Abstract
INTRODUCTION
Esophageal variceal bleeding is a severe complication of portal hypertension with significant morbidity and mortality. While traditional screening and grading of esophageal varices has been performed by endogastroduodenoscopy (EGD), wireless video capsule endoscopy provides a minimally-invasive alternative that may improve screening and surveillance compliance.
AIM
To perform a systematic review and structured meta-analysis of all eligible studies to evaluate the efficacy of wireless capsule endoscopy for screening and diagnosis of esophageal varices among patients with portal hypertension.
METHODS
Searches of PubMed, EMBASE, Web of Science, and the Cochrane Library databases were performed through December 2015. Bivariate and hierarchical models were used to compute the pooled sensitivity and specificity, and to plot the summary receiver operating characteristics curve with summary point and corresponding 95 % confidence region. Bias of included studies was assessed using the quality assessment of diagnostic accuracy studies (QUADAS-2).
RESULTS
Seventeen studies from 2005 to 2015 were included in this meta-analysis (n=1,328). The diagnostic accuracy of wireless capsule endoscopy in the diagnosis of esophageal varices was 90% (95% CI, 0.88–0.93). The diagnostic pooled sensitivity and specificity were 83% (95% CI, 0.76 – 0.89) and 85% (95% CI, .75–0.91), respectively. The diagnostic accuracy of wireless capsule endoscopy for the grading of medium to large varices was 92% (95% CI, 0.90–0.94). The pooled sensitivity and specificity were 72% (95% CI, 0.54–0.85) and 91% (95% CI, 0.86–0.94), respectively, for the grading of medium to large varices. The use of capsule demonstrated only mild adverse events. A sensitivity analysis limited to only high quality studies revealed similar results.
DISCUSSION
Wireless esophageal capsule endoscopy is well tolerated and safe in patients with liver cirrhosis and suspicion of portal hypertension. The sensitivity of capsule endoscopy is not currently sufficient to replace EGD as a first exploration in these patients, but given its high accuracy, it may have a role in cases of refusal or contraindication to EGD.
Keywords: Esophageal varices, Capsule, Cirrhosis, Portal hypertension, Endogastroduodenoscopy
INTRODUCTION
Esophageal varices are common sequelae of both compensated and decompensated liver disease secondary to increased portal pressures. At the time of cirrhosis diagnosis, varices may be present in 30–40% of compensated cirrhotic patients and in as many as 60% of individuals with ascites and decompensated disease.1–3 In those patients in whom varices are not present at the time of diagnosis, the annual incidence of new varices is 5–10%.4–8 The risk of a bleed is a major concern in patients with varices. Esophageal variceal bleeding is a severe complication of portal hypertension with significant morbidity and mortality. Furthermore, the annual incidence of a first episode of bleeding among patients with varices is 12%, with each bleeding episode carrying an estimated 15–20% risk of mortality.2,9–12 Therefore, the ability to appropriately screen at risk patients with a history of elevated portal pressures and liver disease is paramount to improving outcomes in liver disease patients.
To avoid the significant morbidity and mortality associated with variceal bleeding, screening, traditionally with an endogastroduodenoscopy (EGD), has been performed. If varices are present, primary prophylaxis with non-selective beta-blockers (i.e. propanolol or nadolol) or endoscopic band ligation has been shown to reduce the risk of first variceal hemorrhage and bleeding related mortality.2 Though the role of non-selective beta-blockers in preventing varices in patients with cirrhosis and portal hypertension has been called in to question recently, use of this medication still dominates clinical practice.7 While guidelines for the American Association for the Study of Liver Diseases (AASLD) currently recommend EGD at the time of diagnosis of cirrhosis and subsequent EGD every 2 to 3 years for compensated and annually for decompensated liver disease, compliance and timely screening remain a challenge in the U.S. and in Europe.2,13
Wireless video capsule endoscopy, originally designed for evaluation of small bowel pathology and occult bleeding, provides an alternative means for screening for esophageal varices.14 This minimally-invasive esophageal capsule endoscopy has been approved by the United States (U.S.) Food and Drug Administration (FDA) for the detection of esophageal varices. Although capsule endoscopy has been studied and not recommended to be a viable replacement for EGD given the lack of a therapeutic basis, the minimally-invasive approach, potential for widespread screening, and diagnostic capability warrant further exploration amongst a cirrhotic population at risk for significant variceal hemorrhage associated morbidity and mortality.15
The primary aim of this study was to perform a systematic review and structured meta-analysis of all eligible studies to evaluate the efficacy of wireless capsule endoscopy for screening and diagnosis of esophageal varices among patients with portal hypertension.
METHODS
Literature Search
A comprehensive search of the literature was performed to identify articles that examined the utility of wireless esophageal capsule for the screening and grading of esophageal varices. Systematic searches of PubMed, EMBASE, Web of Science, and the Cochrane Library databases were performed through December 2015 using the following search terms “wireless capsule endoscopy”, “esophagus and capsule”, “capsule and varices”, and “capsule and variceal bleeding.” All relevant articles irrespective of language, year of publication, type of publication, or publication status were included. The titles and abstracts of all potentially relevant studies were screened for eligibility. The reference lists of studies of interest were then manually reviewed for additional articles by cross checking bibliographies. Two reviewers (TRM and BN) independently screened the titles and abstracts of all the articles according to predefined inclusion and exclusion criteria. Any differences were resolved by mutual agreement and in consultation with the third reviewer (YA). In the case of studies with incomplete information, contact was attempted with the principal authors to obtain additional data.
