Abstract
The pharmacokinetics of ertapenem and ceftriaxone were investigated in an open, randomized, two-period crossover study after single- and multiple-dose administration in 10 healthy volunteers (five women and five men). Both antibiotics were administered intravenously once daily for 7 days at dosages of 1 g (ertapenem) and 2 g (ceftriaxone). The concentrations of the antibiotics in serum and urine were quantified by the agar well diffusion method bioassay and, in addition, for ertapenem only, by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). For ertapenem the maximum concentration of the drug in plasma (Cmax) was 256 mg/liter, the half-life was 20.7 h, and the area under the plasma concentration-time curve (AUC) was 830 mg · h/liter. The concentrations in fecal samples were (mean value) 37.2 and 32.7 mg/kg on day 4 and day 8, respectively. Ceftriaxone exhibited a mean Cmax of 315 mg/liter, a half-life of 7.6 h, and an AUC of 1,556 mg · h/liter. The mean concentrations in fecal samples were 153 and 258 mg/kg on day 4 and day 8, respectively. No accumulation of ertapenem or ceftriaxone was detected at steady state. A slightly but significantly decreased AUC for ertapenem was detected for the female volunteers. No serious adverse event was observed. Both antibiotics induced a marked decrease in the anaerobic microflora (4-log-unit decreases in lactobacilli, bifidobacteria, clostridia, and bacteroides) and Escherichia coli, whereas the number of enterococci increased (4 log units). A slight overgrowth of yeasts was observed with both regimens. In all cases the microflora returned to normal levels on days 21 to 35.
Ertapenem, recently described as the first class 1 carbapenem (11), is a parenteral broad-spectrum beta-1-methyl-carbapenem with a long half-life in serum. It has been shown to be effective for the treatment of community-acquired pneumonia (9); intra-abdominal infections, skin and skin structure infections, and acute pelvic infections (14); and urinary tract infections (15). In contrast to imipenem and meropenem, ertapenem lacks sufficient activity against Pseudomonas aeruginosa, enterococci, and Acinetobacter spp.; but clinical trials have shown that Pseudomonas infections can be treated with ertapenem.
Ertapenem can be administered once daily due to its long half-life in plasma. The long half-life in plasma reflects its high level of plasma protein binding.
Ceftriaxone matches ertapenem in both its pharmacokinetics and its antibacterial spectrum for the treatment of community-acquired pneumonia and has been used as a comparator drug for ertapenem in clinical trials (9, 17). Ceftriaxone is a broad-spectrum parenteral cephalosporin with a long half-life that also requires administration only once daily.
The application of antibacterial agents for the treatment of infections may have a number of potentially adverse effects on the normal oropharyngeal and intestinal microflora. The normal microflora acts as a barrier against colonization by potentially pathogenic microorganisms and against overgrowth of microorganisms which are already present. This is termed colonization resistance (16). Disturbance of the ecological balance between the host and the host's microflora caused by antibiotics can result in the development of antibiotic resistance among the bacteria in the normal microflora and the overgrowth of indigenous microorganisms, such as yeasts, which may produce systemic infections in immunocompromised patients, and Clostridium difficile, which may cause diarrhea and severe toxic colitis. These adverse effects are particularly common among broad-spectrum antibiotics. In addition, antibiotics with long half-lives in serum are believed to induce resistance more frequently since their pharmacokinetic profiles cause them to exhibit long-term subinhibitory concentrations at the end of therapy. Since ertapenem and ceftriaxone are both broad-spectrum antibiotics with long biological half-lives, there might be a potential for the development of antibacterial resistance.
The aim of this study was to investigate the pharmacokinetic profile of ertapenem, for which only limited data are available, and to investigate its impact on the intestinal flora in comparison to those of ceftriaxone.
MATERIALS AND METHODS
Volunteers.
Ten healthy Caucasian volunteers (five men and five women; age, 35 ± 5 years; weight, 70 ± 13 kg; height, 173 ± 9 cm [the values are means ± standard deviations]) participated in the study. The medical histories of the volunteers were taken; and physical examinations, electrocardiograms, and laboratory tests for hematological and biochemical parameters, including a screening for the use of illicit drugs as well as urinalysis, were performed before enrollment, during every study period, and afterwards. Only volunteers with normal values met the inclusion criteria. Further inclusion criteria were as follows: no blood donation in the 4 weeks prior to the study, negative serological test results for human immunodeficiency virus and hepatitis B and C viruses, and no allergy or intolerance to any of the investigational drugs. The use of any additional medication except oral contraceptives within the 2 weeks prior to study entry as well as during the study was not permitted. No yogurt products with live organisms or probiotics were allowed. Antimicrobial drugs were not allowed from 4 weeks prior to study entry. The protocol was approved by the local ethics committee, according to German law. Informed written consent was obtained from all subjects prior to enrollment.
