Abstract
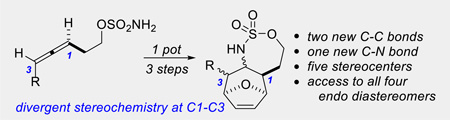
A tandem allene aziridination/[4+3]/reduction sequence converts simple homoallenic sulfamates to densely functionalized aminated cycloheptenes, where the relative stereochemistry at five contiguous asymmetric centers can be controlled through the choice of the solvent and the reductant. The products resulting from this chemistry can be readily transformed into complex molecular scaffolds that contain up to seven contiguous stereocenters.
Keywords: allenes, [4 + 3] cycloaddition, aziridination, stereodivergence
Graphical Abstract
A tandem allene aziridination/[4 + 3]/reduction sequence converts homoallenic sulfamates to aminated cycloheptanes, where the relative stereochemistry at five contiguous chiral carbons can be controlled through the choice of solvent and reductant.
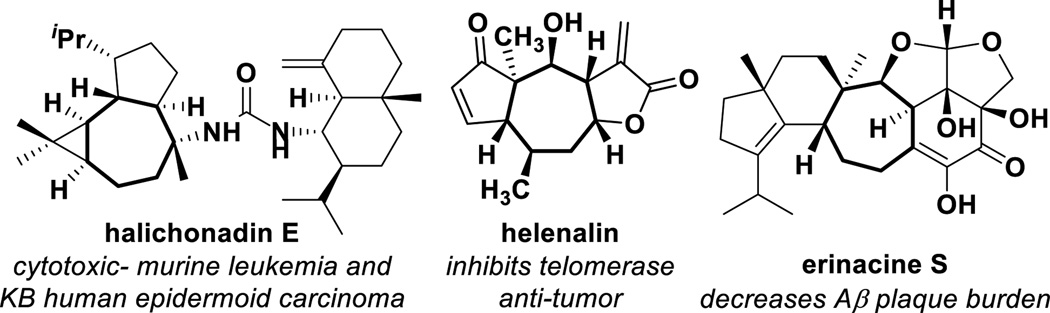
Densely functionalized seven-membered rings are valuable synthetic targets occurring in a range of bioactive natural products and their analogs (Figure 1). Several approaches to address the challenge of controlling the stereochemical outcome in the synthesis of seven-membered rings have been developed, including annulations, ring-closing metatheses and an array of cycloaddition reactions.[1] One powerful strategy involves the [4+3] reaction of a 1,3-diene with a suitable three-carbon coupling partner; analogous to the venerable Diels-Alder reaction, potential exists to form multiple new stereocenters in a highly controlled fashion.[2,3]
Figure 1.
Bioactive natural products with seven-membered carbocyclic cores.
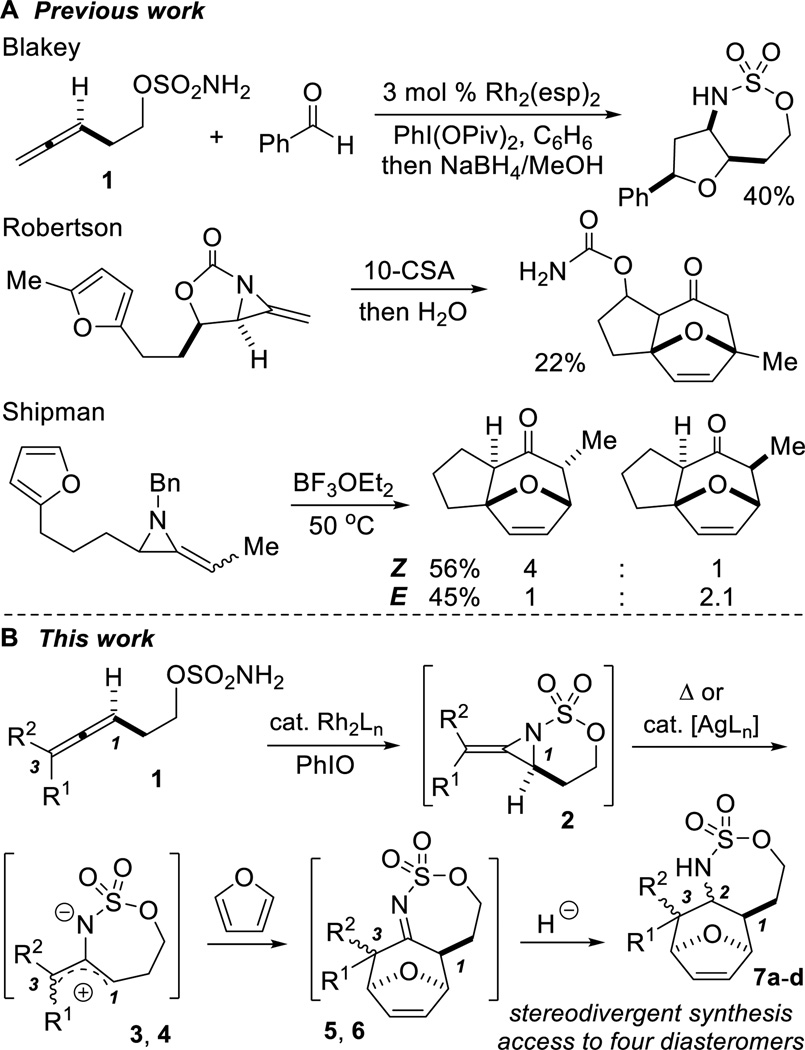
Oxyallyl cations are popular coupling partners in [4+3] cyclo-additions; however, use of their nitrogen counterparts is far less common. Reactive 2-amidoallyl cations for reported intramolecular [4+3] reactions have been generated from α-chloroenamines, α-chloroimines, allenes and methylene aziridines (Scheme 1A).