Study Selection Criteria
Only studies investigating the use of wireless esophageal capsule for the screening or surveillance of esophageal varices were included. Studies involving alternative endoscopic or interventional radiologic modalities were not included. Only human subject studies were considered in the analysis. A particular study was excluded if deemed to have insufficient data, as were the review articles, editorials, and correspondence letters that did not report their own data. Case series and reported studies with fewer than 5 patients were excluded. Participants included patients of any age in whom the presence of esophageal varices was suspected based on chronic liver disease or portal vein thrombosis, irrespective of the etiology or duration of illness.
Patients with cirrhosis of Child Pugh Class A, B, or C were included as well as patients with portal vein thrombosis. Some studies included patients with varices of any size; however, some focused on detection of varices of medium or large size specifically. Studies that included patients with known varices were not excluded from the analysis. Patients who were found to have undergone a transjugular intrahepatic portosystemic shunt (TIPS) procedure, previous endoscopic band ligation, or endoscopic sclerotherapy of esophageal varices were excluded. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement outline for reporting systematic reviews and meta-analyses was used to report findings.16
Outcome Measures
The primary outcome measurements in this study were the diagnostic accuracy, sensitivity, and specificity of wireless capsule endoscopy in identifying esophageal varices in patients with portal hypertension. Secondary outcomes were the assessment of capsule endoscopy in establishing the presence of medium or large esophageal varices and the rates of complications related to capsule endoscopy.
Statistical Analysis
Included studies were analyzed according to the Cochrane DTA Working Group methodology. Measured outcomes included sensitivity and specificity of capsule endoscopy. Data were analyzed using the Stata 13.0 software package (Stata Corp LP, College Station, TX). Bivariate and hierarchical models were used to compute the pooled sensitivity and specificity and to plot the summary receiver operating characteristics curve with summary point and corresponding 95 % confidence region. Briefly, this method fits a two-level model, with binomial distributions for the combined TP and the TN conditional on study-specific sensitivity and specificity, and a bivariate normal model for the logit transformations for sensitivity and specificity between studies. Positive likelihood ratio (LR) and negative LR were also determined. A Fagan plot was also employed to determine the meaningfulness or clinical utility. The Fagan nomogram is a graphical tool for estimating how much the result on a diagnostic test changes the probability that a patient has a disease.17
Index Test
The index test was the use of wireless esophageal capsule endoscopy with studies reporting evidence of esophageal varices in our analysis.
Assessment of methodological quality
Quality assessment of diagnostic accuracy studies (QUADAS-2) was utilized to assess quality in this study. QUADAS-2 is an evidence-based tool for assessment of quality in systematic reviews of diagnostic accuracy studies that is structured so that four key domains are rated for risk of bias and concerns regarding applicability to the research question was used to evaluate the studies. Each key domain has a set of signaling questions to assess bias and applicability.18,19 Disagreement among raters was resolved by consensus with the other authors. We used tabular and graphical displays in Review Manager 5 (RevMan 5.3) to summarize the QUADAS-2 assessments.
RESULTS
Characteristics of Included Studies
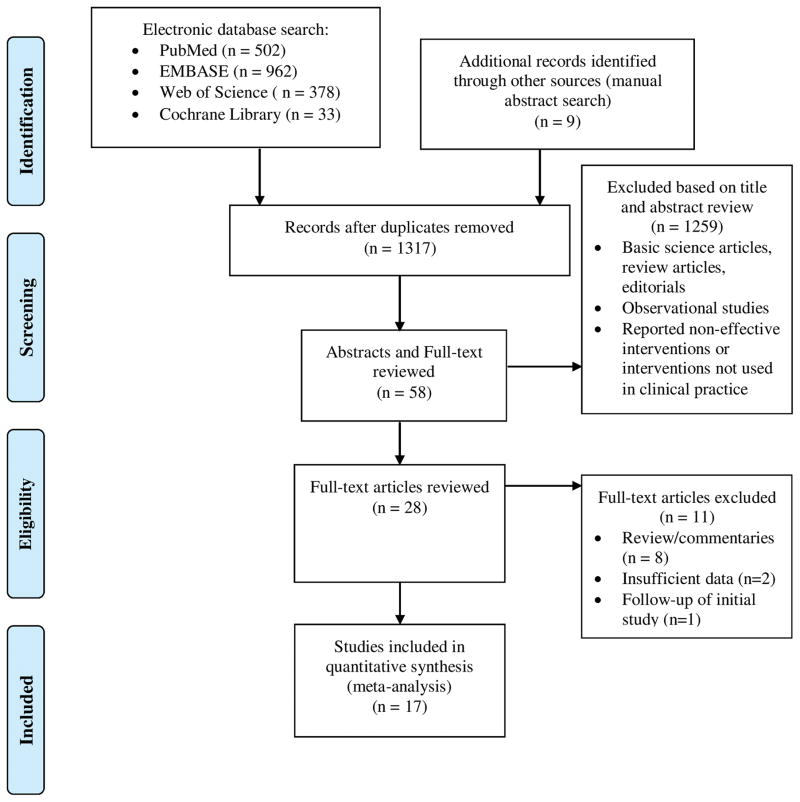
This meta-analysis included 17 studies from 2005 to 2014.20–36 Data on a total of 1,328 patients was assessed. A PRISMA flow chart of search results is shown in Figure 1. Table 1 provides baseline characteristics of included studies including etiology of liver disease, Model for End-Stage Liver Disease (MELD) or Child-Pugh Score, and tolerability or complications if specifically stated. Both male and female patients were included. Twelve of the studies included only patients with parenchymal liver disease as a cause of portal hypertension. However, in studies that included non-cirrhotic causes of portal hypertension and revealed the etiologies of portal hypertension, patients with parenchymal liver disease constituted the majority. For instance, in one study, less than 23% of patients actually had non-cirrhotic cause of their portal hypertension.22 The majority of patients in the studies had alcohol, viral, or NASH-induced liver disease; however patients with primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis were also included. Studies included in this meta-analysis had variable numbers of patients with compensated versus decompensated cirrhosis although the overall majority of patients in the studies had compensated cirrhosis.
