Abstract
Invasive infection due to Scedosporium prolificans is characterized by drug resistance and a high rate of mortality. The effects of posaconazole (POS), an investigational antifungal triazole, murine granulocyte-macrophage colony-stimulating factor (GM-CSF), and their combination against S. prolificans were evaluated ex vivo and in a newly developed murine model of disseminated infection due to this organism. When POS was combined with polymorphonuclear leukocytes from untreated or GM-CSF-treated mice (P < 0.01) ex vivo, it had increased activity in terms of the percentage of hyphal damage. Immunocompetent BALB/c mice were infected with 4 × 104 conidia of S. prolificans via the lateral tail vein. At 24 h postinfection the mice were treated with GM-CSF (5 μg/kg of body weight/day subcutaneously), POS (50 mg/kg/day by gavage), both agents, or saline only. Half of the brain, lung, liver, and kidney from each animal were cultured; and the other half of each organ was processed for histopathology. The mean survival times were 7.0 ± 0.3 days for the controls, 7.4 ± 0.4 days for POS-treated mice, 8.0 ± 0.3 days for GM-CSF-treated mice (P = 0.08 compared with the results for the controls), and 7.3 ± 0.3 days for POS-GM-CSF-treated mice. Fungal burdens (determined as the numbers of CFU per gram of tissue) were found in descending orders of magnitude in the kidneys, brains, livers, and lungs. The burdens were significantly reduced in the brains of GM-CSF-treated mice (P < 0.05) and the livers of POS-treated mice (P < 0.05). The numbers of lesions in the organs closely corresponded to the fungal burdens. GM-CSF tended to prolong survival (P = 0.08 compared with the results for the controls). While the combination of POS and GM-CSF showed enhanced activity ex vivo, it did not increase the activities of the two agents against this highly refractory filamentous fungus in mice.
Scedosporium prolificans is an emerging opportunistic filamentous fungus associated with localized or disseminated infections, particularly in patients with hematologic malignancies or organ transplants (19, 23, 24, 41, 43, 44, 47-49). Systemic infections due to S. prolificans are almost always fatal, whereas bone, soft tissue, and joint S. prolificans infections occur most frequently in children and young adults (10, 19, 23). Antifungal susceptibility studies of conventional and new antifungal drugs and their activities against clinical S. prolificans isolates have demonstrated that the organism has multidrug resistance, indicating an inherently therapy-refractory fungus (6, 26).
Posaconazole (POS), an investigational antifungal triazole (25), inhibits the cytochrome P-450-dependent demethylases of fungi, thus interfering with cell membrane biosynthesis (13, 27, 45). It possesses potent antifungal activity against a broad spectrum of fungal pathogens (5, 9, 11, 12, 21, 22, 31-34, 42). A recent report (16) has demonstrated that POS is active in a murine model of infection due to Scedosporium apiospermum, the second medically important species of the genus Scedosporium.
In the last decade, the combined effects of immune modulators and antifungals have been explored in animal models to determine whether treatment with these agents can be used as an alternative approach to the treatment of disseminated fungal infections (37). In murine models of systemic infections due to Candida or Aspergillus spp., granulocyte colony-stimulating factor (G-CSF) administered in combination with fluconazole or POS was found to improve survival (17, 18; F. Menzel, C. Jackson, A. Patera, J. Halpern, A. Cacciapuoti, R. Hare, and D. Loebenberg, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-858, 2002). Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an agent that enhances the antifungal capacities of polymorphonuclear leukocytes (PMNs), monocytes, and macrophages and that has broader immunoenhancing effects than G-CSF (38). Preclinical studies and a few clinical studies have yielded encouraging results when GM-CSF was used in combination with conventional antifungal agents (3, 7, 28, 37, 39, 46). In this study, a murine model of therapy-refractory invasive infection due to S. prolificans has been developed, and the combined activities of these agents were examined ex vivo and in vivo.
MATERIALS AND METHODS
Organism.
