, DIBBENS LM, OLIVER KL, MALJEVIC S, BAYLY MA, JOENSUU T, CANAFOGLIA L, FRANCESCHETTI S, MICHELUCCI R, MARKKINEN S, HERON SE, HILDEBRAND MS, ANDERMANN E, ANDERMANN F, GAMBARDELLA A, TINUPER P, LICCHETTA L, SCHEFFER IE, CRISCUOLO C, FILLA A, FERLAZZO E, AHMAD J, AHMAD A, BAYKAN B, SAID E, TOPCU M, RIGUZZI P, KING MD, OZKARA C, ANDRADE DM, ENGELSEN BA, CRESPEL A, LINDENAU M, LOHMANN E, SALETTI V, MASSANO J, PRIVITERA M, ESPAY AJ, KAUFFMANN B, DUCHOWNY M, MOLLER RS, STRAUSSBERG R, AFAWI Z, BEN-ZEEV B, SAMOCHA KE, DALY MJ, PETROU S, LERCHE H, PALOTIE A, LEHESJOKI AE. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat Genet. 2015;47:39–46. doi: 10.1038/ng.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAJM J, HORN D, WIMPLINGER I, GOLDEN JA, CHIZHIKOV VV, SUDI J, CHRISTIAN SL, ULLMANN R, KUECHLER A, HAAS CA, FLUBACHER A, CHARNAS LR, UYANIK G, FRANK U, KLOPOCKI E, DOBYNS WB, KUTSCHE K. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40:1065–7. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- NERY S, FISHELL G, CORBIN JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–87. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- NEVES G, SHAH MM, LIODIS P, ACHIMASTOU A, DENAXA M, ROALFE G, SESAY A, WALKER MC, PACHNIS V. The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex. Cereb Cortex. 2013;23:1811–23. doi: 10.1093/cercor/bhs159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIETO-GONZALEZ JL, JENSEN K. BDNF Depresses Excitability of Parvalbumin-Positive Interneurons through an M-Like Current in Rat Dentate Gyrus. PLoS One. 2013;8:e67318. doi: 10.1371/journal.pone.0067318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBREGA-PEREIRA S, KESSARIS N, DU T, KIMURA S, ANDERSON SA, MARIN O. Postmitotic Nkx2–1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–45. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGIWARA I, MIYAMOTO H, MORITA N, ATAPOUR N, MAZAKI E, INOUE I, TAKEUCHI T, ITOHARA S, YANAGAWA Y, OBATA K, FURUICHI T, HENSCH TK, YAMAKAWA K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–14. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA M, CORFAS G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–44. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- OKATY BW, MILLER MN, SUGINO K, HEMPEL CM, NELSON SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–52. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORHAN G, BOCK M, SCHEPERS D, ILINA EI, REICHEL SN, LOFFLER H, JEZUTKOVIC N, WECKHUYSEN S, MANDELSTAM S, SULS A, DANKER T, GUENTHER E, SCHEFFER IE, DE JONGHE P, LERCHE H, MALJEVIC S. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75:382–94. doi: 10.1002/ana.24080. [DOI] [PubMed] [Google Scholar]
- PACIORKOWSKI AR, TRAYLOR RN, ROSENFELD JA, HOOVER JM, HARRIS CJ, WINTER S, LACASSIE Y, BIALER M, LAMB AN, SCHULTZ RA, BERRY-KRAVIS E, PORTER BE, FALK M, VENKAT A, VANZO RJ, COHEN JS, FATEMI A, DOBYNS WB, SHAFFER LG, BALLIF BC, MARSH ED. MEF2C Haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics. 2013;14:99–111. doi: 10.1007/s10048-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARATCHA G, LEDDA F, IBANEZ CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–79. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- PAZ JT, HUGUENARD JR. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci. 2015a;18:351–9. doi: 10.1038/nn.