Abstract
Rationale
Junctional membrane complexes (JMC) in myocytes are critical microdomains, in which excitation-contraction coupling occurs. Structural and functional disruption of JMCs underlies contractile dysfunction in failing hearts. However, the role of newly identified JMC protein ‘striated muscle preferentially expressed gene’ (SPEG) remains unclear.
Objective
To determine the role of SPEG in healthy and failing adult hearts.
Methods and Results
Proteomic analysis of immunoprecipatated JMC-proteins ryanodine receptor type-2 (RyR2) and junctophilin-2 (JPH2) followed by mass spectrometry identified the serine-threonine kinase SPEG as the only novel binding partner for both proteins. Real-time PCR revealed downregulation of SPEG mRNA levels in failing human hearts. A novel cardiac myocyte-specific Speg conditional knockout (MCM-Spegfl/fl) model revealed that adult-onset SPEG-deficiency results in heart failure. Calcium (Ca2+) and transverse-tubule (TT) imaging of ventricular myocytes from MCM-Spegfl/fl mice post heart failure revealed both increased SR Ca2+ spark frequency and disrupted JMC integrity. Additional studies revealed that TT disruption precedes the development of heart failure development in MCM-Spegfl/fl mice. Although total JPH2 levels were unaltered, JPH2 phosphorylation levels were found to be reduced in MCM-Spegfl/fl mice, suggesting that loss of SPEG phosphorylation of JPH2 led to TT disruption, a precursor of heart failure development in SPEG deficient mice.
Conclusion
The novel JMC protein SPEG is downregulated in human failing hearts. Acute loss of SPEG in mouse hearts causes JPH2 dephosphorylation and TT loss associated with downstream Ca2+ mishandling leading to heart failure. Our study suggests that SPEG could be a novel target for the treatment of heart failure.
Keywords: Calcium signaling, heart failure, junctional membrane complex, SPEG, ryanodine receptor
INTRODUCTION
Heart failure (HF) is a deadly and costly disease affecting 5.7 million people in the United States alone.1. Systolic cardiac contraction and diastolic relaxation depend on proper calcium (Ca2+) signaling since cardiac myocytes translate electrical signals to mechanical contraction via Ca2+ signaling, a process known as excitation-contraction coupling (ECC). ECC occurs within specialized subcellular structures called junctional membrane complexes (JMCs), in which voltage-gated L-type Ca2+ channels on the invaginations of the plasmalemma known as transverse tubules (TTs), are positioned near the major cardiac Ca2+ release channels ryanodine receptors (RyR2) on the SR.2 Disruptions in the JMC can lead to hyperactivation of RyR2s causing abnormal Ca2+ release from the SR, which has been implicated in the development of HF.3 We recently demonstrated that junctophilin-2 (JPH2) is a protein essential for the formation and maintenance of JMC structures and TTs within cardiac myocytes.4, 5
In failing hearts, reduced JPH2 protein levels have been associated with a loss of JMCs due to loss of TT number and regularity, resulting in impaired ECC.4 Reduced JPH2 levels can also enhance RyR2 channel activity, since JPH2 binding to RyR2 stabilizes its closed state.6 Numerous studies have shown that altered posttranslational modulation of RyR2 also plays an important role in HF, including enhanced phosphorylation and oxidation of the pore-forming channel subunits.7, 8 This increase in RyR2 activity has been associated with diastolic SR Ca2+ leak, which may contribute to SR Ca2+ depletion and impaired contractility. However, the mechanisms underlying altered RyR2 regulation and JMC deterioration in HF remain incompletely understood.
Here, we used unbiased proteomic analysis of immunoprecipitated RyR2 and JPH2 from adult mouse hearts to identify novel proteins within the JMC. A protein known as ‘striated muscle preferentially expressed protein kinase’ (SPEG)9 was identified as the only protein that binds to both RyR2 and JPH2 in the mouse heart. SPEG - a myosin light chain kinase family protein - contains dual serine/threonine kinase domains unique to the obscurin (OBSCN) subfamily, as well as multiple immunoglobulin (Ig)-like, fibronectin type III (FNIII) domains, and proline rich regions typically involved in protein-protein interactions.9 Speg is a single gene, which is alternatively spliced into several tissue-specific isoforms including APEG (aorta), BPEG (brain), SPEGα and β (skeletal and cardiac muscle). It has been previously demonstrated that Speg is important for cardiac development, as a germline knockout of all Speg isoforms resulted in embryonic dilated cardiomyopathy and early postnatal death.10 Additionally, rare Speg mutations have been implicated in centronuclear myopathy, a genetic disease of skeletal and cardiac muscle.11 Importantly, however, nothing is known to date about the potential role of Speg in the adult heart, or the mechanisms by which altered Speg levels modulate cardiac dysfunction.
Based on SPEG’s interaction with key proteins within the JMC and its importance in cardiac development, we hypothesized that cardiac isoforms of SPEG are necessary for normal adult cardiac function and excitation-contraction coupling. Therefore, we tested the role of SPEG in adult hearts and non-genetic forms of HF using a novel inducible cardiac myocyte-restricted Speg knockout mouse. Our data revealed reduced SPEG levels in patients with HF, and development of HF in inducible cardiac-specific Speg knockout mice. These data demonstrate for the first time that SPEG plays a critical role in the maintenance of JMC integrity and SR Ca2+ handling in adult mammalian hearts, and suggest that loss of Speg contributes to the development of excitation-contraction deficits in heart failure. In addition, our data revealed that loss of SPEG causes disruption of the T-tubule network prior to HF development, despite unaltered JPH2 levels. Loss of SPEG-mediated JPH2 phosphorylation was found to be associated with T-tubule disarray, which suggests that defective post-translational modulation of JPH2 by SPEG represents a key early event in the pathogenesis of HF.
METHODS
Study animals
All mice studies were performed according to protocols approved by the Baylor College of Medicine Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. For the proteomics experiments, whole ventricles were harvested from wild-type (WT) C57BL/6J mice and HA-tagged JPH2 overexpressing mice6 between the ages of 3–6 months and immediately flash-frozen in liquid nitrogen. Speg chimeric mice were obtained from the Knockout mouse project at UC Davis (Davis, CA). These mice were crossed to Flippase mice (The Jackson Laboratory, Bar Harbor, ME) in order to develop a conditional Speg allele. Conditional Speg mice were crossed to Myh6 driven mER-Cre-mER (MCM) mice (kindly provided by Dr. Jeffrey Molkentin) in order to create cardiac myocyte-specific tamoxifen-inducible Speg knockout mice. Male MCM-Spegfl/fl and MCM controls injected with tamoxifen for 5 days were used for the studies described in our paper.