S. prolificans isolate CM906 (CBS-467.74) was originally recovered from a patient with osteomyelitis and was kindly donated by Juan Luis Rodriguez-Tudela. The isolate was resistant to both polyenes and triazoles, including POS (MICs ≥ 16 μg/ml), as determined by the National Committee for Clinical Laboratory Standards M38-P microdilution method (29). This organism was used in all experiments and was maintained as a frozen stock at −20°C. For both the ex vivo and the in vivo experiments, CM906 was grown by culturing it on potato dextrose agar (Merck, Darmstadt, Germany) at 37°C for 7 days. The inoculum was prepared by flooding the plate with sterile phosphate-buffered saline (PBS; Biochrom KG, Berlin, Germany), scraping the surface to detach the fungal cells, and filtering the suspension through sterile gauze to remove the hyphae and agar particles. The conidia were washed, counted with a hemacytometer, and suspended in PBS at 4 × 105 per ml. The number of conidia was checked by plating serial dilutions of the conidial suspension and recording the CFU counts.
Drugs.
POS was provided by the Schering-Plough Research Institute (Kenilworth, N.J.) in powder form. The drug was suspended in 0.4% methylcellulose-0.5% Tween 80-0.9% NaCl and prepared as described previously (21). Recombinant murine GM-CSF was purchased from Sigma Chemical Company (St. Louis, Mo.) as a lyophilized preparation and was dissolved in PBS containing 1% bovine serum albumin at 1 μg/ml.
Animals.
Inbred healthy female and male BALB/c mice (age, 6 to 8 weeks; weight, 20 to 25 g) were used for the studies. Three female or male mice were housed in each cage, which contained wood bedding, and had free access to water and food. All rules and regulations of the Aristotle University Committee for Animal Welfare were observed in the animal facility and for the care procedures.
Ex vivo studies.
Sixty-four animals divided into two groups were studied. The first group received 0.1-ml injections of 5 μg of GM-CSF/kg of body weight/day subcutaneously (s.c.); and the second group, the control group, received 0.1-ml injections of saline s.c. for four consecutive days. The GM-CSF dosages used were selected on the basis of previously reported data (8, 15) on the activities of murine PMNs pretreated with GM-CSF against Scedosporium sp. hyphae. On the fifth day, the animals received an intraperitoneal injection of 1 ml of 10% peptone, and 3 h later PMNs were recovered by peritoneal lavage with 3 to 4 ml of PBS. The cells were resuspended in the same buffer solution and counted on a hemacytometer. Staining with May-Grunwald Giemsa stain showed that >95% of the cells recovered in this manner were PMNs. PMNs from GM-CSF-treated mice as well as PMNs from saline-treated mice were subsequently tested for their abilities to damage hyphae of S. prolificans alone or in combination with 1 μg of POS per ml.
XTT assay.
PMN-induced hyphal damage was assessed by using the dye 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide sodium salt (XTT; Sigma) and coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone; Sigma) (14). Briefly, 200 μl of a suspension containing 7.5 × 104 conidia/ml in yeast nitrogen broth (YNB) was seeded in a 96-well flat-bottom microplate, and the conidia were allowed to grow at 32°C for 18 h. The growth medium was replaced with 200 μl of fresh YNB containing 1 μg of POS per ml, and the hyphae were subsequently incubated at 32°C for 4 h. The medium was then replaced by Hanks balanced salt solution (Gibco), and PMNs were added at a 5:1 effector cell-to-target cell (E:T) ratio. After incubation at 37°C in 5% CO2 for 2 h, the PMNs were lysed by three washes in 200 μl of H2O and vigorous shaking for 5 min per wash, before the addition of 150 μl of PBS containing 0.25 mg of XTT per ml and 40 μg of coenzyme Q0 per ml. After incubation at 37°C in 5% CO2 for 1 h, 100 μl was transferred to a new plate and the absorbance was read at a wavelength of 450 nm with reference to a wavelength of 690 nm. Antihyphal activity was calculated as percent hyphal damage, which was equal to (1 − X/C) × 100, where X is the absorbance of the test wells and C is the absorbance of the control wells, which contained only hyphae. Each set of conditions was tested in quadruplicate, and the results were averaged.