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZ JT, HUGUENARD JR. Optogenetics and epilepsy: past, present and future. Epilepsy Curr. 2015b;15:34–8. doi: 10.5698/1535-7597-15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEKCEC A, MUHLENHOFF M, GERARDY-SCHAHN R, POTSCHKA H. Impact of the PSA-NCAM system on pathophysiology in a chronic rodent model of temporal lobe epilepsy. Neurobiol Dis. 2007;27:54–66. doi: 10.1016/j.nbd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- PETERS HC, HU H, PONGS O, STORM JF, ISBRANDT D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- PFEFFER CK, XUE M, HE M, HUANG ZJ, SCANZIANI M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–76. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PI HJ, HANGYA B, KVITSIANI D, SANDERS JI, HUANG ZJ, KEPECS A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–4. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINAL CS, TOBIN AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol. 1998;5:109–18. [PubMed] [Google Scholar]
- PITCHER GM, KALIA LV, NG D, GOODFELLOW NM, YEE KT, LAMBE EK, SALTER MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–8. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK E, EVEREST M, BROWN A, POULTER MO. Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis. 2014;70:21–31. doi: 10.1016/j.nbd.2014.06.003. [DOI] [PubMed] [Google Scholar]
- POWELL EM. Interneuron development and epilepsy: early genetic defects cause long-term consequences in seizures and susceptibility. Epilepsy Currents. 2013;13:172–176. doi: 10.5698/1535-7597-13.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL EM, CAMPBELL DB, STANWOOD GD, DAVIS C, NOEBELS JL, LEVITT P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–31. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL EM, MARS WM, LEVITT P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- POZAS E, IBANEZ CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–13. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- RACINE RJ, ADAMS B, OSEHOBO P, FAHNESTOCK M. Neural growth, neural damage and neurotrophins in the kindling model of epilepsy. Adv Exp Med Biol. 2002;497:149–70. doi: 10.1007/978-1-4615-1335-3_16. [DOI] [PubMed] [Google Scholar]
- RENZI MJ, FEINER L, KOPPEL AM, RAPER JA. A dominant negative receptor for specific secreted semaphorins is generated by deleting an extracellular domain from neuropilin-1. J Neurosci. 1999;19:7870–80. doi: 10.1523/JNEUROSCI.19-18-07870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSATO-SIRI MD, ZAMBELLO E, MUTINELLI C, GARBATI N, BENEDETTI R, ALDEGHERI L, GRAZIANI F, VIRGINIO C, ALVARO G, LARGE CH. A Novel Modulator of Kv3 Potassium Channels Regulates the Firing of Parvalbumin-Positive Cortical Interneurons. J Pharmacol Exp Ther. 2015;354:251–60. doi: 10.1124/jpet.115.225748. [DOI] [PubMed] [Google Scholar]
- ROSSIGNOL E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. doi: 10.1155/2011/649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSIGNOL E, KOBOW K, SIMONATO M, LOEB JA, GRISAR T, GILBY KL, VINET J, KADAM SD, BECKER AJ. WONOEP appraisal: New genetic approaches to study epilepsy. Epilepsia. 2014;55:1170–86. doi: 10.1111/epi.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSIGNOL E, KRUGLIKOV I, VAN DEN MAAGDENBERG AM, RUDY B, FISHELL G. CaV 2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann Neurol. 2013;74:209–22. doi: 10.1002/ana.23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHBARD JB, BRACKENBURY R, CUNNINGHAM BA, EDELMAN GM. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982;257:11064–9. [PubMed] [Google Scholar]