Study design.
The study had an open, randomized, two-period crossover design and was divided into two periods of 35 days. The treatment regimens were (i) 1 g of ertapenem and (ii) 2 g of ceftriaxone. Both drugs were administered intravenously over a period of 30 min once daily for 7 days.
In a randomized order, each volunteer received first one treatment regimen and then the crossover regimen. The washout period between the administrations of the two antibiotic regimens was 4 weeks. Strenuous physical activity, smoking, and the intake of alcohol and stimulating beverages containing xanthine derivatives (tea, coffee, and soft drinks containing caffeine) were prohibited from 24 h before to 48 h after drug administration to avoid analytical interference.
Sampling.
Fecal flora was evaluated on days −2 (baseline), −1 (baseline), 4, 8, 14, 21, and 35, with a washout period of 4 weeks between each treatment period.
Serum (ceftriaxone) and plasma (ertapenem) concentration-versus-time data were generated by collecting blood samples (approximately 6 ml each) before infusion (−0.5 h), at the end of infusion (0 h), and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the end of infusion for each study drug on day 1 and day 7.
All urine samples were collected during the period of blood sampling (0 to 6, 6 to 12, and 12 to 24 h). The total volume was noted at the end of each period, and an aliquot of well-mixed urine was analyzed for determination of the drug concentration.
Determination of ertapenem and ceftriaxone concentrations in blood and urine.
The concentrations of ceftriaxone in serum and urine were quantified by an agar well diffusion method (bioassay). The concentrations of ertapenem in plasma and urine were determined by bioassay and, in addition, by liquid chromatography (LC) coupled with tandem mass spectrometry (LC-MS/MS).
Plasma and serum concentration-versus-time curves were calculated for the first and last days of dosing for each study drug. The level of drug excretion in urine was measured simultaneously with the plasma and serum drug concentrations.
LC-MS/MS.
Plasma samples were analyzed by LC-MS/MS following protein precipitation with acetonitrile. Urine samples were analyzed following dilution. The calibration curve of the plasma assay was linear over a concentration range of 1 mg/liter (lower limit of quantification) to 500 mg/liter (correlation coefficient, 0.9702). The urine samples were prepared for analysis by dilution. The calibration curve of the urine assay was linear over a concentration range of 16 mg/liter (lower limit of quantification) to 1,020 mg/liter (correlation coefficient, 0.9702). An intra-assay coefficient of variation could not be established since the instability of the thawed samples did not allow the measurements to be redetermined with identical samples. The lower limits of detection of the assays for ertapenem in plasma and urine were 0.2 and 1 mg/liter, respectively. LC was performed as described by Musson et al. (8).
Bioassay.
The bioassay was based on an agar plate diffusion technique previously described in detail by Andrews (2). Plasma (ertapenem) and serum (ceftriaxone) samples were assayed against standards prepared in activity-free pooled human serum. Four serum or four urine samples, one control sample, and five standard samples were tested in triplicate on each agar plate. After prediffusion for 30 min at room temperature, the agar plates were incubated for 18 h at 30°C. The baseline sample from each patient, collected before the administration of ertapenem, was tested for antibacterial activity. None of them revealed an inhibition zone. The concentrations of ertapenem and ceftriaxone were determined in relation to the diameters of the inhibition zones caused by the known concentrations from the standard series. The test strains were Escherichia coli (ATCC 25922) for ceftriaxone and Bacillus subtilis (ATCC 6633) for ertapenem. The detection limits in both urine and plasma or serum were 0.12 mg/liter for ertapenem and 0.15 mg/liter for ceftriaxone. The coefficients of variation for the quantification of ertapenem, determined on three different days, were 4.41% for plasma and 4.06% for urine. The coefficients of variation for the quantification of ceftriaxone were 5.07% for serum and 6.39% for urine.
Determination of ertapenem and ceftriaxone concentrations in fecal samples.
The concentrations of ertapenem and ceftriaxone in fecal samples were also determined by bioassay. The test medium was antibiotic medium 1 (Difco), and the indicator strain was E. coli (ATCC 25922). Samples were run in duplicate, and a concomitant standard series was inoculated on each agar plate. The plates were incubated for 18 h at 37°C. The concentrations of ertapenem and ceftriaxone were determined in relation to the diameters of the inhibition zones caused by the known concentrations from the standard series. The detection limit was 0.5 mg/kg. The coefficient of variation was less than 5%.
Processing of fecal samples for microbiological analysis.