In vivo studies.
A total of 168 mice were submitted to the in vivo studies. In each experiment, at 24 h after infection, groups of six mice (three males and three females) either were not treated (controls) or were treated with single daily doses of GM-CSF, POS, or the combination. On day 10 after the initiation of treatment, the surviving mice were killed by cervical dislocation. The inoculum and the drugs were administered in 0.1-ml volumes by use of sterile 1-ml syringes with 27-gauge needles. The mice were infected via the lateral tail vein with different concentrations of conidial suspensions ranging from 2 × 104 to 500 × 104 conidia per 0.1 ml. An inoculum of 4 × 104 was chosen for subsequent studies because it resulted in 100% mortality of the control mice by day 10.
The GM-CSF dosage was chosen on the basis of the results of our ex vivo studies and previously published data (8). Furthermore, doses of GM-CSF of 5, 12.5, and 25 μg/kg/day were administered to the mice s.c. While there was a trend for the fungal burdens to be lower when 12.5 μg/kg/day was administered, these were not significantly different from the fungal burdens obtained by administration of 5 and 25 μg/kg/day. Therefore, 5 μg/kg/day was used in the subsequent experiments. In preliminary experiments 50, 100, or 250 mg of POS/kg/day was administered to the mice by gavage. Since there was no dose-dependent response in the clearance of S. prolificans from the organs of the mice and previous pharmacokinetic studies of POS (30) showed that it has good oral bioavailability over a concentration range of 40 to 80 mg/kg, the concentration of 50 mg/kg/day was chosen for use in the survival and tissue burden studies.
Organ fungal cultures.
Mice were dissected under aseptic conditions; and the livers, kidneys, lungs, and brains were removed. Half of each organ was weighed and homogenized in 2 ml of saline solution. After dilution of each homogenized tissue 1:10 and 1:100, samples of 0.1 ml were plated on potato dextrose agar plates containing 10 μg of chloramphenicol per ml and 25 μg of gentamicin per ml. The plates were incubated at 37°C for 3 to 4 days, the numbers of CFU were counted, and the values obtained were converted to log CFU per gram per organ for statistical evaluation.
Histopathology.
The other halves of the livers, kidneys, lungs, and brains were fixed in 10% neutral formalin solution, embedded in paraffin, and stained with hematoxylin-eosin and Gomori's methenamine-silver nitrate stains. Tissue sections through the entire hemiorgan were prepared and microscopically inspected by a blinded investigator (S.L., an animal pathologist) for the presence of inflammatory foci and the extent of organ damage caused by S. prolificans. Scores of 0 to 3 were assigned as follows: 0, no focal lesions in the total section; 1, one to two focal lesions per section; 2, three to five focal lesions per section; and 3, more than five focal lesions per section.
Statistical evaluation.
Statistical assessments were performed with InStat (version 3.0) software (Graphpad Inc., San Diego, Calif.). The ex vivo data were evaluated by analysis of variance (ANOVA) with the Tukey test for multiple comparisons. The Kruskal-Wallis test with Dunn's posttest was used for the fungal burden and histopathological studies. Prior to statistical analysis, all values of fungal burdens were transformed to their corresponding natural logarithms and expressed as the means ± standard errors of the mean. The log rank test was used to analyze the survival data. A two-sided P value of ≤0.05 was taken to denote statistical significance.
RESULTS
Ex vivo studies.
