Abstract
Mitochondria, an integral component of cellular energy metabolism and other key functions, are extremely vulnerable to damage by environmental stressors. Although methods to measure mitochondrial function in vitro exist, sensitive, medium- to high-throughput assays that assess respiration within physiologically-relevant whole organisms are needed to identify drugs and/or chemicals that disrupt mitochondrial function, particularly at sensitive early developmental stages. Consequently, we have developed and optimized an assay to measure mitochondrial bioenergetics in zebrafish larvae using the XFe24 Extracellular Flux Analyzer. To prevent larval movement from confounding oxygen consumption measurements, we relied on MS-222-based anesthetization. We obtained stable measurement values in the absence of effects on average oxygen consumption rate and subsequently optimized the use of pharmacological agents for metabolic partitioning. To confirm assay reproducibility we demonstrated that triclosan, a positive control, significantly decreased spare respiratory capacity. We then exposed zebrafish from 5 hours post-fertilization (hpf) – 6 days post-fertilization (dpf) to three polycyclic aromatic hydrocarbons (PAHs) – benzo(a)pyrene (BaP), phenanthrene (Phe), and fluoranthene (FL) – and measured various fundamental parameters of mitochondrial respiratory chain function, including maximal respiration, spare respiratory capacity, mitochondrial and non-mitochondrial respiration. Exposure to all three PAHs decreased spare respiratory capacity and maximal respiration. Additionally, Phe exposure increased non-mitochondrial respiration and FL exposure decreased mitochondrial respiration and increased non-mitochondrial respiration. Overall, this whole organism-based assay provides a platform for examining mitochondrial dysfunction in vivo at critical developmental stages. It has important implications in biomedical sciences, toxicology and ecophysiology, particularly to examine the effects of environmental chemicals and/or drugs on mitochondrial bioenergetics.
Keywords: bioenergetics, development, mitochondria, oxygen consumption, polycyclic aromatic hydrocarbons (PAHs), respiration, triclosan, zebrafish
1. Introduction
Mitochondria are important organelles that regulate many critical biological processes, including ATP production, redox signaling, intracellular calcium signaling, and apoptosis (Dumollard et al., 2007; Meyer et al., 2013). Many human diseases, such as cancer, diabetes, and neurological, cardiovascular, and gastrointestinal disorders, have been associated with mitochondrial DNA (mtDNA) mutations or mitochondrial dysfunction (Ballinger, 2005; Chapman et al., 2014; Coskun et al., 2012; Dumollard et al., 2007; Rolo and Palmeira, 2006; Singh, 2006), and several drugs that cause numerous off-target mitochondrial effects have also been identified. Increasing evidence suggests that mitochondrial structure and function are highly vulnerable to damage by environmental contaminants. The high lipid content of mitochondria facilitates accumulation of lipophilic chemicals, and cytochrome P450 (CYP) enzymes found in mitochondria have the potential to activate previously nonreactive chemicals. Moreover, mitochondria lack some of the DNA repair mechanisms present for nuclear DNA (nDNA) damage repair and may accumulate mitochondrial DNA (mtDNA) mutations over time. Additionally, mitochondria constantly change their morphology, number, and composition depending on cell type, developmental stage, environmental cues, and metabolic demands, especially in response to environmental stressors (Meyer et al., 2013). Thus, there is a need to examine the mitochondrial effects of environmental chemicals, particularly for more sensitive early life stages, as many of these compounds may be undiscovered mitochondrial toxicants.
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental contaminants formed from incomplete combustion of organic material. They originate from both natural and anthropogenic sources such as volcanoes, forest fires, cigarette smoke, and motorized vehicles (ATSDR, 1995). PAHs can enter aquatic ecosystems through soil erosion or runoff, atmospheric depositions, industrial effluent, or oil spills, and tend to accumulate in aquatic sediments over time (Cousin and Cachot, 2014). Due to urban expansion and increased use of automobiles, the concentration of these compounds in aquatic environments is steadily increasing (Lima et al., 2003). Potential adverse impacts of PAHs include carcinogenesis, effects on the reproductive, neurologic, and immune systems, and developmental abnormalities (Arkoosh and Kaattari, 1991; Brown et al., 2016; Hawkins et al., 1990; Incardona et al., 2011; Johnson, 1988; Van Tiem and Di Giulio, 2011; Vignet et al., 2014a; Vignet et al., 2014b). In fish, the most notable adverse developmental effects occur due to bioactivation of PAHs via the aryl hydrocarbon receptor (AHR) pathway, disrupting normal cardiovascular development and resulting in deformities such as elongated “stringy” hearts, impaired heart looping, decreased blood flow, and pericardial effusion (Billiard et al., 2006; Incardona et al., 2004; Van Tiem and Di Giulio, 2011; Wassenberg and Di Giulio, 2004).
Several studies indicate that mitochondria are an important target of PAH toxicity. This is not unexpected, as these very hydrophobic compounds tend to be attracted to lipid-rich mitochondrial membranes inside the cell (Eisler, 1987). PAHs and their metabolites have been shown to localize in mitochondria (Li et al., 2003), and a truncated form of CYP1A, the enzyme that predominantly metabolizes PAHs, is found in the inner mitochondrial membrane in mammals, suggesting that PAHs may be directly metabolized in the mitochondria (Addya et al., 1997). In addition, induction of mitochondrial CYP1A enzyme activity is observed in killifish following benzo(a)pyrene exposure (Jung and Di Giulio, 2010), and it has been demonstrated that bulky DNA adducts formed by PAH metabolites interact with mtDNA (Jung et al., 2009). Furthermore, exposure to PAHs in mammals is associated with effects on nDNA and mtDNA, changes in membrane potential, induction of apoptosis, decreases in ATP production, mitochondrial morphological changes and structural damage, and oxidative stress (Backer and Weinstein, 1980; Graziewicz et al., 2004; Li et al., 2003; Pavanello et al., 2013; Pieters et al., 2013; Xia et al., 2004; Zhu et al., 1995).
Traditionally, mitochondrial respiration has been assessed in vitro and in various animal models using the Clark-type oxygen electrode, which is limited to measuring one sample at a time and lacks sensitivity and throughput (Chance and Williams, 1955; Gruber et al., 2011; Stackley et al., 2011). However, recent technological advances, such as the development of the XFe24 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA, USA), have allowed for a more streamlined method to analyze mitochondrial respiration using a 24- or 96-well microplate format. Despite significant advancements in development and application of assays that evaluate mitochondrial function in vitro and ex vivo (Jayasundara et al., 2015a; Tiernan et al., 2015; Wills et al., 2015), there is an ongoing need to develop medium- to high-throughput assays that rapidly assess mitochondrial function within whole organisms to ensure physiologically-relevant responses are measured. Therefore, the goals of this study were to 1) develop and optimize a reliable, robust assay using the XFe24 Extracellular Flux Analyzer and pharmacological agents to obtain in vivo measurements of mitochondrial respiratory chain parameters in zebrafish larvae, and 2) apply this assay to examine how developmental exposure to subteratogenic concentrations of three PAHs – benzo(a)pyrene, phenanthrene, and fluoranthene - affects normal mitochondrial function.
2. Materials and Methods
2.1. Animals
Laboratory-reared adult wildtype (5D) zebrafish (founder fish provided by Dr. David Volz, University of California, Riverside) were raised and maintained within a recirculating Aquatic Habitats® Z-Hab system (Pentair Aquatic Eco-systems, Inc., Apopka, FL, USA) containing conditioned reverse osmosis (RO) water (27–28°C) on a 14 h:10 h light:dark cycle. Adult females and males were bred directly on-system using in-tank breeding traps suspended within 3-l tanks. For all experiments described below, newly fertilized eggs were staged according to previously described methods (Kimmel et al., 1995). All fish were handled and treated in accordance with approved Institutional Animal Care and Use Committee (IACUC) protocols at Duke University
2.2. Chemicals
Benzo(a)pyrene (BaP), phenanthrene (Phe), fluoranthene (FL), triclosan (Catalog #: 72779), tricaine methanosulfonate (MS-222), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) and sodium azide (NaN3) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of BaP, Phe or FL (1000 mg/l) and triclosan (289.5 mg/l [1 mM]) were prepared by dissolving chemicals in dimethyl sulfoxide (DMSO), and then performing serial dilutions into DMSO to create stock solutions for each working solution. These stock solutions were stored at room temperature within 2-ml amber glass vials containing polytetrafluoroethylene (PTFE)-lined caps. MS-222 (4000 mg/l) and NaN3 (62.5 mM) stock solutions were prepared in ultrapure water, and FCCP stock solutions (2.5 mM) were prepared in DMSO. These stock solutions were stored at −20°C in 0.5 ml microcentrifuge tubes.