Mass spectrometry
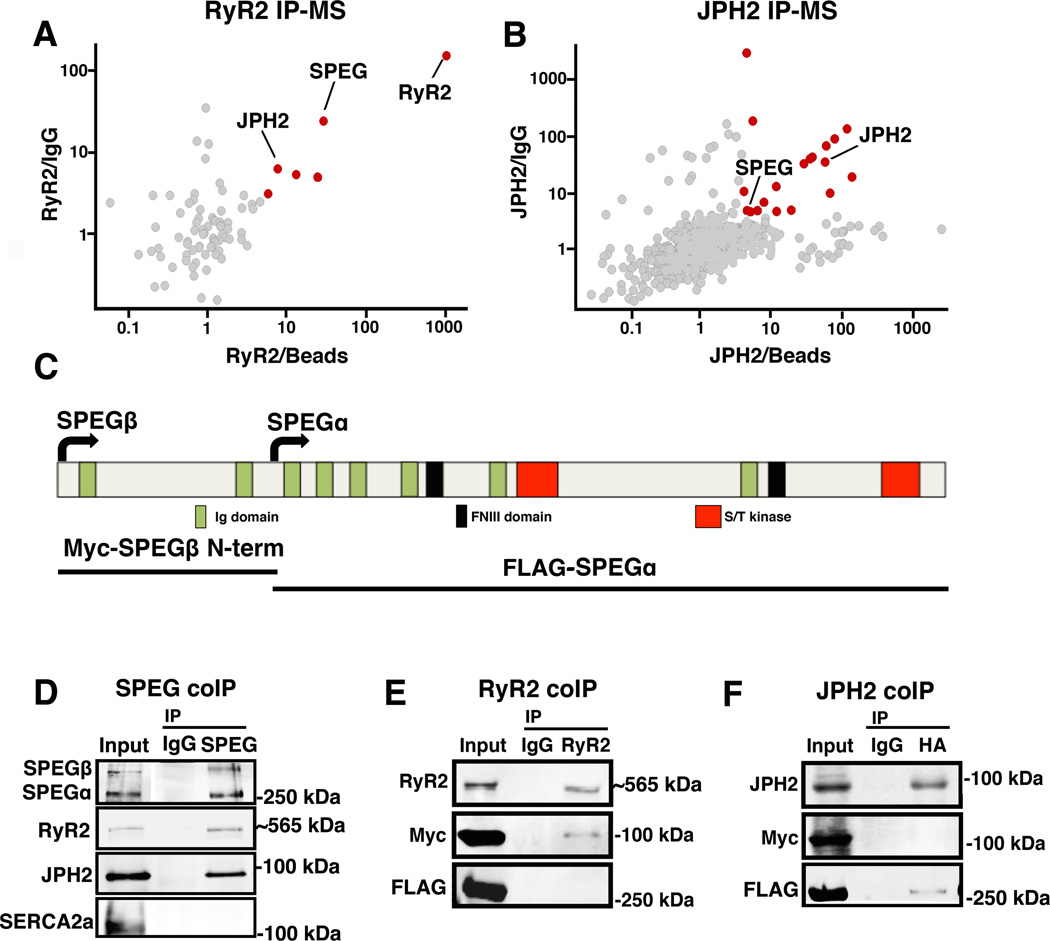
Co-immunoprecipitation followed by mass spectrometry (IP-MS) experiments was carried out as previously described with few modifications.12 Briefly, RyR2 was immunoprecipitated from WT mouse hearts using RyR2 monoclonal antibody (MA3–916, Thermo Fisher Scientific, Waltham, MA) and JPH2 was immunoprecipitated from JPH2 overexpressing hearts using monoclonal HA antibody (Sigma-Aldrich, St. Louis, MO). Murine non-specific IgG (Sigma-Aldrich) and beads were used as negative controls. Immunoprecipitates were run on a gel and each lane was cut and trypsinized followed by tandem MS/MS mass spectrometry and analysis with Maxquant software. In order to distinguish between specific and nonspecific binding, we used a cutoff ratio of 3 for the intensity of each protein that came down with the RyR2 IP vs. IgG IP and RyR2 IP vs. beads only IP, and a ratio of 4.5 for the same with JPH2 IP vs. IgG IP and JPH2 IP vs. beads only IP. A table of putative binding partners (Online Tables I and II) was generated with the most specific binding partners highlighted in grey (red dots in Figure 1 A–B).
Figure 1. SPEG directly binds to RyR2 and JPH2 within JMCs.
(A–B) Unbiased proteomics analyses of putative RyR2 and JPH2 binding partners based on adult mouse heart co-immunoprecipitation of RyR2 and JPH2, respectively, coupled to mass spectrometry (IP-MS). Specific binding partners (red dots) were defined based on cut-off ratios of 3 and 4, respectively, of the MS intensity of each protein that came down with RyR2 or JPH2 IP vs. control IgG IP (y-axis) and RyR2 or JPH2 IP vs. beads only IP (x-axis). (C) Schematic overview of SPEG protein structure with key functional domains marked, and SPEG fragments used for binding-site analysis. (D) Western blots of reciprocal SPEG co-IP experiment from mouse heart showing that both RyR2 and JPH2 but not SERCA2a pulled down with SPEG. (E) RyR2, Myc-tagged SPEGβ N-terminal peptide, and FLAG-tagged SPEGα were co-expressed in HEK293 cells. Only SPEGβ N-terminal peptide was shown to bind the RyR2 pull-down. (F) JPH2 was co-expressed with Myc-tagged SPEGβ N-terminal peptide and FLAG-tagged SPEGα; only FLAG-tagged SPEGα was shown to bind the JPH2 pull-down.
Cloning
Spegα and the N-terminal 861 amino acids of Spegβ were cloned from cDNA from RNA isolated from WT mouse ventricular lysate. Speg inserts were amplified using polymerase chain reaction with KOD Xtreme Hot Start Kit (EMD, Millipore) and the following primers: Spegα + N-terminal Flag-tag: Fwd-5’CTGAATGATATCGCCACCATGGATTACAAGGATG-ACGATGACAAGGACGTCAAGCTCAGTCCCAGCCAGGATCATGATTCC-3’; Rev-5’-CTGAATGCGG-CCGCCTAGGGGCTGCCAGGGTAGGAGCGG-3’. Spegα was cloned into a pcDNA3.1+ vector using restriction sites EcoRV and NotI. HA-tagged JPH2 was cloned from mouse heart using the following primers: Fwd-5’-AAGAATTCGCCACCATGTACCCATACGACGTCCCAGACTACGCTAGCGGGGGCC-GCTTTGACTTTG-3’ and Rev-5’-AATCTAGATCAAGCGTAGTCTGGGACGTCGTATGGGTA AGTCAGGAGGTGAACAAATAGG-3’ using EcoRI and XbaI restriction digest. Constructs were cloned into a pcDNA3.1+ vector using T4 ligase (NEB). The Spegβ N-terminus was amplified using the following primers: Fwd-5’- ATCTAAGCTTCAGAAAGCCCGGGGCACG-3’ and Rev-5’- ATTAGCGGCCGCCT-ACTGGCTGGGACTGAGCTTC-3’ and was cloned into a cMyc-tagged pcDNA3.1+ vector using the HindIII/ NotI restriction digest (NEB) followed by ligation with T4 ligase for 10 minutes at room temperature. Constructs were transformed into MAX Efficiency DH5α (Thermofisher Scientific) competent cells. Recombinant pCMV5-hRyR2 was kindly provided by Dr. Andrew Marks.
Cell culture and recombinant DNA expression
HEK293T cells were seeded into 10cm plates and grown in DMEM supplemented with 10% FBS, 1% Penicillin-streptomycin, and 1% L-glutamine. Plasmids encoding full length RyR2, HA-tagged JPH2, cMyc-tagged Spegβ N-terminus (amino acids 1–861), or full length Flag-tagged Spegα were co-expressed in HEK293T cells. Cells were washed in ice cold PBS and harvested by scraping 60 hours post transfection. Cells were spun down and resuspended in a modified radioimmunoprecipitation assay (RIPA) buffer containing 1% CHAPS, phosphostop and complete mini protease inhibitor cocktail (Roche), 20mM NaF and 1mM Na3VO4. Samples were sonicated 3× for 1 second each on ice and centrifuged at 16,000g 15 minutes. Supernatants were collected as the cell lysate.
Co-Immunoprecipitation (co-IP) and Western blots (WB)
Reciprocal SPEG coIP from mouse heart lysate was performed using the same protocol as the coIP for mass spec, except that SPEG polyclonal, rabbit antibody (custom-made by Yenzyme) was used to immunoprecipitate SPEG and rabbit IgG was used as a negative control. After washing 2× with modified RIPA buffer beads were incubated with 2× Lamelli’s Buffer at room temp for 30 minutes then gel electrophoresed, transferred to a PVDF membrane and immunoblotted. For JPH2 phospho-serine Western blots, Dynabeads (Invitrogen) and JPH2 custom antibody (yenzyme) were used to co-immunoprecipitate JPH2 from MCM and MCM-Spegfl/fl heart lysates prior to Western blot and probing with phosphoserine followed by total JPH2 antibody.