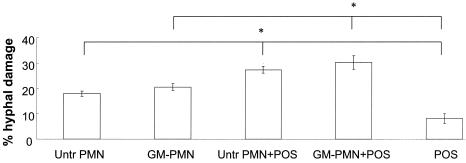
Eight experiments with eight mice in each experiment and two treatments were conducted. PMNs isolated from saline-treated (control) or GM-CSF-treated mice were used to evaluate their antifungal activities alone or in combination with POS against S. prolificans hyphae. Incubation of the hyphae with the combination of POS and PMNs from saline-treated mice induced damage significantly greater than that induced by each component alone (P < 0.01). Furthermore, the incubation of hyphae with the combination of POS plus PMNs from GM-CSF-treated mice showed enhanced activity against S. prolificans hyphae (P < 0.01). Specifically, POS alone induced 8.2% ± 2.0% hyphal damage, PMNs from saline-treated mice alone induced 18.0% ± 1.0% hyphal damage, PMNs from GM-CSF-treated mice induced 20.6% ± 1.3% hyphal damage, the combination of POS plus PMNs from saline-treated mice induced 27.4% ± 1.3% hyphal damage, and the combination of POS plus PMNs from GM-CSF-treated mice induced 30.3% ± 2.6% hyphal damage (Fig. 1). There was no significant difference between the percent hyphal damage induced by PMNs isolated from mice treated with four daily s.c. doses of 5 μg of GM-CSF per kg and that induced by PMNs from control mice treated with saline (Fig. 1).
FIG. 1.
Percent hyphal damage induced by PMNs from saline-treated mice (Untr PMN) or from mice treated with GM-CSF (GM-PMN) alone, POS alone, or the combination at an effector cell-to-target cell ratio of 5:1. Bars indicate means ± standard errors of the mean of eight experiments. The asterisks indicate statistical differences (P < 0.01) found by ANOVA with the Tukey posttest for multiple comparisons between the combination of PMNs from saline-treated mice and mice treated with POS and PMNs from either saline-treated mice or mice treated with POS alone and between the combination of PMNs from mice treated with GM-PMN and POS and PMNs from mice treated with either GM-PMN or POS alone.
In vivo studies. (i) Organ fungal burdens.
Seven in vivo experiments were conducted. A murine model of disseminated infection due to S. prolificans was developed with an inoculum of 4 × 104 conidia/mouse. The infection was characterized by changes in fur consistency and weight loss and the onset of lethargy and neurological symptoms, such as leaning to one side, jumping, and the complete loss of balance. The most infected organ was the kidney, with 3.77 ± 0.19 log CFU/g of organ, followed by the brain, with 3.41 ± 0.10 log CFU/g, and the liver, with 1.99 ± 0.10 log CFU/g. Even though no lung infections were apparent with the inoculum used in this study, conidial suspensions of 7 × 104 to 8 × 104 did cause lung infections in saline-treated mice. However, the lungs had the lowest fungal burden among all organs tested (data not shown).
Mice treated with 50 mg of POS/kg/day demonstrated significantly reduced CFU counts in the livers (P < 0.05). GM-CSF treatment produced no significant reductions in the liver and kidney fungal burdens but resulted in decreased brain fungal burdens compared to those in the controls (P < 0.05) (Table 1).
TABLE 1.
Fungal burdens in the brains, livers, and kidneys of mice treated with saline (control), POS, GM-CSF, or the combination of POS and GM-CSF
| Organ | Fungal burden (log CFU/g) in mice treated witha:
|
|||
|---|---|---|---|---|
| Saline | POS | GM-CSF | POS-GM-CSF | |
| Brain | 3.41 ± 0.10 | 3.25 ± 0.11 | 2.97 ± 0.18b | 3.23 ± 0.14 |
| Liver | 1.99 ± 0.10 | 1.70 ± 0.09b | 2.05 ± 0.14 | 1.78 ± 0.07 |
| Kidneys | 3.77 ± 0.19 | 3.08 ± 0.25 | 4.08 ± 0.19 | 3.53 ± 0.19 |
All values are expressed as the means ± standard errors of the means for each organ. The Kruskal-Wallis nonparametric ANOVA with Dunn's posttest was used to evaluate the differences from saline-treated (control) mice.
P < 0.05.