2.3 Chemical exposures
Newly fertilized eggs were collected within 1 hour after spawning and placed in groups of approximately 100 per petri dish within a light- and temperature-controlled incubator until 5 hours post-fertilization (hpf). On the basis of previously published data assessing mitochondrial bioenergetics in 24 hpf zebrafish embryos, triclosan was selected as a positive control to evaluate assay reproducibility (Shim et al., 2016). For triclosan exposures, viable 5D larvae were exposed at 6 days post-fertilization (dpf) to vehicle (0.1% DMSO) or non-teratogenic concentrations of triclosan (144.7 µg/l [0.5 µM] or 289.5 µg/l [1 µM]) in 65 mg/l ASW within 24-well plates. Larvae were incubated under static conditions at 28°C for 1 hour and remained in the exposure solution during heart rate or oxygen consumption rate (OCR) measurements, similar to the protocol used in Shim et al. (2016).
Using our established assay, we also assessed mitochondrial function in 6 dpf zebrafish larvae developmentally exposed (5 hpf-6 dpf) to three PAHs, which were chosen based on their known abundance in aquatic environments and demonstrated effects on heart development and physiology at higher concentrations (Incardona et al., 2005; Incardona et al., 2011; Latimer and Zheng, 2003). For developmental PAH exposures, viable 5D embryos were exposed in small glass petri dishes (10 per dish), each containing 10 ml Danieau (58 mM NaCl, 0.7 mM KCl, 0.6 mM Ca(NO3)2, 0.4 mM MgSO4, 5 mM HEPES, pH 7.6), to vehicle (0.1% DMSO) or non-teratogenic concentrations of BaP (100–1000 µg/l), Phe (100–1000 µg/l), or FL (500–1000 µg/l) under static conditions at 28°C from 5 hpf–6 dpf. At 6 dpf, 5–8 randomly selected larvae were removed from exposure solutions, rinsed once in 65 mg/l ASW, and oxygen consumption rates or heart rates were subsequently assessed. ASW and Danieau solution were chosen based on similar studies previously conducted in our laboratory (Jayasundara et al, 2015a, 2015b) to facilitate comparison of results across experiments. The presence of HEPES and other buffering chemicals can affect OCR measurements by the extracellular flux analyzer. Therefore all OCR measurements were conducted using 65 mg/l ASW, as there is very little buffering capacity in this solution. OCR experiments were replicated five independent times and heart rate experiments were replicated three independent times.
2.4. Bioenergetic measurements using the XFe24 Extracellular Flux Analyzer
First, one larva (6 dpf) + 450 µl of 65 mg/l ASW was added to each well of a 24-well islet plate, and capture screens were carefully placed on top. Each plate contained two “blank” wells, which received only 450 µl of 65 mg/l ASW. To measure oxygen consumption within each well of the islet plate, the sensor cartridge creates a temporary 7 µl microchamber with limited oxygen diffusion to rapidly assess significant changes in oxygen concentration, followed by re-equilibration of the media when the probe returns to its original position. For optimization of MS-222 concentration, basal OCR was initially measured for ~80 minutes (20 cycles, each consisting of a 1-minute mix, 1-minute wait, and 2-minute measurement), following a 12-minute equilibration period. Then, MS-222 was injected into the plate to obtain final concentrations of 75, 125, or 175 mg/l, and OCR measurements were recorded for ~80 additional minutes (20 cycles, each consisting of a 1-minute mix, 1-minute wait, and 2-minute measurement). For metabolic partitioning with pharmacological agents, we first optimized the concentrations of FCCP and NaN3 required to successfully stimulate maximal respiration or block the respiratory chain in 6 dpf zebrafish larvae, as these drugs have been previously used in zebrafish embryos up to 48 hpf (Stackley et al., 2011). MS-222 was injected immediately prior to loading each plate to obtain a final well concentration of 125 mg/l. After a 12-minute equilibration period, basal OCR was measured for ~ 24 minutes (6 cycles, each consisting of a 1-minute mix, 1-minute wait, and 2-minute measurement). Sequential injections of 2.5 µM FCCP and 6.25 mM NaN3 were subsequently performed, and OCR measurements were recorded for ~32 minutes (8 cycles, each consisting of a 1-minute mix, 1-minute wait, and 2-minute measurement) and ~80 minutes (20 cycles, each consisting of a 1-minute mix, 1-minute wait, and 2-minute measurement), respectively. DMSO concentrations within each well remained less than 0.1% following injection of FCCP. The internal temperature of the XFe24 Extracellular Flux Analyzer remained between 28–28.5°C for the duration of all assays.
2.5. Heart rate measurements
Heart rates were assessed in 6 dpf larvae as an index of overall physiological performance and circulation of the organism, to confirm that the addition of MS-222 or the chemical compounds did not significantly affect larval health. For these experiments, larvae were placed in a 24-well plate (1 per well) with 450 µl of 65 mg/l ASW. MS-222 was manually added into each individual well to obtain final concentrations of 75, 125, or 175 mg/l. To assess heart rate after exposure to triclosan, BaP, Phe, or FL, larvae were first anesthetized with 125 mg/l MS-222. Heart rates were then assessed at 36, 68, and 148 minutes, corresponding to the end of the basal, FCCP, and NaN3 measurement segments on the XFe24 Extracellular Flux Analyzer. Heart rate measurements were completed by visual inspection under a Nikon SMZ-1500 zoom stereomicroscope. For each larva, heart rates were counted once for 15 seconds and multiplied by 4 to get the total number of beats per minute (bpm).
2.6. Statistical analysis
All statistical procedures were performed using GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA, USA). To compare basal respiration in larvae with and without MS-222, all “before” and all “after” OCR measurements were averaged to estimate basal respiration for each treatment concentration. The coefficient of variation [defined as (standard deviation/mean)*100] was also calculated for each individual larva within the various treatment groups before and after MS-222 addition in order to compare the inter-individual variance associated with optimization experiments, as this measure takes into account the differences in mean OCR values. To obtain change in OCR due to pharmacological agents, the three highest OCR measurements were averaged post FCCP treatment, and the three lowest OCR measurements were averaged for basal or NaN3 treatment. Maximal respiration [defined as (final FCCP OCR measurement – final NaN3 OCR measurement)], spare respiratory capacity [defined as (final FCCP OCR measurement – final basal OCR measurement)], mitochondrial respiration [defined as (final basal OCR measurement – final NaN3 OCR measurement)], and non-mitochondrial respiration [defined as (final NaN3 OCR measurement)] were calculated. The flux ratio [defined as (mitochondrial respiration/maximal respiration)] was also determined and these data are included in the supplemental section. OCR data for each cell respiratory parameter, RCR data, and heart rate data were analyzed for statistical significance using a two-way analysis of variance (ANOVA) (α = 0.05) to determine overall effects, followed by Tukey’s post-hoc test to correct for multiple comparisons and identify significant treatment-related effects. All data are presented as mean +/− SEM.