[3–5] Typically, nitrogen is not retained in the product and the stereochemical outcome is dictated by the nature of the substrate. With these limitations in mind, we wanted to develop an intermolecular, stereodivergent [4+3] cycloaddition protocol with the ability to rapidly increase molecular complexity from simple allene precursors.
Scheme 1.
Stereochemical diversity via allene aziridination.
Our group has reported a suite of highly chemo-, regio- and stereoselective oxidative aminations that transform allenes into a diverse array of amine stereotriads with control over both the identity and relative stereochemistry of the three heteroatoms at C1–3.[6] We envisaged extending this concept to controlling relative stereochemistry at each of the three original allene carbons in the synthesis of aminated cycloheptenes through a tandem aziridination/[4+3]/reduction sequence (Scheme 1B). This strategy features: 1) a one-pot allene aziridination/ring-opening of the C-N bond of 2 to yield 2-amidoallyl cation 3–4, 2) intermolecular trapping with inexpensive furan, where the reaction conditions control the relative stereochemistry at C1 and C3 in 5–6 and 3) reagent-controlled reduction of the imine to yield stereodivergent syntheses of all four stereoisomers of 7, containing functionality for further elaboration into useful building blocks.[7]
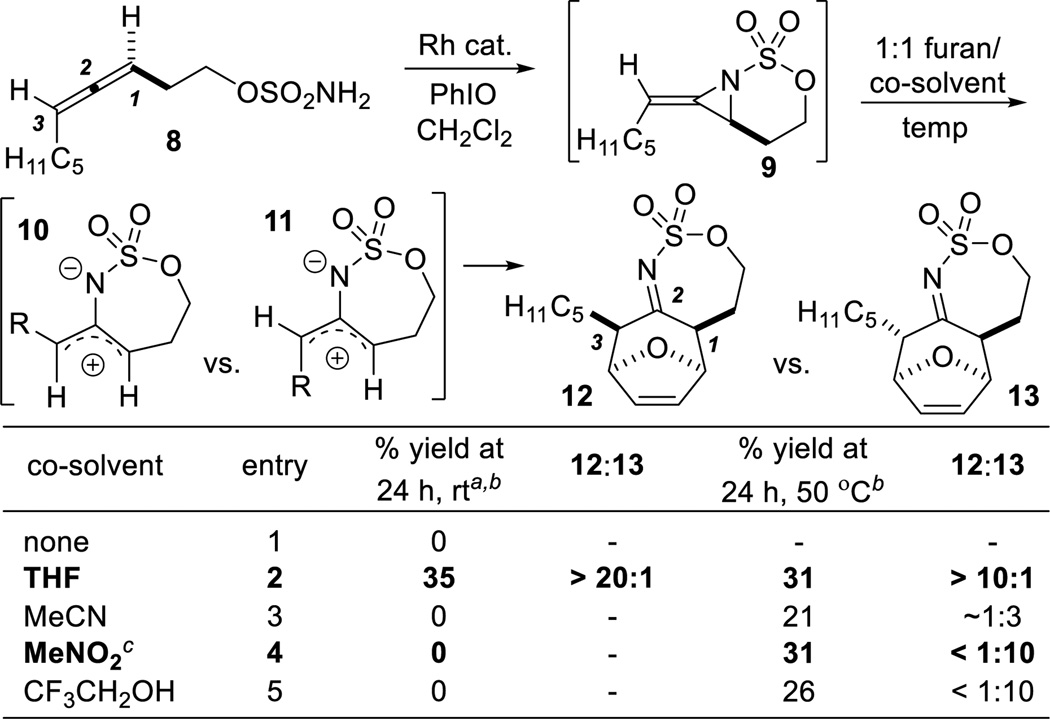
Initial efforts to convert 8 to imines 12 and 13 through the intermediacy of 10 and 11 (Table 1) showed a 1:1 THF/furan mixture successfully transformed 9 to the endo [4+3] adduct 12 at rt (entry 2). The syn C1–C3 relationship was established by X-ray crystallography (see the SI for details). Reaction in polar aprotic solvents, such as MeCN and MeNO2 (entries 3–4) did not occur at rt, but heating to 50 °C resulted in endo cyclization to furnish 13 with a 1,3-anti relationship. The yield of 13 was increased to 45% over two steps by heating in MeNO2 at 65 °C, albeit in slightly lower dr.