(ii) Histopathology.
Microscopic examination of tissue sections showed various numbers of pyogranulomatous inflammatory foci, depending on the treatment received and the organ examined. The lesions were characterized by focal necrosis of the organ tissue and infiltration by PMNs, monocytes (or neuroglial cells in the brain), and a small number of lymphocytes. S. prolificans hyphae were apparent within the inflammatory foci.
The mean lesion scores for control and treated mice are shown in Table 2. While fewer focal lesions were observed in the livers than in the brains and the kidneys, there were no significant differences in the focal lesion scores assigned to any organ by histopathology between the different treatment groups and the control mice.
TABLE 2.
Histopathological scores assigned to the focal lesions observed in the brains, livers, and kidneys of mice treated with saline (control), POS, GM-CSF, or the combination of POS and GM-CSF
| Organ | Focal lesion score for mice treated witha:
|
|||
|---|---|---|---|---|
| Saline | POS | GM-CSF | POS-GM-CSF | |
| Brain | 1.50 ± 0.22 | 1.48 ± 0.22 | 0.84 ± 0.16 | 1.59 ± 0.28 |
| Liver | 0.11 ± 0.06 | 0.21 ± 0.08 | 0.30 ± 0.09 | 0.08 ± 0.05 |
| Kidneys | 1.43 ± 0.20 | 1.56 ± 0.21 | 1.32 ± 0.23 | 1.15 ± 0.22 |
All values are expressed as the means ± standard errors of means of the focal lesion scores for each organ. Lesion scores were calculated as described in Materials and Methods. Data were analyzed by the Kruskal-Wallis ANOVA with Dunn's posttest for multiple comparisons. No significant differences were seen among the treatment groups.
(iii) Survival.
The mean survival times for the four groups of mice are shown in Table 3. The inoculum size used in this study caused 100% mortality of control mice by day 10. Compared to the 0% rate of survival among the 36 saline-treated animals, the rates of survival were 22% among the 32 POS-treated mice, 30% among the 33 GM-CSF-treated mice (P = 0.08 compared to the results for the controls), and 15% among the 34 animals treated with the combination of POS and GM-CSF. While GM-CSF-treated mice tended to survive longer, treatment with POS alone or in combination with GM-CSF did not significantly prolong the survival of the mice.
TABLE 3.
Survival of S. prolificans-infected mice treated with saline (control), POS, GM-CSF, or the combination of POS and GM-CSF
| Treatment | No. of dead mice/no. of infected mice | Mean survival time (days)a | P |
|---|---|---|---|
| Saline (control) | 36/36 | 7.00 ± 0.35 | |
| POS | 25/32 | 7.34 ± 0.35 | NSb |
| GM-CSF | 23/33 | 8.00 ± 0.32 | 0.08 |
| POS-GM-CSF | 29/34 | 7.32 ± 0.33 | NS |
All values are expressed as the means ± standard errors of the means. Differences in mean survival times between POS-, GM-CSF-, or POS-GM-CSF-treated and saline-treated (control) mice were evaluated by the log rank test.
NS, no significant difference from the results for saline-treated mice.
DISCUSSION
This study is the first to examine the activities of POS in combination with GM-CSF, a cytokine with relatively broad activity in enhancing the antifungal capacities of phagocytes (38), against S. prolificans, a therapy-refractory filamentous fungus. While the two agents combined showed enhanced activity against S. prolificans hyphae ex vivo, they did not show parallel enhanced efficacy in a newly established immunocompetent murine model of disseminated infection due to this organism.