3. Results
3.1. Optimization of MS-222-based anesthesia
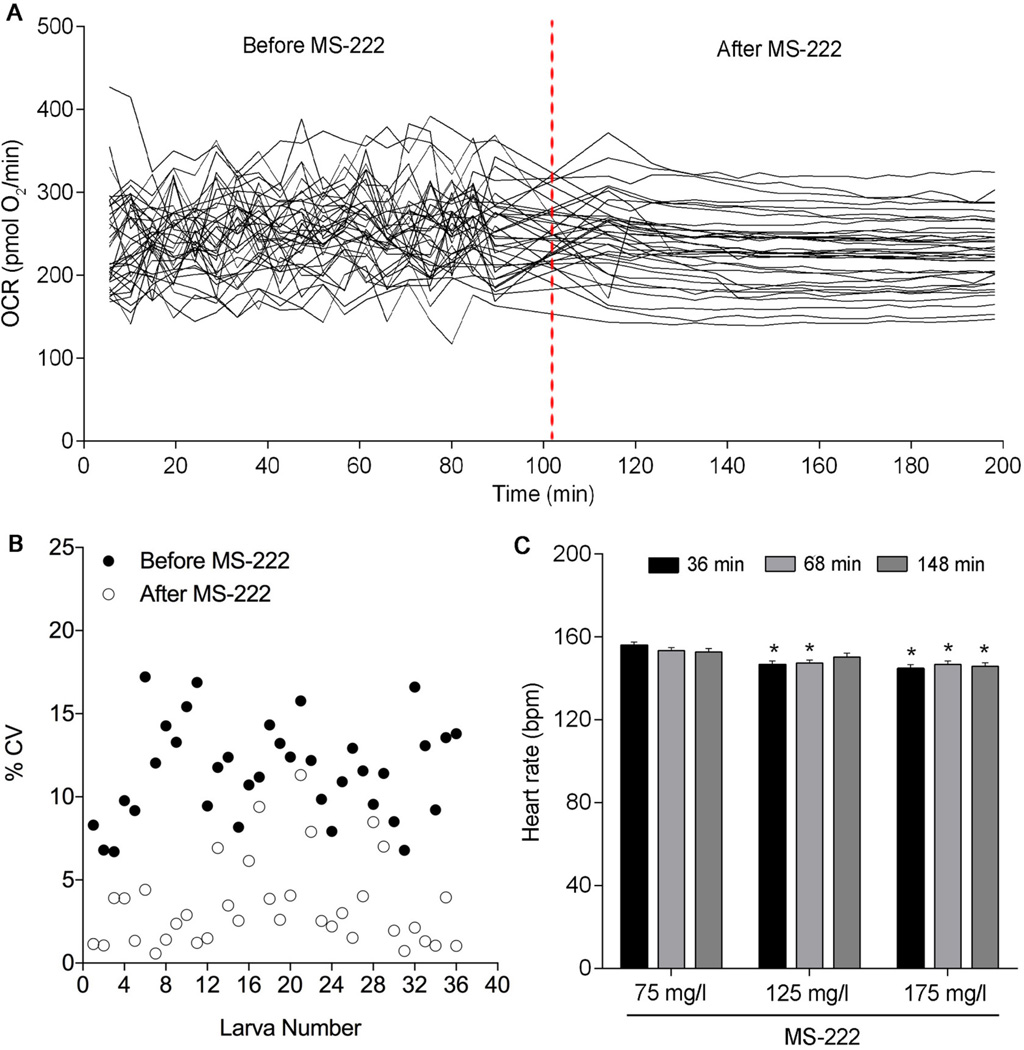
To determine whether MS-222 affected basal OCR in 6 dpf larvae, we analyzed six independent plates containing 5–6 individual larvae per treatment per plate on the XFe24 Extracellular Flux Analyzer. Prior to addition of MS-222 at all concentrations, basal OCR measurements for an individual larva were extremely variable over time (Figure 1A, 125 mg/l treatment shown). Average basal OCR before MS-222 injection was 257.4 pmol O2/min (75 mg/l), 250.3 pmol O2/min (125 mg/l), and 245.3 pmol O2/min (175 mg/l). The coefficient of variation (CV) for an individual larva prior to MS-222 addition ranged from 4.8–17.6% at 75 mg/l, 6.7–17.2% at 125 mg/l (Figure 1B), and 5.2–21% at 175 mg/l. However, after injection of 125 mg/l MS-222, basal OCR measurements significantly stabilized (Figure 1A), producing more repeatable and reliable measurement values with less variation. Following anesthetization with 125 mg/l MS-222, the CV for an individual larva ranged from 0.58–11.3% in the absence of significant effects on average basal OCR (231.7 pmol O2/min) (Figure 1B). Injection of MS-222 at 75 mg/l did not anesthetize the larvae enough to reduce OCR measurement variation (average basal OCR = 278.4 pmol O2/min; CV range for an individual larva = 3.1–21.3%), while injection of MS-222 at 175 mg/l stabilized and reduced OCR measurement variation in a similar manner as 125 mg/l (average basal OCR = 230.5 pmol O2/min; CV range for an individual larva = 0.74–6.3%).
Figure 1.
MS-222 injection results in stable baseline basal oxygen consumption rate (OCR) in 6 dpf zebrafish larvae. (A) Basal OCR before and after 125 mg/l MS-222 injection. The dotted line indicates injection of MS-222. N = six independent plates with 6 larvae per treatment per plate. (B) Larva number and % coefficient of variation (% CV) for each individual larva before and after 125 mg/l MS-222 injection. N = six independent plates with 6 larvae per treatment per plate. (C) Average heart rate of 6 dpf larvae after exposure to MS-222 (75, 125, or 175 mg/l) for 36, 68, and 148 minutes. N = three independent plates with 8 larvae per treatment per plate. Asterisk (*) denotes significant difference from 75 mg/l MS-222 treatment at each individual time point. Error bars are mean +/− SEM.
To assess heart rates at 75, 125, and 175 mg/l MS-222, we analyzed four independent plates containing 8 individual larvae per treatment per plate. Attempts to reliably count heart rates in nonanesthetized larvae were unsuccessful due to the presence of an inflated swim bladder at 6 dpf; therefore, 75 mg/l MS-222 was treated as the control group. Across all time points and MS-222 concentrations, average heart rates ranged from 142–156 bpm. Compared to 75 mg/l, mean heart rates were significantly decreased by 6.3% (p = 0.0003) and 3.9% (p = 0.0329) after exposure to 125 mg/l MS-222 for 36 and 68 minutes, respectively (Figure 1C). Furthermore, exposure to 175 mg/l MS-222 significantly decreased mean heart rates by 7.7% (p < 0.0001), 4.4% (p = 0.0150), and 7.6% (p = 0.0079) at 36, 68, and 148 minutes, respectively (Figure 1C). Although we detected a minor effect of MS-222 on heart rate in our study, mean heart rates across all MS-222 concentrations tested were similar to heart rates of nonanesthetized 72 hpf zebrafish larvae incubated at the same temperature (28°C) without MS-222 (Burns et al., 2005; Yozzo et al., 2013). Therefore, we selected 125 mg/l MS-222 for all subsequent exposures since this concentration (1) represented the lowest concentration necessary to fully anesthetize hatched 6 dpf larvae, (2) produced consistent, reliable OCR measurements over time in the absence of effects on average OCR.