The stool samples were suspended in prereduced peptone-yeast extract medium, diluted to 10−7, and inoculated on nonselective and selective media, as described previously (4a). The following agar media were used: brucella blood agar (Kemila; LabM, Bury, United Kingdom) for total aerobes and anaerobes, CLED agar (Merck, Darmstadt, Germany) for detection of members of the family Enterobacteriaceae, Enterococcosel agar (BBL, Cockeysville, Md.) for detection of enterococci, Sabouraud agar (Difco, Detroit, Mich.) for detection of yeasts, Rogosa agar (Difco) for cultivation of lactobacilli, BL agar (Difco) for cultivation of bifidobacteria, kanamycin-vancomycin-blood agar for cultivation of Bacteroides and Prevotella species, neomycin-vancomycin-blood agar for cultivation of fusobacteria, veillonella agar (Difco) for cultivation of Veillonella cocci, egg yolk agar (Oxoid, Basingstoke, United Kingdom) for cultivation of clostridia, and taurocholate-cycloserine-cefoxitin-fructose agar (peptone from casein and proteose peptone no. 3 [40 mg/ml], sodium hydrogen phosphate [5 mg/ml], potassium-dihydrogen phosphate [1 mg/ml], sodium chloride [2 mg/ml], sodium sulfate [0.2 mg/ml], Bacto Agar/Agar-Agar [20 mg/ml], taurocholic acid [1 mg/ml], neutral red [0.03 mg/ml], 15% fructose, C. difficile supplement d-cycloserine, and cefoxitin) for detection of C. difficile. The aerobic agar plates were incubated for 24 h at 37°C, and the anaerobic agar plates were incubated for 48 h at 37°C in GasPak anaerobic jars (BBL). The anaerobic agar plates were reexamined after 5 to 7 days. After incubation, the different colony types were counted, isolated in pure culture, and identified to the genus level. All isolates were analyzed by the Gram stain reaction and determination of cell and colony morphologies, followed by different biochemical tests. An API 20E test kit (BioMérieux, Marcy l'Etoile, France) was used for the identification of the members of the family Enterobacteriaceae. The anaerobic microorganisms were identified by gas-liquid chromatography of metabolites from glucose. The lower limit of detection was 102 microorganisms/g of feces.
Examination of intestinal microflora.
For each volunteer, the posttreatment results of quantitative fecal flora and clinical evaluations were compared with the baseline values for each study drug. Adverse events related to the gastrointestinal tract, i.e., changes in fecal flora and drug levels in feces, in addition to clinical laboratory data, were evaluated.
Statistics. (i) Pharmacokinetics.
Single-dose and steady-state pharmacokinetics were determined by standard noncompartmental methods with WinNonlin Professional software (version 4.0.1, 1998-2002; Pharsight, Palo Alto, Calif.). For the maximum concentration in plasma or serum (Cmax), the time to reach Cmax (Tmax), and the minimum concentration at steady state (Cmin), the raw data were used without further calculations. The terminal half-life (t1/2) was estimated by linear regression of the concentration-time curve on a semilogarithmic scale by ln(2)/Lz, where Lz is the slope of the terminal concentration-time curve on a semilogarithmic scale.
The area under the plasma or serum concentration-time curve (AUC) from the start of infusion to the time of the last quantifiable concentration in plasma or serum (AUC0-last) was calculated by using the linear trapezoidal rule. The AUC from time zero to infinity (AUC0-inf) was derived by extrapolating the area to infinity by adding the last quantifiable concentration in plasma or serum/Lz to AUC0-last. The AUC from the start of the infusion to 24 h postdosing (AUC0-24) was calculated by linear interpolation between 12.5 and 24.5 h after the start of infusion.
Total clearance (CLtot) was determined as dose/AUC0-inf for the single-dose data and dose/AUC0-24 for steady-state data. The mean residence time (MRT), the volume of distribution during the terminal phase (Vz), and the volume of distribution at steady-state (VSS) were estimated by the use of standard noncompartmental formulas, as implemented in WinNonlin Professional.
The urine drug concentration and urine volume data were used to calculate urinary excretion, where fu describes the fraction of the dose cumulatively excreted into urine. Renal clearance (CLR) was determined as the ratio of the amount of drug cumulatively excreted in urine up to the time that the concentration was last quantifiable in urine and the respective AUC up to that time point. Nonrenal clearance (CLNR) was derived as the difference between CLtot and CLR.
(ii) Estimation of differences by sex.
All pharmacokinetic parameters (except Tmax) were tested for possible differences by sex by using WinNonlin Professional. For each drug and each pharmacokinetic parameter, average bioequivalence statistics were calculated according to a parallel group design for the comparison of data for both sexes. Pharmacokinetic parameters were log transformed, and due to the absence of differences between single-dose (day 1) and steady-state (day 7) pharmacokinetic parameters, data for both dosing states were analyzed jointly. With sex as the factor to be tested, the point estimates and 90% confidence intervals for the ratio of the value for female volunteers/value for male volunteers for each pharmacokinetic parameter were estimated. The P values determined by analysis of variance provided an additional test of significance.
