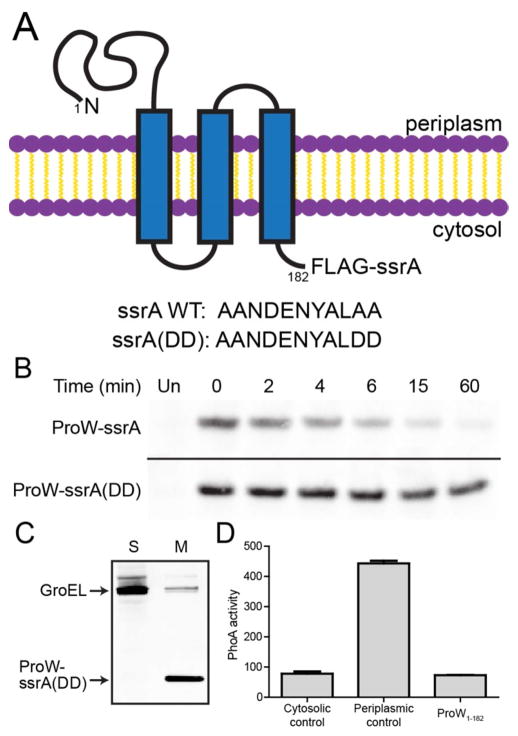
Figure 1.
An ssrA-tagged model membrane protein is degraded in E. coli. (A) Topology of ProW1-182-FLAG-ssrA (top) and amino-acid sequences of wild-type and mutant ssrA tags (bottom). (B) Expression of ProW1-182-FLAG-ssrA or ProW1-182-FLAG-ssrA(DD) in E. coli X90 was induced, pulsed with 35S-labeled methionine and cysteine, and chased with unlabeled amino acids. Samples taken at different times were immunoprecipitated and analyzed by SDS-PAGE and autoradiography. “Un” indicates an uninduced control sample. (C) E. coli cells expressing ProW1-182-FLAG-ssrA(DD) were lysed and fractionated into soluble (S) and membrane (M) fractions, which were analyzed by SDS-PAGE and immunoblotted using antibodies against GroEL (a cytosolic protein) and the FLAG tag. (D) Activity (in Miller units) of C-terminal FLAG-ssrA-PhoA fusions of a cytosolic protein (λ repressor), a periplasmic protein (cytochrome b562), and ProW1-182.