3.2. Assay reproducibility
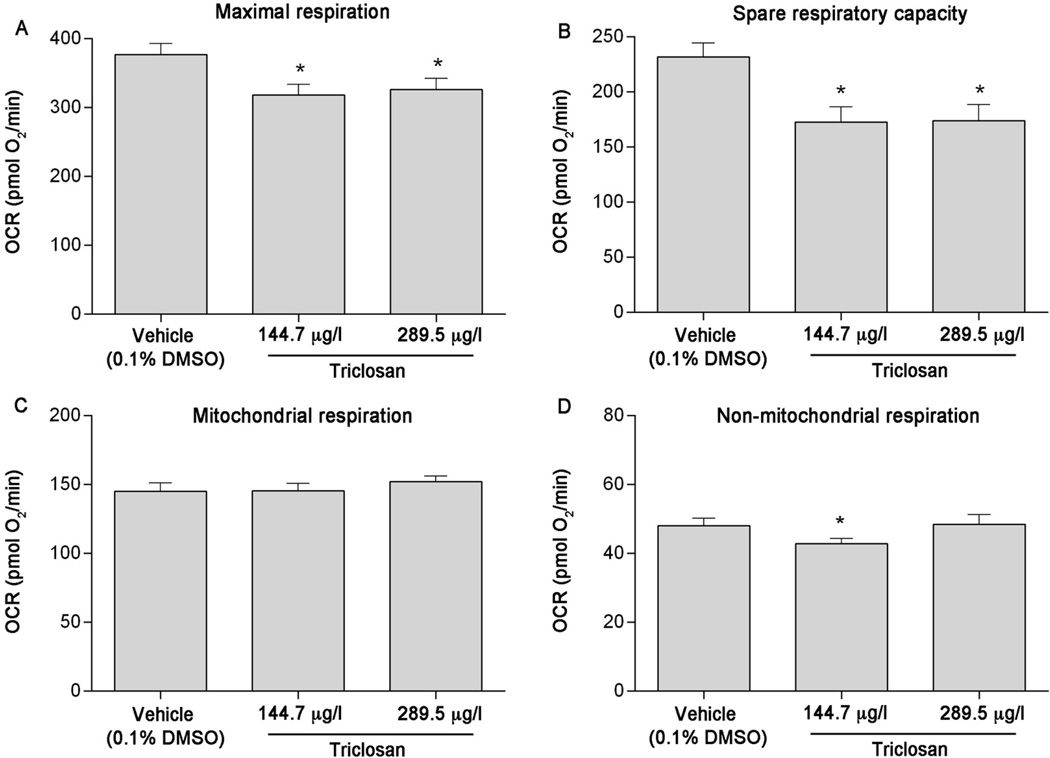
Using pharmacological agents, five independent plates containing 5–7 individual larvae per treatment per plate were analyzed to assess triclosan (positive control) impacts on various cell respiratory parameters. Maximal respiration was significantly decreased after a 1-hour exposure to 144.7 or 289.5 µg/l (0.5 or 1 µM) triclosan (p = 0.0143 and 0.0365, respectively) (Figure 2A). In addition, spare respiratory capacity was also significantly decreased after a 1-hour exposure to 144.7 or 289.5 µg/l (0.5 or 1 µM) triclosan (p = 0.0030 and 0.0034, respectively) (Figure 2A). Triclosan exposure did not affect mitochondrial respiration, though a slight significant decrease in non-mitochondrial respiration was observed at 144.7 µg/l (Figure 2C & 2D). On the basis of results from MS-222 optimization and positive control experiments, we concluded that this assay is reproducible based on a sample size of 5–7 initial larvae per treatment per plate.
Figure 2.
Acute triclosan exposure significantly decreases maximal respiration and spare respiratory capacity in 6 dpf zebrafish larvae. (A) Maximal respiration, (B) spare respiratory capacity, (C) mitochondrial respiration, and (D) non-mitochondrial respiration following exposure to 144.7 or 289.5 µg/l (0.5 or 1 µM) triclosan. Asterisk (*) denotes significant difference from vehicle control (p<0.05). N = five independent plates with 5–6 larvae per treatment per plate. Error bars are mean +/− SEM.
3.3. Developmental PAH exposure and mitochondrial bioenergetics
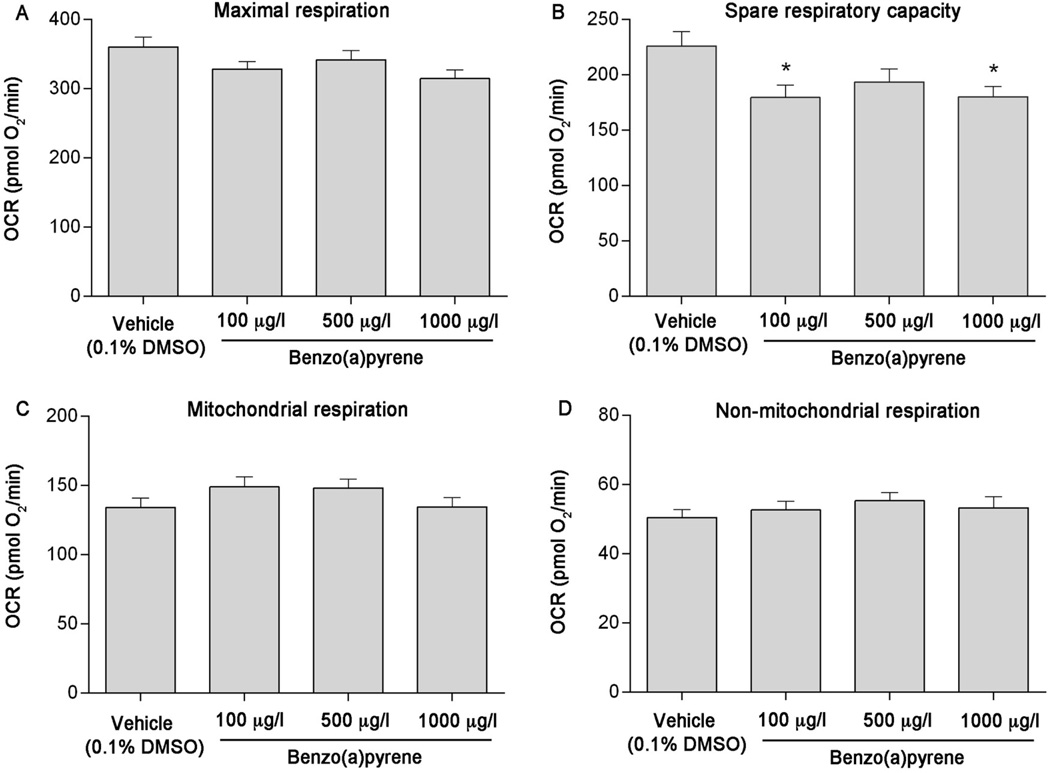
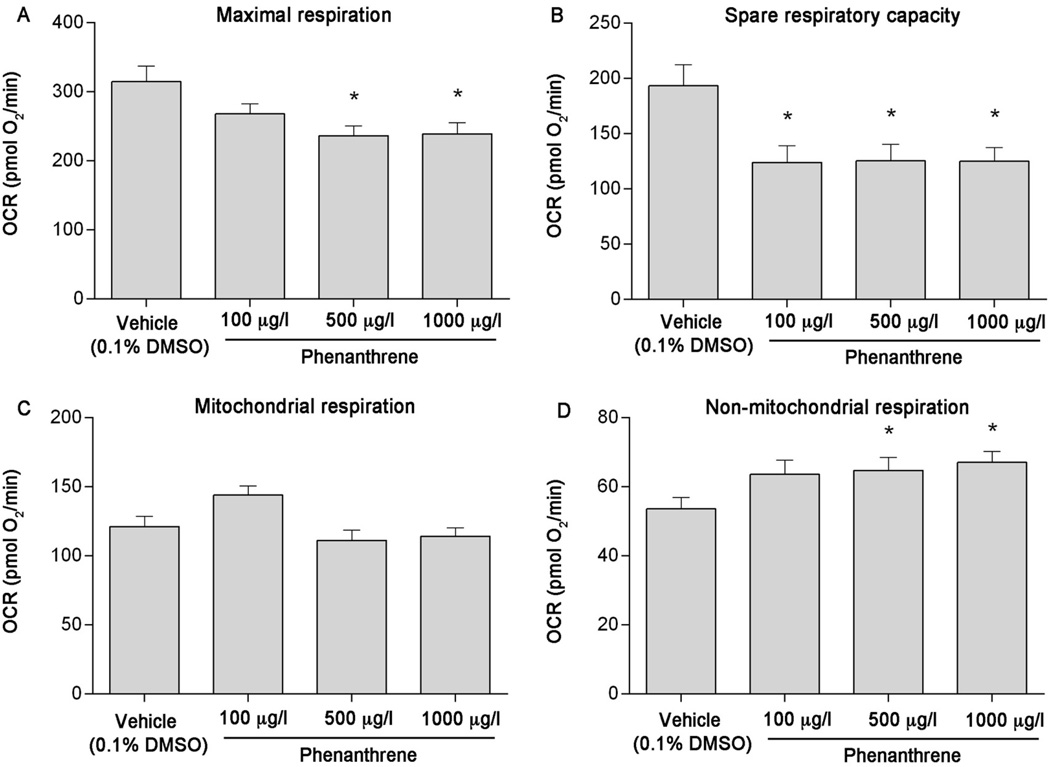
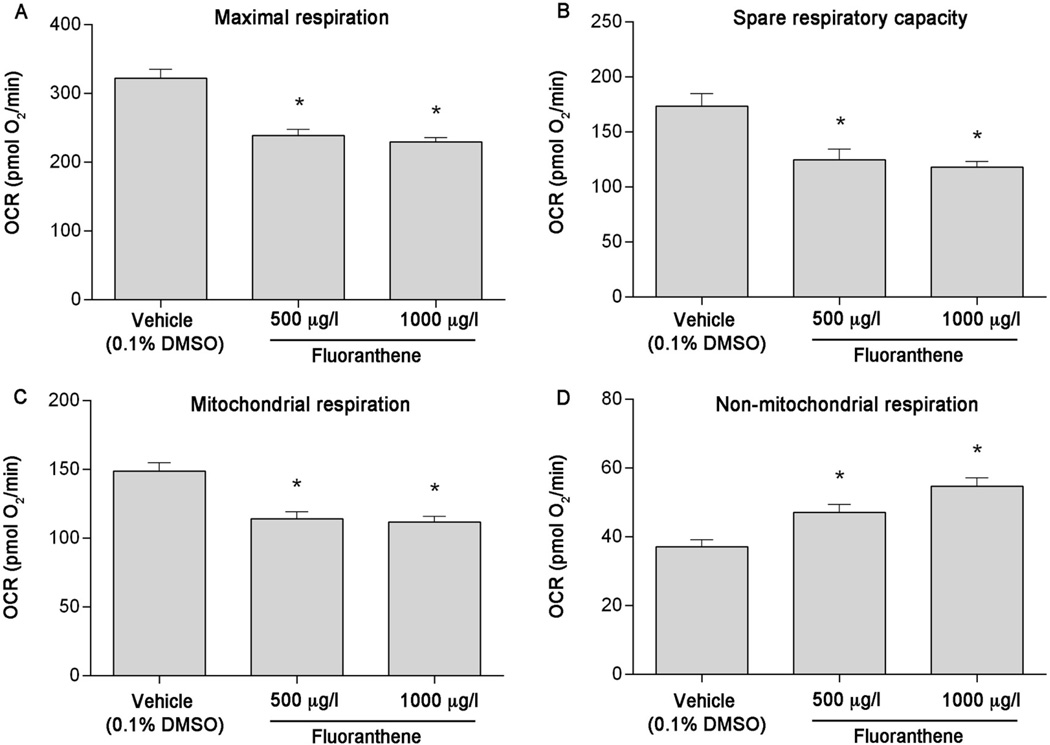
Using pharmacological agents, five independent plates containing 5–7 individual larvae per treatment per plate were analyzed to assess impacts of developmental BaP, Phe, and FL exposure on mitochondrial bioenergetics parameters. While developmental exposure to BaP did not affect mitochondrial or non-mitochondrial respiration (Figure 3C & 3D), a slight decrease in maximal respiration was observed (Figure 3A). In addition, compared to vehicle control, spare respiratory capacity was significantly decreased by 21% at 100 µg/l BaP (p = 0.0187) and 22% at 1000 µg/l BaP (p = 0.0252) (Figure 3B). Developmental exposure to Phe also resulted in significant effects on maximal respiration (~24% decrease from vehicle at 500 and 1000 µg/l, p = 0.0004 and 0.0008, respectively, Figure 4A) and spare respiratory capacity (~35% decrease from vehicle at all concentrations tested, p = 0.0004, Figure 4B). Although no differences in mitochondrial respiration were detected (Figure 4C), a significant increase in non-mitochondrial respiration was also observed (20% at 500 µg/l [p = 0.0251] and 25% at 1000 µg/l [p = 0.0049], Figure 4D). Developmental exposure to 500 and 1000 µg/l FL significantly reduced maximal respiration (by 25% and 28%, respectively [p < 0.0001]), spare respiratory capacity (by 28% and 32%, respectively [p < 0.0001]), and mitochondrial respiration (by 23% and 25%, respectively [p < 0.0001]), and increased non-mitochondrial respiration (by 27% [p = 0.0002] and 47% [p < 0.0001], respectively) (Figure 5A–D).