- RUDY B, CHOW A, LAU D, AMARILLO Y, OZAITA A, SAGANICH M, MORENO H, NADAL MS, HERNANDEZ-PINEDA R, HERNANDEZ-CRUZ A, ERISIR A, LEONARD C, VEGA-SAENZ DE MIERA E. Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci. 1999;868:304–43. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- RUDY B, FISHELL G, LEE S, HJERLING-LEFFLER J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDY B, MCBAIN CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–26. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- RUTHERFORD LC, DEWAN A, LAUER HM, TURRIGIANO GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–35. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTHERFORD LC, NELSON SB, TURRIGIANO GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–30. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- SAGANICH MJ, MACHADO E, RUDY B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–24. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHAY A, MOLLIVER ME, GINTY DD, KOLODKIN AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–80. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITSU H, AKITA T, TOHYAMA J, GOLDBERG-STERN H, KOBAYASHI Y, COHEN R, KATO M, OHBA C, MIYATAKE S, TSURUSAKI Y, NAKASHIMA M, MIYAKE N, FUKUDA A, MATSUMOTO N. De novo KCNB1 mutations in infantile epilepsy inhibit repetitive neuronal firing. Sci Rep. 2015;5:15199. doi: 10.1038/srep15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITSU H, KATO M, KOIDE A, GOTO T, FUJITA T, NISHIYAMA K, TSURUSAKI Y, DOI H, MIYAKE N, HAYASAKA K, MATSUMOTO N. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann Neurol. 2012a;72:298–300. doi: 10.1002/ana.23620. [DOI] [PubMed] [Google Scholar]
- SAITSU H, KATO M, MIZUGUCHI T, HAMADA K, OSAKA H, TOHYAMA J, URUNO K, KUMADA S, NISHIYAMA K, NISHIMURA A, OKADA I, YOSHIMURA Y, HIRAI S, KUMADA T, HAYASAKA K, FUKUDA A, OGATA K, MATSUMOTO N. De novo mutations in the gene encoding STXBP1 (MUNC18–1) cause early infantile epileptic encephalopathy. Nat Genet. 2008;40:782–8. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- SAITSU H, KATO M, OSAKA H, MORIYAMA N, HORITA H, NISHIYAMA K, YONEDA Y, KONDO Y, TSURUSAKI Y, DOI H, MIYAKE N, HAYASAKA K, MATSUMOTO N. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia. 2012b;53:1441–9. doi: 10.1111/j.1528-1167.2012.03548.x. [DOI] [PubMed] [Google Scholar]
- SATO K, IWAI M, NAGANO I, SHOJI M, ABE K. Changes of localization of highly polysialylated neural cell adhesion molecule (PSA-NCAM) in rat hippocampus with exposure to repeated kindled seizures. Brain Res. 2002;946:323–7. doi: 10.1016/s0006-8993(02)02616-1. [DOI] [PubMed] [Google Scholar]
- SAUNDERS JA, TATARD-LEITMAN VM, SUH J, BILLINGSLEA EN, ROBERTS TP, SIEGEL SJ. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 2013;6:69–77. doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFMAN HE. Brain-derived neurotrophic factor and epilepsy--a missing link? Epilepsy Curr. 2005;5:83–8. doi: 10.1111/j.1535-7511.2005.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEFFER IE, WALLACE RH, PHILLIPS FL, HEWSON P, REARDON K, PARASIVAM G, STROMME P, BERKOVIC SF, GECZ J, MULLEY JC. X-linked myoclonic epilepsy with spasticity and intellectual disability: mutation in the homeobox gene ARX. Neurology. 2002;59:348–56. doi: 10.1212/wnl.59.3.348. [DOI] [PubMed] [Google Scholar]
- SCHEVON CA, WEISS SA, MCKHANN G, JR, GOODMAN RR, YUSTE R, EMERSON RG, TREVELYAN AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWALLER B, TETKO IV, TANDON P, SILVEIRA DC, VREUGDENHIL M, HENZI T, POTIER MC, CELIO MR, VILLA AE. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004;25:650–63. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- SCOTTI AL, KALT G, BOLLAG O, NITSCH C. Parvalbumin disappears from GABAergic CA1 neurons of the gerbil hippocampus with seizure onset while its presence persists in the perforant path. Brain Res. 1997;760:109–17. doi: 10.1016/s0006-8993(97)00309-0. [DOI] [PubMed] [Google Scholar]