(iii) Correlation of analytical methods.
The concentrations of ertapenem in plasma and urine were quantified by LC-MS/MS and bioassay. Statistical measures were applied to compare the concentrations derived by both analytical methods. For this, weighted linear regressions with the concentration determined by bioassay as the dependent variable and the concentration determined by LC-MS/MS as the independent variable were calculated for the data for plasma and urine. The weight of 1/the concentration determined by LC-MS/MS was applied to account for the large concentration range so that the influences of higher concentrations were not overemphasized.
It should additionally be estimated whether the LC-MS/MS and bioassay data were influenced by dosing state (single dose versus steady state, equivalent to the data for day 1 versus day 7, respectively) and the effects of individual subjects. In order to test for these possible effects, in addition to weighted linear regression, analysis of covariance statistics were applied to account for the categorical factors subject and dosing state.
Statistical tests for alterations of intestinal microflora.
Quantitative alterations in the cultivable bacteria were statistically compared within groups between pretreatment and at the end of treatment as well as between pretreatment and 4 weeks after treatment by the Wilcoxon signed rank test by the use of SPSS (version 11.0) software.
RESULTS
Pharmacokinetics.
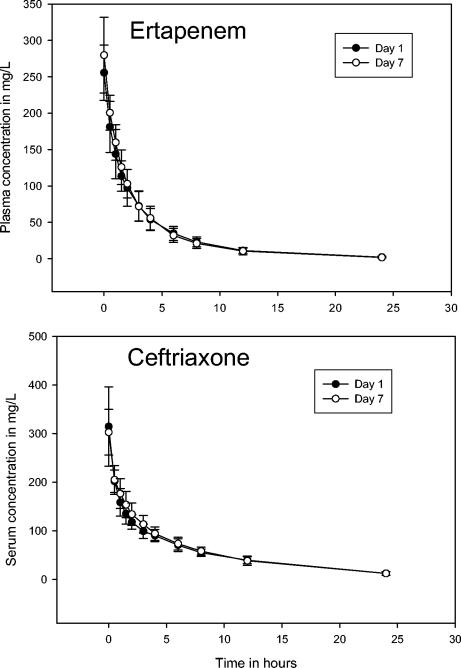
The plasma and serum concentration-time curves are displayed in Fig. 1. Table 1) provides the pharmacokinetic parameters for ertapenem (LC-MS/MS data) and ceftriaxone (bioassay data). Single-dose and steady-state data were comparable for each drug. Thus, the data reveal that neither of the drugs accumulates at steady state.
FIG. 1.
Arithmetic means (± standard deviations) of measured concentrations in plasma after the administration of single (day 1) and multiple (day 7) intravenous doses of 1 g of ertapenem (top) and 2 g of ceftriaxone (bottom).
TABLE 1.
Single-dose (day 1) and steady-state (day 7) pharmacokinetic parameters for ertapenem (LC-MS/MS data) and ceftriaxone (bioassay data) for both sexes
| Drug and day | Cmax (mg/liter) | AUC doseb (mg · h/liter) | Tmax (h) | CLtot (ml/min) | CLR (ml/min) | CLNR (ml/min) | t1/2 (h) | MRT (h) | Vss (liters) | Vz (liters) | fU (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ertapenem | |||||||||||
| Day 1 | 253 (15) | 817 (20) | 0.5 (0) | 20.4 (18) | 9.38 (37) | 9.63 (38) | 4.5 (23) | 4.7 (13) | 5.7 (18) | 8.0 (25) | 45.1 (36) |
| Day 7 | 275 (19) | 823 (19) | 0.5 (0) | 20.2 (16) | 8.62 (46) | 11.0 (33) | 4.3 (11) | 4.1 (18) | 5.0 (18) | 7.5 (20) | 41.2 (42) |
| Ceftriaxone | |||||||||||
| Day 1 | 306 (26) | 1,557 (15) | 0.5 (0) | 21.4 (18) | 8.63 (9) | 12.5 (31) | 7.5 (17) | 9.1 (16) | 11.7 (16) | 13.9 (15) | 36.7 (18) |
| Day 7 | 300 (16) | 1,463 (15) | 0.5 (0) | 22.8 (18) | 9.60 (11) | 13.0 (31) | 3.1 (12) | 8.5 (14) | 11.6 (16) | 14.0 (15) | 42.3 (15) |
Data are reported as geometric mean (coefficient of variation [in percent]).
AUC0-inf for single-dose data (day 1) and AUC0-24 for steady-state data (day 7).