Figure 3.
Developmental benzo(a)pyrene (BaP) exposure causes mitochondrial toxicity in 6 dpf zebrafish larvae. (A) Maximal respiration, (B) spare respiratory capacity, (C) mitochondrial respiration, and (D) non-mitochondrial respiration following exposure to 100, 500, or 1000 µg/l BaP. Asterisk (*) denotes significant difference from vehicle control (p<0.05). N = five independent plates with 5–6 larvae per treatment per plate. Error bars are mean +/− SEM.
Figure 4.
Developmental phenanthrene (Phe) exposure causes mitochondrial toxicity in 6 dpf zebrafish larvae. (A) Maximal respiration, (B) spare respiratory capacity, (C) mitochondrial respiration, and (D) non-mitochondrial respiration following exposure to 100, 500, or 1000 µg/l Phe. Asterisk (*) denotes significant difference from vehicle control (p<0.05). N = five independent plates with 5–6 larvae per treatment per plate. Error bars are mean +/− SEM.
Figure 5.
Developmental fluoranthene (FL) exposure causes mitochondrial toxicity in 6 dpf zebrafish larvae. (A) Maximal respiration, (B) spare respiratory capacity, (C) mitochondrial respiration, and (D) non-mitochondrial respiration following exposure to 500 or 1000 µg/l FL. Asterisk (*) denotes significant difference from vehicle control (p<0.05). N = five independent plates with 5–6 larvae per treatment per plate. Error bars are mean +/− SEM.
3.4. Heart rate measurements
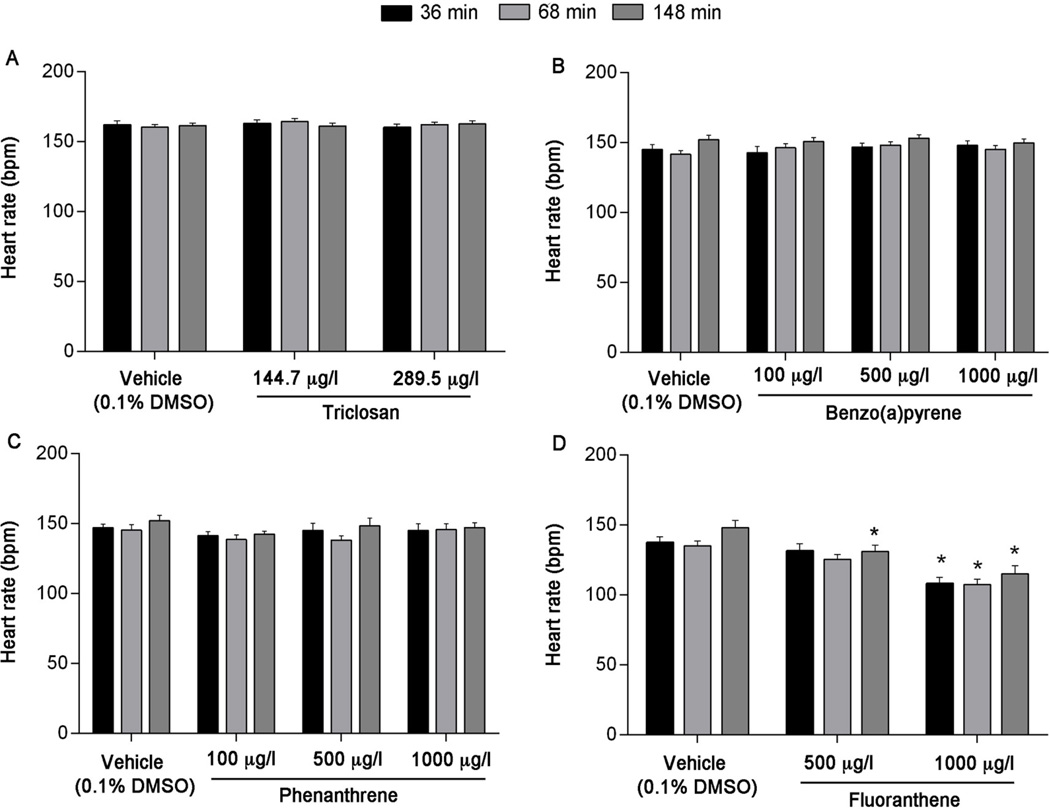
To determine whether heart rate was adversely impacted by exposure to triclosan, BaP, Phe, or FL, we analyzed the heart rates of individual larvae after immersion in 125 mg/l MS-222 for 36, 68, and 148 minutes, time points corresponding to the end of the basal, FCCP, and NaN3 segments on the XFe24 Extracellular Flux Analyzer. Exposure to triclosan, BaP, and Phe did not affect heart rate (Figure 6A–C). Across all time points and concentrations, heart rates of triclosan exposed larvae ranged from 160–164 bpm, with no more than a 2.5% change compared to vehicle control at any time point. For BaP, average heart rates ranged from 142–153 bpm across all time points and concentrations, with no more than a 4.5% change compared to vehicle control at any time point. Phe exposure resulted in average heart rates that ranged from 136–149 bpm across all time points and concentrations, with no more than a 4.8% change observed compared to vehicle control at any time point. However, larvae exposed to 1000 µg/l, but not 500 µg/l FL, resulted in significantly decreased heart rates at 36 and 68 minutes (p < 0.0001 and p = 0.0001, respectively) (Figure 6D). At 148 minutes, significantly decreased heart rates were observed at 500 and 1000 µg/l (p = 0.0268 and p < 0.0001, respectively) (Figure 6D). Across all time points, average heart rates ranged from 139–146 bpm for vehicle control, 132–138 bpm for 500 µg/l FL and 106–115 bpm for 1000 µg/l FL. Compared to vehicle control, this corresponds to a 1.3–5.2% and 20.7–23.3% change in heart rate for 500 and 1000 µg/l, respectively.