Figure 1.
PRISMA Flow Chart of Search Results for Capsule Endoscopy
Table 1.
Included Studies to Assess Diagnosis and Grading of Esophageal Varices By Capsule Endoscopy
| Author | Year | No of Pts |
Age (Range) |
Sex (M/F) |
Location of Study |
Type of Liver Disease | Average MELD (Range) |
Child Pugh Score (Range) |
Interobserver variability |
No with Side Effects or Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Aoyama et al | 2014 | 119 | 66.9 (23–88) | 73/46 | Japan | 18 HBV, 70 HCV, 13 EtOH, 6 NASH, 12 Other | - | 56 CPA, 56 CPB, 7CPC | - | - |
| Chavalitdhamrong et al | 2012 | 65 | 54.6 (35–79) | 43/22 | USA | 37 HCV, 13 EtOH, 5 HBV, 4 NASH, 3 AIH, 3 PBC | 10.6 | 27 CPA, 27 CPB, 11 CPC | - | - |
| de Franchis et al | 2008 | 288 | 56 (21–81) | - | Italy, Spain, USA, Israel | 58 EtOH, 26 HBV, 101 HCV, 38 EtOH + HBV/HCV, 65 Other | - | 198 CPA, 73 CPB, 23 CPB | - | 4 (1 severe pain, 1 diarrhea, 1 nausea with capsule retention, 1 vomiting with capsule retention) |
| Donnelly et al | 2006 | 8 | - | 5/3 | UK | 5 EtOH, 1 HCV, 1 NASH, 1 PSC | - | - | - | - |
| Eisen et al | 2006 | 32 | 57.2 | 20/12 | Italy, Israel, USA | - | - | - | - | - |
| Gerson et al | 2008 | 24 | 52 (36–70) | 14/10 | - | 19 HCV, 5 Other | - | 17 CPB | k=0.55 | - |
| Groce et al | 2007 | 21 | - | - | - | - | - | - | - | 5 difficulty swallowing capsule |
| Ishiguro et al | 2012 | 29 | 66 | 9/20 | - | 5 HCV, 4 EtOH, 1 PBC, 17 HCC, 2 Other | - | 15 CPA, 14 CPB, 1 CPC | - | - |
| Lapalus et al | 2006 | 21 | 62 (49–79) | - | France | 5 HCV, 15 EtOH, 2 AIH, 1 NASH, 1 Other | 10.5 | 13 CPA, 6 CPB, 2 CPC | - | 2 difficulty swallowing capsule |
| Lapalus et al | 2009 | 120 | 58 (23–84) | 72/48 | France | 17 HCV, 78 EtOH, 14 NASH, 9 Other | 11.5 | 58 CPA, 36 CPB, 26 CPC | k=0 | - |
| Pena et al | 2008 | 20 | 50.7 (34–61) | 14/6 | USA | 5 HCV, 6 NASH, 2 EtOH, 7 combined | 12.9 (7–25) | Average 7.9 (5–12) | - | - |
| Ramirez et al | 2005 | 30 | 54.4 (43–69) | 30/0 | USA | 14 HCV, 8 EtOH, 7 EtOH+HCV, 1 Other | 12.5 | Average 6.3 | - | 6 difficulty swallowing |
| Sacher-Huvelin et al | 2015 | 330 | 56 (20–81) | 216/84 | France | 134 EtOH, 81 HBV or HCV, 16 NASH, 34 EtOH+HBV/HCV, 18 Other | - | - | - | 2 difficulty swallowing capsule |
| Schreibman et al | 2011 | 37 | 56 (21–78) | 28/9 | USA | 11 EtoH, 8 NASH, 7 HCV, 5 EtOH+HCV, 6 Other | - | 23 CPA, 9 CPB, 5 CPC | - | - |
| Sharma et al | 2009 | 34 | - | - | - | - | - | - | - | - |
| Stipho et al | 2012 | 100 | 55 | 99/1 | USA | 91 EtOH alone or + HCV, 9 Other | 10.8 | Average 5.9 | - | - |
| Frennette et al | 2008 | 50 | 58 (25–74) | 34/16 | USA | 24 HCV, 7 HCV + EtOH, 6 EtOH, 6 NASH, 27 Other | 9.48 (6–23) | Average 6.8 (5–13) | k=0.56 | 10 difficulty swallowing capsule |
EtOH = Alcoholic liver disease; HCV = Hepatitis C liver disease; HBV = Hepatitis B liver disease; PBC = Primary biliary cirrhosis; AID = Autoimmune hepatitis; NASH = Non-alcoholic steatohepatitis; CPA = Child Pugh A; CPB = Child Pugh B
The capsule device used in these studies was the first or second generation Pillcam capsule. In 16 studies, the accuracy of capsule endoscopy in assessing the presence of any varices regardless of size was estimated.20–35 The remaining one study assessed the accuracy of capsule endoscopy for only large, high-risk esophageal varices.37 A total of 7 studies evaluated the use of wireless capsule endoscopy for medium or large varices.22,27,28,32–34,37 A forest plot is shown in Figure 2. The accuracy of each individual study assessing the diagnosis and grading of esophageal varices is illustrated in Figure 3.