- SEBE JY, BARABAN SC. The promise of an interneuron-based cell therapy for epilepsy. Dev Neurobiol. 2010;71:107–17. doi: 10.1002/dneu.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SESSOLO M, MARCON I, BOVETTI S, LOSI G, CAMMAROTA M, RATTO GM, FELLIN T, CARMIGNOTO G. Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J Neurosci. 2015;35:9544–57. doi: 10.1523/JNEUROSCI.5117-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN C, RATHORE SS, YU H, GULBRANSON DR, HUA R, ZHANG C, SCHOPPA NE, SHEN J. The trans-SNARE-regulating function of Munc18–1 is essential to synaptic exocytosis. Nat Commun. 2015;6:8852. doi: 10.1038/ncomms9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERR EH. The ARX story (epilepsy, mental retardation, autism, and cerebral malformations): one gene leads to many phenotypes. Curr Opin Pediatr. 2003;15:567–71. doi: 10.1097/00008480-200312000-00004. [DOI] [PubMed] [Google Scholar]
- SHIRI Z, MANSEAU F, LEVESQUE M, WILLIAMS S, AVOLI M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol. 2015;77:541–6. doi: 10.1002/ana.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRI Z, MANSEAU F, LEVESQUE M, WILLIAMS S, AVOLI M. Activation of specific neuronal networks leads to different seizure onset types. Ann Neurol. 2016;79:354–65. doi: 10.1002/ana.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISODIYA S, CROSS JH, BLUMCKE I, CHADWICK D, CRAIG J, CRINO PB, DEBENHAM P, DELANTY N, ELMSLIE F, GARDINER M, GOLDEN J, GOLDSTEIN D, GREENBERG DA, GUERRINI R, HANNA M, HARRIS J, HARRISON P, JOHNSON MR, KIROV G, KULLMAN DM, MAKOFF A, MARINI C, NABBOUT R, NASHEF L, NOEBELS JL, OTTMAN R, PIRMOHAMED M, PITKANEN A, SCHEFFER I, SHORVON S, SILLS G, WOOD N, ZUBERI S. Genetics of epilepsy: epilepsy research foundation workshop report. Epileptic Disord. 2007;9:194–236. doi: 10.1684/epd.2007.0107. [DOI] [PubMed] [Google Scholar]
- SISODIYA SM, MEFFORD HC. Genetic contribution to common epilepsies. Curr Opin Neurol. 2011;24:140–5. doi: 10.1097/WCO.0b013e328344062f. [DOI] [PubMed] [Google Scholar]
- SMART SL, LOPANTSEV V, ZHANG CL, ROBBINS CA, WANG H, CHIU SY, SCHWARTZKROIN PA, MESSING A, TEMPEL BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- SMETS K, DUARRI A, DECONINCK T, CEULEMANS B, VAN DE WARRENBURG BP, ZUCHNER S, GONZALEZ MA, SCHULE R, SYNOFZIK M, VAN DER AAN, DE JONGHE P, VERBEEK DS, BAETS J. First de novo KCND3 mutation causes severe Kv4.3 channel dysfunction leading to early onset cerebellar ataxia, intellectual disability, oral apraxia and epilepsy. BMC Med Genet. 2015;16:51. doi: 10.1186/s12881-015-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH KM, MARAGNOLI ME, PHULL PM, TRAN KM, CHOUBEY L, VACCARINO FM. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS One. 2014;9:e103696. doi: 10.1371/journal.pone.0103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOH H, PANT R, LOTURCO JJ, TZINGOUNIS AV. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J Neurosci. 2014;34:5311–21. doi: 10.1523/JNEUROSCI.3919-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOHAL VS, HUGUENARD JR. Inhibitory coupling specifically generates emergent gamma oscillations in diverse cell types. Proc Natl Acad Sci U S A. 2005;102:18638–43. doi: 10.1073/pnas.0509291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOHAL VS, ZHANG F, YIZHAR O, DEISSEROTH K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLEMAN S, FILIPPOV MA, DITYATEV A, FAWCETT JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253:194–213. doi: 10.1016/j.neuroscience.2013.08.050. [DOI] [PubMed] [Google Scholar]