Figure 6.
Average heart rates in 6 dpf zebrafish larvae following exposure to (A) triclosan, (B) benzo(a)pyrene, (C) phenanthrene, or (D) fluoranthene. Asterisk (*) denotes significant difference from vehicle control (p<0.05) within each time point. N = three independent plates with 6 larvae per treatment per plate. Error bars are mean +/− SEM.
4. Discussion
The objectives of the current study were to 1) develop and optimize a bioenergetics assay to assess mitochondrial health in zebrafish larvae, and 2) apply this assay to assess mitochondrial toxicity of PAHs. Although a few assays have evaluated mitochondrial function in zebrafish embryos and larvae (Gibert et al., 2013; Grone et al., 2016; Kumar et al., 2016; Stackley et al., 2011), our assay adds significant value to these existing assays by using pharmacological agents to perturb metabolism in larvae, providing additional information on how environmental chemical exposure impacts normal mitochondrial function at this critical developmental stage. This is particularly important, as the larval life stage may be more sensitive to chemical toxicity due to the absence of a protective chorion (Ansari and Ansari, 2011, 2015). After reducing assay variability and confirming reproducibility using triclosan, a positive control known to affect mitochondrial function, we exposed zebrafish to three PAHs, benzo(a)pyrene, phenanthrene, and fluoranthene – ubiquitous environmental chemicals – from 5 hpf-6 dpf, and found that exposure to all of these chemicals resulted in mitochondrial dysfunction.
Contrary to the consistent oxygen consumption measurements observed in chorionated zebrafish embryos over time (data not shown), significant fluctuations in measured oxygen consumption values were observed for a single individual larva over the 80-minute measurement period without anesthetization (Figure 1A). Prior to anesthetization with 125 mg/l MS-222, the lowest or highest measurement value for an individual larva was up to 41% from the average (range = 11–41%; median = 21%), compared to up to 30% from the average (range = 1–30%; median = 4%) following anesthetization. Due to the way oxygen consumption is measured using the sensor cartridge, the variation in oxygen consumption measurements observed prior to anesthetization was likely due to larval movement, resulting in incorrect estimation of the oxygen concentration at different time points. Furthermore, without anesthetization, OCR measurement values for an individual larva were expected to be even more variable due to increased movement in response to addition of the drug. Therefore, to ensure acquisition of reliable oxygen consumption rates and reduce any further measurement variation that might occur when adding pharmacological agents to nonanesthetized larvae, we relied on MS-222-based anesthetization. This enabled us to use FCCP and NaN3 to stimulate maximal respiration and block the respiratory chain, respectively, and facilitated the detection of OCR changes with altered mitochondrial function upon exposure to environmental chemicals. Additionally, although inter-individual variation in oxygen consumption rates was expected in our assay, attempts to decrease this variation by normalizing data to larval body weight were unsuccessful, as body weight differences were too insignificant. Future studies may also benefit from calculating the flux ratio by normalizing mitochondrial respiration data to maximal respiration data. However, these calculations did not significantly alter our overall conclusions (Supplemental Figure 1).
Following MS-222-based anesthetization to control for measurement variation, our assay detected significant adverse effects on mitochondrial function after exposure to triclosan, a positive control. Similar to previous studies in 24 hpf zebrafish embryos (Shim et al., 2016), we observed a significant decrease in spare respiratory capacity at 6 dpf, indicating mitochondrial dysfunction (Brand and Nicholls, 2011). In Shim et al. (2016), the authors also observed a significant increase in proton leak and decrease in ATP-linked respiration; however, our attempts to repeat this in larvae were unsuccessful, as we were unable to identify an oligomycin concentration that optimally inhibited ATP synthase at this developmental stage. We also observed a decrease in maximal respiration, slight decrease in non-mitochondrial respiration, and no effect on mitochondrial respiration in our assay, endpoints that were not specifically reported in Shim et al. Despite the difference in developmental stage used in these two assays, similar results were obtained, demonstrating the reproducibility of our assay as well as the usefulness of the extracellular flux analyzer for successful identification of environmental chemicals that target mitochondria in vivo in zebrafish.
All three PAHs were found to impact mitochondrial function at 6 dpf, though the effects were compound-specific. Exposure to Phe and FL significantly reduced both maximal respiration and spare respiratory capacity. However, while a significant reduction in spare respiratory capacity was also observed following BaP exposure, only a slight decrease in maximal respiration was observed. Spare respiratory capacity calculated based on maximal respiration rates induced by FCCP, an uncoupling agent that collapses the proton gradient, is an indicator of the total mitochondrial capacity when mitochondrial demand is increased. Consequently, our results indicate that exposure to PAHs decreased maximum respiration rates and reduced the ability of larvae to respond to stressful conditions, indicating reduced fitness.
To date, only a few studies, primarily focused on adult fish, have observed adverse effects of PAH exposure on bioenergetics. One study discovered that sublethal exposure to a complex PAH mixture derived from crude oil during early development in zebrafish reduced aerobic capacity and altered cardiac morphology in adults (Hicken et al., 2011). Decreased active metabolic rate, aerobic scope, and cardiac output were also observed in adult zebrafish exposed to β-naphthoflavone (BNF), an AHR agonist, for 48 hours (Gerger et al., 2015). In addition, PAH exposure during early zebrafish development was found to affect mitochondrial bioenergetics ex vivo in adult heart tissue (Jayasundara, unpublished results). Nevertheless, an inability to meet an increased metabolic demand upon exposure to environmental stressors at any developmental stage can affect survival, growth, reproduction, and/or swimming performance (Brown et al., in review; Jung et al., 2009; Nisbet, 2000). However, our current data on BaP and Phe suggest that mitochondrial dysfunction is independent of any cardiac abnormalities, considering no significant changes in heart rate were detected with exposure to these PAHs. In contrast, heart rates as well as overall mitochondrial function decreased in larvae developmentally exposed to FL at 1000 µg/l. However, prior to the addition of NaN3, only mitochondrial function was affected in larvae exposed to 500 µg/l FL, suggesting that FL may lead to mitochondrial dysfunction irrespective of any systemic failure or circulatory limitations due to reduced heart rates. Further research is required to partition PAH effects on cardiac function leading to organismal mitochondrial effects from direct mitochondrial effects. Alternatively, the altered mitochondrial function may play a role in cardiac dysfunction (reduced heart rates) and warrants further research.