For co-IP from HEK cells, 500 µg of cell lysate was rotated overnight at 4 °C in 500 µL of radioimmunoprecipitation assay buffer (RIPA) buffer with 2 µL of either monoclonal anti-RyR2 (ma3–916) to pull down RyR2, anti-Flag (M2, Sigma-Aldrich), or anti-HA (Sigma-Aldrich) to pull down JPH2.6 Murine IgG non-specific antibody was used as a negative control. Next, 40 µL of PBS-washed protein G beads (Pierce) re-suspended in RIPA were incubated with lysate-antibody complexes for 1.5 hours at 4 °C. Bead-antibody-protein complexes were washed 2× using the same modified RIPA buffer containing 5% CHAPS. Beads were incubated in 2× Lamelli buffer for 10 min at 70 °C. The immunoprecipitates and 75 µg of input lysate were gel electrophoresed and immunoblotted with the appropriate antibodies to confirm pulldown and either anti-cMyc (9E10, Sigma-Aldrich), anti-Flag, or anti-JPH2 to determine which SPEG isoform co-immunoprecipitated with RyR2 and JPH2.
For WB from mouse heart, mouse heart lysate was made by first flash freezing hearts in liquid nitrogen and homogenizing them in the modified RIPA buffer listed above prior to sonication for 3× 1 sec. After gel electrophoresis on a 6–12% acrylamide gel, proteins were transferred to a PVDF membrane overnight. Membranes were blocked 30 minutes with 3% BSA-TBS and incubated overnight at 4C in custom antibodies (Yenzyme) for SPEG p-S2808 and p-S2814, or anti-phosphoserine (Sigma) diluted in 3% BSA-TBS. JPH2 (Yenzyme) and SERCA (ma3–919, Thermo Fisher) were diluted in 5% milk-TBS and applied SPEG IP Western blots. Blots were then incubated in the appropriate fluorescent secondary antibodies (anti-rabbit, Rockland; anti-mouse, Thermo Fisher Scientific) diluted in 5% milk-TBS and visualized. Total RyR2 (MA3–916) or total JPH2 (custom, Yenzyme) were subsequently assessed.
Human heart samples
Left ventricular samples were obtained from patients undergoing clinically indicated cardiac transplantation for end-stage heart failure (HF) after obtaining informed consent (Online Table III). Seven non-failing (NF) human hearts (which could not be used for transplantation) were obtained from the ‘International Institute for the Advancement of Medicine’ (IIAM). All studies were performed with IRB approval from Baylor College of Medicine and Texas Heart Institute (H31918).
Quantitative real time polymerase chain reaction (qRT-PCR)
RNA isolation and qRT-PCR were performed as previously described.13 RNA was isolated from human left ventricles samples using the Zymo Directzol miniprep kit. cDNA was prepared from the resulting RNA using iScript. qRT-PCR was performed using the following human SPEG primers: exons 9–10 Fwd-5’-GCGGTCAATGAGTATGGTGC-3’ and Rev -5’-CATTTGAGCAGTGCAGGCTG-3’. Samples were assayed in triplicate and normalized to L7.
Histology
Mouse cardiac samples were fixed in 10% buffered formalin and dehydrated in an ethanol series. Histoclear and paraffin washes were used to paraffinize the samples. 5µm sections were cut, placed on slides, and deparaffinized. Sections were stained with Masson’s trichrome (Thermo Fisher Scientific) and imaged on a light microscope to highlight gross morphology and fibrosis.
Echocardiography
Echocardiography was performed using the Vevo 770 and 2100 imaging systems (VisualSonics, Toronto, Canada) equipped with a high frequency probe.14 Measurements were made while the mouse was anesthetized using 2%± .5% isoflurane and had a heart rate >400 bpm. In addition, body temperature was maintained in a range (37.0 ± 1.0 °C) to avoid confounding effects of hypothermia.
Calcium imaging studies
Mice from each group were anesthetized with 2% isoflurane and humanely euthanized by cervical dislocation. Cells were isolated from hearts by enzymatic digestion with liberase using Langendorff perfusion and mechanical shearing of the ventricles. Ventricular myocytes were then incubated in either di-8-anneps or Fluo-4 AM and J127 and subsequently imaged by confocal microscopy.5 Line scans were used to obtain Ca spark data and transient amplitudes. Caffeine was used to determine the SR load. Data were analyzed using Image J and the SparkMaster plug-in.
T-Tubule imaging and analysis
TTs were visualized in ventricular myocytes using Di-8-ANEPPS staining (10 µM for 10 min) in normal Tyrode solution containing 1.8 mM Ca2+. The spatial integrity of TTs were quantified using methods described in previous studies,4 and was performed with ImageJ software (http://rsb.info.nih.gov/ij/). The regions of interest were selected within a cell, and excluded the nucleus. The power spectrum was computed using Fast Fourier Transform (FFT). The normalized power at a spatial frequency of ~0.55 µm−1 (peak power at ~0.55 µm−1 normalized to average power at a spatial frequency of 0.2 to 0.4 µm−1) was used as an index of the spatial integrity of TTs (TT-power).
Immunocytochemistry in isolated myocytes
Cardiac myocytes were isolated from C57Bl/6 WT mice as described for the Ca2+ imaging studies. Cells were then attached to a slide with laminin and fixed using 4% paraformaldehyde. Tritin-X was used to permeabilize the cells, which were blocked with NGS before incubating in primary antibody. Primary antibodies against SPEG (Yenzyme) and RyR2 (MA3–916) diluted in 1%NGS/1%BSA were incubated with cells overnight at 4deg C. Cells were washed in PBS and incubated with Alexa 488 goat-anti-mouse and 568 goat-anti-rabbit secondary antibodies. Cells were imaged by confocal microscopy.
Statistical analysis
Statistical differences in the survival of MCM and MCM-Spegfl/fl mice were assessed using the log-rank test. For all other statistics, values were expressed as mean +/- SEM. Statistical differences between MCM and MCM-Spegfl/fl mice were tested for by unpaired t-test for a particular time point or multiple t-tests between time points. A value of P <0.05 was considered statistically significant.
RESULTS
SPEG is a novel JMC protein associated with RyR2 and JPH2
To identify novel regulatory proteins within the JMC, we performed unbiased proteomic analyses of proteins that bind to RyR2 or JPH2, respectively. RyR2 and JPH2 were immunoprecipitated separately from mouse ventricular lysates. Next, binding partners of RyR2 and JPH2 were identified using mass spectrometry (MS). About 20 proteins were found to be associated with RyR2 or JPH2 in adult mouse hearts (Figure 1A–B). Since conditions of the IP were quite stringent, we did not recover many of the known members of the RyR2 macromolecular complex, presumably due to their weak interactions. Instead, several new binding partners were recovered with higher confidence (see Online Tables I and II). The most relevant finding for this investigation, however, was the identification of SPEG as the only protein that bound specifically to both RyR2 and JPH2.