Table 1.
Solvent screen for the formal [4 + 3] cycloaddition.
The differing stereochemical outcomes in 12 and 13 using THF or MeNO2 were intriguing; control experiments showed neither product epimerized under the reaction conditions. Shimizu noted solvent effects on the dr of [4+3] cycloadditions of 2-oxyallyl cations; in contrast to our results, MeNO2 favored 1,3-syn products in typical dr of 2:1, while THF:Et2O favored the 1,3-anti products, also in low dr.[8] We hypothesize under our reaction conditions, 12 results from rapid addition of furan to 11, while THF permits equilibration of cation 11 to 10 to relieve A1,3 strain, resulting in the syn product 12. This could occur through rapid reversible addition of the ethereal oxygen to the allyl cation, permitting bond rotation and equilibration to 11 before addition of furan takes place.
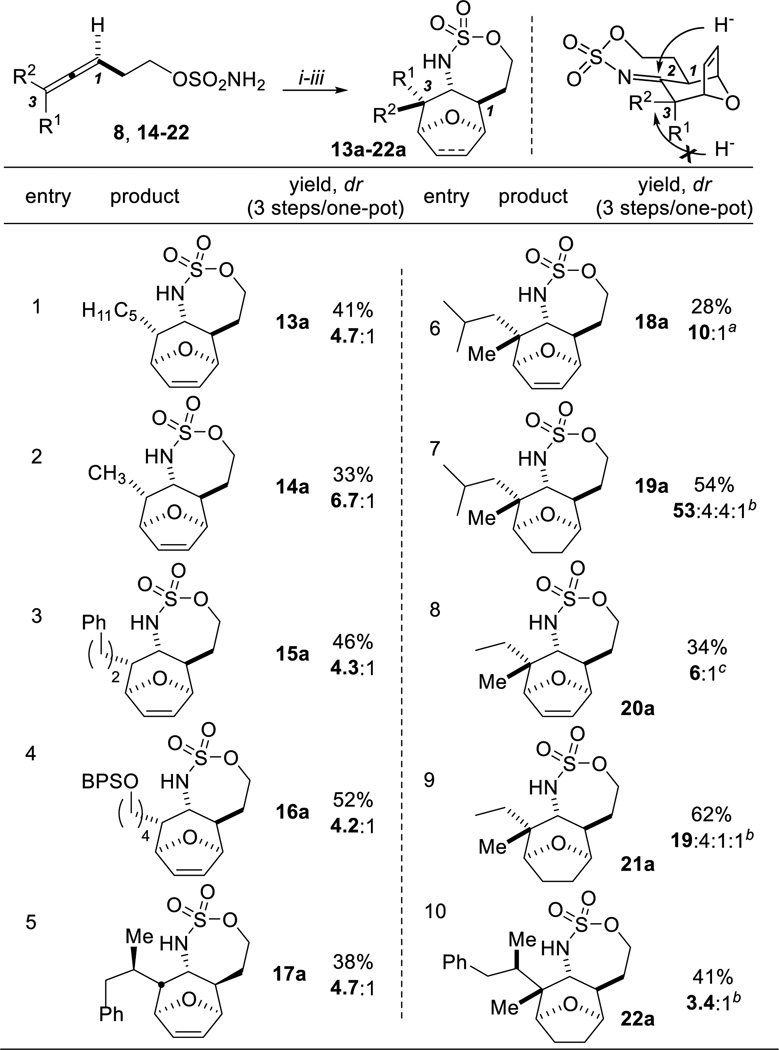
The scope of the thermal [4+3] cycloaddition in MeNO2 was explored first (Table 2). The intermediate imines were reduced using NaBH3CN, resulting in attack on the imine via a pseudo-axial trajectory. Allenes 8 and 14–16, with monosubstitution at C3 (entries 1–4), delivered 13a–16a in moderate-to-good dr. The additional stereocenter α to C3 in allene 17 (entry 5) exerted an influence on the dr of 17a, resulting in only two major diastereomers in a 4.7:1 ratio.[9,10]
Table 2.