The influence of sex on the pharmacokinetic parameters is shown in Table 2. The AUC for ertapenem differed significantly (P < 0.004) between the female and the male volunteers (in the single-dose study, 711 ± 45 μg · h/ml for the female volunteers and 950 ± 151 μg · h/ml for the male volunteers; in the steady-state study, 770 ± 88 μg · h/ml for the female volunteers and 901 ± 192 μg · h/ml for the male volunteers), while there were no significant differences in the AUCs for ceftriaxone. The CLtot for ertapenem was approximately 24% higher for the female volunteers. This was primarily based on a 51% higher CLR for the female volunteers.
TABLE 2.
Differences in the pharmacokinetic parameters of ertapenem by sex
| Parameter | Ratio of point estimates for male volunteers/female volunteers (90% CIa) | P value for differences by sexb |
|---|---|---|
| Cmax (mg/liter) | 97 (84-111) | 0.661 |
| AUC dose (mg · h/l) | 81 (72-90) | 0.004 |
| CLtot (ml/min) | 124 (11-138) | 0.004 |
| CLR (ml/min) | 151 (112-205) | 0.030 |
| CLNR (ml/min) | 108 (73-160) | 0.736 |
| t1/2 | 88 (78-100) | 0.095 |
| MRT (h) | 82 (74-91) | 0.003 |
| Vss (liter) | 101 (87-118) | 0.884 |
| Vz (liter) | 109 (89-113) | 0.461 |
| Cmin (mg/liter) | 54 (37-79) | 0.017 |
| fu (%) | 126 (93-170) | 0.195 |
CI, confidence interval.
Boldface indicates a statistically significant difference.
The concentrations in urine are given in Table 3. The levels of recovery (fu) of ertapenem in urine, as determined by bioassay, were 77.7% in the single-dose study and 70.2% in the steady-state study.
TABLE 3.
Urine ertapenem and ceftriaxone concentrations after administration of a single dose (day 1) and multiple doses (day 7)
| Drug (day) | Concn (mg/liter)
|
||
|---|---|---|---|
| 0-6 h | 6-12 h | 12-24 h | |
| Ceftriaxone (day 1)a | 941 ± 612 | 152 ± 53 | 154 ± 66 |
| Ceftriaxone (day 7)a | 1,135 ± 611 | 177 ± 61 | 142 ± 41 |
| Ertapenem (day 1)a | 1,099 ± 600 | 195 ± 105 | 61 ± 29 |
| Ertapenem (day 7)a | 1,253 ± 115 | 143 ± 53 | 54 ± 15 |
| Ertapenem (day 1)b | 768 ± 600 | 102 ± 66 | 29 ± 12 |
| Ertapenem (day 7)b | 634 ± 210 | 97 ± 35 | 36 ± 13 |
As determined by bioassay.
As determined by LC-MS/MS.
The mean concentrations of ertapenem and ceftriaxone in feces are shown in Table 4. No antimicrobial activity was detected in fecal samples collected on days −2, −1, 14, 21, and 35.
TABLE 4.
Concentrations of ertapenem and ceftriaxone in feces on days 4 and 8 in 10 subjects
| Drug | Concn (mg/kg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 4
|
Day 8
|
|||||||
| Mean | SD | Median | Range | Mean | SD | Median | Range | |
| Ertapenema | 37.2 | 110 | 0.3 | 0-330 | 32.7 | 97.3 | 0 | 0-292 |
| Ceftriaxonea | 152 | 253 | 2.4 | 0-657 | 258 | 296 | 161 | 0-806 |
As determined by bioassay.
Analytical method correlation.
Since pretesting revealed that there was no significant dosing state (single dose versus steady state) or subject effect, a simple weighted linear regression model was applied. For the data for plasma, the slope of 1.09 ± 0.0141 reveals that the concentrations determined by bioassay are, on average, approximately 9% higher than those determined by LC-MS/MS, which should be acceptable on the basis of the overall variability of the data and the analytical techniques. The slope of the data for urine was 1.49 ± 0.135, which indicates that the concentrations in urine determined by bioassay are roughly 50% higher than those determined by LC-MS/MS.
The small and not significantly different intercepts for the data for plasma and urine indicate that there was no bias in the correlation of both methods.
The corresponding coefficients of correlation (r) showed a very good correlation between both assays for the data for plasma (r = 0.982) and a moderate correlation for the data for urine (r = 0.825).
In summary, these data show that the plasma ertapenem concentrations determined by bioassay are well comparable to those determined by LC-MS/MS.
Impact of ertapenem on intestinal microflora.