To validate that SPEG directly binds to RyR2 and JPH2, we performed the reciprocal co-IP experiment in WT mouse heart lysate. SPEG was immunoprecipitated from C57Bl/6N mouse hearts lysate and immunoblot confirmed that SPEG binds both RyR2 and JPH2 but not another SR membrane protein sarcoplasmic endoplasmic reticulum ATPase type 2a (SERCA2a) (Figure 1D). Additionally, we demonstrated that SPEG co-localizes with RyR2 within isolated cardiac myocytes (Online Figure I). To further examine RyR2 and JPH2 binding to SPEG, we performed co-expression and co-IP studies in HEK293 cells of full length RyR2 or JPH2 with SPEG fragments. Myc-tagged SPEGβ N-terminal and Flag-tagged SPEGα fragments were co-expressed with full-length RyR2 or HA-tagged JPH2. RyR2 was pulled down from HEK293 lysates with non-specific mouse IgG as negative control. Western blots demonstrated RyR2 interaction with the N-terminal SPEGβ fragment (Figure 1E). In contrast, JPH2 pull-down with anti-HA antibody revealed an interaction with SPEGα (Figure 1F). These experiments demonstrate that SPEG binds RyR2 and JPH2 independently and via distinct domains, which corresponds to the SPEG isoforms identified in the IP-MS experiment.
Reduced SPEG levels in patients with heart failure
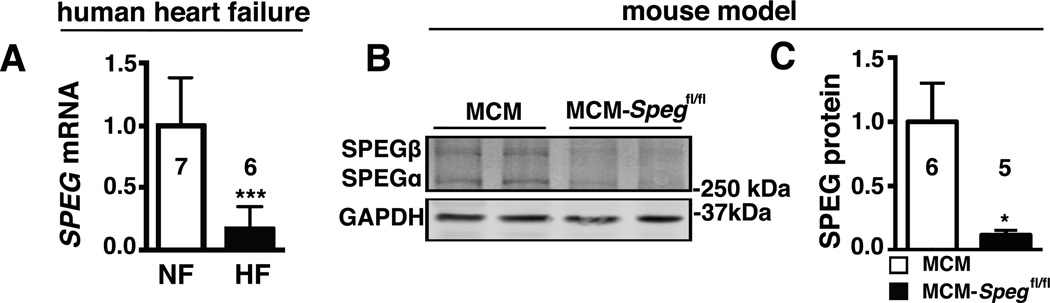
Recent studies linked rare loss-of-function mutations in SPEG to dilated cardiomyopathy in humans.11 We measured SPEG mRNA levels in patients with non-genetic forms of HF (Online Table III). Quantitative PCR revealed an 83% reduction in SPEG transcripts compared to non-failing (NF) human hearts (Figure 2A). To circumvent prenatal lethality in germline Speg knockout mice10 and study the role of SPEG in cardiac myocytes, we obtained generated conditional cardiac myocyte-restricted tamoxifen-inducible Speg knockout (MCM-Spegfl/fl) mice by crossing Speg-floxed (fl) mice with Myh6-driven mER-Cre-mER (MCM) mice. Upon administration of tamoxifen for 5 days, SPEG protein levels were assessed using Western blotting in the hearts of MCM-Spegfl/fl and MCM control mice (Figure 2B). Quantification of the protein levels at 2 weeks after tamoxifen revealed a robust 80% reduction in cardiac isoforms of SPEG in the hearts of MCM-Spegfl/fl mice compared to controls (Figure 2C).
Figure 2. SPEG is downregulated in human heart failure and a mouse model of cardiac myocyte specific inducible Speg knockout.
(A) Quantitative real-time PCR analysis of SPEG mRNA transcript abundance in myocardium from patients suffering from HF or patients with normal heart function (NF). (B) SPEG western blots in hearts from inducible cardiac-specific SPEG-deficient mice (MCM-Spegfl/fl) and MCM-control mice, and (C) Quantification of SPEG levels in MCM and MCM-Spegfl/fl knockout mice. n, number of hearts, mice. * P<0.05, ***P<0.001.
Inducible cardiac myocyte-specific SPEG deficiency causes heart failure
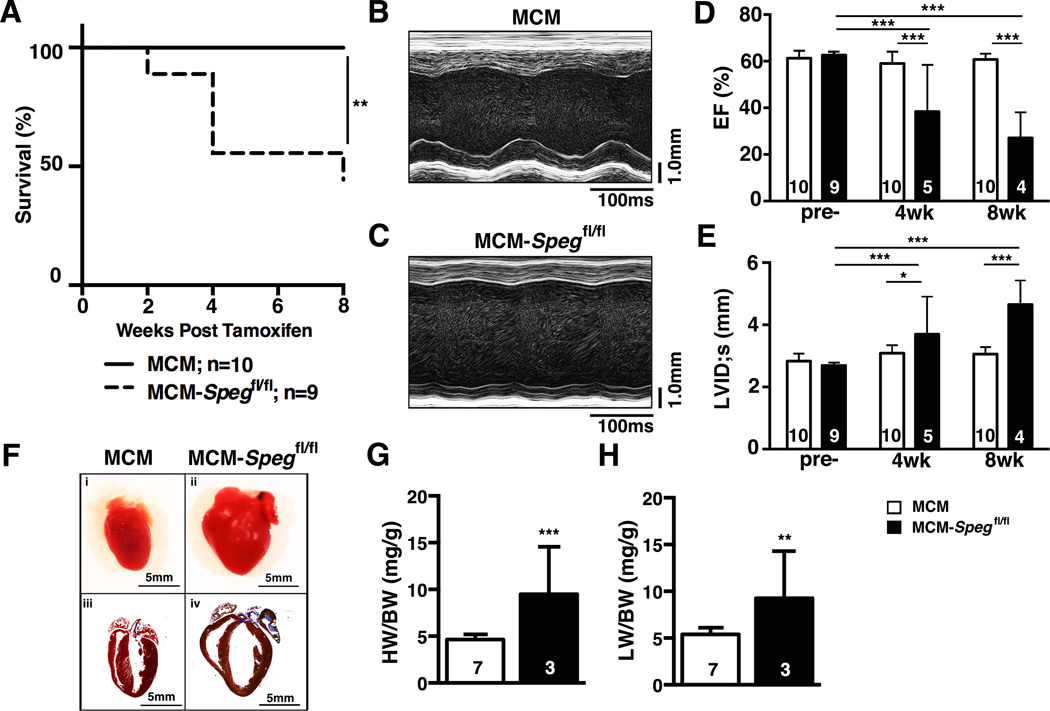
To assess the effects of cardiac myocyte-specific SPEG deficiency on heart function, we prospectively followed cohorts of MCM-Spegfl/fl (n=9) and MCM control mice (n=10) following tamoxifen injections. Kaplan-Meier survival curve analysis revealed that the loss of SPEG leads to premature death in the MCM-Spegfl/fl (44 % death by 8 weeks after tamoxifen induction; Figure 3A). Representative images of M-mode echocardiography performed at 8 weeks after tamoxifen showed evidence of weakened contractility and cardiac dilation in MCM-Spegfl/fl (Figure 3B–C). Quantification of serial echocardiography measurements before, 4 and 8 weeks after tamoxifen revealed that at baseline (before tamoxifen), there were no differences between both groups of mice (Figure 3D–E). Starting at 4 weeks and progressively deteriorating at 8 weeks post-tamoxifen, SPEG deficiency led to development of contractile dysfunction as evidenced by a decline in the ejection fraction (EF; Figure 3D). In addition, there was evidence of left ventricular dilatation at 4 weeks, which became much more pronounced at 8 weeks post-tamoxifen (Figure 3E). Online Table IV demonstrates that cardiac dilatation of the anterior and posterior wall, as well as the intraventricular septum occurred by 8 weeks post-tamoxifen. Finally, histological analysis of Masson’s trichrome-stained sections of hearts obtained at 8 weeks revealed evidence of severe dilated cardiomyopathy with thinning of the ventricular walls, and pronounced left atrial fibrosis (Figure 3F). Together, these findings demonstrate that conditional loss of SPEG is causally linked to HF development in MCM-Spegfl/fl mice.