Scope of the [4+3] reaction in MeNO2using NaBH3CN as reductant.
Gratifyingly, trisubstituted allene 18 (Table 2, entry 6) proved a good substrate for tandem aziridination/cycloaddition to set the all-carbon quaternary stereocenter. While NaBH3CN gave low conversion, LiBH4 yielded 18a in 10:1 dr. When the three-step transformation was performed in one pot, residual Rh catalyzed the reduction of the olefin with LiBH4 to furnish 19a. Ultimately, we found olefin hydrogenation (entry 7, using Pd/C and H2) prior to imine reduction gave reproducible high yields of 19a in excellent dr.[10] The chemistry could even distinguish between a Me and an Et group at C3 of allene 20 to yield 20a (entry 8) or 21a (entry 9, Pd/C and H2 to reduce the alkene) in good dr.[10] Finally, trisubstituted allene 22, containing an additional stereocenter α to C3, gave 22a (entry 12) in dr comparable to the disubstituted allene 17 (entry 5).
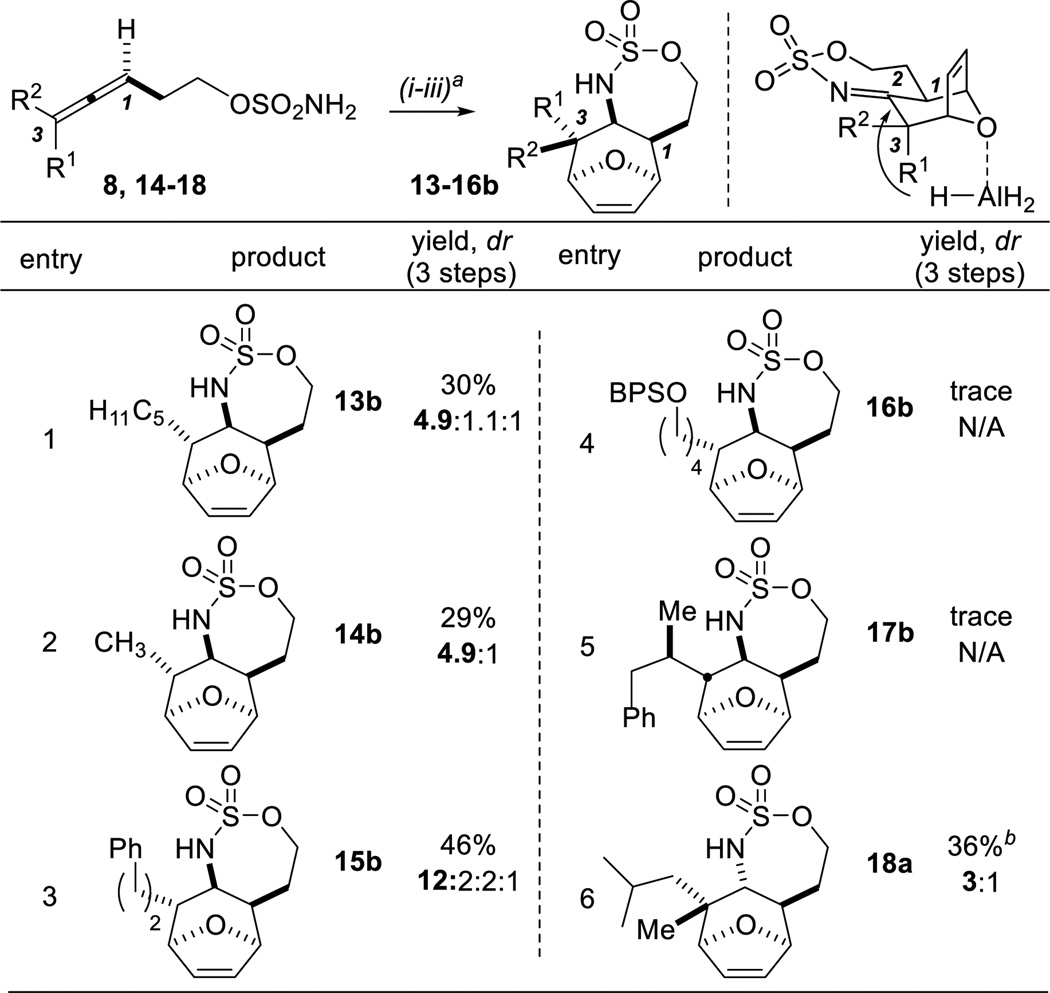
Access to the 1,2-syn:2,3-anti diastereomer 13b requires the hydride to approach the 1,3-anti imine from the same face as any substituents at C3 of the allene precursor (Table 3). DIBAL-H and triisobutylaluminum gave 13b as the minor diastereomer; however, AlH3•Me2NEt furnished the desired stereochemical outcome. Presumably, AlH3 binds to the oxygen of the [3.2.1] bicyclic ring to direct reduction to the hindered imine face, although coordination to the sulfamate O or to the imine is also possible. The challenge of overriding substrate control is reflected in lower dr and scope (Table 3, entries 1–3) compared to the 1,2-anti:2,3-syn diastereomers. Alane did not tolerate a Si-protected oxygen in 16 or branching α to C3 in 17 as 16b and 17b were observed only in trace amounts (entry 4 and 5). Axial reduction was noted for trisubstituted allenes (entry 6); despite the lower dr, the use of AlH3•Me2NEt gave more reproducible access to 18a, as compared to the use of LiBH4 (Table 2, entry 6).