Exposure to Phe and FL, but not BaP, also significantly altered non-mitochondrial and/or total mitochondrial respiration. Interestingly, 10% of cellular oxygen uptake in mammal and fish cells is due to non-mitochondrial, rather than mitochondrial sources (Brand and Nicholls, 2011; Rolfe et al., 1999). The increase in non-mitochondrial respiration observed after Phe and FL exposure could be due to contributions from oxidase enzymes within other intracellular organelles including the endoplasmic reticulum, peroxisomes, or plasma membrane (Affourtit and Brand, 2009; Bishop and Brand, 2000; Herst et al., 2004; van den Bosch, 1992). Based on in vitro data obtained from mammalian cells exposed to various concentrations of oxygen, Bishop & Brand (2000) have proposed that an increase in non-mitochondrial oxygen consumption might serve as a protective mechanism to remove oxygen when it is present at potentially harmful concentrations. This suggestion provides a plausible explanation for the significant increase in non-mitochondrial oxygen consumption observed in our study, as it would help delay or prevent PAH-induced increases in ROS generation and oxidative stress (Arzuaga and Elskus, 2010; Timme-Laragy et al., 2009; Wilk et al., 2013). Since we are currently unable to partition total mitochondrial respiration due to ATP turnover and proton leak in larvae, future research will aim to determine why mitochondrial respiration was decreased after FL exposure.
It is well known that the toxic responses of some PAHs, particularly BaP, are mediated through the AHR, a ligand-activated transcription factor. Recent studies have demonstrated that the AHR might influence mitochondrial function through its interaction with the ATP5α1 subunit of the ATP synthase complex and mitochondrial ribosomal protein L40 (Tappenden et al., 2013; Tappenden et al., 2011). In addition, previous research has demonstrated mitochondrial membrane potential and electron transport chain activities are modified by AHR ligands, impacting mitochondrial function (Senft et al., 2002; Tappenden et al., 2011). Furthermore, TCDD, a strong AHR agonist, has been found to alter cellular respiration in an AHR-dependent manner (Hwang et al., 2016). However, we also found that exposure to Phe and FL, two non-AHR agonists, affected mitochondrial function, suggesting that PAHs might cause mitochondrial toxicity via an AHR-independent mechanism. In fact, a recent study demonstrated that exposure to crude oil-derived PAHs disrupted excitation-contraction coupling of cardiomyocytes isolated from juvenile tuna (Brette et al., 2014). This process is highly controlled by Ca2+ and K+ ions, both of which play central roles in the regulation of mitochondrial metabolism (Santo-Domingo and Demaurex, 2010). Overall, we speculate that the reduced FCCP response is potentially due to a compensatory increase in basal mitochondrial function or ATP synthesis that may be decreased by PAH exposure via above described processes. This would lead to reduced maximal respiratory rates, thus reduced spare capacity. Further research will help determine the specific mechanism of PAH-induced mitochondrial dysfunction and elucidate whether the observed effects are due to a direct molecular interaction (e.g. with the AHR) or indirect systemic toxicity.
In summary, we have successfully developed a reliable, robust assay to assess mitochondrial function in vivo in zebrafish larvae, and subsequently identified three PAHs as mitochondrial toxicants. This assay has important implications across multiple fields of biology, considering the wide use of zebrafish from toxicology to biomedical sciences and extending to ecological studies (Engeszer et al., 2007; Lieschke and Currie, 2007; Parichy, 2015; Spence et al., 2008). It can be used to screen environmental chemicals and identify potential mitochondrial toxicants, explore the effects of environmental stressors on organismal fitness, and help understand the pathogenesis of mitochondrial diseases. In the future, we envision that this assay can be used to develop hypothesis-driven studies to uncover mechanisms of mitochondrial toxicity using a variety of different molecular approaches. This will provide a better understanding of how early life stage sublethal exposure to environmental stressors affects later life stages, allowing for better evaluation of the impacts and potential risks associated with chemical exposure.
Supplementary Material
Acknowledgments
We gratefully thank Dr. David Volz for providing us with founder fish to establish our wildtype (5D) zebrafish colony. This research was supported by Duke University’s Superfund Research Center (NIEHS P42-ES010356).
Footnotes
Conflict of Interest Statement
All authors declare no conflict of interest.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) Atlanta, GA: Department of Health and Human Services, Public Health Service; 1995. [PubMed] [Google Scholar]
- Addya S, Anandatheerthavarada HK, Biswas G, Bhagwat SV, Mullick J, Avadhani NG. Targeting of NH2-terminal-processed microsomal protein to mitochondria: a novel pathway for the biogenesis of hepatic mitochondrial P450MT2. J Cell Biol. 1997;139:589–599. doi: 10.1083/jcb.139.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affourtit C, Brand MD. Measuring mitochondrial bioenergetics in INS-1E insulinoma cells. Methods Enzymol. 2009;457:405–424. doi: 10.1016/S0076-6879(09)05023-X. [DOI] [PubMed] [Google Scholar]
- Ansari S, Ansari BA. Embryo and Fingerling Toxicity of Dimethoate and Effect on Fecundity, Viability, Hatchability and Survival of Zebrafish, Danio rerio. World Journal of Fish and Marine Sciences. 2011;3:167–173. [Google Scholar]
- Ansari S, Ansari BA. Effects of Heavy Metals on the Embryo and Larvae of Zebrafish, Danio rerio (Cyprinidae) Sch. Acad. J. Biosci. 2015;3:52–56. [Google Scholar]
- Arkoosh MR, Kaattari SL. Development of immunological memory in rainbow trout (Oncorhynchus mykiss). I. An immunochemical and cellular analysis of the B cell response. Dev Comp Immunol. 1991;15:279–293. doi: 10.1016/0145-305x(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Arzuaga X, Elskus A. Polluted-site killifish (Fundulus heteroclitus) embryos are resistant to organic pollutant-mediated induction of CYP1A activity, reactive oxygen species, and heart deformities. Environ Toxicol Chem. 2010;29:676–682. doi: 10.1002/etc.68. [DOI] [PubMed] [Google Scholar]
- Backer JM, Weinstein IB. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980;209:297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Bishop T, Brand MD. Processes contributing to metabolic depression in hepatopancreas cells from the snail Helix aspersa. J Exp Biol. 2000;203:3603–3612. doi: 10.1242/jeb.203.23.3603. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette F, Machado B, Cros C, Incardona JP, Scholz NL, Block BA. Crude oil impairs cardiac excitation-contraction coupling in fish. Science. 2014;343:772–776. doi: 10.1126/science.1242747. [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Thompson J, Chernick M, Hinton DE, Di Giulio RT. Later life swimming performance and persistent heart damage following subteratogenic PAH mixture exposure in the Atlantic killifish Fundulus heteroclitus) Environ Toxicol Chem. doi: 10.1002/etc.3877. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylationIKinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Chapman TP, Hadley G, Fratter C, Cullen SN, Bax BE, Bain MD, Sapsford RA, Poulton J, Travis SP. Unexplained gastrointestinal symptoms: think mitochondrial disease. Dig Liver Dis. 2014;46:1–8. doi: 10.1016/j.dld.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin X, Cachot J. PAHs and fish--exposure monitoring and adverse effects--from molecular to individual level. Environ Sci Pollut Res Int. 2014;21:13685–13688. doi: 10.1007/s11356-014-3161-8. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007;77:21–49. doi: 10.1016/S0070-2153(06)77002-8. [DOI] [PubMed] [Google Scholar]
- Eisler R. Polycyclic aromatic hydrocarbon hazards to fish, wildlife, and invertebrates: A synoptic review. U.S. Fish and Wildlife Service, Patuxent Wildlife Research Center; 1987. [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- Gerger CJ, Thomas JK, Janz DM, Weber LP. Acute effects of beta-naphthoflavone on cardiorespiratory function and metabolism in adult zebrafish (Danio rerio) Fish Physiol Biochem. 2015;41:289–298. doi: 10.1007/s10695-014-9982-z. [DOI] [PubMed] [Google Scholar]