Figure 2.
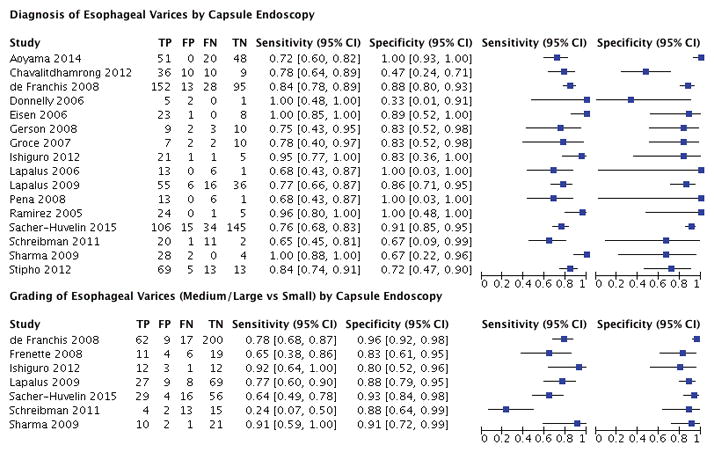
Forest Plot for the Diagnosis and Grading of Esophageal Varices By Capsule Endoscopy
Figure 3.
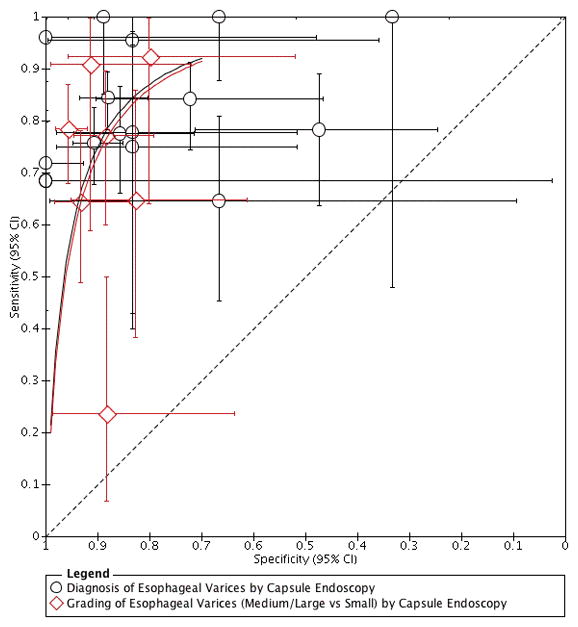
Accuracy of Individual Studies Assessing the Diagnosis and Grading of Esophageal Varices
Quality Assessment of Included Studies
The quality of the eligible studies was assessed by QUADAS- 2 criteria and is reported Figure 4. In most studies, there was a low risk of bias regarding the selection of patients; however, 8 studies in total demonstrated a high-risk of bias. There was no risk of bias issues of the index test in any of the studies. In most studies there was a low risk of bias to determine whether an appropriate reference standard was used or its applicability.
Figure 4.
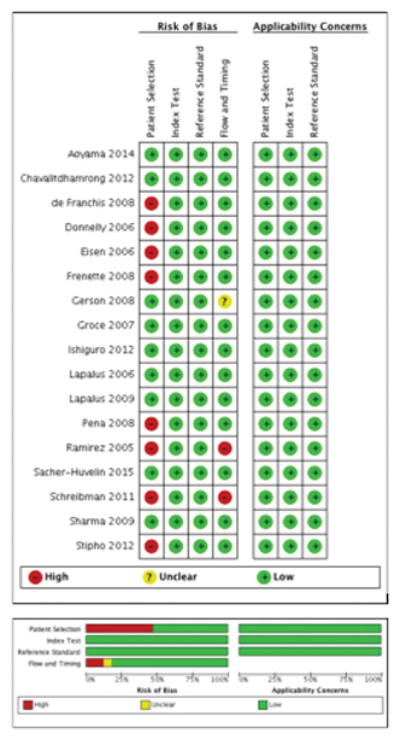
Quality Assessment of Included Studies
Capsule Endoscopy for Screening
The pooled estimates of sensitivity and specificity of wireless capsule endoscopy in the diagnosis of esophageal varices were calculated as above. The diagnostic accuracy of capsule endoscopy was 90% (95% CI, 0.88–0.93), Figure 5. Sixteen total studies assessing varices of any size demonstrated pooled of sensitivity and specificity of 83% (95% CI, 0.76 – 0.89) and 85% (95% CI, .75–0.91), respectively. The pooled positive LR and negative LR were 5.4 (95% CI, 3.3–9.0) and 0.20 (95% CI, 0.14–0.28), respectively. The diagnostic odds ratio was 27 (95% CI, 14–54). When the 8 studies with high-risk of bias were excluded, the pooled sensitivity and specificity of capsule endoscopy for screening of esophageal varices was similar [sensitivity of 80% (95% CI, 0.73–0.85) and specificity of 86% (95% CI, 0.68–0.94), Supplemental Figure 1]. This separate analysis resulted in a lower diagnostic accuracy of 85% (95% CI, 0.81–0.88), Supplemental Figure 2.
Figure 5.