Table 3.
Scope of the [4+3] in MeNO2 with AlH3•Me2NEt as the reductant.
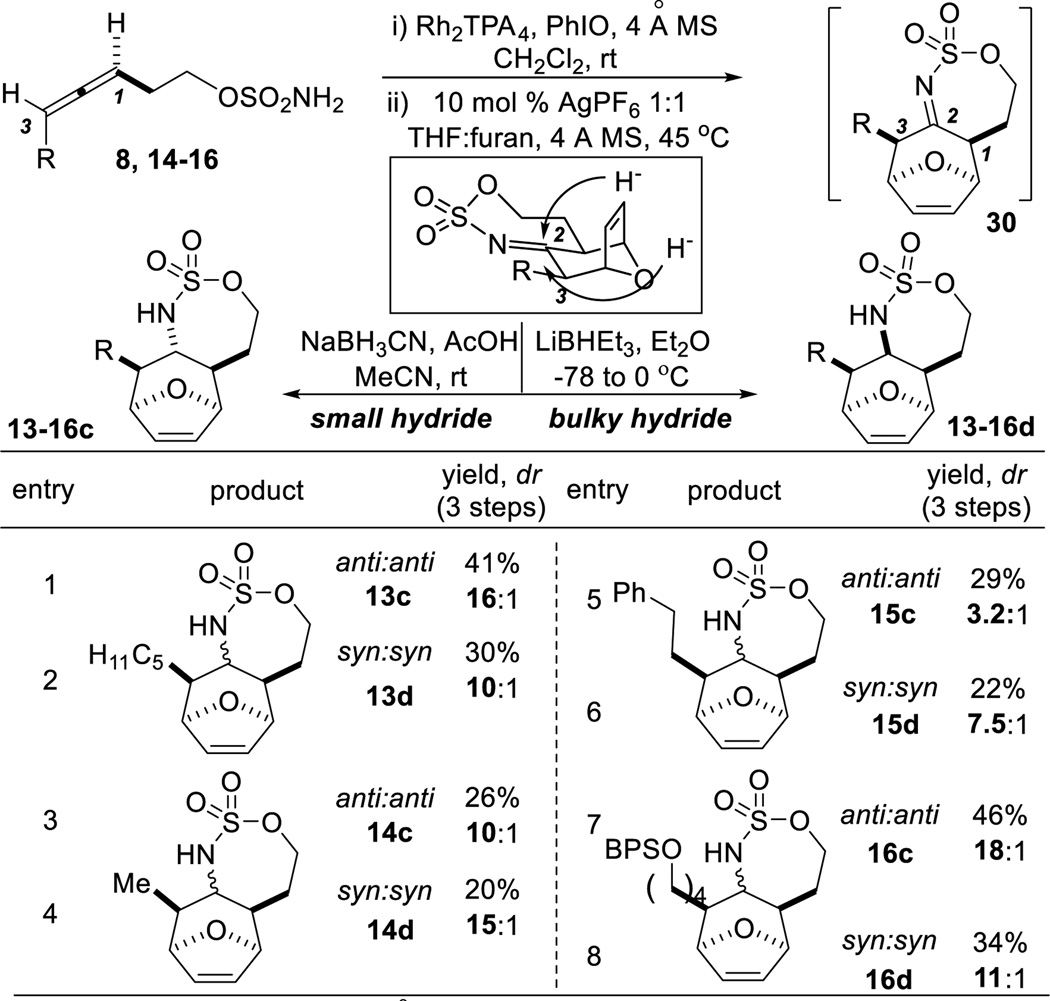
The next goal was to improve access to 1,3-syn imines of the form 12 (Table 1). Reaction of 8 with a Rh catalyst, followed by [4+3] in THF at 50 °C (entry 2) resulted in only a 31% yield of 12. A variety of Lewis acids (see the SI for details) were tested, with 10 mol % of AgPF6 providing optimal results. Adoption of these conditions furnished the 1,3-syn imine 30 (Table 4) in typical dr >10:1. To achieve tunable reduction of 30, we postulated that less bulky hydride sources (NaBH3CN) should approach from the top face of 30 to favor the 1,2-anti:2,3-anti diastereomers. In contrast, bulkier reductant such as LiBHEt3 would be expected to favor reduction from the face opposite the alkene bridge to give the all syn stereotriads. The 1,3-syn imines were much easier to reduce than the corresponding 1,3-anti imines. For example, NaBH3CN reduced syn imines to the anti:anti products 13–16c (Table 4, entries 1, 3, 5, 7) in good-to-excellent dr. Switching the reductant to LiBHEt3 delivered the syn:syn products 13–16d (entries 2, 4, 6, 8), also in good dr.