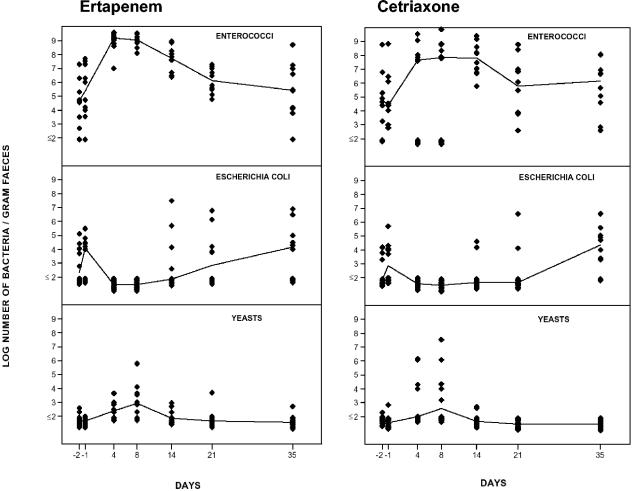
The number of enterococci increased significantly (P < 0.05) during the administration of ertapenem, while the number of E. coli organisms decreased (Fig. 2).
FIG. 2.
Effects of ertapenem (left) and ceftriaxone (right) on the intestinal aerobic microflora in 10 volunteers. The lines indicate the median values of the logarithmic number of microorganisms per gram of feces.
There was an overgrowth of low levels of yeasts on day 8 compared with the pretreatment levels.
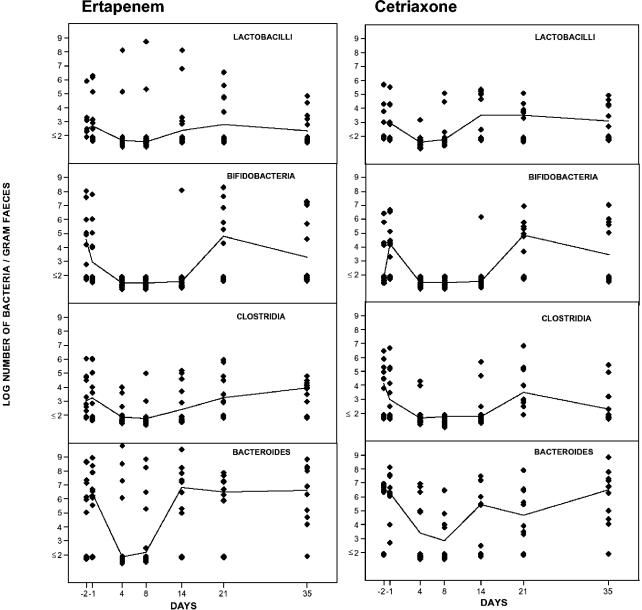
The aerobic microflora was normalized on day 35. The total number of anaerobic bacteria decreased during ertapenem administration (Fig. 3). The numbers of bifidobacteria and Bacteroides fragilis group strains were significantly reduced (P < 0.05), while there were minor alterations in the number of lactobacilli and clostridia. On day 35 the anaerobic microflora had returned to normal levels.
FIG. 3.
Effects of ertapenem (left) and ceftriaxone (right) on the intestinal anaerobic microflora in 10 volunteers. The lines indicate the median values of the logarithmic number of microorganisms per gram of feces.
Impact of ceftriaxone on intestinal microflora.
Figure 2 shows the impact of ceftriaxone on the aerobic microflora. The number of enterococci increased markedly during the administration of ceftriaxone, while the number of E. coli organisms decreased significantly (P < 0.05). No significant overgrowth of yeasts was noticed on day 8. The aerobic microflora had returned to pretreatment levels on day 35.
Ceftriaxone caused significant decreases in the total number of anaerobic bacteria (P < 0.05) (Fig. 3). Lactobacilli, bifidobacteria, clostridia, and B. fragilis group strains were markedly reduced in number during ceftriaxone administration. On day 35 the microflora was normalized.
Safety.
No severe adverse events were recorded. Diarrhea and loose stools occurred at the same frequency during both study periods (n = 9 volunteers). Headache was more frequently reported after the administration of ceftriaxone (n = 7; for ertapenem, n = 4), and a metallic taste occurred more often after ertapenem administration (n = 4; for ceftriaxone, n = 1). During both study periods only one subject each complained about local irritation at the site of injection.
DISCUSSION
Ertapenem is a novel broad-spectrum 1-beta-methyl carbapenem with a long t1/2 attributable to its high level of protein binding. According to the manufacturer, the drug binds to albumin, which is dependent on ertapenem's negative charge (6). In contrast, on the basis of in vitro studies investigating the decrease in the ertapenem MIC after the addition of either human serum or albumin, Kiem and Craig (S. Kiem and W. A. Craig, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 493, 2002) suggested that ertapenem may be bound to a component other than albumin. The calculation of the pharmacokinetic parameters for ertapenem on the basis of the data from our study revealed higher values than those published by Majumdar et al. (6). However, all data in that study were obtained by high-pressure liquid chromatography, whereas in our study the data for ertapenem were confirmed by two independent methods, LC-MS/MS and the agar well diffusion test; and the correlation of the results of both methods was good, with both methods revealing similar plasma ertapenem concentrations. A possible explanation for the higher values in our study is that the mean body weight of our subjects was only 70 ± 13 kg. Majumdar et al. (6) did not provide such details about their subjects. Our results also revealed slight differences in ertapenem pharmacokinetics by sex, with a decreased ertapenem AUC for the female volunteers, probably due to an increased CLR. These differences by sex are not considered of clinical importance and have also been observed with other drugs (13).