- Gibert Y, McGee SL, Ward AC. Metabolic profile analysis of zebrafish embryos. J Vis Exp. 2013:e4300. doi: 10.3791/4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziewicz MA, Sayer JM, Jerina DM, Copeland WC. Nucleotide incorporation by human DNA polymerase gamma opposite benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyguanosine and deoxyadenosine. Nucleic Acids Res. 2004;32:397–405. doi: 10.1093/nar/gkh213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Marchese M, Hamling KR, Kumar MG, Krasniak CS, Sicca F, Santorelli FM, Patel M, Baraban SC. Epilepsy, Behavioral Abnormalities, and Physiological Comorbidities in Syntaxin-Binding Protein 1 (STXBP1) Mutant Zebrafish. PLoS One. 2016;11:e0151148. doi: 10.1371/journal.pone.0151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Ng LF, Fong S, Wong YT, Koh SA, Chen CB, Shui G, Cheong WF, Schaffer S, Wenk MR, Halliwell B. Mitochondrial changes in ageing Caenorhabditis elegans--what do we learn from superoxide dismutase knockouts? PLoS One. 2011;6:e19444. doi: 10.1371/journal.pone.0019444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins WE, Walker WW, Overstreet RM, Lytle JS, Lytle TF. Carcinogenic effects of some polycyclic aromatic hydrocarbons on the Japanese medaka and guppy in waterborne exposures. Sci Total Environ. 1990;94:155–167. doi: 10.1016/0048-9697(90)90370-a. [DOI] [PubMed] [Google Scholar]
- Herst PM, Tan AS, Scarlett DJ, Berridge MV. Cell surface oxygen consumption by mitochondrial gene knockout cells. Biochim Biophys Acta. 2004;1656:79–87. doi: 10.1016/j.bbabio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK, Scholz NL, Incardona JP. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci U S A. 2011;108:7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HJ, Dornbos P, Steidemann M, Dunivin TK, Rizzo M, LaPres JJ. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol Appl Pharmacol. 2016;304:121–132. doi: 10.1016/j.taap.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Linbo TL, Scholz NL. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol. 2011;257:242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Jayasundara N, Kozal JS, Arnold MC, Chan SS, Di Giulio RT. High-Throughput Tissue Bioenergetics Analysis Reveals Identical Metabolic Allometric Scaling for Teleost Hearts and Whole Organisms. PLoS One. 2015a;10:e0137710. doi: 10.1371/journal.pone.0137710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasundara N, Van Tiem Garner L, Meyer JN, Erwin KN, Di Giulio RT. AHR2-Mediated Transcriptomic Responses Underlying the Synergistic Cardiac Developmental Toxicity of PAHs. Toxicol Sci. 2015b;143:469–481. doi: 10.1093/toxsci/kfu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LL, Casillas E, Collier TK, McCain BB, Varanasi U. Contaminant effects on ovarian development in english sole (Parophrys vetulus) from Puget Sound, Washington. Can J Fish Aquat Sci. 1988;45:2133–2146. [Google Scholar]
- Jung D, Cho Y, Collins LB, Swenberg JA, Di Giulio RT. Effects of benzo[a]pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquat Toxicol. 2009;95:44–51. doi: 10.1016/j.aquatox.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Di Giulio RT. Identification of mitochondrial cytochrome P450 induced in response to polycyclic aromatic hydrocarbons in the mummichog (Fundulus heteroclitus) Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:107–112. doi: 10.1016/j.cbpc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kumar MG, Rowley S, Fulton R, Dinday MT, Baraban SC, Patel M. Altered Glycolysis and Mitochondrial Respiration in a Zebrafish Model of Dravet Syndrome. eNeuro. 2016;3(2) doi: 10.1523/ENEURO.0008-16.2016. ENEURO.0008–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer JS, Zheng J. The Sources, Transport, and Fate of PAHs in the Marine Environment. In: Douben E, editor. PAHs: An Ecotoxicological Perspective. Chichester, UK: John Wiley & Sons, Ltd; 2003. [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lima AL, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet RM, Muller EB, Lika K, Kooijman S. From molecules to ecosystems through dynamic energy budget models. Journal of Animal Ecology. 2000;69:913–926. [Google Scholar]
- Parichy DM. Advancing biology through a deeper understanding of zebrafish ecology and evolution. Elife. 2015;4:e05635. doi: 10.7554/eLife.05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Dioni L, Hoxha M, Fedeli U, Mielzynska-Svach D, Baccarelli AA. Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 2013;22:1722–1729. doi: 10.1158/1055-9965.EPI-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters N, Koppen G, Smeets K, Napierska D, Plusquin M, De Prins S, Van De Weghe H, Nelen V, Cox B, Cuypers A, Hoet P, Schoeters G, Nawrot TS. Decreased mitochondrial DNA content in association with exposure to polycyclic aromatic hydrocarbons in house dust during wintertime: from a population enquiry to cell culture. PLoS One. 2013;8:e63208. doi: 10.1371/journal.pone.0063208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Newman JM, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. Am J Physiol. 1999;276:C692–C699. doi: 10.1152/ajpcell.1999.276.3.C692. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Shim J, Weatherly LM, Luc RH, Dorman MT, Neilson A, Ng R, Kim CH, Millard PJ, Gosse JA. Triclosan is a mitochondrial uncoupler in live zebrafish. J Appl Toxicol. 2016 doi: 10.1002/jat.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006;1067:182–190. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Stackley KD, Beeson CC, Rahn JJ, Chan SS. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011;6:e25652. doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden DM, Hwang HJ, Yang L, Thomas RS, Lapres JJ. The Aryl-Hydrocarbon Receptor Protein Interaction Network (AHR-PIN) as Identified by Tandem Affinity Purification (TAP) and Mass Spectrometry. J Toxicol. 2013;2013:279829. doi: 10.1155/2013/279829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden DM, Lynn SG, Crawford RB, Lee K, Vengellur A, Kaminski NE, Thomas RS, LaPres JJ. The aryl hydrocarbon receptor interacts with ATP5alpha1, a subunit of the ATP synthase complex, and modulates mitochondrial function. Toxicol Appl Pharmacol. 2011;254:299–310. doi: 10.1016/j.taap.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiernan CT, Edwin EA, Hawong HY, Rios-Cabanillas M, Goudreau JL, Atchison WD, Lookingland KJ. Methylmercury impairs canonical dopamine metabolism in rat undifferentiated pheochromocytoma (PC12) cells by indirect inhibition of aldehyde dehydrogenase. Toxicol Sci. 2015;144:347–356. doi: 10.1093/toxsci/kfv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci. 2009;109:217–227. doi: 10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H, Schutgens RBH, Wanders RJA, Tager JM. Biochemistry of Peroxisomes. Annual Review of Biochemistry. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- Van Tiem LA, Di Giulio RT. AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 2011;254:280–287. doi: 10.1016/j.taap.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignet C, Devier MH, Le Menach K, Lyphout L, Potier J, Cachot J, Budzinski H, Begout ML, Cousin X. Long-term disruption of growth, reproduction, and behavior after embryonic exposure of zebrafish to PAH-spiked sediment. Environ Sci Pollut Res Int. 2014;21:13877–13887. doi: 10.1007/s11356-014-2585-5. [DOI] [PubMed] [Google Scholar]
- Vignet C, Le Menach K, Lyphout L, Guionnet T, Frere L, Leguay D, Budzinski H, Cousin X, Begout ML. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish--part II: behavior. Environ Sci Pollut Res Int. 2014b;21:13818–13832. doi: 10.1007/s11356-014-2762-6. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk A, Waligorski P, Lassak A, Vashistha H, Lirette D, Tate D, Zea AH, Koochekpour S, Rodriguez P, Meggs LG, Estrada JJ, Ochoa A, Reiss K. Polycyclic aromatic hydrocarbons-induced ROS accumulation enhances mutagenic potential of T-antigen from human polyomavirus JC. J Cell Physiol. 2013;228:2127–2138. doi: 10.1002/jcp.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Beeson GC, Hoover DB, Schnellmann RG, Beeson CC. Assessment of ToxCast Phase II for Mitochondrial Liabilities Using a High-Throughput Respirometric Assay. Toxicol Sci. 2015;146:226–234. doi: 10.1093/toxsci/kfv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozzo KL, Isales GM, Raftery TD, Volz DC. High-content screening assay for identification of chemicals impacting cardiovascular function in zebrafish embryos. Environ Sci Technol. 2013;47:11302–11310. doi: 10.1021/es403360y. [DOI] [PubMed] [Google Scholar]
- Zhu H, Li Y, Trush MA. Characterization of benzo[a]pyrene quinone-induced toxicity to primary cultured bone marrow stromal cells from DBA/2 mice: potential role of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1995;130:108–120. doi: 10.1006/taap.1995.1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.