Table 4.
Scope of the [4 + 3] cycloaddition/reduction in THF.
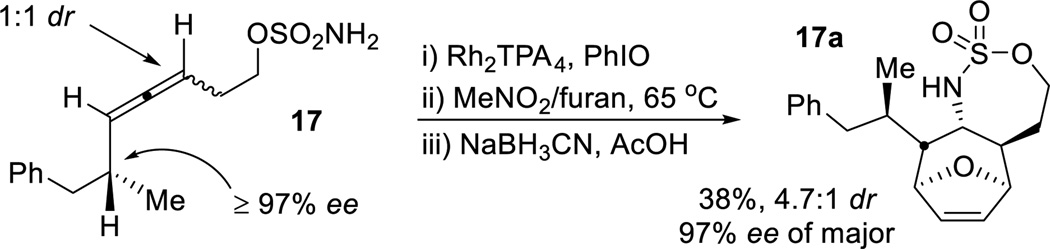
In our previous work, aziridination and subsequent functionalization of enantioenriched allenes gave excellent transfer of axial-to-point chirality.[6a,c] However, the intermediacy of an amidoallyl cation in the [4+3] precludes chirality transfer (see SI for details). Nonetheless, the ability to racemize the axial chirality of the allene during the cycloaddition process could be used to advantage to transfer chirality at the sp3 carbon of 31 to the three carbons of the initial racemic allene to yield 32 (Scheme 2) in moderate dr and excellent ee.[10,11]
Scheme 2.
Enantioenriched cycloheptenes via [4 + 3] reactions.
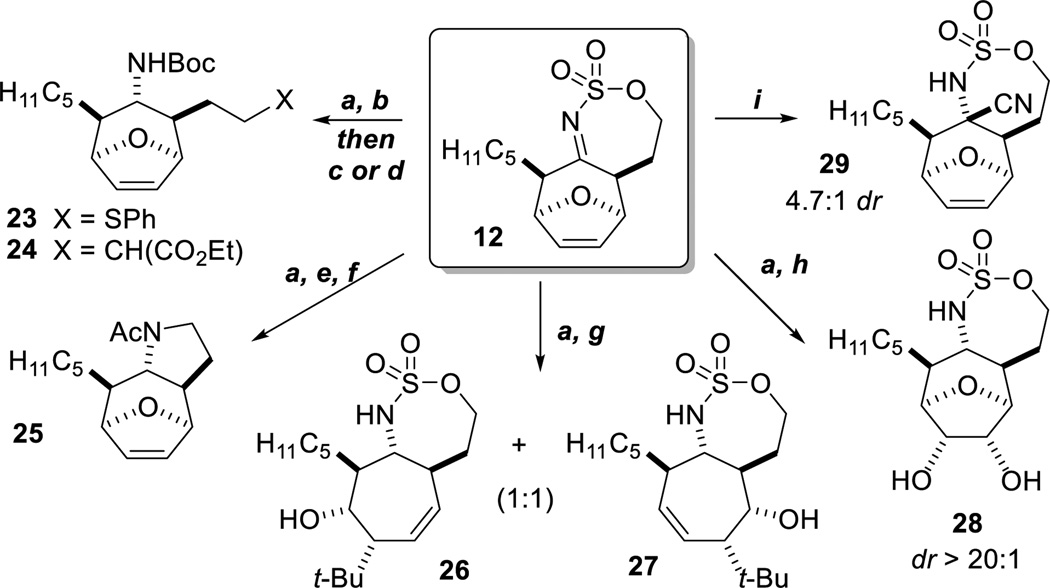
The cycloheptenes from the tandem allene aziridination/formal [4+3] are flexible scaffolds for further diversification, as 12 has four primary reactive functional handles that can be manipulated (Scheme 3). For example, reduction of 12, activation of the sulfamate and nucleophilic attack with thiophenol or diethyl malonate delivers 23 and 24 in good yields.[11,12] Ring contraction to pyrrolidine 25[13] can be achieved or the ether bridge cleaved in an SN2' fashion using t-BuLi as a nucleophile to give a 1:1 mixture of regioisomers 26 and 27.[14] Dihydroxylation of the alkene yields 28 in high dr; this sequence sets seven contiguous stereocenters in high dr over four steps from a simple allene.[10,15] Carbon nucleophiles also add to imine 12, as evidenced by a Strecker reaction to afford 29.