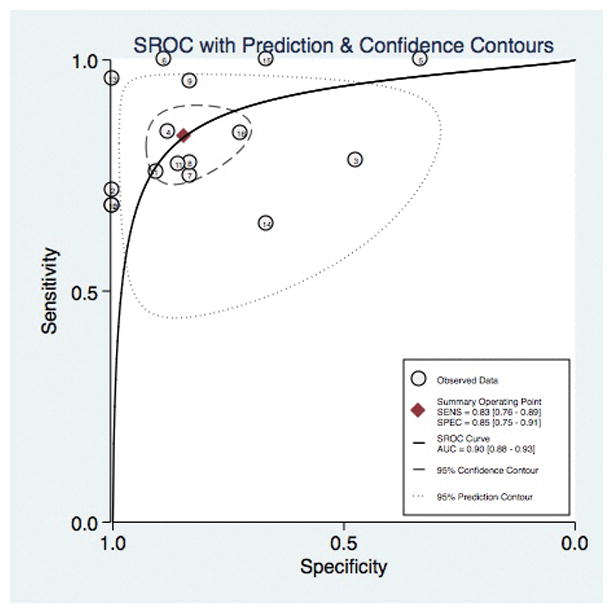
Diagnostic Accuracy of Capsule Endoscopy for the Diagnosis of Esophageal Varices
The associated Fagan nomogram is shown in Figure 6. With a low pre-test probability (20%) of varices in a patient with liver disease, if capsule endoscopy demonstrated the presence of varices, the post-test probability that the patient truly has varices would be approximately 60%. Alternatively, if the patient tests negative (i.e. no varices are seen on capsule endoscopy), the post-test probability that the patient truly has varices would be less than 5%. Again, when studies with a high-risk of bias were excluded from analysis, the Fagan nomogram demonstrated similar results, Supplemental Figure 3.
Figure 6.
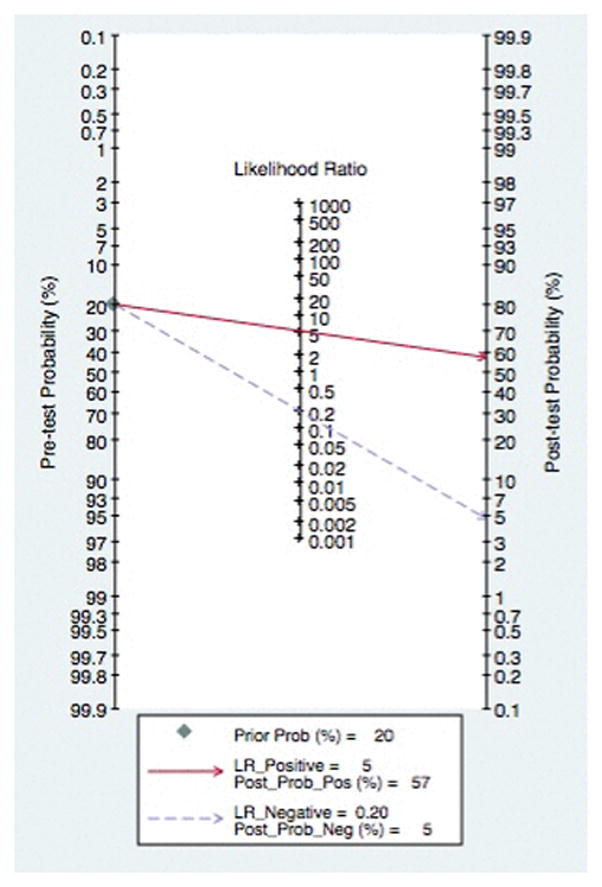
Fagan Nomogram for Diagnosis of Esophageal Varices
Capsule Endoscopy for Grading
Subgroup analysis was performed for studies specifically evaluating the grading of medium and large varices. The diagnostic accuracy of wireless capsule endoscopy for medium to large varices was 92% (95% CI, 0.90–0.94), Figure 7. Of the 7 included studies, the pooled sensitivity was 72% (95% CI, 0.54–0.85). The pool specificity in grading medium to large varices was 91% (95% CI, 0.86–0.94). The positive LR, negative LR, and odds ratio were 8.1 (95% CI, 4.8–13.8), 0.31 (95% CI, 0.18–0.54), and 26 (95% CI, 10–69), respectively. Again, excluding studies with a high-risk of bias yielded an improved sensitivity though slightly lower specificity [sensitivity of 79% (95% CI, 0.63–0.89) and specificity of 89% (95% CI, 0.83–0.94), Supplemental Figure 4]. The diagnostic accuracy for medium to large varices when studies with a high-risk of were excluded resulted in a similar accuracy of 92% (95% CI, 0.89–0.94), Supplemental Figure 5.
Figure 7.
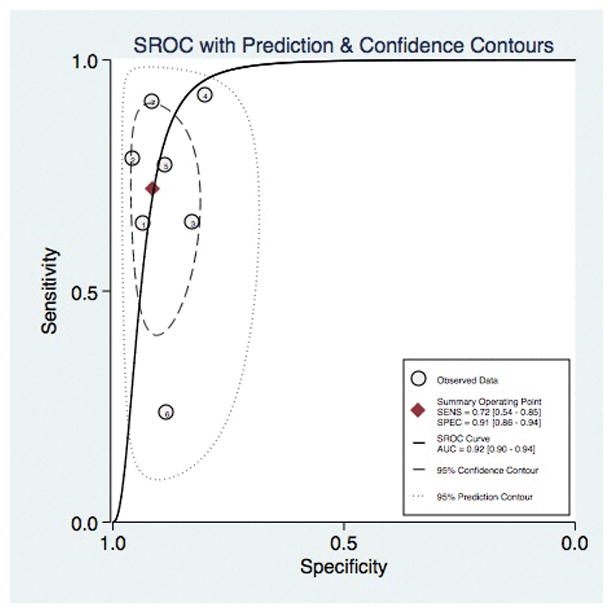
Diagnostic Accuracy of Capsule Endoscopy for the Grading of Esophageal Varices
A Fagan plot with regard to grading of esophageal varices demonstrated that with a low pre-test probability (20%) of medium to large varices, if capsule revealed medium to large varices, the post-test probability that the patient truly has varices would be approximately 70%. In the face of a negative test, the post-test probability that the patient actually has medium to large varices would be less than 7%, Figure 8. The Fagan nomogram was also analyzed after studies with a high-risk of bias were excluded and resulted in a similar clinical utility, Supplemental Figure 6.