- SOMOGYI P, KLAUSBERGER T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG C, XU W, ZHANG X, WANG S, ZHU G, XIAO T, ZHAO M, ZHAO C. CXCR4 Antagonist AMD3100 Suppresses the Long-Term Abnormal Structural Changes of Newborn Neurons in the Intraventricular Kainic Acid Model of Epilepsy. Mol Neurobiol. 2016;53:1518–32. doi: 10.1007/s12035-015-9102-9. [DOI] [PubMed] [Google Scholar]
- SOUTHWELL DG, PAREDES MF, GALVAO RP, JONES DL, FROEMKE RC, SEBE JY, ALFARO-CERVELLO C, TANG Y, GARCIA-VERDUGO JM, RUBENSTEIN JL, BARABAN SC, ALVAREZ-BUYLLA A. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–13. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUMM RK, ZHOU C, ARA T, LAZARINI F, DUBOIS-DALCQ M, NAGASAWA T, HOLLT V, SCHULZ S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–30. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULTAN KT, BROWN KN, SHI SH. Production and organization of neocortical interneurons. Front Cell Neurosci. 2013;7:221. doi: 10.3389/fncel.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN QQ, HUGUENARD JR, PRINCE DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–30. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSEL L, MARIN O, KIMURA S, RUBENSTEIN JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–70. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- SYRBE S, HEDRICH UB, RIESCH E, DJEMIE T, MULLER S, MOLLER RS, MAHER B, HERNANDEZ-HERNANDEZ L, SYNOFZIK M, CAGLAYAN HS, ARSLAN M, SERRATOSA JM, NOTHNAGEL M, MAY P, KRAUSE R, LOFFLER H, DETERT K, DORN T, VOGT H, KRAMER G, SCHOLS L, MULLIS PE, LINNANKIVI T, LEHESJOKI AE, STERBOVA K, CRAIU DC, HOFFMAN-ZACHARSKA D, KORFF CM, WEBER YG, STEINLIN M, GALLATI S, BERTSCHE A, BERNHARD MK, MERKENSCHLAGER A, KIESS W, EURO ER, GONZALEZ M, ZUCHNER S, PALOTIE A, SULS A, DE JONGHE P, HELBIG I, BISKUP S, WOLFF M, MALJEVIC S, SCHULE R, SISODIYA SM, WECKHUYSEN S, LERCHE H, LEMKE JR. De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet. 2015;47:393–9. doi: 10.1038/ng.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABADICS J, LORINCZ A, TAMAS G. Beta and gamma frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. J Neurosci. 2001;21:5824–31. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABUCHI K, BIEDERER T, BUTZ S, SUDHOF TC. CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J Neurosci. 2002;22:4264–73. doi: 10.1523/JNEUROSCI.22-11-04264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMAMAKI N, FUJIMORI K, NOJYO Y, KANEKO T, TAKAUJI R. Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–48. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- TAMAS G, BUHL EH, LORINCZ A, SOMOGYI P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–71. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- TAN GH, LIU YY, HU XL, YIN DM, MEI L, XIONG ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2012;15:258–66. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- TANAKA T, SERNEO FF, HIGGINS C, GAMBELLO MJ, WYNSHAW-BORIS A, GLEESON JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165:709–21. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANSEY EP, CHOW A, RUDY B, MCBAIN CJ. Developmental expression of potassium-channel subunit Kv3.2 within subpopulations of mouse hippocampal inhibitory interneurons. Hippocampus. 2002;12:137–48. doi: 10.1002/hipo.1104. [DOI] [PubMed] [Google Scholar]
- TASKER JG, HOFFMAN NW, KIM YI, FISHER RS, PEACOCK WJ, DUDEK FE. Electrical properties of neocortical neurons in slices from children with intractable epilepsy. J Neurophysiol. 1996;75:931–9. doi: 10.1152/jn.1996.75.2.931. [DOI] [PubMed] [Google Scholar]