Figure 3. SPEG downregulation causes heart failure.
(A) Kaplan Meier survival plot showing percent survival of MCM and MCM-Spegfl/fl mice for 8 weeks after tamoxifen injection.(C–D) Representative M-mode echocardiography images from MCM and MCM-Spegfl/fl knockout mice at 8-weeks post-tamoxifen. Summary bar graphs showing (E) left ventricular ejection fraction (EF) and (F) left ventricular internal diameter in systole (LVID;s) at baseline (pre-) and 4 or 8 weeks after tamoxifen injection. (F) (i-ii) Representative images of whole hearts from MCM(i) and MCM-Spegfl/fl (ii) mice after tamoxifen and (iii-iv) Masson’s trichrome histological images of MCM(iii) and MCM-Spegfl/fl(iv) hearts. n, number of hearts/mice. * P<0.05, *** P<0.001 versus MCM control.
Aberrant systolic SR Ca2+ handling in SPEG deficient mice
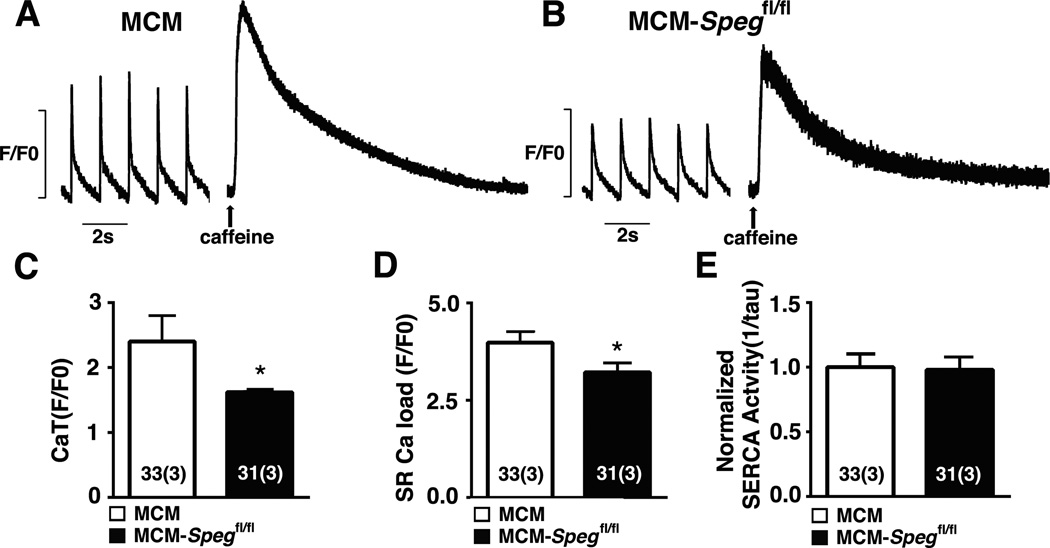
Since we found that SPEG binds to RyR2, we determined functional effects of SPEG deficiency on SR Ca2+ handling and RyR2 activity in situ. Ventricular myocytes were isolated from MCM-Spegfl/fl mice 8-weeks after tamoxifen injection, and paced at 1-Hz to obtain steady-state Ca2+ handling. There was a reduction in steady-state Ca2+ transient amplitude in SPEG-deficient myocytes consistent with systolic dysfunction in Speg conditional knockout mice (Figure 4A–C). Rapid application of caffeine revealed a modest but significant reduction in SR Ca2+ load (Figure 4D). Quantification of the Ca2+ transient decline indicated that the reduced SR load was not due to a reduced SERCA2a activity (Figure 4E). Overall, these findings indicate that loss of SPEG negatively impacts SR Ca2+ load and systolic SR Ca2+ release, consistent with reduced contractility in SPEG deficient mice.
Figure 4. Abnormal systolic SR Ca2+ release in SPEG deficient cardiac myocytes.
(A–B) Representative tracings of Ca2+ transient recordings after 1Hz pacing, subjected to caffeine at the end, in myocytes from MCM and MCM-Spegfl/fl knockout mice 8 weeks post tamoxifen. (C) Quantifications of Ca2+ transient amplitude, (D) sarcoplasmic reticulum (SR) Ca2+ load, and (E) SERCA2a activity. n, number of sparks (mice). * P<0.05.
Loss of SPEG leads to diastolic Ca2+ dysregulation
To investigate whether diastolic Ca2+ handling was also affected in SPEG deficient mice, we measured spontaneous SR Ca2+ release events in isolated cardiac myocytes using confocal microscopy (Figure 5A–B). Quantification of the data revealed an elevated Ca2+ spark frequency in SPEG-deficient myocytes, consistent with enhanced RyR2-mediated SR Ca2+ leak (Figure 5C). Further analysis of the Ca2+ spark properties revealed a slight increase in Ca2+ spark amplitude (Figure 5D) and a reduction in the full-width half-maximum (spatial width) (Figure 5E). These data suggest that loss of SPEG directly alters RyR2 activity, which is associated with HF development. Although RyR2 hyper-phosphorylation at S2808 and S2814 has been shown to contribute to Ca2+ dysregulation in heart failure progression,14–16 the phosphorylation states of these sites were unaltered in MCM-Spegfl/fl hearts (Online Figure II). Thus, enhanced SR Ca2+ leak through hyperactive RyR2 may contribute to a reduced SR Ca2+ load and contractile dysfunction in SPEG deficient hearts.
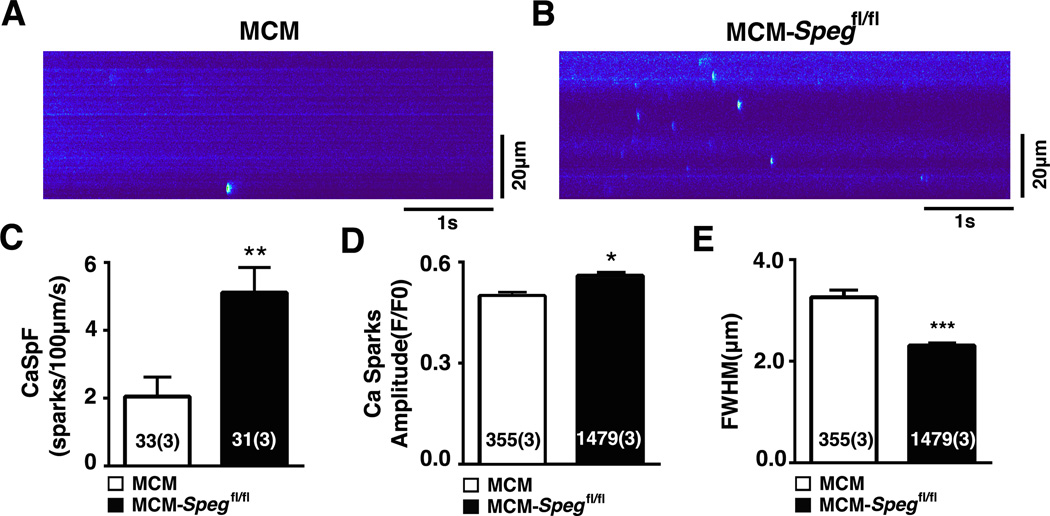
Figure 5. Spontaneous RyR2-mediated Ca2+ release in cardiac myocytes with loss of SPEG.
(A–B) Confocal microscopy line scans revealing increased number of Ca2+ sparks in MCM-Spegfl/fl mice 8 weeks post tamoxifen. (C) Bar graphs showing quantification of Ca2+ spark frequency (CaSpF), (D) Ca2+ spark amplitude, and (E) full-width of half max (FWHM). n, number of sparks (mice). * P<0.05, **P<0.01, ***P<0.001.