Figure 8.
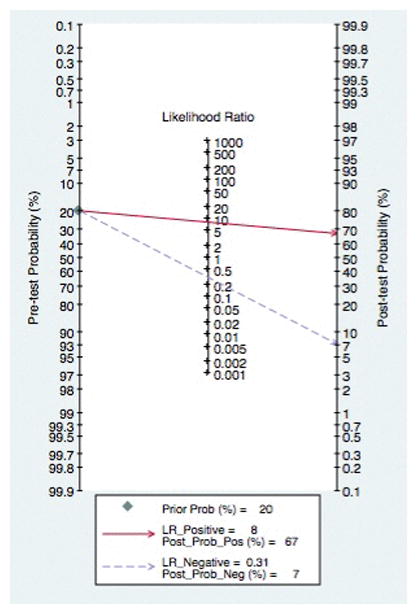
Fagan Nomogram for Grading of Esophageal Varices
Adverse Events
Although participants reported minor discomfort due to the process of swallowing the capsule in the majority of studies, only 2 significant adverse events were reported. These included 2 episodes of nausea or vomiting secondary to capsule retention as caused by an unsuspected esophageal stricture. Of these 2 significant adverse events, only one required EGD for the recovery of capsule.22,26,29,31,32,37
DISCUSSION
The results of this study confirm the feasibility, safety, and tolerance of wireless capsule endoscopy in the diagnosis and grading of esophageal varices among patients with liver cirrhosis and suspicion of portal hypertension. This study also demonstrated that the sensitivity of capsule endoscopy is not currently sufficient to replace EGD as a first exploration in these patients, but given its high accuracy, it may have a role in cases of refusal or contraindication to EGD.
The need for appropriate screening and surveillance stems from the significant morbidity and mortality of variceal hemorrhage. Variceal bleeding accounts for 70% of all upper gastrointestinal bleeding (UGIB) episodes in patients with liver cirrhosis.38 While variceal size is the most useful predictor for incidence of variceal bleeding, other important factors for the development of variceal bleeding include severity of liver dysfunction (i.e. Child-Pugh classification and MELD score) and red wale signs.3 The ability to accurately grade varices is paramount as it changes surveillance recommendations.2 Though the sensitivity of capsule endoscopy is not sufficient to replace EGD, capsule demonstrated a diagnostic accuracy of 92% in assessing medium to large grade varices–those that require treatment with beta-blockers or band ligation.
At this time, the PillCam ESO2 capsule is the only wireless capsule endoscopic modality currently available for esophageal application.39 While similar in design to the second generation Pillcam SB2 (designed for small bowel observation), the battery life is significantly shorter (20 minutes versus 8–12 hours, respectively). The minimally-invasive nature of the modality provides a particularly important aspect of expanding its use, provided the patient is able to swallow the capsule–the most frequent adverse event reported in this cohort of studies. Further barriers to clinical application and expanded use in practice include capsule interpretation. At present, the American Society For Gastrointestinal Endoscopy (ASGE) recommends formal capsule training during fellowship or completion of a formal society–endorsed training course with proctoring of the first 10 capsule interpretations.40 Although required formal training presents a barrier to expansion of this modality, the increased use of capsule endoscopy in the evaluation of both esophageal and small bowel pathology necessitates that gastroenterologists become more adept at interpretation.
Further discussion regarding capsule interpretation requires an understanding of positive and negative results. The Fagan nomogram for capsule demonstrated the clinical utility of this modality. Even in patients with a modest-to-low pre-test probability, wireless capsule endoscopy is an effective modality for ruling out the presence of varices as well as grading medium to large varices. When studies with a high-risk of bias were excluded, a similar clinical utility was demonstrated. It is important to consider, additional factors that may influence the use of EGD over capsule or vice versa including the cost of the modality, availability, as well as the likelihood for therapeutic intervention. Further cost effectiveness analysis should be performed in order to best estimate the true utility of capsule in the diagnosis and grading of esophageal varices in patients with liver disease.
Aside from the small, inherent heterogeneity limitations of meta-analyses that exist between studies, a major limitation of this study is the modality of choice. Only one study, by Sacher-Huvelin and colleagues, used the second generation Pillcam.32 All other studies included used the first generation modality. In this French study, the sensitivity of capsule endoscopy in correctly diagnosing and staging esophageal varices was 76% and 64%, respectively; the specificity of the test was 91% and 93%, demonstrating that although capsule endoscopy is not sufficient at this time for the initial diagnosis of esophageal varices, the high specificity of the test may allow its use in cases where patients are refusing or are unable to undergo EGD.32 The accuracy of capsule for diagnosis and grading was 84% and 81%, respectively. This lower value may indicate the pooled accuracy of this study may be overestimated; thereby requiring more studies with the newer generation before a change in guidelines or surveillance recommendations are made. Additionally, of the included studies, there was a high-risk of bias (8 of the total 17 studies) that may limit generalizability of these results; however, separate analysis excluded these studies was performed and yielded similar results. Overall, based upon the current quality of evidence, more studies are needed to further evaluate the role of wireless capsule endoscopy for patients with portal hypertension.