Scheme 3.
Synthetic utility of cycloheptene products.
a: NaBH3CN, AcOH, MeCN; 41%, 16:1 dr from 8. b: Boc2O, cat. DMAP, Et3N, CH2Cl2, rt. c: thiophenol, K2CO3, CH3CN, rt; 75% from 13. d: diethyl malonate, TBAB, Cs2CO3, MeCN, rt; 58% from 13. e: KOt-Bu, DMAP, CH2CL2, 0 °C, then Ac2O, rt; 96%. f. Nal, DMF, 60 °C, then NaH, 40 to 60 °C ; 83%. g: t-BuLi, THF, −40 °C; 61% of a 1:1 mixture of regioisomers. h: 10 mol% OsO4, NMO, acetone, H2O, t-BuOH, rt; 65%, >20:1 dr. i: n-Bu4NCN, MeCN, rt; 80% yield from 12.
In conclusion, we have described the first examples of intermolecular [4+3] reactions occurring via 2-amidoallyl cations arising from direct allene aziridination. The ability to manipulate the stereochemistry of the intermediate amidoallyl cation leads to stereodivergent syntheses of all four possible diastereomeric cycloheptenes resulting from endo cyclization. The functional group diversity of the products enables access to an array of densely functionalized synthetic building blocks in a few simple steps. While the stereoablative nature of the chemistry prevents direct transfer of axial-to-point chirality, the presence of an additional stereocenter can be employed to yield enantioenriched aminated carbocycles. Future work is focused on expanding the scope of both allenes and coupling partners, as well as applying this methodology to the syntheses of both bioactive natural products and their aminated analogs.
Supplementary Material
Acknowledgments
JMS thanks the NIH 1R01GM111412-01 and NSF-CAREER Award 1254397 for funding. The NMR facilities at UW-Madison are funded by NSF (CHE-9208463, CHE-9629688) and NIH (RR08389-01). The National Magnetic Resonance Facility at UW-Madison is supported by NIH (P41GM103399, S10RR08438, S10RR029220) and NSF (BIR-0214394). Lu Liu, Josh Taylor and Nick Dolan of UW-Madison are thanked for starting materials and assistance with NOE studies. Adrian Amador is acknowledged for his assistance with SFC analyses.
Contributor Information
Nels C. Gerstner, Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706 (United States)
Christopher S. Adams, Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706 (United States)
Dr. Maik Tretbar, Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706 (United States)
Prof. Jennifer M. Schomaker, Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706 (United States)
References
- 1.For selected reviews, see: Nguyen TV, Hartmann JM, Enders D. Synthesis. 2013;45:845. Battiste MA, Pelphrey PM, Wright DL. Chem. Eur. J. 2006;12:3438. doi: 10.1002/chem.200501083. Pellissier H. Adv. Synth. Catal. 2011;353:189. Foley DA, Maguire AR. Tetrahedron. 2010;66:1131.
- 2.For reviews dedicated to [4+3] cycloadditions, see Hartung IV, Hoffmann HMR. Angew. Chem. Int. Ed. 2004;43:1934. doi: 10.1002/anie.200300622. Harmata M. Acc. Chem. Res. 2001;34:595. doi: 10.1021/ar000064e. Harmata M. Chem. Comm. 2010;46:8886. doi: 10.1039/c0cc03620j. Hoffmann HMR. Angew. Chem. Int. Ed. 1984;23:1.
- 3.For selected reviews on heteroatom-substituted allyl cations in [4+3] cycloadditions, see: Lohse AG, Hsung RP. Chem. Eur. J. 2011;17:3812. doi: 10.1002/chem.201100260. Harmata M. Chem. Comm. 2010;46:8904. doi: 10.1039/c0cc03621h.