SPEG is essential for JMC integrity
Our data also revealed that SPEG directly binds to JPH2, a protein critical for JMC stability in cardiac myocytes.4 To assess potential changes in JMC organization in SPEG-deficient ventricular myocytes, we stained the structure of the T-tubular (TT) network using di-8-ANEPPS. A significant disruption of the normal TT structure was apparent in tamoxifen-treated MCM-Spegfl/fl mice after the onset of HF (Figure 6A–D). Image quantification revealed that SPEG deficiency causes cellular hypertrophy (Figure 6E), and a pronounced loss of TT power and TT area (Figure 6F–G) consistent with an overall loss of JMC integrity. Together, these data indicate that heart failure in MCM-Spegfl/fl mice is caused by knockdown of SPEG, and that SPEG is required for JMC integrity and normal ECC in cardiac myocytes.
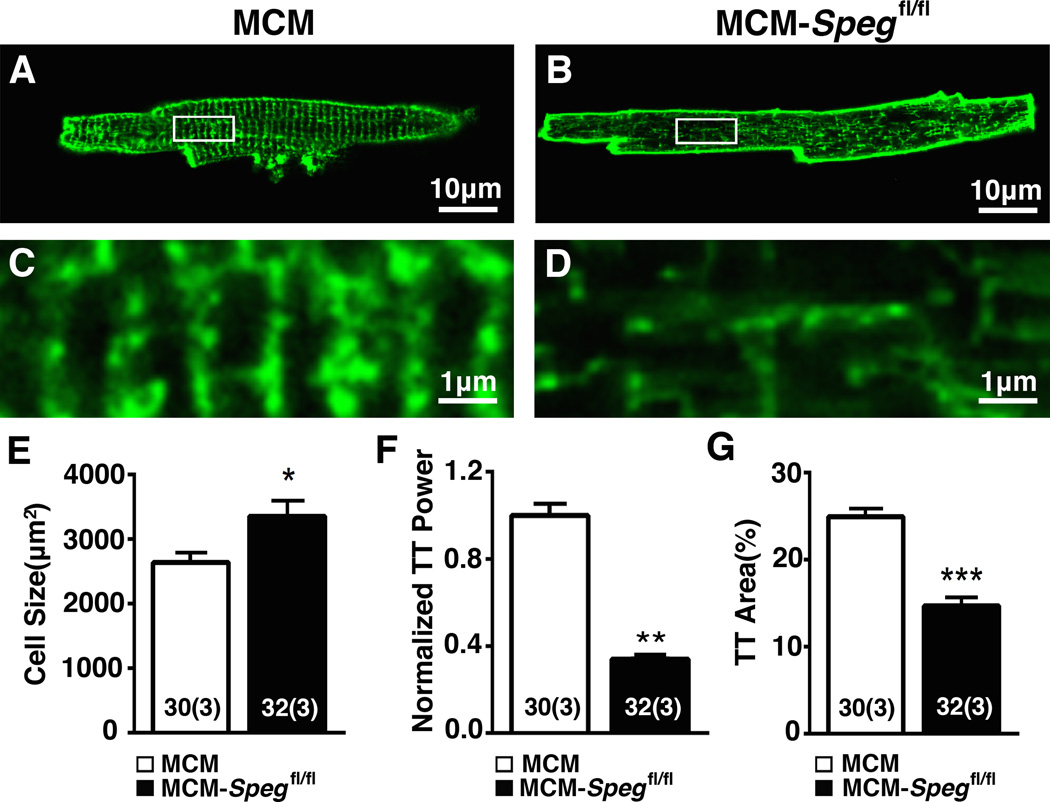
Figure 6. SPEG knockdown causes disrupted JMCs.
(A–B) Ventricular myocytes isolated from MCM-Spegfl/fl mice or MCM-controls 8 weeks post tamoxifen stained with di-8-ANEPPS to visualize TT organization. (C–D) Enlarged image (corresponding to white box in panels A,B) showing disrupted TT structures in MCM-Spegfl/fl knockout mice. (E) Quantification of ventricular myocyte size, (F) normalized TT power, and (G) and TT area. n, number of sparks (mice). * P<0.05, **P<0.01, ***P<0.001.
Loss of SPEG phosphorylation of JPH2 causes TT disruption preceding HF development
To determine whether the aberrant Ca2+ handling and/or TT loss associated with SPEG deficiency are causally linked to HF development, or merely a secondary consequence of HF, we studied another group of tamoxifen-treated MCM-Spegfl/fl mice at 2 weeks post-tamoxifen. At this earlier time point, the average ejection fraction of MCM-Spegfl/fl mice was 47.4±3.3 compared to 55.7±1.7 in MCM controls (P<0.05; Online Figure III). Despite this small but significant decline in cardiac contractility, the MCM-Spegfl/fl mice were not in heart failure. Consistent with these findings, there was a modest reduction in the Ca2+ transient amplitude in ventricular myocytes from MCM-Spegfl/fl mice compared to MCM controls (Online Figure IV A–C). However, there were no significant changes in SR Ca2+ load (Online Figure IV D), SERCA2a activity (Online Figure IV E), or SR Ca2+ spark frequency (Online Figure IV F–H).
In contrast, di-8-ANEPPS staining revealed severely disrupted TT structures in ventricular myocytes from MCM-Spegfl/fl mice at 2 weeks post-tamoxifen (Figure 7A–E). The normalized TT power was reduced to 0.42±0.03 in MCM-Spegfl/fl mice compared to 1.00±0.04 in MCM controls (P<0.001). Given that JPH2 was previously shown to be essential for TT formation and maintenance,5 we measured JPH2 levels in these hearts. Surprisingly, total JPH2 protein levels were unaltered in myocytes from MCM-Spegfl/fl mice (Figure 7F–G). Since SPEG is a Ser/Thr-kinase, we assessed whether the loss of SPEG altered JPH2 phosphorylation levels. Interestingly, we found that JPH2 phosphorylation was significantly decreased by about 51% in MCM-Spegfl/fl hearts (P<0.05; Figure 7H–I). To confirm that SPEG can phosphorylate JPH2, we transfected JPH2 cDNA in HEK293 cells together with SPEG or pcDNA3.1 empty vector as a negative control (Online Figure V). While baseline JPH2 phosphorylation in the absence of SEPG was very low, co-expression of SPEG with JPH2 led to a clear increase in JPH2 phosphorylation. Together, our data suggest that defective post-translational modulation of JPH2 by SPEG might play a role in the pathogenesis of HF.
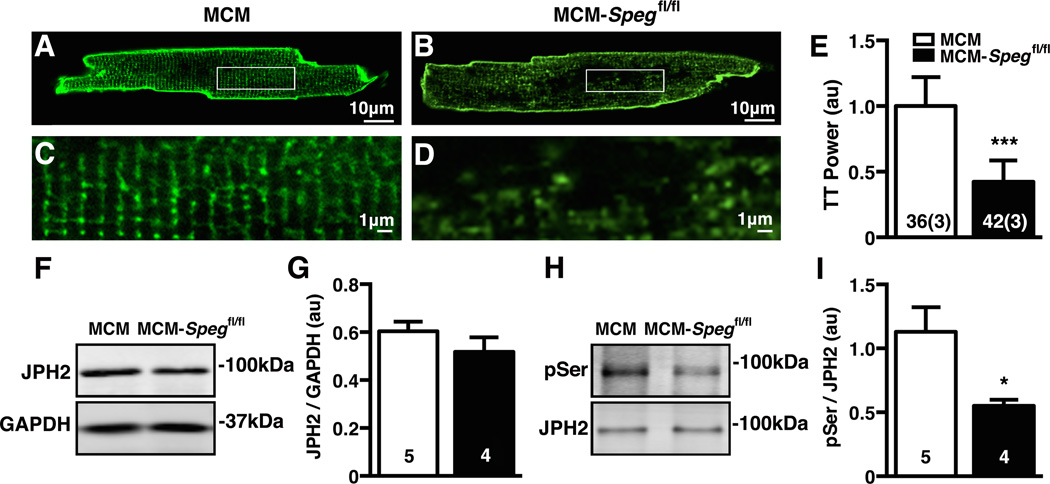
Figure 7. Loss of TTs and JPH2 phosphorylation precedes heart failure in MCM-Spegfl/fl mice.