Despite these limitations, this systematic review and meta-analysis has several strengths. Apart from summarizing all methodologically rigorous data on wireless capsule endoscopy for the diagnosis and grading of esophageal varices in patients with portal hypertension, this study allowed for determination of a pooled sensitivity, specificity, and accuracy to support the use of capsule as an accurate modality in patients with decreased access to endoscopic screening or contraindication to EGD. While our reported results are similar to a previous Cochrane Database Systematic Review by Colli et al, the addition of more recent studies, implementation of the Fagan nomogram, and assessment of clinical utility are paramount to reporting the best evidence on the effectiveness of capsule endoscopy.15 Furthermore, this pooled data coupled with an understanding of clinical utility, may provide a pivotal tool to guide primary care physicians, practicing gastroenterologists, and hepatologists caring for these patients with portal hypertension. At present, there is a paradigm shift in the field of gastroenterology and hepatology with regard to the diagnosis and screening of liver disease (i.e. less invasive testing for liver fibrosis and less reliance on traditional liver biopsy). With this changing landscape to identify liver disease and degree of fibrosis, the assessment of liver-associated sequalae such as esophageal varices from a less invasive means will become pivotal.41
In summary, given the significant morbidity and mortality associated with variceal hemorrhage, alternative screening and diagnostic measures are needed to expand beyond the use of simple endoscopy. While EGD remains the gold standard, and should continue to be standard of care for the diagnosis and management of patients with known varices, this study demonstrated that wireless capsule endoscopy is an effective alternative modality for the diagnosis and grading of esophageal varices and may have a role in cases of refusal or contraindication to EGD. The role for wireless esophageal capsule endoscopy remains limited as this modality lacks any potential therapeutic intervention. Furthermore, based upon the current quality of overall evidence, more studies are needed to evaluate the use of wireless capsule endoscopy for screening and grading of esophageal varices. Nonetheless, this minimally-invasive approach may improve overall compliance with AASLD screening and surveillance recommendations and thus expand the indication to provide prophylactic pharmacologic therapy to patients with portal hypertension.
Supplementary Material
Forest Plot for the Diagnosis of Esophageal Varices By Capsule Endoscopy - High Quality Studies Only
Diagnostic Accuracy of Capsule Endoscopy for the Diagnosis of Esophageal Varices - High Quality Studies Only
Fagan Nomogram for Diagnosis of Esophageal Varices - High Quality Studies Only
Forest Plot for the Grading of Esophageal Varices By Capsule Endoscopy - High Quality Studies Only
Diagnostic Accuracy of Capsule Endoscopy for the Grading of Esophageal Varices - High Quality Studies Only
Fagan Nomogram for Grading of Esophageal Varices – High Quality Studies Only
Acknowledgments
Financial Support: Supported by NIH 5 T32 DK 7356-37 (BN)
Footnotes
Author Contributions – Study concept and design: TRM, YA, BN; Acquisition and analysis of data: BN; Interpretation of data: TRM, YA, BN; Initial draft: TRM; Critical revision of manuscript: YA, BN. All authors approved the final draft submitted.
Potential Conflicts of Interest: The authors have no potential conflicts of interest to report.
References
- 1.Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Seminars in liver disease. 1991;11:31–9. doi: 10.1055/s-2008-1040420. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD Practice Guidelines Committee of American Association for Study of Liver D, Practice Parameters Committee of American College of G. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. The American journal of gastroenterology. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 3.North Italian Endoscopic Club for the S, Treatment of Esophageal V. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. The New England journal of medicine. 1988;319:983–9. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 4.Christensen E, Fauerholdt L, Schlichting P, Juhl E, Poulsen H, Tygstrup N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology. 1981;81:944–52. [PubMed] [Google Scholar]
- 5.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clinics in liver disease. 2001;5:645–63. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 6.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. Journal of hepatology. 2003;38:266–72. doi: 10.1016/s0168-8278(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 7.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. The New England journal of medicine. 2005;353:2254–61. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Bailliere's clinical gastroenterology. 1997;11:243–56. doi: 10.1016/s0950-3528(97)90038-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith JL, Graham DY. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology. 1982;82:968–73. [PubMed] [Google Scholar]
- 10.D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611–24. doi: 10.1053/j.gastro.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. The American journal of gastroenterology. 2003;98:653–9. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. The American journal of gastroenterology. 2000;95:3566–73. doi: 10.1111/j.1572-0241.2000.03376.x. [DOI] [PubMed] [Google Scholar]
- 13.Lapalus MG, Saurin JC. Complications of gastrointestinal endoscopy: gastroscopy and colonoscopy. Gastroenterologie clinique et biologique. 2003;27:909–21. [PubMed] [Google Scholar]