- THEODORE WH, SPENCER SS, WIEBE S, LANGFITT JT, ALI A, SHAFER PO, BERG AT, VICKREY BG. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006;47:1700–22. doi: 10.1111/j.1528-1167.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- TIAN N, PETERSEN C, KASH S, BAEKKESKOV S, COPENHAGEN D, NICOLL R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–6. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIVERON MC, ROSSEL M, MOEPPS B, ZHANG YL, SEIDENFADEN R, FAVOR J, KONIG N, CREMER H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–8. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIVODAR S, KALEMAKI K, KOUNOUPA Z, VIDAKI M, THEODORAKIS K, DENAXA M, KESSARIS N, DE CURTIS I, PACHNIS V, KARAGOGEOS D. Rac-GTPases Regulate Microtubule Stability and Axon Growth of Cortical GABAergic Interneurons. Cereb Cortex. 2015;25:2370–82. doi: 10.1093/cercor/bhu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUB RD, CUNNINGHAM MO, GLOVELI T, LEBEAU FE, BIBBIG A, BUHL EH, WHITTINGTON MA. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci U S A. 2003;100:11047–52. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUB RD, KOPELL N, BIBBIG A, BUHL EH, LEBEAU FE, WHITTINGTON MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–86. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN AJ, SUSSILLO D, WATSON BO, YUSTE R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–55. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN AJ, SUSSILLO D, YUSTE R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–7. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROPEA D, KREIMAN G, LYCKMAN A, MUKHERJEE S, YU H, HORNG S, SUR M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–8. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- TROTTER SA, KAPUR J, ANZIVINO MJ, LEE KS. GABAergic synaptic inhibition is reduced before seizure onset in a genetic model of cortical malformation. J Neurosci. 2006;26:10756–67. doi: 10.1523/JNEUROSCI.2323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI LH, GLEESON JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–8. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- TUNCDEMIR SN, WAMSLEY B, STAM FJ, OSAKADA F, GOULDING M, CALLAWAY EM, RUDY B, FISHELL G. Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron. 2016;89:521–35. doi: 10.1016/j.neuron.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBAN DJ, ROTH BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- VALIENTE M, MARTINI FJ. Migration of cortical interneurons relies on branched leading process dynamics. Cell Adh Migr. 2009;3:278–80. doi: 10.4161/cam.3.3.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN BERGHE V, STAPPERS E, VANDESANDE B, DIMIDSCHSTEIN J, KROES R, FRANCIS A, CONIDI A, LESAGE F, DRIES R, CAZZOLA S, BERX G, KESSARIS N, VANDERHAEGHEN P, VAN IJCKEN W, GROSVELD FG, GOOSSENS S, HAIGH JJ, FISHELL G, GOFFINET A, AERTS S, HUYLEBROECK D, SEUNTJENS E. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- VIDA I, BARTOS M, JONAS P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–17. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- VIDAKI M, TIVODAR S, DOULGERAKI K, TYBULEWICZ V, KESSARIS N, PACHNIS V, KARAGOGEOS D. Rac1-dependent cell cycle exit of MGE precursors and GABAergic interneuron migration to the cortex. Cereb Cortex. 2012;22:680–92. doi: 10.1093/cercor/bhr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULLHORST D, NEDDENS J, KARAVANOVA I, TRICOIRE L, PETRALIA RS, MCBAIN CJ, BUONANNO A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–64. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACLAW RR, EHRMAN LA, PIERANI A, CAMPBELL K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 2010;30:6944–53. doi: 10.1523/JNEUROSCI.5772-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG D, FAWCETT J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–60. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- WANG HS, PAN Z, SHI W, BROWN BS, WYMORE RS, COHEN IS, DIXON JE, MCKINNON D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- WANG Y, DYE CA, SOHAL V, LONG JE, ESTRADA RC, ROZTOCIL T, LUFKIN T, DEISSEROTH K, BARABAN SC, RUBENSTEIN JL. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J Neurosci. 2010;30:5334–45. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, GUPTA A, TOLEDO-RODRIGUEZ M, WU CZ, MARKRAM H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- WECKHUYSEN S, IV, ANOVIC V, HENDRICKX R, VAN COSTER R, HJALGRIM H, MOLLER RS, GRONBORG S, SCHOONJANS AS, CEULEMANS B, HEAVIN SB, ELTZE C, HORVATH R, CASARA G, PISANO T, GIORDANO L, ROSTASY K, HABERLANDT E, ALBRECHT B, BEVOT A, BENKEL I, SYRBE S, SHEIDLEY B, GUERRINI R, PODURI A, LEMKE JR, MANDELSTAM S, SCHEFFER I, ANGRIMAN M, STRIANO P, MARINI C, SULS A, DE JONGHE P, GROUP KS. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81:1697–703. doi: 10.1212/01.wnl.0000435296.72400.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WECKHUYSEN S, MANDELSTAM S, SULS A, AUDENAERT D, DECONINCK T, CLAES LR, DEPREZ L, SMETS K, HRISTOVA D, YORDANOVA I, JORDANOVA A, CEULEMANS B, JANSEN A, HASAERTS D, ROELENS F, LAGAE L, YENDLE S, STANLEY T, HERON SE, MULLEY JC, BERKOVIC SF, SCHEFFER IE, DE JONGHE P. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- WEN L, LU YS, ZHU XH, LI XM, WOO RS, CHEN YJ, YIN DM, LAI C, TERRY AV, JR, VAZDARJANOVA A, XIONG WC, MEI L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–6. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICHTERLE H, TURNBULL DH, NERY S, FISHELL G, ALVAREZ-BUYLLA A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–71. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- WILLIAMS CA, BATTAGLIA A. Molecular biology of epilepsy genes. Exp Neurol. 2013;244:51–8. doi: 10.1016/j.expneurol.2011.12.001. [DOI] [PubMed] [Google Scholar]
- WONDERS CP, ANDERSON SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- WONDERS CP, TAYLOR L, WELAGEN J, MBATA IC, XIANG JZ, ANDERSON SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–36. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO RS, LI XM, TAO Y, CARPENTER-HYLAND E, HUANG YZ, WEBER J, NEISWENDER H, DONG XP, WU J, GASSMANN M, LAI C, XIONG WC, GAO TM, MEI L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- WU X, FU Y, KNOTT G, LU J, DI CRISTO G, HUANG ZJ. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci. 2012;32:331–43. doi: 10.1523/JNEUROSCI.3189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WULFF P, PONOMARENKO AA, BARTOS M, KOROTKOVA TM, FUCHS EC, BAHNER F, BOTH M, TORT AB, KOPELL NJ, WISDEN W, MONYER H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–6. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYKES RC, KULLMANN DM, PAVLOV I, MAGLOIRE V. Optogenetic approaches to treat epilepsy. J Neurosci Methods. 2016;260:215–20. doi: 10.1016/j.jneumeth.2015.06.004. [DOI] [PubMed] [Google Scholar]
- XU H, JEONG HY, TREMBLAY R, RUDY B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–67. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Q, COBOS I, DE LA CRUZ E, RUBENSTEIN JL, ANDERSON SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAKAWA K. Molecular and cellular basis: insights from experimental models of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):70–1. doi: 10.1111/j.1528-1167.2011.03006.x. [DOI] [PubMed] [Google Scholar]