Clinical isolates of S. prolificans are almost universally resistant to amphotericin B, fluconazole, and ketoconazole and are often resistant to itraconazole and miconazole (2, 4, 20, 35, 40). The activity of POS has been shown to be equivalent or superior to those of amphotericin B, fluconazole, voriconazole, and itraconazole against both common and new emerging pathogens (5, 9, 31, 32, 34). The particular isolate used for this study was resistant to both polyenes and triazoles. Ex vivo, 1 μg of POS per ml was found to exert a low level of activity in damaging S. prolificans hyphae. However, it exerted significantly enhanced activity when it was combined with PMNs derived from either saline-treated or GM-CSF-treated mice. This result is in agreement with the findings of previous in vitro studies with human PMNs (14), and its mechanism needs further elucidation. The antihyphal effect of this concentration of POS, as determined by the XTT viability assay, cannot be compared to the MICs obtained by the NCCLS assay, since the methods are totally different.
We also sought to enhance the activity of POS in combination with GM-CSF in a murine model of disseminated scedosporiosis achieved after healthy mice were infected with the conidia of S. prolificans. We hypothesized that GM-CSF may enhance the destructive effect of PMN antifungal metabolites on the surface of the fungus in such a way that POS can more efficiently act on its ergosterol synthesis target(s). POS administered 24 h after infection at a concentration of 50 mg/kg/day appears to be selectively effective in reducing fungal burdens in the liver but not in the kidneys or the brain. In contrast to the ex vivo result of enhanced activity, no significant differences in the rates of survival among mice infected with S. prolificans were observed between those treated with the combination and those treated with either POS or GM-CSF alone. This discordance may be related to the animal model used as well as to pharmacodynamic and pharmacokinetic factors related to the two agents in mice. Even though the therapeutic regimen used in this study was chosen on the basis of pharmacokinetic studies of POS in mice (1, 16, 17, 21, 30, 31), the resilient nature of the isolate that we used resulted in deep infections that progressively damaged the tissues studied.
By comparison, GM-CSF significantly decreased the fungal burdens in the brains of mice and also tended to prolong the survival of the infected animals. However, it did not affect the fungal burdens in other organs. While brain damage appeared to determine the outcome in this model, the concentrations of either of the two agents in the brain that may have influenced the superior effect of GM-CSF are unknown. The concentrations in organs or other subtle differences may be responsible for this difference.
On the other hand, histopathologic sections of the organs showed various numbers of pyogranulomatous inflammatory foci, depending on the treatment received and the organ examined. Although pneumonia is a frequent finding of disseminated infection due to S. prolificans in immunocompromised patients (2, 36), lung involvement is achieved only after the administration of higher inocula in this model of immunocompetent mice. The kidneys and the brains were the most affected organs. Nevertheless, there were no significant differences in the number of focal lesions between treated and control mice.
In conclusion, this study demonstrated that POS and GM-CSF have a combined effect in damaging S. prolificans hyphae ex vivo. When POS and GM-CSF are administered to mice with invasive infection due to S. prolificans, they have selective beneficial effects on the burdens in certain organs but offer no additional benefit to survival. These studies need to be extended further by adopting modified therapeutic strategies for invasive infections due to S. prolificans in immunocompetent and immunocompromised mice.
Acknowledgments
This study was supported by Schering-Plough Research Institute.