- 14.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 15.Colli A, Gana JC, Turner D, et al. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. The Cochrane database of systematic reviews. 2014;10:CD008760. doi: 10.1002/14651858.CD008760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 17.Fagan TJ. Letter: Nomogram for Bayes theorem. The New England journal of medicine. 1975;293:257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 18.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Statistics in medicine. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC medical research methodology. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoyama T, Oka S, Aikata H, et al. Is small-bowel capsule endoscopy effective for diagnosis of esophagogastric lesions related to portal hypertension? Journal of gastroenterology and hepatology. 2014;29:511–6. doi: 10.1111/jgh.12372. [DOI] [PubMed] [Google Scholar]
- 21.Chavalitdhamrong D, Jensen DM, Singh B, et al. Capsule endoscopy is not as accurate as esophagogastroduodenoscopy in screening cirrhotic patients for varices. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:254–8. e1. doi: 10.1016/j.cgh.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 22.de Franchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595–603. doi: 10.1002/hep.22227. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly S, Campbell N, Forrest EH, et al. Wireless capsule oesophagoscopy (PilCam ESO) compared to upper GI endoscopy in the detection of oesophageal varices. Gut. 2006;55:A58. [Google Scholar]
- 24.Eisen GM, Eliakim R, Zaman A, et al. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy. 2006;38:31–5. doi: 10.1055/s-2005-921189. [DOI] [PubMed] [Google Scholar]
- 25.Gerson L, Kamal A, Ullah N, Ahmed A. Randomized controlled tiral of esophageal capsule endoscopy versus standard endoscopy for screening in patietns pre-liver transplantation. Assessment of inter-observer variability and patient preferences. Gastroenterology. 2008;134(4):A-63. [Google Scholar]
- 26.Groce JR, Raju GS, Sood GK, Snyder N. A prospective single blinded comparative trial of capsule esophagoscopy vs traditional EGD for variceal screening. Gastroenterology. 2007;132:A-802. [Google Scholar]
- 27.Ishiguro H, Saito S, Imazu H, Aihara H, Kato T, Tajiri H. Esophageal Capsule Endoscopy for Screening Esophageal Varices among Japanese Patients with Liver Cirrhosis. Gastroenterology research and practice. 2012;2012:946169. doi: 10.1155/2012/946169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapalus MG, Ben Soussan E, Gaudric M, et al. Esophageal capsule endoscopy vs. EGD for the evaluation of portal hypertension: a French prospective multicenter comparative study. The American journal of gastroenterology. 2009;104:1112–8. doi: 10.1038/ajg.2009.66. [DOI] [PubMed] [Google Scholar]
- 29.Lapalus MG, Dumortier J, Fumex F, et al. Esophageal capsule endoscopy versus esophagogastroduodenoscopy for evaluating portal hypertension: a prospective comparative study of performance and tolerance. Endoscopy. 2006;38:36–41. doi: 10.1055/s-2006-924975. [DOI] [PubMed] [Google Scholar]
- 30.Pena LR, Cox T, Koch AG, Bosch A. Study comparing oesophageal capsule endoscopy versus EGD in the detection of varices. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2008;40:216–23. doi: 10.1016/j.dld.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez FC, Hakim S, Tharalson EM, Shaukat MS, Akins R. Feasibility and safety of string wireless capsule endoscopy in the diagnosis of esophageal varices. The American journal of gastroenterology. 2005;100:1065–71. doi: 10.1111/j.1572-0241.2005.41037.x. [DOI] [PubMed] [Google Scholar]
- 32.Sacher-Huvelin S, Cales P, Bureau C, et al. Screening of esophageal varices by esophageal capsule endoscopy: results of a French multicenter prospective study. Endoscopy. 2015;47:486–92. doi: 10.1055/s-0034-1391393. [DOI] [PubMed] [Google Scholar]
- 33.Schreibman I, Meitz K, Kunselman AR, Downey M, Le T, Riley T. Defining the threshold: new data on the ability of capsule endoscopy to discriminate the size of esophageal varices. Digestive diseases and sciences. 2011;56:220–6. doi: 10.1007/s10620-010-1272-8. [DOI] [PubMed] [Google Scholar]
- 34.Sharma NR, Socoloff DN, Hartlage M, et al. A comparative evaluation of esophageal capsule endoscopy versus esophagogastroduodenoscopy for assessing esophageal varices in a veteran population. Gastroenterology. 2009;136(Suppl I):A825. [Google Scholar]
- 35.Stipho S, Tharalson E, Hakim S, Akins R, Shaukat M, Ramirez FC. String capsule endoscopy for screening and surveillance of esophageal varices in patients with cirrhosis. Journal of interventional gastroenterology. 2012;2:54–60. doi: 10.4161/jig.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frenette CT, Kuldau JG, Hillebrand DJ, et al. Comparison of esophageal capsule endoscopy and esophagogastroduodenoscopy for diagnosis of esophageal varices. World Journal of Gastroenterology. 2008;14(28):4480–5. doi: 10.3748/wjg.14.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frenette CT, Kuldau JG, Hillebrand DJ, Lane J, Pockros PJ. Comparison of esophageal capsule endoscopy and esophagogastroduodenoscopy for diagnosis of esophageal varices. World journal of gastroenterology. 2008;14:4480–5. doi: 10.3748/wjg.14.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Amico G, De Franchis R Cooperative Study G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 39.Committee AT, Wang A, Banerjee S, et al. Wireless capsule endoscopy. Gastrointestinal endoscopy. 2013;78:805–15. doi: 10.1016/j.gie.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Faigel DO, Baron TH, Adler DG, et al. ASGE guideline: guidelines for credentialing and granting privileges for capsule endoscopy. Gastrointestinal endoscopy. 2005;61:503–5. doi: 10.1016/s0016-5107(04)02781-6. [DOI] [PubMed] [Google Scholar]
- 41.Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970–9. 9 e1–3. doi: 10.1053/j.gastro.2011.02.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest Plot for the Diagnosis of Esophageal Varices By Capsule Endoscopy - High Quality Studies Only
Diagnostic Accuracy of Capsule Endoscopy for the Diagnosis of Esophageal Varices - High Quality Studies Only
Fagan Nomogram for Diagnosis of Esophageal Varices - High Quality Studies Only
Forest Plot for the Grading of Esophageal Varices By Capsule Endoscopy - High Quality Studies Only
Diagnostic Accuracy of Capsule Endoscopy for the Grading of Esophageal Varices - High Quality Studies Only
Fagan Nomogram for Grading of Esophageal Varices – High Quality Studies Only