- YAU HJ, WANG HF, LAI C, LIU FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–64. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- YEKHLEF L, BRESCHI GL, LAGOSTENA L, RUSSO G, TAVERNA S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophysiol. 2015;113:1616–30. doi: 10.1152/jn.00841.2014. [DOI] [PubMed] [Google Scholar]
- YI F, BALL J, STOLL KE, SATPUTE VC, MITCHELL SM, PAULI JL, HOLLOWAY BB, JOHNSTON AD, NATHANSON NM, DEISSEROTH K, GERBER DJ, TONEGAWA S, LAWRENCE JJ. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol. 2014;592:3463–94. doi: 10.1113/jphysiol.2014.275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YI F, DECAN E, STOLL K, MARCEAU E, DEISSEROTH K, LAWRENCE JJ. Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia. 2015;56:297–309. doi: 10.1111/epi.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU FH, MANTEGAZZA M, WESTENBROEK RE, ROBBINS CA, KALUME F, BURTON KA, SPAIN WJ, MCKNIGHT GS, SCHEUER T, CATTERALL WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- YU J, PRODDUTUR A, SWIETEK B, ELGAMMAL FS, SANTHAKUMAR V. Functional Reduction in Cannabinoid-Sensitive Heterotypic Inhibition of Dentate Basket Cells in Epilepsy: Impact on Network Rhythms. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN J, WANG LY, LI JM, CAO NJ, WANG L, FENG GB, XUE T, LU Y, WANG XF. Altered expression of the small guanosine triphosphatase RhoA in human temporal lobe epilepsy. J Mol Neurosci. 2010;42:53–8. doi: 10.1007/s12031-010-9330-4. [DOI] [PubMed] [Google Scholar]
- YUTSUDO N, KITAGAWA H. Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp Neurol. 2015;274:126–133. doi: 10.1016/j.expneurol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- ZAITSEV AV, POVYSHEVA NV, LEWIS DA, KRIMER LS. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J Neurophysiol. 2007;97:3567–73. doi: 10.1152/jn.01293.2006. [DOI] [PubMed] [Google Scholar]
- ZAMECNIK J, KRSEK P, DRUGA R, MARUSIC P, BENES V, TICHY M, KOMAREK V. Densities of parvalbumin-immunoreactive neurons in non-malformed hippocampal sclerosis-temporal neocortex and in cortical dysplasias. Brain Res Bull. 2006;68:474–81. doi: 10.1016/j.brainresbull.2005.10.008. [DOI] [PubMed] [Google Scholar]
- ZEISEL A, MUNOZ-MANCHADO AB, CODELUPPI S, LONNERBERG P, LA MANNO G, JUREUS A, MARQUES S, MUNGUBA H, HE L, BETSHOLTZ C, ROLNY C, CASTELO-BRANCO G, HJERLING-LEFFLER J, LINNARSSON S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–42. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, LIU J, LUAN G, WANG X. Inhibition of the small GTPase Cdc42 in regulation of epileptic-seizure in rats. Neuroscience. 2015;289:381–91. doi: 10.1016/j.neuroscience.2014.12.059. [DOI] [PubMed] [Google Scholar]
- ZHAO Y, FLANDIN P, LONG JE, CUESTA MD, WESTPHAL H, RUBENSTEIN JL. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU FW, CHEN HX, ROPER SN. Balance of inhibitory and excitatory synaptic activity is altered in fast-spiking interneurons in experimental cortical dysplasia. J Neurophysiol. 2009;102:2514–25. doi: 10.1152/jn.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU FW, ROPER SN. Reduced chemical and electrical connections of fast-spiking interneurons in experimental cortical dysplasia. J Neurophysiol. 2014;112:1277–90. doi: 10.1152/jn.00126.2014. [DOI] [PubMed] [Google Scholar]
- ZUBERI SM, EUNSON LH, SPAUSCHUS A, DE SILVA R, TOLMIE J, WOOD NW, MCWILLIAM RC, STEPHENSON JB, KULLMANN DM, HANNA MG. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122(Pt 5):817–25. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]