REFERENCES
- 1.Al-Abdely, H. M., L. Najvar, R. Bocanegra, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and J. R. Graybill. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, J. V. Martinez-Suarez, et al. 1997. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Medicine (Baltimore) 76:256-265. [DOI] [PubMed] [Google Scholar]
- 3.Bodey, G. P., E. Anaissie, J. Gutterman, and S. Vadhan-Raj. 1994. Role of granulocyte-macrophage colony-stimulating factor as adjuvant treatment in neutropenic patients with bacterial and fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 13(Suppl. 2):S18-S22. [DOI] [PubMed] [Google Scholar]
- 4.Bouza, E., P. Munoz, L. Vega, M. Rodriguez-Creixems, J. Berenguer, and A. Escudero. 1996. Clinical resolution of Scedosporium prolificans fungemia associated with reversal of neutropenia following administration of granulocyte colony-stimulating factor. Clin. Infect. Dis. 23:192-193. [DOI] [PubMed] [Google Scholar]
- 5.Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, Jr., E. L. Moss, Jr., C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall, A., and L. A. Pirofski. 2001. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 33:1048-1056. [DOI] [PubMed] [Google Scholar]
- 8.Deepe, G. S., and R. Gibbons. 2000. Recombinant murine granulocyte-macrophage colony-stimulating factor modulates the course of pulmonary histoplasmosis in immunocompetent and immunodeficient mice. Antimicrob. Agents Chemother. 44:3328-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis, M. 2002. Invasive fungal infections: evolving challenges for diagnosis and therapeutics. Mol. Immunol. 38:947-957. [DOI] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galgiani, J. N., and M. L. Lewis. 1997. In vitro studies of activities of the antifungal triazoles SCH56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob. Agents Chemother. 41:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil-Lamaignere, C., E. Roilides, J. Mosquera, A. Maloukou, and T. J. Walsh. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil-Lamaignere, C., R. Vitale, J. Curfs, P. Verweij, and E. Roilides. 2001. In vivo treatment of mice with interferon-gamma and granulocyte-monocyte colony-stimulating factor enhances hyphal damage of Scedosporium spp. by murine neutrophils. Clin. Microbiol. Infect. 7:125.11318810 [Google Scholar]
- 16.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. G. Rinaldi, D. Loebenberg, and J. R. Graybill. 2003. Activity of posaconazole against Pseudallescheria boydii: in vitro and in vivo assays. Antimicrob. Agents Chemother. 47:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graybill, J. R., R. Bocanegra, L. K. Najvar, D. Loebenberg, and M. F. Luther. 1998. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression. Antimicrob. Agents Chemother. 42:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graybill, J. R., R. Bocanerga, and M. Luther. 1995. Antifungal combination with G-CSF and fluconazole in experimental disseminated candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 14:700-703. [DOI] [PubMed] [Google Scholar]
- 19.Groll, A. H., and T. J. Walsh. 2001. Uncommon opportunistic fungi: new nosocomial threats. Clin. Microbiol. Infect. 7(Suppl. 2):8-24. [DOI] [PubMed] [Google Scholar]
- 20.Hopwood, V., E. G. Evans, J. Matthews, and D. W. Denning. 1995. Scedosporium prolificans, a multi-resistant fungus, from a U.K. AIDS patient. J. Infect. 30:153-155. [DOI] [PubMed] [Google Scholar]
- 21.Lozano-Chiu, M., S. Arikan, V. L. Paetznick, E. J. Anaissie, D. Loebenberg, and J. H. Rex. 1999. Treatment of murine fusariosis with SCH 56592. Antimicrob. Agents Chemother. 43:589-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz, J. E., K. V. Clemons, B. H. Aristizabal, and D. A. Stevens. 1997. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob. Agents Chemother. 41:1558-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maertens, J., K. Lagrou, H. Deweerdt, I. Surmont, G. E. Verhoef, J. Verhaegen, and M. A. Boogaerts. 2000. Disseminated infection by Scedosporium prolificans: an emerging fatality among haematology patients. Case report and review. Ann. Hematol. 79:340-344. [DOI] [PubMed] [Google Scholar]
- 24.Marin, J., M. A. Sanz, G. F. Sanz, J. Guarro, M. L. Martinez, M. Prieto, E. Gueho, and J. L. Menezo. 1991. Disseminated Scedosporium inflatum infection in a patient with acute myeloblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 10:759-761. [DOI] [PubMed] [Google Scholar]