The main pharmacokinetic-pharmacodynamic parameter of carbapenems, which are antibiotics with time-dependent killing activities, is the proportion of the time during the dosing interval that the concentration in plasma exceeds the MIC (T > MIC) (3). For carbapenems it has previously been suggested that a T > MIC of 30 to 40% of the dosing interval is sufficient (7) because of their rapid bactericidal activities, whereas for cephalosporins maximal bactericidal activity is seen when levels are above the MIC for 60 to 70% of the dosing interval (as reviewed by Andes and Craig [1]). Both ertapenem and ceftriaxone show high levels of protein binding. Some researchers believe that only the unbound antibiotic is active. However, other investigators suggest that the plasma-bound fraction can be seen as a reservoir, since there is an equilibration between the bound and the unbound fractions (10). By taking into account the fact that a T > MIC of 30 to 40% is required for ertapenem and that a T > MIC of 60 to 70% is required for ceftriaxone and the fact that both antibiotics exhibit free fractions between 5 and 10% in the therapeutic concentration range (6, 10), the directly measured concentration of ertapenem at 12 h (11.8 mg/liter) can be compared with the concentration of ceftriaxone at 24 h (10.3 mg/liter). The interpretation of these data in the context of the MICs of ceftriaxone and ertapenem at which 90% of isolates are inhibited, obtained in a large multicenter surveillance study (5), revealed that both antibiotics have comparable pharmacokinetic-pharmacodynamic parameters for Moraxella spp., pneumococci, and Haemophilus influenzae, the most frequent pathogens causing community-acquired pneumonia. The pharmacokinetic-pharmacodynamic parameters for ertapenem were particularly favorable for activity against Staphylococcus aureus, a more frequent cause of pneumonia in elderly people and patients with comorbidities. This might be one reason for the pronounced clinical efficacy of ertapenem observed in that particular group of patients (17).
Ertapenem has been shown to be clinically successful for the treatment of urinary tract infections (15). In comparison to ceftriaxone, the urine ertapenem concentration was slightly higher immediately after intravenous administration and decreased faster, reflecting the shorter biological half-life and the primarily renal excretion of ertapenem. There was a pronounced discrepancy between the urine ertapenem concentrations determined by bioassay and those determined by LC-MS/MS. Majumdar at al. (6) reported a rate of recovery of the unchanged drug in urine of 44%. This closely matches the level of recovery in urine obtained by LC-MS/MS, which did not detect metabolites. Determination by bioassay revealed 77% recovery in urine, which is similar to the 80% obtained by liquid scintillation spectrometry after application of radioactively labeled ertapenem (B. Wong, D. Musson, and K. Birk, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 938, 2001). This is somewhat surprising, since the main ertapenem metabolite is excreted as a ring-opened metabolite (4) and is supposed to be inactive, according to the manufacturer. Therefore, it should not be detected by bioassay. In contrast, the plasma ertapenem concentrations measured by both methods showed good agreement. Thus, this discrepancy can be explained by the fact that ertapenem metabolites might have had some activities against the test strain used for the bioassay.
In the same study, Wong et al. (Wong et al., 41st ICAAC) recovered only 10% of the labeled ertapenem in feces. Carbapenems are usually eliminated in feces at a very low level, with only a moderate impact on the intestinal flora (as reviewed by Sullivan et al. [12]). However, we found considerable concentrations of ertapenem in feces, which was reflected by marked decreases in the numbers of E. coli, Bacteroides spp., and bifidobacteria organisms and a corresponding overgrowth of yeasts and enterococci. In contrast to other carbapenems, there was no difference between ceftriaxone and ertapenem with regard to the impact on the intestinal microflora. This was also reflected by the same number of cases of diarrhea as a side effect in both arms of the study.
In conclusion, by taking into consideration the different pharmocodynamics of the carbapenems and the cephalosporins, the pharmacokinetic-pharmacodynamic parameters of ertapenem seem to be comparable to those of ceftriaxone, which might be reflected in part by the similar clinical efficacies of the two drugs demonstrated in clinical trials. However, the effects of the two drugs on the intestinal microflora were also comparable, which was not expected in the case of ertapenem, since earlier studies with imipenem and meropenem demonstrated that carbapenems have only very moderate effects on the intestinal microflora.