(A–B) Representative images of ventricular myocytes isolated from MCM-Spegfl/fl mice or MCM controls 2 weeks post tamoxifen stained with di-8-ANEPPS and zoom (C–D) showing TT structure. (E) Quantification of TT Power. (F) Representative blot of total JPH2 with GAPDH as loading control from mouse heart lysates 2 weeks post tamoxifen and (G) corresponding quantification. (H) Representative image of JPH2 phosphoserine (pSer) Western blot normalized to JPH2 from immunoprecipitates from mice 2 weeks post tamoxifen and (I) quantification of phosphoserine normalized to total JPH2. Number in bars = number cells (mice); number hearts. * P<0.05.
DISCUSSION
This study identified the serine-threonine kinase SPEG as a novel protein within the JMC of cardiac myocytes that has critical functions in regulating excitation-contraction coupling. SPEG was found to be a unique binding partner of both the SR Ca2+ release channel RyR2 and JMC-structural protein JPH2. In human failing hearts, SPEG levels are downregulated. Using a novel cardiac-specific, inducible Speg knockout mouse model, we established a causal role between SPEG downregulation and HF development coincident with dysregulated SR Ca2+ release and disruption of TTs and the JMC.
The first goal of this study was to identify novel proteins within the JMC, a key subcellular structure in adult cardiac myocytes in which excitation-contraction coupling is regulated. To identify new JMC proteins, we performed an unbiased proteomic approach involving IP-MS analysis of two key proteins within the JMC, namely the SR Ca2+ release channel RyR2 and structural protein JPH2.17 We used stringent filtering settings to eliminate any potential weak or aspecific interacting proteins. As a result of this approach, we did not find several common binding partners of JPH2 and RyR2 (Online Table I and II). However, this level of stringency gave us great confidence that SPEG is indeed a specific binding partner of both RyR2 and JPH2 in vivo in the mouse heart. Moreover, the fact that another SR membrane protein SERCA2a did not associate with both RyR2 and JPH2 provides additional evidence for the specificity of our proteomic findings. The IP-MS experiment also revealed that two different SPEG isoforms bound to RyR2 and JPH2. This data is consistent with our in vitro co-expression and IP studies in which we found that the N-terminal region of SPEGβ alone binds RyR2 and that SPEGα binds JPH2. Since SPEGβ and α are the same with the exception of a truncated N-terminus in SPEGβ, RyR2 can only bind to SPEGβ, whereas JPH2 can bind both SPEGα and SPEGβ. This combination of data obtained in native tissue and a heterologous expression system makes a compelling argument for the specific binding of SPEG to both RyR2, and JPH2.
Our data for the first time demonstrate that SPEG is downregulated by about 80% in patients with non-genetic forms of cardiomyopathy and HF. These findings are consistent with the prior observation that rare Speg mutations are associated with a genetic disease called centronuclear myopathy, which is often associated with cardiomyopathy.11 Since complete SPEG deficiency is embryonically lethal,10 we developed a novel inducible, tissue-specific Speg knockout model. By crossing the tamoxifen-inducible cardiac myocyte-specific Cre transgenic model (αMHC-MerCreMer) with a floxed SPEGfl/fl mouse, we were able to quickly downregulate SPEG levels by 80% in the mouse heart. The fact that there was still some residual SPEG left 2 weeks after tamoxifen treatment may be a testament to the protein turnover dynamics of this protein. Alternatively, it is possible that SPEG expression in non-myocytes contributes to the residual expression in cardiac tissue. It is interesting that in humans with heart failure, SPEG levels were also downregulated by about 80%. Therefore, the data obtained in our MCM-Spegfl/fl mice are not only clinically relevant, but also allowed us to establish a causal relationship between induced SPEG downregulation and congestive heart failure.
Previous studies have focused on the role of SPEG in myocardial development, although no distinction between the function of SPEGα or SPEGβ has been reported to date. Disruption of the SPEG gene locus in developing mouse hearts resulted in cardiac myocyte hypertrophy, myofibril degeneration, and impaired cardiac function, leading to increased neonatal mortality.10 It had yet to be determined whether loss of SPEG in mature myocardium affected cardiac contractility and cardiac myocyte organization. Our study is the first to show that deletion of SPEG in the adult heart has a profound impact on cardiac function and calcium handling. In particular, our results demonstrated that SPEG is required for normal EC coupling. In addition, we found that cardiac myocytes of MCM-Spegfl/fl mice displayed increased Ca2+ spark frequency indicative of increased activity of RyR2, as well as decreased Ca2+ transient amplitudes due to reduced SR Ca2+ stores. Since MCM-Spegfl/fl cardiac myocytes exhibit increased Ca2+ spark frequency without any change in SERCA2a activity, the SR Ca2+ store reduction is likely due to RyR2 leakage, rather than the result of decreased SERCA2a activity. This is consistent with the finding that SPEG does not bind directly to SERCA2a (Fig 1D), indicating that SPEG exerts its effects by regulating RyR2 function in the macromolecular complex.
The mechanism by which SPEG regulates RyR2 function remains largely unknown. SPEGα and SPEGβ, along with obscurin, are members of the myosin light-chain kinases (MLCKs) that are required for cytoskeletal remodeling in myocytes.9 Since the MLCKs including SPEG contain unique dual serine/threonine kinase domains as well as multiple protein-protein interaction domains (Fig 1),18 it is possible that enhanced SR Ca2+ leak in SPEG-deficient mice is caused by reduced SPEG-RyR2 binding or decreased SPEG-mediated phosphorylation of RyR2 leading to HF. Indeed, loss of function of Unc-89, an obscurin-MLCK found in Drosophila and Caenorhabditis elegans, led to RyR dysregulation and contractile dysfunction in C. elegans.18, 19 Our data revealed an elevated Ca2+ spark frequency in SPEG-deficient myocytes after the onset of HF, consistent with enhanced RyR2-mediated SR Ca2+ leak, indicating that SPEG is essential for normal RyR2 function. However, prior to HF, SPEG deficiency did not alter Ca2+ handling or the phosphorylation state of S2808 or S2814, two RyR2 phosphorylation sites linked to Ca2+ dysregulation in heart failure progression.14–16 Taken together SPEG loss seems to have a downstream effect on Ca2+ handling secondary to another primary insult.
Our studies revealed that SPEG also directly binds to JPH2. Previous work from our lab showed that JPH2 is critical for TT maintenance and JPH2 knockdown leads to TT disruption in developing myocardium and in the adult mouse heart.4, 5 For this reason, we used TTs as a functional readout for JPH2 function. Our findings showed that SPEG downregulation led to loss of TT regularity and area (Fig. 6). Additional experiments showed that this severe disruption of TTs occurred after acute SPEG knockdown prior to the onset of HF (Fig. 7). This TT loss was not due to a decrease in total levels of JPH2 (Fig. 7). However, we found that the TT loss was due to altered phosphorylation of JPH2. In line with this theory, previous work showed that JPH2 may be regulated by phosphorylation in HL-1 cells, and loss of JPH2 phosphorylation can lead to cell hypertrophy and Ca2+ dysregulation.20 Here we demonstrated that SPEG loss prior to HF leads to decreased JPH2 phosphorylation and TT disruption, suggesting that SPEG indeed regulates JPH2 by phosphorylation. Studies in HEK293 cells confirmed that SPEG overexpression increases JPH2 phosphorylation (Online Fig. V). Finally, it is possible that targets of SPEG other than RyR2 or JPH2 may contribute to HF pathogenesis.