- 4.a) Kende AS, Huang H. Tetrahedron Lett. 1997;38:3353. [Google Scholar]; b) Schmid R, Schmid H. Helv. Chim. Acta. 1981;64:813. [Google Scholar]; c) Robertson J, Feast GC, White LV, Steadman VA, Claridge TDW. Org. Biomol. Chem. 2010;8:3060. doi: 10.1039/c003693e. [DOI] [PubMed] [Google Scholar]; d) Prié G, Prévost N, Twin H, Fernandes SA, Hayes JF, Shipman M. Angew. Chem. Int. Ed. 2004;43:6517. doi: 10.1002/anie.200461084. [DOI] [PubMed] [Google Scholar]
- 5.For recent examples of reactions believed to occur through the intermediacy of 2-amidoallyl cations, see: Stoll AH, Blakey SB. Chem. Sci. 2011;2:112. doi: 10.1039/C0SC00577K. Stoll AH, Blakey SB. J Am. Chem. Soc. 2010;132:2108. doi: 10.1021/ja908538t. Thorton AR, Martin VI, Blakey SB. J Am. Chem. Soc. 2009;131:2434. doi: 10.1021/ja809078d.
- 6.a) Adams CS, Grigg RD, Schomaker JM. Chem. Sci. 2014;5:3046. [Google Scholar]; b) Adams CS, Grigg RD, Schomaker JM. Tetrahedron. 2014;70:4128. [Google Scholar]; c) Adams CS, Boralsky LA, Guzei IA, Schomaker JM. J Am. Chem. Soc. 2012;134:10807. doi: 10.1021/ja304859w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For examples of generating 2-oxyallyl cations from allenes and their use in [4+3] cycloadditions, see: Fernández I, Mascareñas JL. Org. Biomol. Chem. 2012;10:699. doi: 10.1039/c1ob06604h. Xiong H, Hsung RP, Berry CR, Rameshkumar C. J Am. Chem. Soc. 2001;123:7174. doi: 10.1021/ja0108638. Huang J, Hsung RP. J Am. Chem. Soc. 2005;127:50. doi: 10.1021/ja044760b. Lohse AG, Hsung RP, Leider MD, Ghosh SK. J Org. Chem. 2011;76:3246. doi: 10.1021/jo200147h.
- 8.Shimizu N, Tanaka M, Tsuno Y. J Am. Chem. Soc. 1982:1330. [Google Scholar]
- 9.For examples of stereocenters adjacent to either the diene or the 2-oxyallyl cation affecting the dr of a [4+3] cycloaddition, see: Giguere RJ, Tasseley SM, Tose MI. Tetrahedron Lett. 1990;31:4577. Lautens M, Aspiotis R, Colucci J. J Am. Chem. Soc. 1996;118:10930. Chung WK, Lam SK, Lo B, Liu LL, Wong W-T, Chiu P. J Am. Chem. Soc. 2009;131:4556. doi: 10.1021/ja807566t. Cho SY, Lee JC, Cha JK. J Org. Chem. 1999;64:3394. doi: 10.1021/jo990262n. Harmata M, Rashatasakhon P. Org. Lett. 2000;2:2913. doi: 10.1021/ol006390b. Stark CBW, Eggert U, Hoffmann HMR. Angew. Chem. Int. Ed. 1998;37:1266. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1266::AID-ANIE1266>3.0.CO;2-9. Harmata M, Jones DE, Kahraman M, Sharma U, Barnes CL. Tetrahedron Lett. 1999;40:1831. Montaña AM, Grima PM. Tetrahedron Lett. 2002;43:2017.
- 10.The relative stereochemistry of product was determined by NOE studies.
- 11.Duran F, Leman L, Ghini A, Burton G, Dauban P, Dodd RH. Org. Lett. 2002;4:2481. doi: 10.1021/ol0200899. [DOI] [PubMed] [Google Scholar]
- 12.a) Bower JF, Švenda J, Williams AJ, Charmant JPH, Lawrence RM, Szeto P, Gallagher T. Org. Lett. 2004;6:4727. doi: 10.1021/ol048036+. [DOI] [PubMed] [Google Scholar]; b) Moss TA, Alonso B, Fenwick DR, Dixon DJ. Angew. Chem. Int. Ed. 2010;49:568. doi: 10.1002/anie.200905329. [DOI] [PubMed] [Google Scholar]
- 13.For examples of pyrrolidine formation, see ref. [6a] Thorton AR, Martin VI, Blakey SB. J Am. Chem. Soc. 2009;131:2434. doi: 10.1021/ja809078d.
- 14.Lautens M, Abd-El-Aziz AS, Lough A. J Org. Chem. 1990;55:5305. [Google Scholar]
- 15.He S, Hsung RP, Presser WR, Ma Z-X, Haugen BJ. Org. Lett. 2014;16:2180. doi: 10.1021/ol5006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.