Acknowledgments
The study was supported by a grant from Merck Sharpe & Dohme, Germany. Mathias W. R. Pletz was supported by the German Research Foundation.
The technical assistance of M. Rau and G. Schreiber is gratefully acknowledged. We thank Fritz Sörgel, Patrick J. Halvey, and Lesley McGee for critical reading of the manuscript.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. Pharmacokinetics and pharmacodynamics of outpatient intravenous antimicrobial therapy. Infect. Dis. Clin. N. Am. 12:849-860, vi. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, J. M. 1999. Microbiological assays, p. 35-44. In B. S. Reeves, R. Wise, J. M. Andrews, and L. O. White (ed.), Clinical antimicrobial assays. Oxford University Press, New York, N.Y.
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Curran, M., D. Simpson, and C. Perry. 2003. Ertapenem: a review of its use in the management of bacterial infections. Drugs 63:1855-1878. [DOI] [PubMed] [Google Scholar]
- 4a.Jousime-Somers, H. R., P. Summanen, D. M. Ditron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual, 6th ed. Star Publishing Co., Belmont, Calif.
- 5.Livermore, D. M., M. W. Carter, S. Bagel, B. Wiedemann, F. Baquero, E. Loza, H. P. Endtz, N. van Den Braak, C. J. Fernandes, L. Fernandes, N. Frimodt-Moller, L. S. Rasmussen, H. Giamarellou, E. Giamarellos-Bourboulis, V. Jarlier, J. Nguyen, C. E. Nord, M. J. Struelens, C. Nonhoff, J. Turnidge, J. Bell, R. Zbinden, S. Pfister, L. Mixson, and D. L. Shungu. 2001. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob. Agents Chemother. 45:1860-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumdar, A. K., D. G. Musson, K. L. Birk, C. J. Kitchen, S. Holland, J. McCrea, G. Mistry, M. Hesney, L. Xi, S. X. Li, R. Haesen, R. A. Blum, R. L. Lins, H. Greenberg, S. Waldman, P. Deutsch, and J. D. Rogers. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouton, J. W., D. J. Touzw, A. M. Horrevorts, and A. A. Vinks. 2000. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin. Pharmacokinet. 39:185-201. [DOI] [PubMed] [Google Scholar]
- 8.Musson, D. G., C. J. Kitchen, J. Y. Hsieh, and K. L. Birk. 2002. Modified high-performance liquid chromatographic method for the determination of ertapenem in human urine: enhanced selectivity and automation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 779:341-346. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz-Ruiz, G., J. Caballero-Lopez, I. R. Friedland, G. L. Woods, and A. Carides. 2002. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin. Infect. Dis. 34:1076-1083. [DOI] [PubMed] [Google Scholar]
- 10.Perry, T. R., and J. J. Schentag. 2001. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin. Pharmacokinet. 40:685-694. [DOI] [PubMed] [Google Scholar]
- 11.Shah, P. M., and R. D. Isaacs. 2003. Ertapenem, the first of a new group of carbapenems. J. Antimicrob. Chemother. 52:538-542. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 13.Swan, S. K., and M. J. Hursting. 2000. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 20:318-329. [DOI] [PubMed] [Google Scholar]
- 14.Tellado, J., G. L. Woods, R. Gesser, K. McCarroll, and H. Teppler. 2002. Ertapenem versus piperacillin-tazobactam for treatment of mixed anaerobic complicated intra-abdominal, complicated skin and skin structure, and acute pelvic infections. Surg. Infect 3:303-314. [DOI] [PubMed] [Google Scholar]
- 15.Tomera, K. M., E. A. Burdmann, O. G. Reyna, Q. Jiang, W. M. Wimmer, G. L. Woods, and R. M. Gesser. 2002. Ertapenem versus ceftriaxone followed by appropriate oral therapy for treatment of complicated urinary tract infections in adults: results of a prospective, randomized, double-blind multicenter study. Antimicrob. Agents Chemother. 46:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Waaij, D., and C. E. Nord. 2000. Development and persistence of multi-resistance to antibiotics in bacteria; an analysis and a new approach to this urgent problem. Int. J. Antimicrob. Agents 16:191-197. [DOI] [PubMed] [Google Scholar]
- 17.Vetter, N., E. Cambronero-Hernandez, J. Rohlf, S. Simon, A. Carides, T. Oliveria, and R. Isaacs. 2002. A prospective, randomized, double-blind multicenter comparison of parenteral ertapenem and ceftriaxone for the treatment of hospitalized adults with community-acquired pneumonia. Clin. Ther. 24:1770-1785. [DOI] [PubMed] [Google Scholar]