Conclusions
Here we demonstrated that SPEG loss results in hypo-phosphorylated JPH2, which is a novel mechanism underlying the disruption of T-tubules. This JMC disruption effectively de-couples RyR2 from the TTs containing voltage-gated Ca2+ channels, ultimately leading to diastolic Ca2+ leak, impaired ECC, and contractile dysfunction associated with heart failure. Future studies are warranted to explore whether SPEG within JMCs might represent a novel therapeutic target for heart failure.
Supplementary Material
Novelty and Significance.
What Is Known?
Excitation-contraction coupling occurs in junctional membrane complexes (JMCs) within cardiac myocytes.
Loss of JMC integrity and abnormal intracellular Ca2+ handling are associated with heart failure development.
The molecular basis of abnormal sarcoplasmic reticulum Ca2+ handling remains unclear, in part due to a lack of understanding of JMC-targeted proteins.
What New Information Does This Article Contribute?
The junctional membrane protein SPEG is downregulated in failing human hearts.
Reduced SPEG-mediated phosphorylation of JPH2 causes T-tubule disarray, which is an early step during the pathogenesis of heart failure.
Junctional membrane complexes (JMC) in myocytes are critical microdomains, in which excitation-contraction coupling occurs. Structural and functional disruption of JMCs underlies contractile dysfunction in failing hearts. In our study, we found a novel serine-threonine kinase called ‘striated muscle preferentially expressed gene’ (SPEG) within the JMC. In human failing hearts, SPEG protein levels were found to be downregulated. Conditional SPEG knockout in mouse hearts established a causal link between SPEG downregulation, SPEG-mediated phosphorylation of JPH2, JMC disruption, and heart failure development.
Acknowledgments
The Speg mouse strain used for this research project was created from ES cell clone EPD0180_2_A07 obtained from KOMP Repository (www.komp.org) and generated by the Wellcome Trust Sanger Institute.
SOURCES OF FUNDING
This study was supported by NIH/NHLBI grants HL089598, HL091947, HL117641, HL129570 (XHTW), T32HL07676 (APQ), American Heart Association grants 14PRE20490083 (APQ), 12PRE11700012 (DYC), and 13EIA14560061 (XHTW), the European Society of Cardiology Basic Research Fellowship for 2015 (DYC). This project was also supported by the Mouse Phenotyping Core at Baylor College of Medicine with funding from the NIH (U54 HG006348), and the Leducq foundation grant 08CVD01.
Nonstandard Abbreviations and Acronyms
- Ca2+
Calcium
- ECC
Excitation-contraction coupling
- HF
Heart failure
- JMC
Junctional membrane complex
- MLCK
Myosin light-chain kinase
- RyR2
Ryanodine receptor type 2
- SERCA2a
Sarcoplasmic endoplasmic reticulum ATPase type 2a
- SPEG
Striated muscle preferentially expressed gene
- SR
Sarcoplasmic reticulum
- TT
Transverse tubule
Footnotes
DISCLUSORES
X.H.T.W. is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Houser SR. Does protein kinase a-mediated phosphorylation of the cardiac ryanodine receptor play any role in adrenergic regulation of calcium handling in health and disease? Circ Res. 2010;106:1672–1674. doi: 10.1161/CIRCRESAHA.110.221853. [DOI] [PubMed] [Google Scholar]
- 3.Ather S, Respress JL, Li N, Wehrens XH. Alterations in ryanodine receptors and related proteins in heart failure. Biochim Biophys Acta. 2013;1832:2425–2431. doi: 10.1016/j.bbadis.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ, Wehrens XH. Junctophilin-2 is necessary for t-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100:44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, Dobrev D, Ackerman MJ, Wehrens XH. Mutation e169k in junctophilin-2 causes atrial fibrillation due to impaired ryr2 stabilization. J Am Coll Cardiol. 2013;62:2010–2019. doi: 10.1016/j.jacc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrev D, Wehrens XH. Role of ryr2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houser SR. Role of ryr2 phosphorylation in heart failure and arrhythmias: Protein kinase a-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ Res. 2014;114:1320, 1327. doi: 10.1161/CIRCRESAHA.114.300569. discussion 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh CM, Fukumoto S, Layne MD, Maemura K, Charles H, Patel A, Perrella MA, Lee ME. Striated muscle preferentially expressed genes alpha and beta are two serine/threonine protein kinases derived from the same gene as the aortic preferentially expressed gene-1. J Biol Chem. 2000;275:36966–36973. doi: 10.1074/jbc.M006028200. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Ramjiganesh T, Chen YH, Chung SW, Hall SR, Schissel SL, Padera RF, Jr, Liao R, Ackerman KG, Kajstura J, Leri A, Anversa P, Yet SF, Layne MD, Perrella MA. Disruption of striated preferentially expressed gene locus leads to dilated cardiomyopathy in mice. Circulation. 2009;119:261–268. doi: 10.1161/CIRCULATIONAHA.108.799536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal PB, Pierson CR, Joshi M, Liu X, Ravenscroft G, Moghadaszadeh B, Talabere T, Viola M, Swanson LC, Haliloglu G, Talim B, Yau KS, Allcock RJ, Laing NG, Perrella MA, Beggs AH. Speg interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am J Hum Genet. 2014;95:218–226. doi: 10.1016/j.ajhg.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang DY, Lebesgue N, Beavers DL, Alsina KM, Damen JM, Voigt N, Dobrev D, Wehrens XH, Scholten A. Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients. J Am Coll Cardiol. 2015;65:163–173. doi: 10.1016/j.jacc.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang DY, Zhang M, Voigt N, Alsina KM, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N. Identification of microrna-mrna dysregulations in paroxysmal atrial fibrillation. Int J Cardiol. 2015;184:190–197. doi: 10.1016/j.ijcard.2015.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of ryr2 phosphorylation at s2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Pka phosphorylation dissociates fkbp12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 16.Fischer TH, Herting J, Tirilomis T, Renner A, Neef S, Toischer K, Ellenberger D, Forster A, Schmitto JD, Gummert J, Schondube FA, Hasenfuss G, Maier LS, Sossalla S. Ca2+/calmodulin-dependent protein kinase ii and protein kinase a differentially regulate sarcoplasmic reticulum ca2+ leak in human cardiac pathology. Circulation. 2013;128:970–981. doi: 10.1161/CIRCULATIONAHA.113.001746. [DOI] [PubMed] [Google Scholar]
- 17.Beavers DL, Landstrom AP, Chiang DY, Wehrens XH. Emerging roles of junctophilin-2 in the heart and implications for cardiac diseases. Cardiovasc Res. 2014;103:198–205. doi: 10.1093/cvr/cvu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutter SB, Raeker MO, Borisov AB, Russell MW. Orthologous relationship of obscurin and unc-89: Phylogeny of a novel family of tandem myosin light chain kinases. Dev Genes Evol. 2004;214:352–359. doi: 10.1007/s00427-004-0413-5. [DOI] [PubMed] [Google Scholar]
- 19.Spooner PM, Bonner J, Maricq AV, Benian GM, Norman KR. Large isoforms of unc-89 (obscurin) are required for muscle cell architecture and optimal calcium release in caenorhabditis elegans. PLoS One. 2012;7:e40182. doi: 10.1371/journal.pone.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, Wehrens XH, Claycomb WC, Ko JK, Hwang M, Pan Z, Ma J, Ackerman MJ. Mutations in jph2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42:1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.