- 25.Masia Canuto, M., and F. Gutierrez Rodero. 2002. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2:550-563. [DOI] [PubMed] [Google Scholar]
- 26.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellado, E., T. M. Diaz-Guerra, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2001. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 39:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan, U., N. Randhawa, E. Brummer, and D. A. Stevens. 1998. Effect of granulocyte-macrophage colony-stimulating factor on candidacidal activity of neutrophils, monocytes or monocyte-derived macrophages and synergy with fluconazole. J. Med. Microbiol. 47:359-363. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Nomeir, A. A., P. Kumari, M. J. Hilbert, S. Gupta, D. Loebenberg, A. Cacciapuoti, R. Hare, G. H. Miller, C. C. Lin, and M. N. Cayen. 2000. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakley, K. L., G. Morrissey, and D. W. Denning. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, S. Piscitelli, M. Candelario, A. Field-Ridley, N. Avila, J. Bacher, and T. J. Walsh. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabodonirina, M., S. Paulus, F. Thevenet, R. Loire, E. Gueho, O. Bastien, J. F. Mornex, M. Celard, and M. A. Piens. 1994. Disseminated Scedosporium prolificans (S. inflatum) infection after single-lung transplantation. Clin. Infect. Dis. 19:138-142. [DOI] [PubMed] [Google Scholar]
- 36.Revankar, S. G., J. E. Patterson, D. A. Sutton, R. Pullen, and M. G. Rinaldi. 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 34:467-476. [DOI] [PubMed] [Google Scholar]
- 37.Roilides, E., C. G. Lamaignere, and E. Farmaki. 2002. Cytokines in immunodeficient patients with invasive fungal infections: an emerging therapy. Int. J. Infect. Dis. 6:154-163. [DOI] [PubMed] [Google Scholar]
- 38.Roilides, E., C. A. Lyman, P. Panagopoulou, and S. Chanock. 2003. Immunomodulation of invasive fungal infections. Infect. Dis. Clin. N. Am. 17:193-219. [DOI] [PubMed] [Google Scholar]
- 39.Root, R. K., and D. C. Dale. 1999. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients. J. Infect. Dis. 179(Suppl. 2):S342-S352. [DOI] [PubMed] [Google Scholar]
- 40.Salkin, I. F., M. R. McGinnis, M. J. Dykstra, and M. G. Rinaldi. 1988. Scedosporium inflatum, an emerging pathogen. J. Clin. Microbiol. 26:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segal, B. H., E. J. Bow, and F. Menichetti. 2002. Fungal infections in nontransplant patients with hematologic malignancies. Infect. Dis. Clin. N. Am. 16:935-964, vii. [DOI] [PubMed] [Google Scholar]
- 42.Sugar, A. M., and X. P. Liu. 1996. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob. Agents Chemother. 40:1314-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadros, T. S., K. A. Workowski, R. J. Siegel, S. Hunter, and D. A. Schwartz. 1998. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum. Pathol. 29:1266-1272. [DOI] [PubMed] [Google Scholar]
- 44.Tapia, M., C. Richard, J. Baro, R. Salesa, J. Figols, F. Zurbano, and A. Zubizarreta. 1994. Scedosporium inflatum infection in immunocompromised haematological patients. Br. J. Haematol. 87:212-214. [DOI] [PubMed] [Google Scholar]
- 45.Vanden Bossche, H., and L. Koymans. 1998. Cytochromes P450 in fungi. Mycoses 41(Suppl. 1):32-38. [DOI] [PubMed] [Google Scholar]
- 46.Vora, S., N. Purimetla, E. Brummer, and D. A. Stevens. 1998. Activity of voriconazole, a new triazole, combined with neutrophils or monocytes against Candida albicans: effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 42:907-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westerman, D. A., B. R. Speed, and H. M. Prince. 1999. Fatal disseminated infection by Scedosporium prolificans during induction therapy for acute leukemia: a case report and literature review. Pathology 31:393-394. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, C. M., E. J. O'Rourke, M. R. McGinnis, and I. F. Salkin. 1990. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J. Infect. Dis. 161:102-107. [DOI] [PubMed] [Google Scholar]
- 49.Wood, G. M., J. G. McCormack, D. B. Muir, D. H. Ellis, M. F. Ridley, R. Pritchard, and M. Harrison. 1992. Clinical features of human infection with Scedosporium inflatum. Clin. Infect. Dis. 14:1027-1033. [DOI] [PubMed] [Google Scholar]